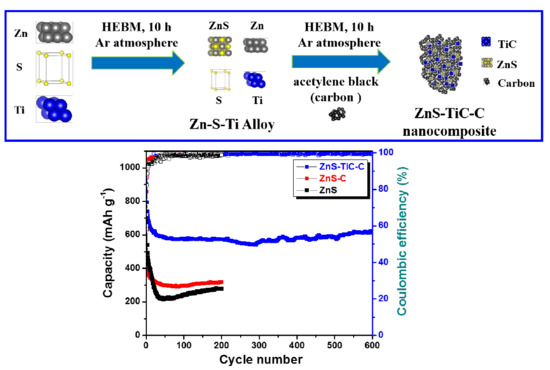
Enhanced Cycle Stability of Zinc Sulfide Anode for High-Performance Lithium-Ion Storage: Effect of Conductive Hybrid Matrix on Active ZnS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
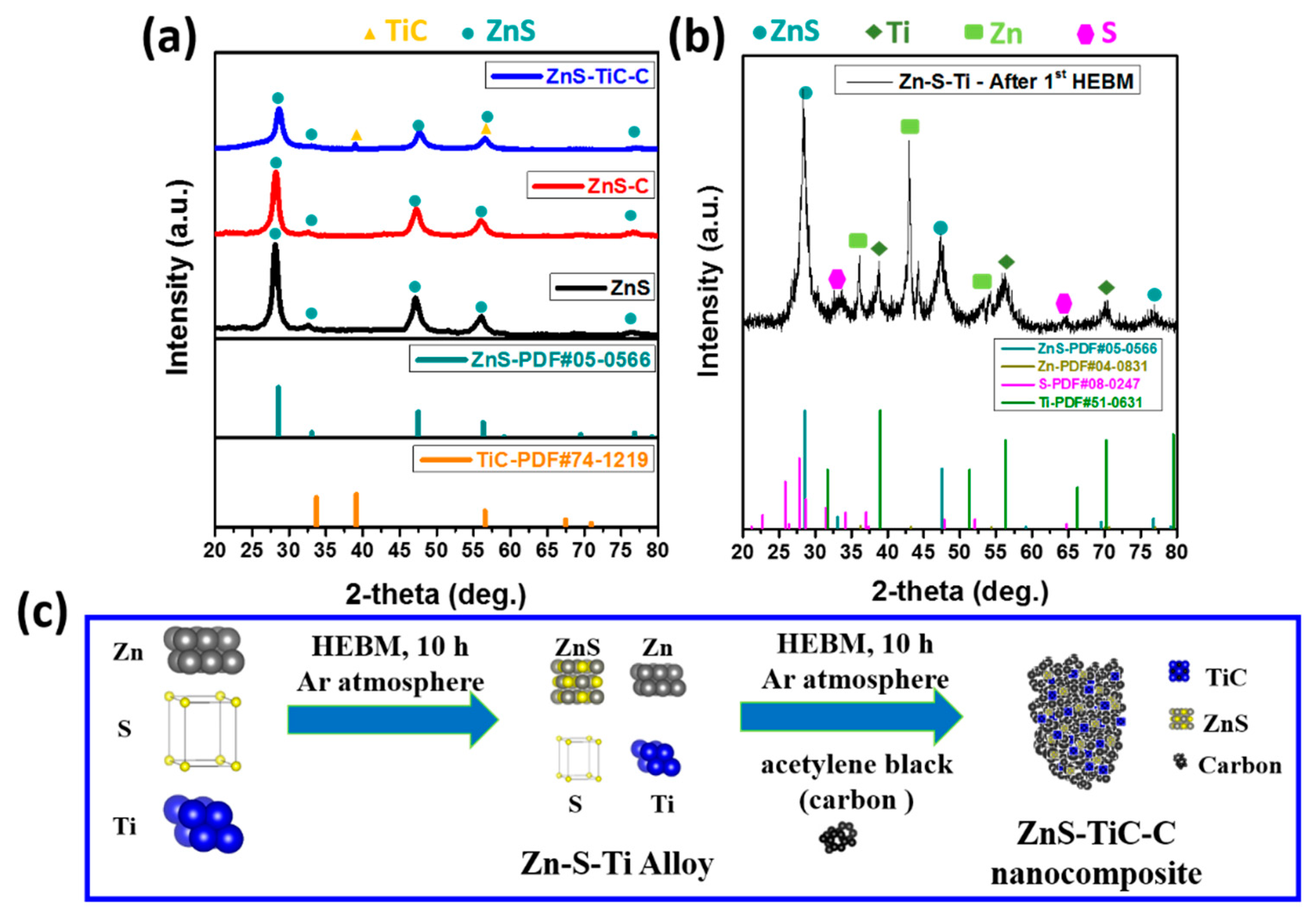
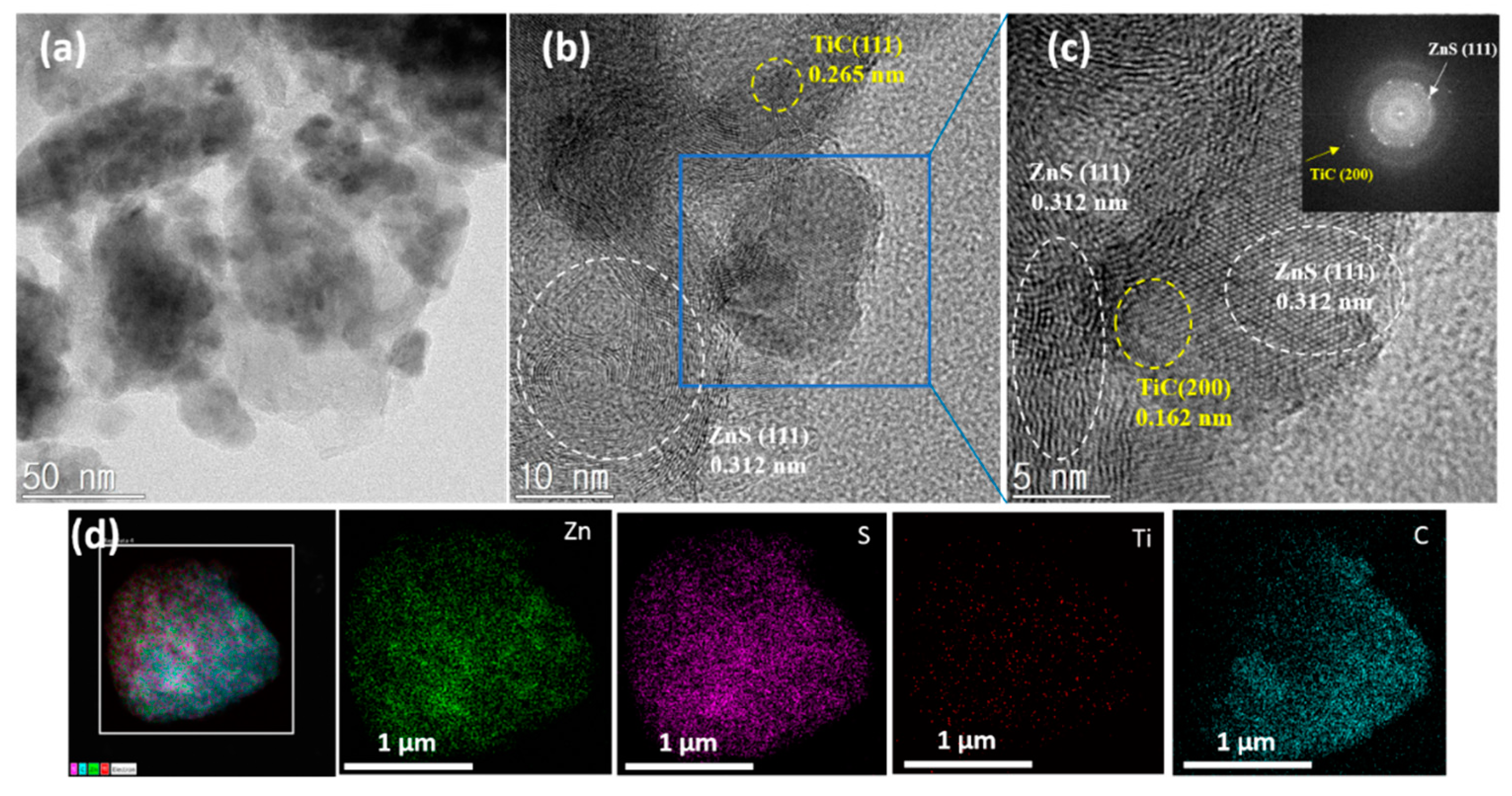
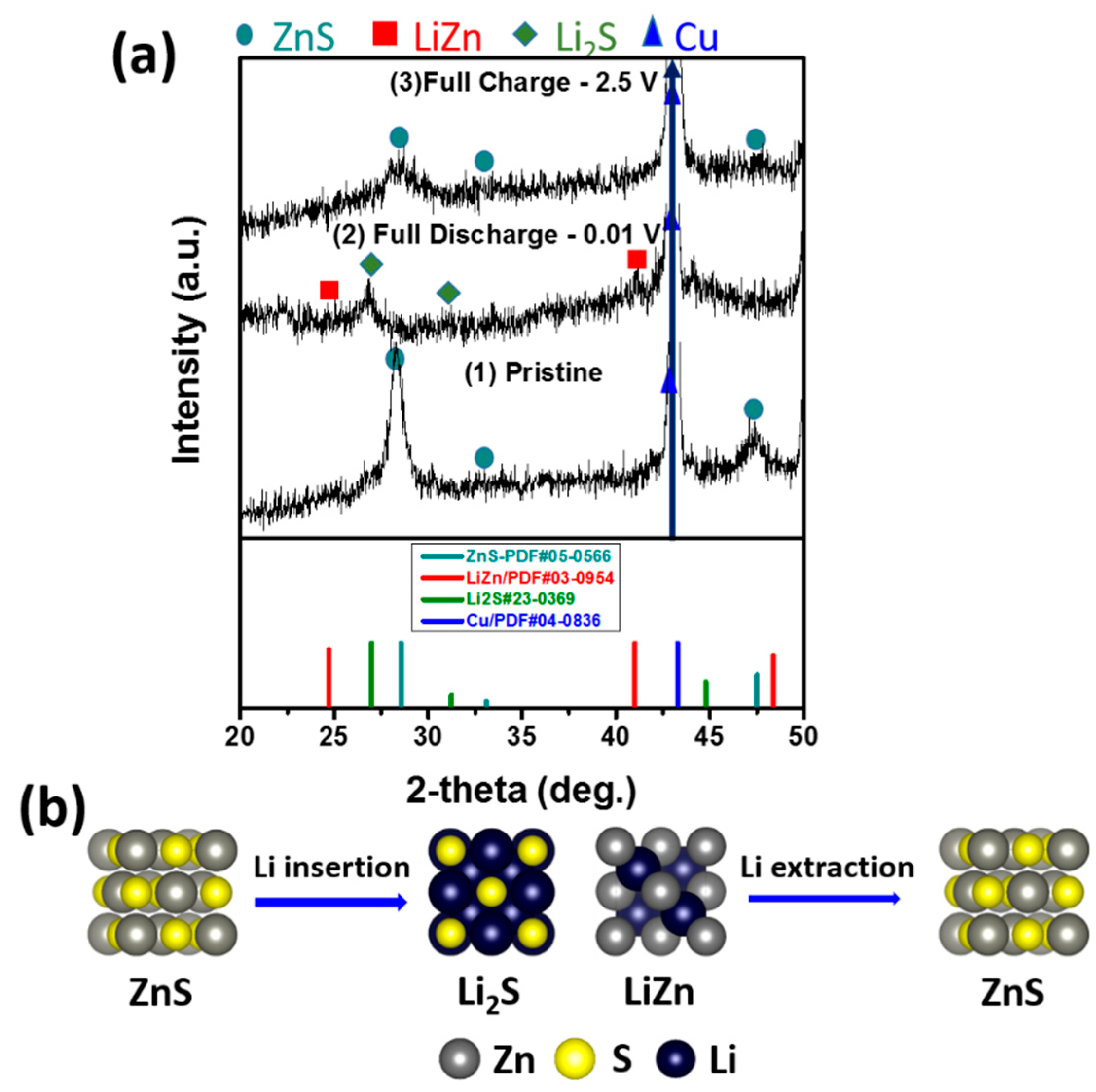
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy. Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Yim, C.H.; Courtel, F.M.; Abu-Lebdeh, Y. A high capacity silicon–graphite composite as anode for lithium-ion batteries using low content amorphous silicon and compatible binders. J. Mater. Chem. A 2013, 1, 8234–8243. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Doh, C.H.; Jin, B.S.; Lim, J.H.; Moon, S.I. Electrochemical characteristics of lithium transition-metal oxide as an anode material in a lithium secondary battery. Korean J. Chem. Eng. 2002, 19, 749–755. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jung, D.W.; Kong, B.S.; Shin, C.M.; Oh, E.S. The effect of graphene nanosheets as an additive for anode materials in lithium ion batteries. Korean J. Chem. Eng. 2011, 28, 2202–2205. [Google Scholar] [CrossRef]
- Hai, N.Q.; Kim, H.; Yoo, I.S.; Kim, J.H.; Hur, J. Comparative study of mechanically milled MoS2 and MoSe2 in graphite matrix as anode materials for high-performance lithium-ion batteries. J. Nanosci. Nanotechnol. 2018, 18, 6469–6474. [Google Scholar] [CrossRef]
