Colloidal Self-Assembly of Inorganic Nanocrystals into Superlattice Thin-Films and Multiscale Nanostructures
Abstract
:1. Introduction
2. Self-Assembly of NCs
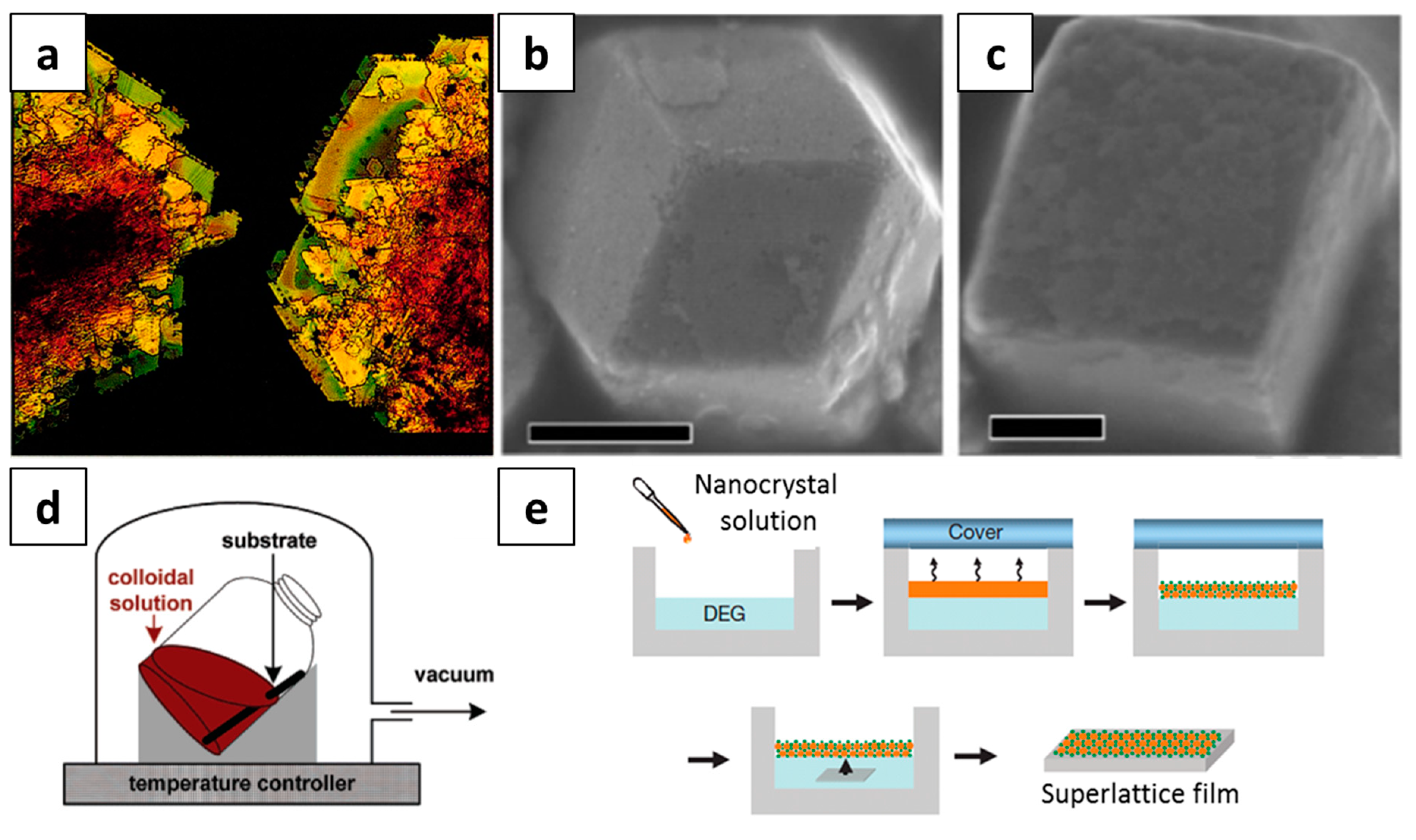
2.1. Methods for the Self-Assembly of NCs
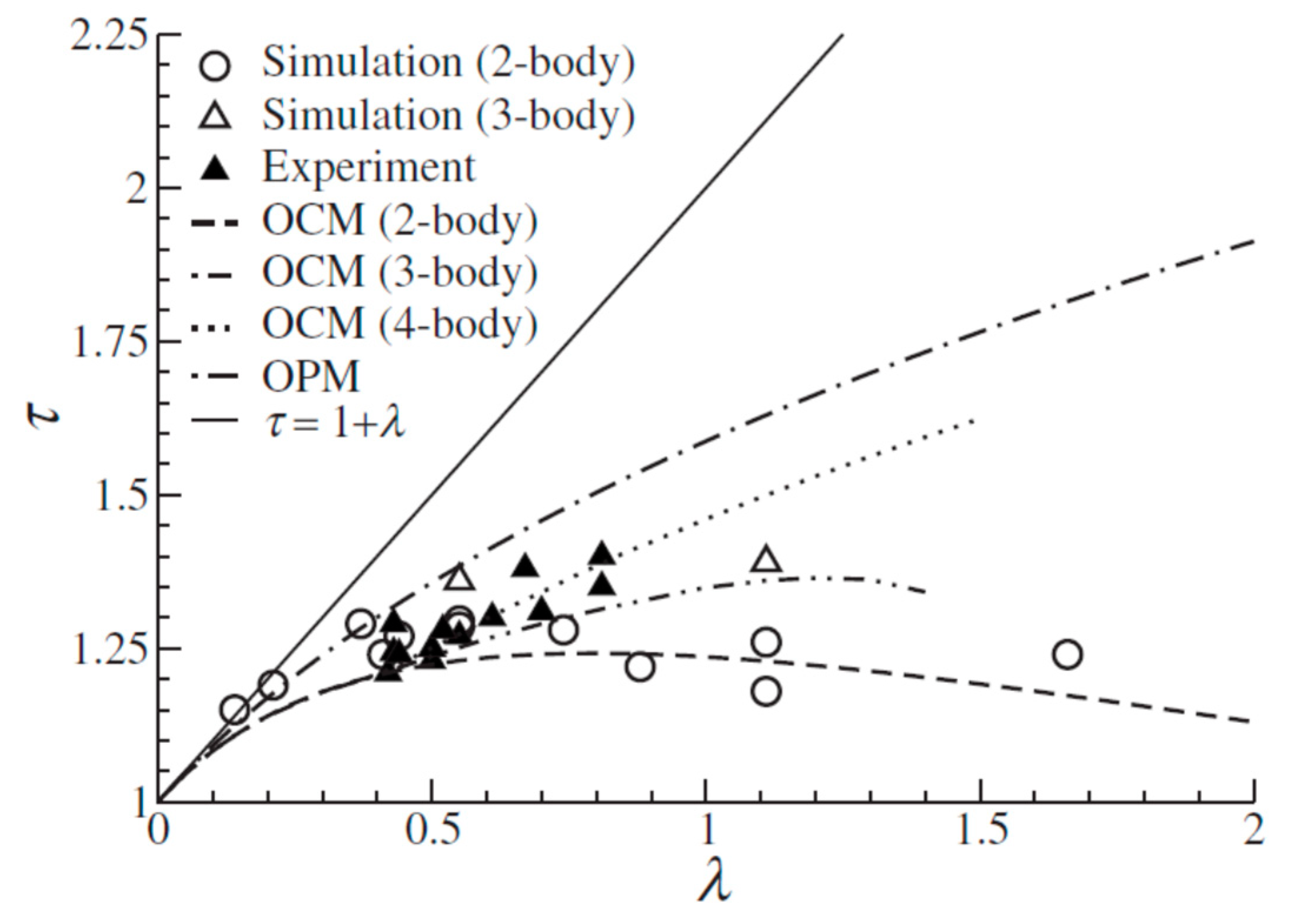
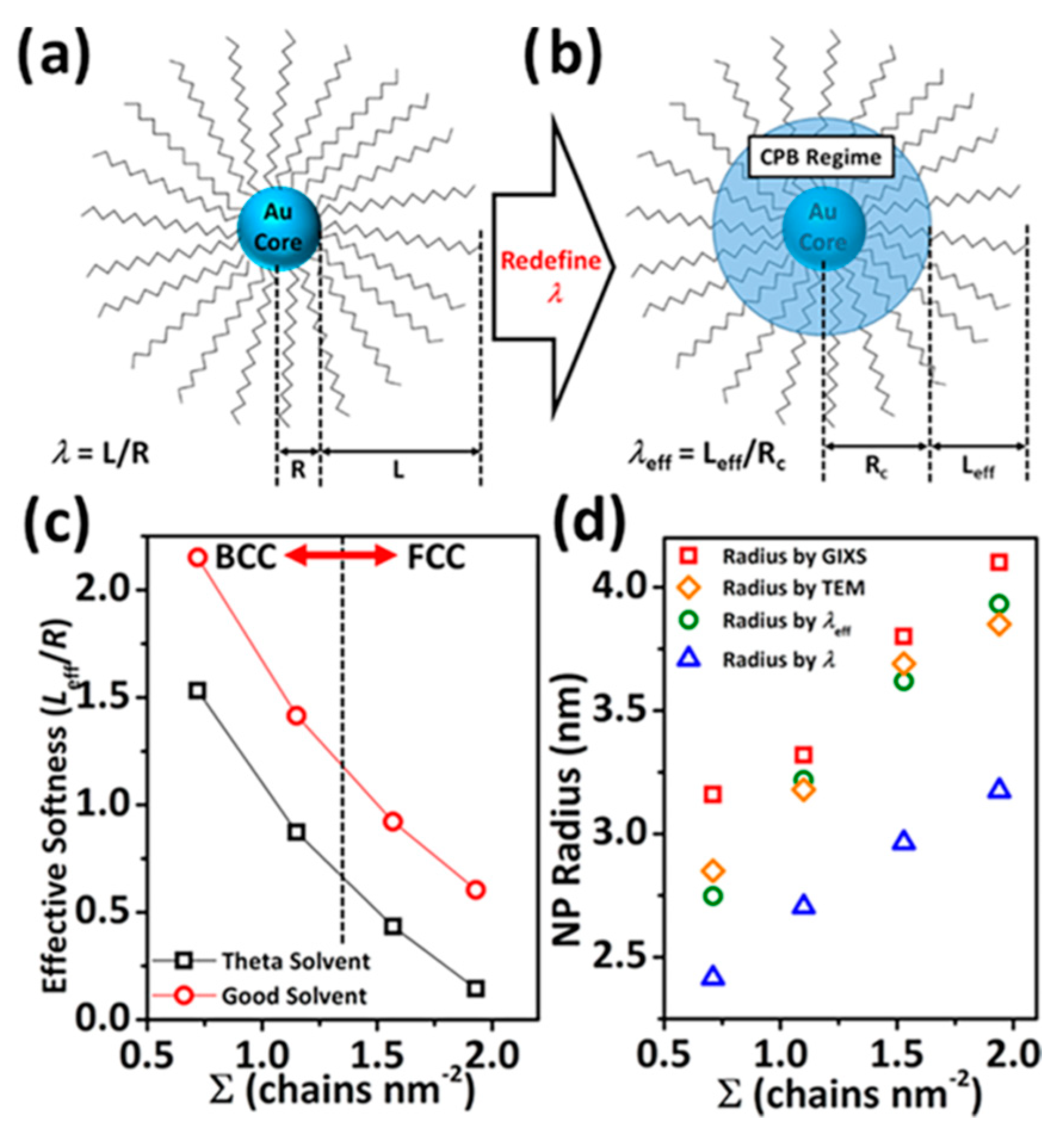
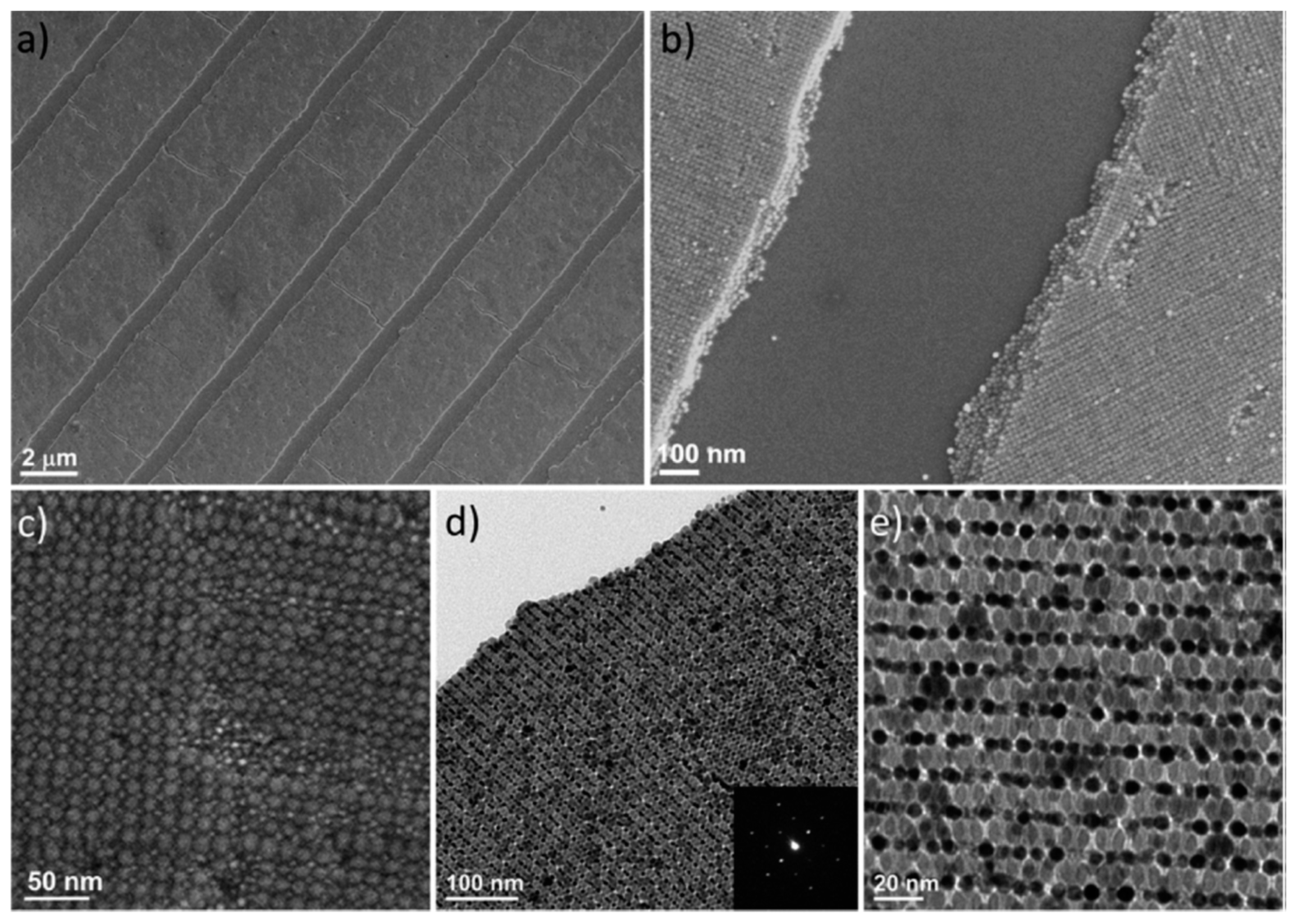
2.2. Self-Assembly of Spherical NCs
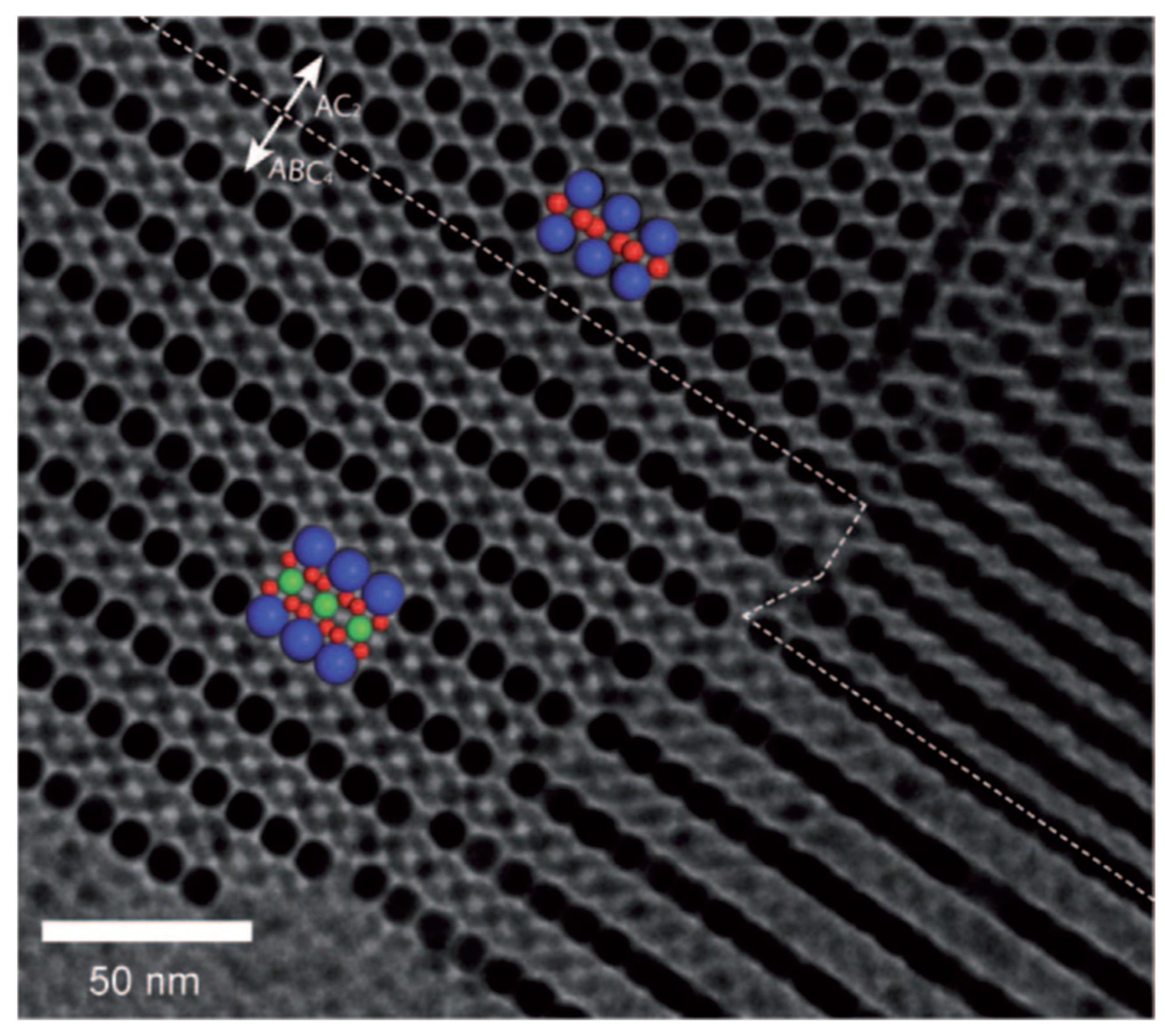
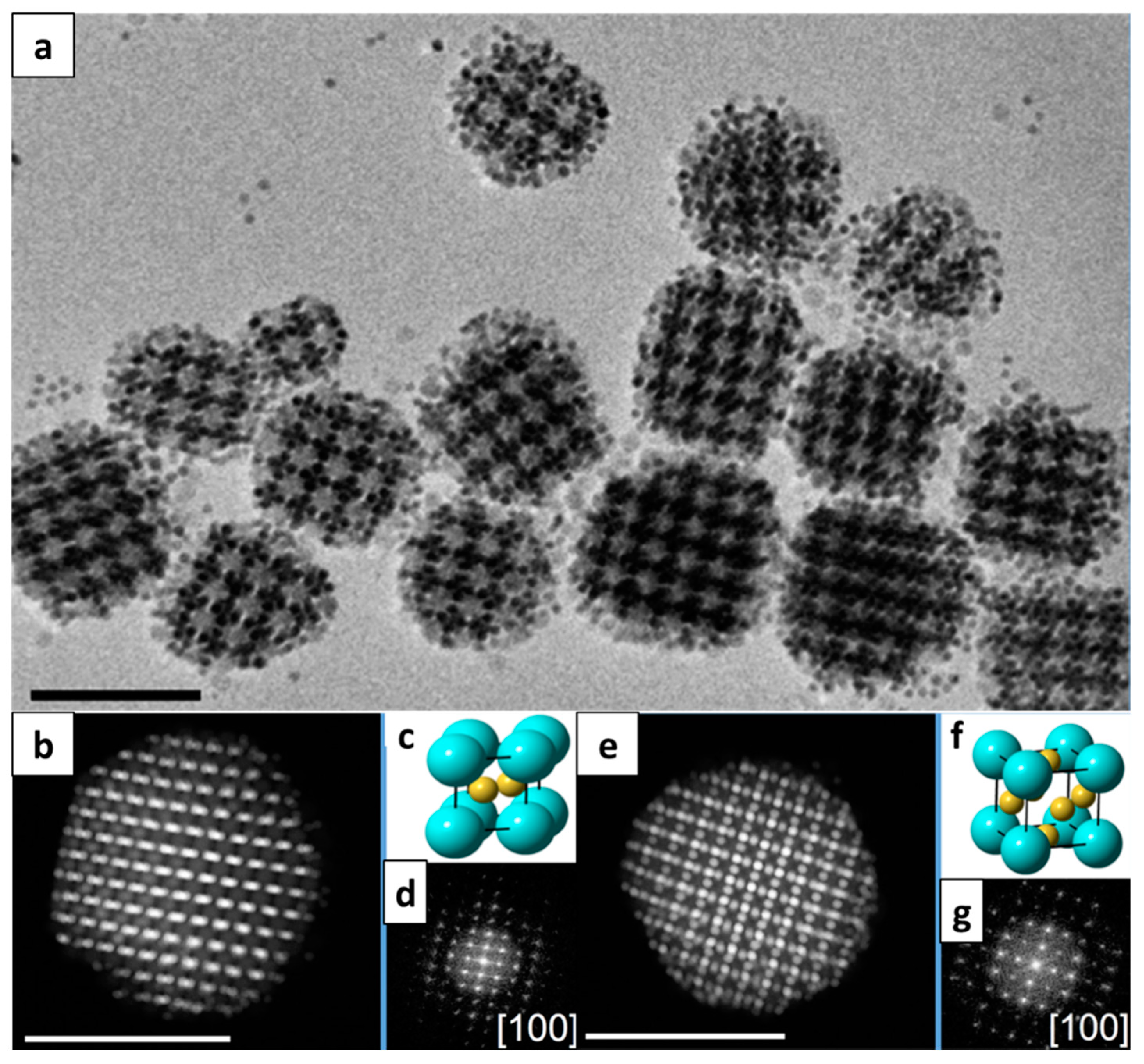
2.3. Self-Assembly of BNSLs
2.4. Self-Assembly of Anisotropic BNSLs
3. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kagan, C.R. Flexible colloidal nanocrystal electronics. Chem. Soc. Rev. 2019, 48, 1626–1641. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-W.; Rodarte, A.L.; Som, M.; Arya, G.; Tao, A.R. Colloidal Plasmonic Nanocomposites: From Fabrication to Optical Function. Chem. Rev. 2018, 118, 3100–3120. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and characterization of monodispersed nanocrystals and close-packed nanocrystals assemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Pileni, M.P. Supracrystals of inorganic nanocrystals: An open challenge for new physical properties. Acc. Chem. Res. 2008, 41, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, E.V.; Talapin, D.V.; Kotov, N.A.; O’Brien, S.; Murray, C.B. Structural diversity in binary nanoparticle superlattices. Nature 2006, 439, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E= S, Se, Te) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Peng, X.; Manna, L.; Yang, W.; Wickham, J. Shape control of CdSe nanocrystals. Nature 2000, 404, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Connolly, S.; Fullam, S.; Korgel, B.; Fitzmaurice, D. Time-resolved small-angle X-ray scattering studies of nanocrystal superlattice self-assembly. J. Am. Chem. Soc. 1998, 120, 2969–2970. [Google Scholar] [CrossRef]