- Hai, N.Q.; Kim, H.; Yoo, I.S.; Hur, J. Facile and Scalable Preparation of a MoS2/carbon nanotube nanocomposite anode for high-performance lithium-ion batteries: effects of carbon nanotube content. J. Nanosci. Nanotechnol. 2019, 19, 1494–1499. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Choi, J.S.; Lee, Y.C.; Kim, I.T.; Hur, J. 3D hierarchical structure of MoS2@ G-CNT combined with post-film annealing for enhanced lithium-ion storage. J. Ind. Eng. Chem. 2019, 69, 116–126. [Google Scholar] [CrossRef]
- Obrovac, M.; Chevrier, V. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 2014, 11, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Hung, N.T.; Park, S.J.; Kim, I.T.; Hur, J. Enhanced performance of carbon-free intermetallic zinc titanium alloy (Zn-ZnxTiy) anode for lithium-ion batteries. Electrochim. Acta 2019, 301, 229–239. [Google Scholar] [CrossRef]
- Hai, N.Q.; Kwon, S.H.; Kim, H.; Kim, I.T.; Lee, S.G.; Hur, J. High-performance MoS2-based nanocomposite anode prepared by high-energy mechanical milling: The effect of carbonaceous matrix on MoS2. Electrochim. Acta 2018, 260, 129–138. [Google Scholar] [CrossRef]
- Hung, N.T.; Bae, J.; Kim, J.H.; Son, H.B.; Kim, I.T.; Hur, J. Facile preparation of a zinc-based alloy composite as a novel anode material for rechargeable lithium-ion batteries. Appl. Surf. Sci. 2018, 429, 210–217. [Google Scholar] [CrossRef]
- Park, C.M.; Kim, J.H.; Kim, H.; Sohn, H.J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liao, X.Z.; Yang, K.; He, Y.S.; Wen, W.; Ma, Z.F. Electrochemical characteristics and intercalation mechanism of ZnS/C composite as anode active material for lithium-ion batteries. Electrochim. Acta 2011, 56, 1213–1218. [Google Scholar] [CrossRef]
- Park, A.R.; Jeon, K.J.; Park, C.M. Electrochemical mechanism of Li insertion/extraction in ZnS and ZnS/C anodes for Li-ion batteries. Electrochim. Acta 2018, 265, 107–114. [Google Scholar] [CrossRef]
- Saadat, S.; Tay, Y.Y.; Zhu, J.; Teh, P.F.; Maleksaeedi, S.; Shahjamali, M.M.; Shakerzadeh, M.; Srinivasan, M.; Tay, B.Y.; Hng, H.H. Template-free electrochemical deposition of interconnected ZnSb nanoflakes for li-ion battery anodes. Chem. Mater. 2011, 23, 1032–1038. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Quasi-Intercalation and facile amorphization in layered ZnSb for Li-ion batteries. Adv. Mater. 2010, 22, 47–52. [Google Scholar] [CrossRef]
- Park, M.G.; Lee, C.K.; Park, C.M. Amorphized ZnSb-based composite anodes for high-performance Li-ion batteries. RSC Adv. 2014, 4, 5830–5833. [Google Scholar] [CrossRef]
- Hwa, Y.; Sung, J.H.; Wang, B.; Park, C.M.; Sohn, H.J. Nanostructured Zn-based composite anodes for rechargeable Li-ion batteries. J. Mater. Chem. 2012, 22, 12767–12773. [Google Scholar] [CrossRef]
- Seo, J.U.; Park, C.M. ZnTe and ZnTe/C nanocomposite: A new electrode material for high-performance rechargeable Li-ion batteries. J. Mater. Chem. A 2014, 2, 20075–20082. [Google Scholar] [CrossRef]
- Kwon, H.T.; Park, C.M. Electrochemical characteristics of ZnSe and its nanostructured composite for rechargeable Li-ion batteries. J. Power Sources 2014, 251, 319–324. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Yang, X.; Qu, Y.; Li, Q. Nanostructured ZnSe anchored on graphene nanosheets with superior electrochemical properties for lithium ion batteries. Electrochim. Acta 2015, 168, 285–291. [Google Scholar] [CrossRef]
- Besenhard, J.; Hess, M.; Komenda, P. Dimensionally stable Li-alloy electrodes for secondary batteries. Solid State Ion. 1990, 40, 525–529. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Liu, Q.; Duan, H. Recent progress in Zn-based anodes for advanced lithium ion batterie. Mater. Chem. Front. 2018, 2, 1414–1435. [Google Scholar] [CrossRef]