- Murray, C.; Kagan, C.; Bawendi, M. Self-organization of CdSE nanocrystallites into three-dimensional quantum dot superlattices. Science 1995, 270, 1335–1338. [Google Scholar] [CrossRef]
- Kang, Y.; Li, M.; Cai, Y.; Cargnello, M.; Diaz, R.E.; Gordon, T.R.; Wieder, N.L.; Adzic, R.R.; Gorte, R.J.; Stach, E.A.; et al. Heterogeneous catalysts need not be so “Heterogeneous”: Monodisperse Pt nanocrystals by combining shape-controlled synthesis and purification by colloidal recrystallization. J. Am. Chem. Soc. 2013, 135, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, E.V.; Talapin, D.V.; Murray, C.B.; O’Brien, S. Structural characterization of self-assembled multifunctional binary nanoparticle superlattices. J. Am. Chem. Soc. 2006, 128, 3620–3637. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Chen, J.; Vora, P.M.; Kikkawa, J.M.; Murray, C.B. Binary nanocrystal superlattice membranes self-assembled at the liquid-air interface. Nature 2010, 466, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Bigioni, T.P.; Lin, X.M.; Nguyen, T.T.; Corwin, E.I.; Witten, T.A.; Jaeger, H.M. Kinetically driven self assembly of highly ordered nanoparticle monolayers. Nat. Mater. 2006, 5, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Korgel, B.; Fitzmaurice, D. Small-angle x-ray-scattering study of silver-nanocrystal disorder-order phase transitions. Phys. Rev. B 1999, 59, 14191–14201. [Google Scholar] [CrossRef]
- Goodfellow, B.W.; Yu, Y.; Bosoy, C.A.; Smilgies, D.M.; Korgel, B.A. The role of ligand packing frustration in body-centered cubic (bcc) superlattices of colloidal nanocrystals. J. Phys. Chem. Lett. 2015, 6, 2406–2412. [Google Scholar] [CrossRef]