- Leibowitz, J.; Allcorn, E.; Manthiram, A. SnSb–TiC–C nanocomposite alloy anodes for lithium-ion batteries. J. Power Sources 2015, 279, 549–554. [Google Scholar] [CrossRef]
- Son, S.Y.; Hur, J.; Kim, K.H.; Son, H.B.; Lee, S.G.; Kim, I.T. SnTe-TiC-C composites as high-performance anodes for Li-ion batteries. J. Power Sources 2017, 365, 372–379. [Google Scholar] [CrossRef]
- Kim, I.T.; Kim, S.O.; Manthiram, A. Effect of TiC addition on SnSb–C composite anodes for sodium-ion batteries. J. Power Sources 2014, 269, 848–854. [Google Scholar] [CrossRef]
- Allcorn, E.; Manthiram, A. High-rate, high-density FeSb–TiC–C nanocomposite anodes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 3891–3900. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Yoon, Y.H.; Nguyen, Q.H.; Kim, I.T.; Hur, J.; Lee, S.G. Sb2Te3-TiC-C nanocomposites for the high-performance anode in lithium-ion batteries. Electrochim. Acta 2019, 293, 8–18. [Google Scholar] [CrossRef]
- Kim, S.O.; Manthiram, A. High-Performance Zn-TiC-C Nanocomposite Alloy Anode with Exceptional Cycle Life for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 14801–14807. [Google Scholar] [CrossRef] [PubMed]
- Applestone, D.; Manthiram, A. Cu6Sn5–TiC–C nanocomposite alloy anodes with high volumetric capacity for lithium ion batteries. RSC Adv. 2012, 2, 5411–5417. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Hur, J. MoS2-TiC-C Nanocomposites as New Anode Materials for High-Performance Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2019, 19, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, Z.; Qi, Z.; Liu, G.; Bian, X. Formation of nanocrystalline TiC from titanium and different carbon sources by mechanical alloying. J. Alloys Compd. 2009, 472, 97–103. [Google Scholar] [CrossRef]