- Kaushik, A.P.; Clancy, P. Solvent-driven symmetry of self-assembled nanocrystal superlattices—A computational study. J. Comput. Chem. 2013, 34, 523–532. [Google Scholar] [CrossRef]
- Zha, X.; Travesset, A. Stability and free energy of nanocrystal chains and superlattices. J. Phys. Chem. C 2018, 122, 23153–23164. [Google Scholar] [CrossRef]
- Landman, U.; Luedtke, W.D. Small is different: Energetic, structural, thermal, and mechanical properties of passivated nanocluster assemblies. Faraday Discuss. 2004, 125, 1–22. [Google Scholar] [CrossRef]
- Schapotschnikow, P.; Vlugt, T.J.H. Understanding interactions between capped nanocrystals: Three-body and chain packing effects. J. Chem. Phys. 2009, 131, 124705. [Google Scholar] [CrossRef] [Green Version]
- Travesset, A. Soft skyrmions, spontaneous valence and selection rules in nanoparticle superlattices. ACS Nano 2017, 11, 5375–5382. [Google Scholar] [CrossRef] [PubMed]
- Boles, M.A.; Talapin, D.V. Many-body effects in nanocrystal superlattices: Departure from sphere oacking explains stability of binary phases. J. Am. Chem. Soc. 2015, 137, 4494–4502. [Google Scholar] [CrossRef]
- Henzie, J.; Andrews, S.C.; Ling, X.Y.; Li, Z.; Yang, P. Oriented assembly of polyhedral plasmonic nanoparticle clusters. Proc. Natl. Acad. Sci. USA 2013, 110, 6640–6645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhu, C.; Ercius, P.; Raja, S.N.; He, B.; Jones, M.R.; Hauwiller, M.R.; Liu, Y.; Xu, T.; Alivisatos, A.P. Structural diversity in binary superlattices self-assembled from polymer-grafted nanocrystals. Nat. Commun. 2015, 6, 10052. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.W.; Ye, X.; Koshy, D.M.; Vachhani, S.; Hosemann, P.; Alivisatos, A.P. Tolerance to structural disorder and tunable mechanical behavior in self-assembled superlattices of polymer-grafted nanocrystals. Proc. Natl. Acad. Sci. USA 2017, 114, 2836–2841. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.; Yu, J.; Lee, Y.J.; Kim, J.-S.; Park, C.H.; Nam, C.; Han, J.; Heo, T.-Y.; Choi, S.-H.; Lee, D.C.; et al. Symmetry transitions of polymer-grafted nanoparticles: Grafting density effect. Chem. Mater. 2019, 31, 5264–5273. [Google Scholar] [CrossRef]
- Kim, Y.; Macfarlane, R.J.; Jones, M.R.; Mirkin, C.A. Transmutable nanoparticles with reconfigurable surface ligands. Science 2016, 351, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Auyeung, E.; Li, T.I.N.G.; Senesi, A.J.; Schmucker, A.L.; Pals, B.C.; de la Cruz, M.O.; Mirkin, C.A. DNA-mediated nanoparticle crystallization into Wulff polyhedra. Nature 2014, 505, 73–77. [Google Scholar] [CrossRef]
- Jishkariani, D.; Diroll, B.T.; Cargnello, M.; Klein, D.R.; Hough, L.A.; Murray, C.B.; Donnio, B. Dendron-mediated engineering of interparticle separation and self-assembly in dendronized gold nanoparticles superlattices. J. Am. Chem. Soc. 2015, 137, 10728–10734. [Google Scholar] [CrossRef]
- Jishkariani, D.; Lee, J.D.; Yun, H.; Paik, T.; Kikkawa, J.M.; Kagan, C.R.; Donnio, B.; Murray, C.B. The dendritic effect and magnetic permeability in dendron coated nickel and manganese zinc ferrite nanoparticles. Nanoscale 2017, 9, 13922–13928. [Google Scholar] [CrossRef]
- Ye, X.; Chen, J.; Murray, C.B. Polymorphism in self-assembled AB6 binary nanocrystal superlattices. J. Am. Chem. Soc. 2011, 133, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Ye, X.; Chen, J.; Qi, L.; Diaz, R.E.; Doan-nguyen, V.; Xing, G.; Kagan, C.R.; Li, J.; Gorte, R.J.; et al. Engineering catalytic contacts and thermal stability: Gold/iron oxide binary nanocrystal superlattices for CO oxidation. J. Am. Chem. Soc. 2013, 135, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Redl, F.X.; Cho, K.; Murray, C.B.; Brien, S.O. Three-dimensional binary superlattices of magnetic nanocrystals and semiconductor quantum dots. Nautre 2003, 423, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.R.; Sun, S.; Wang, Z.L. Phase transformation, coalescence, and twinning of monodisperse FePt nanocrystals. Nano Lett. 2001, 1, 443–447. [Google Scholar] [CrossRef]
- Dong, A.; Chen, J.; Ye, X.; Kikkawa, J.M.; Murray, C.B. Enhanced thermal stability and magnetic properties in NaCl-type. J. Am. Chem. Soc. 2011, 133, 13296–13299. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Ye, X.; Chen, J.; Murray, C.B. Two-dimensional binary and ternary nanocrystal superlattices: The case of monolayers and bilayers. Nano Lett. 2011, 11, 1804–1809. [Google Scholar] [CrossRef]
- Paik, T.; Yun, H.; Fleury, B.; Hong, S.H.; Jo, P.S.; Wu, Y.; Oh, S.J.; Cargnello, M.; Yang, H.; Murray, C.B.; et al. Hierarchical materials design by pattern transfer printing of self-assembled binary nanocrystal superlattices. Nano Lett. 2017, 17, 1387–1394. [Google Scholar] [CrossRef]
- Murray, M.J.; Sanders, J.V. Close-packed structures of spheres of two different sizes II. The packing densities of likely arrangements. Philos. Mag. A Phys. Condens. Matter Struct. Defects Mech. Prop. 1980, 42, 721–740. [Google Scholar] [CrossRef]