- Mani, A.; Aubert, P.; Mercier, F.; Khodja, H.; Berthier, C.; Houdy, P. Effects of residual stress on the mechanical and structural properties of TiC thin films grown by RF sputtering. Surf. Coat. Technol. 2005, 194, 190–195. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Lu, Y.; Zhu, Y.; Qian, Y.; Wang, D.; Zhou, J.; Lin, N.; Qian, Y. A graphene oxide-wrapped bipyramidal sulfur@ polyaniline core–shell structure as a cathode for Li–S batteries with enhanced electrochemical performance. J. Mater. Chem. A 2016, 4, 6404–6410. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent Advances in Electrolytes for Lithium-Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1500117. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Barghamadi, M.; Kapoor, A.; Wen, C. A review on Li-S batteries as a high efficiency rechargeable lithium battery. J. Electrochem. Soc. 2013, 160, A1256–A1263. [Google Scholar] [CrossRef]
- Du, X.; Zhao, H.; Zhang, Z.; Lu, Y.; Gao, C.; Li, Z.; Teng, Y.; Zhao, L.; Świerczek, K. Synthesis of core-shell-like ZnS/C nanocomposite as improved anode material for lithium ion batteries. Electrochim. Acta 2017, 225, 129–136. [Google Scholar] [CrossRef]
- Du, X.; Zhao, H.; Lu, Y.; Zhang, Z.; Kulka, A.; Świerczek, K. Core-shell structured ZnS-C nanoparticles with enhanced electrochemical properties for high-performance lithium-ion battery anodes. Electrochim. Acta 2017, 228, 100–106. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, B.; Cao, Y.; Wang, X.; Yao, Y.; Yu, W.; Zheng, J.; Zhang, J.; Tong, H. ZnS nanoparticles embedded in porous honeycomb-like carbon nanosheets as high performance anode material for lithium ion batteries. Ceram. Int. 2018, 44, 13706–13711. [Google Scholar] [CrossRef]