- Talapin, D.V.; Shevchenko, E.V.; Bodnarchuk, M.I.; Ye, X.; Chen, J.; Murray, C.B. Quasicrystalline order in self-assembled binary nanoparticle superlattices. Nature 2009, 461, 964–967. [Google Scholar] [CrossRef]
- Evers, W.H.; Friedrich, H.; Filion, L.; Dijkstra, M.; Vanmaekelbergh, D. Observation of a ternary nanocrystal superlattice and its structural characterization by electron tomography. Angew. Chem. Int. Ed. 2009, 48, 9655–9657. [Google Scholar] [CrossRef]
- Wang, P.P.; Qiao, Q.; Zhu, Y.; Ouyang, M. Colloidal binary supracrystals with tunable structural lattices. J. Am. Chem. Soc. 2018, 140, 9095–9098. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, B.; Shen, X.; Yao, L.; Wang, L.; Chen, X.; Xie, S.; Li, T.; Hu, J.; Yang, D.; et al. Scalable assembly of crystalline binary nanocrystal superparticles and their enhanced magnetic and electrochemical properties. J. Am. Chem. Soc. 2018, 140, 15038–15047. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Jin, L.; Caglayan, H.; Chen, J.; Xing, G.; Zheng, C. Improved size-tunable synthesis of monodisperse gold nanorods through the use of aromatic additives. ACS Nano 2012, 6, 2804–2817. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Shevchenko, E.V.; Murray, C.B.; Kornowski, A.; Föraster, S.; Weller, H. CdSe and CdSe/CdS nanorod solids. J. Am. Chem. Soc. 2004, 126, 12984–12988. [Google Scholar] [CrossRef] [PubMed]
- Paik, T.; Gordon, T.R.; Prantner, A.M.; Yun, H.; Murray, C.B. Designing tripodal and triangular gadolinium oxide nanoplates and self-assembled nanofibrils as potential multimodal bioimaging probes. ACS Nano 2013, 7, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Paik, T.; Ko, D.K.; Gordon, T.R.; Doan-Nguyen, V.; Murray, C.B. Studies of liquid crystalline self-assembly of GdF3 nanoplates by in-plane, out-of-plane SAXS. ACS Nano 2011, 5, 8322–8330. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.B.; Ghezelbash, A.; Hanrath, T.; Saunders, A.E.; Lee, F.; Korgel, B.A. Solventless synthesis of monodisperse Cu2S nanorods, nanodisks, and nanoplatelets. J. Am. Chem. Soc. 2003, 125, 16050–16057. [Google Scholar] [CrossRef]
- Chen, M.; Pica, T.; Jiang, Y.; Li, P.; Yano, K.; Liu, J.P.; Datye, A.K.; Fan, H. Synthesis and self-assembly of fcc phase FePt nanorods. J. Am. Chem. Soc. 2007, 129, 6348–6349. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Chan, H.; Baskin, A.; Gelman, E.; Repnin, N.; Král, P.; Klajn, R. Self-assembly of magnetite nanocubes into helical superstructures. Science 2014, 345, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Millan, J.A.; Engel, M.; Chen, J.; Diroll, B.T.; Glotzer, S.C.; Murray, C.B. Shape alloys of nanorods and nanospheres from self-assembly. Nano Lett. 2013, 13, 4980–4988. [Google Scholar] [CrossRef] [PubMed]
- Paik, T.; Diroll, B.T.; Kagan, C.R.; Murray, C.B. Binary and ternary superlattices self-assembled from colloidal nanodisks and nanorods. J. Am. Chem. Soc. 2015, 137, 6662–6669. [Google Scholar] [CrossRef] [PubMed]
- Paik, T.; Murray, C.B. Shape-directed binary assembly of anisotropic nanoplates: A nanocrystal puzzle with shape-complementary building blocks. Nano Lett. 2013, 13, 2952–2956. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, A.; Cai, J.; Ye, X.; Kang, Y.; Kikkawa, J.M.; Murray, C.B. Collective dipolar interactions in self-assembled magnetic binary nanocrystal superlattice membranes. Nano Lett. 2010, 10, 5103–5108. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.J.; Talapin, D.V.; Shevchenko, E.V.; Kagan, C.R.; Murray, C.B. Synergism in binary nanocrystal superlattices leads to enhanced p-type conductivity in self-assembled PbTe/Ag2Te thin films. Nat. Mater. 2007, 6, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Jeong, S. Colloidal quantum dot based solar cells: From materials to devices. Nano Converg. 2017, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, E.V.; Ringler, M.; Schwemer, A.; Talapin, D.V.; Klar, T.A.; Rogach, A.L.; Feldmann, J.; Alivisatos, A.P. Self-assembled binary superlattices of CdSe and Au nanocrystals and their fluorescence properties. J. Am. Chem. Soc. 2008, 130, 3274–3275. [Google Scholar] [CrossRef] [PubMed]
- Gaulding, E.A.; Diroll, B.T.; Goodwin, E.D.; Vrtis, Z.J.; Kagan, C.R.; Murray, C.B. Deposition of wafer-scale single-component and binary nanocrystal superlattice thin films via dip-coating. Adv. Mater. 2015, 27, 2846–2851. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.; Paik, T. Colloidal Self-Assembly of Inorganic Nanocrystals into Superlattice Thin-Films and Multiscale Nanostructures. Nanomaterials 2019, 9, 1243. https://doi.org/10.3390/nano9091243
Yun H, Paik T. Colloidal Self-Assembly of Inorganic Nanocrystals into Superlattice Thin-Films and Multiscale Nanostructures. Nanomaterials. 2019; 9(9):1243. https://doi.org/10.3390/nano9091243
Chicago/Turabian StyleYun, Hongseok, and Taejong Paik. 2019. "Colloidal Self-Assembly of Inorganic Nanocrystals into Superlattice Thin-Films and Multiscale Nanostructures" Nanomaterials 9, no. 9: 1243. https://doi.org/10.3390/nano9091243
APA StyleYun, H., & Paik, T. (2019). Colloidal Self-Assembly of Inorganic Nanocrystals into Superlattice Thin-Films and Multiscale Nanostructures. Nanomaterials, 9(9), 1243. https://doi.org/10.3390/nano9091243