- Wang, L.; Ju, J.; Deng, N.; Wang, G.; Cheng, B.; Kang, W. ZnS nanoparticles anchored on porous carbon nanofibers as anode materials for lithium ion batteries. Electrochem. Commun. 2018, 96, 1–5. [Google Scholar] [CrossRef]
- Qin, W.; Li, D.; Zhang, X.; Yan, D.; Hu, B.; Pan, L. ZnS nanoparticles embedded in reduced graphene oxide as high performance anode material of sodium-ion batteries. Electrochim. Acta 2016, 191, 435–443. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Z.; Yang, X.; Gan, Y.; Chen, W. ZnS nanoparticles embedded in porous carbon matrices as anode materials for lithium ion batteries. RSC Adv. 2015, 5, 86941–86944. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Zhang, X.; Hou, S.; Lu, T.; Yao, Y.; Pan, L. ZnS nanoparticles decorated on nitrogen-doped porous carbon polyhedra: A promising anode material for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2017, 5, 20428–20438. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.; Shi, X.; Xu, Z.; Zhang, Z. Urchinlike ZnS Microspheres decorated with nitrogen-doped carbon: a superior anode material for lithium and sodium Storage. Chem. Eur. J. 2017, 23, 157–166. [Google Scholar] [CrossRef]
- Manthiram, A. Materials challenges and opportunities of lithium ion batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Qin, M.; Li, Y.; Lv, X.J. Preparation of Ce-and La-doped Li4Ti5O12 nanosheets and their electrochemical performance in Li half cell and Li4Ti5O12/LiFePO4 full cell batteries. Nanomaterials 2017, 7, 150. [Google Scholar] [CrossRef] [PubMed]


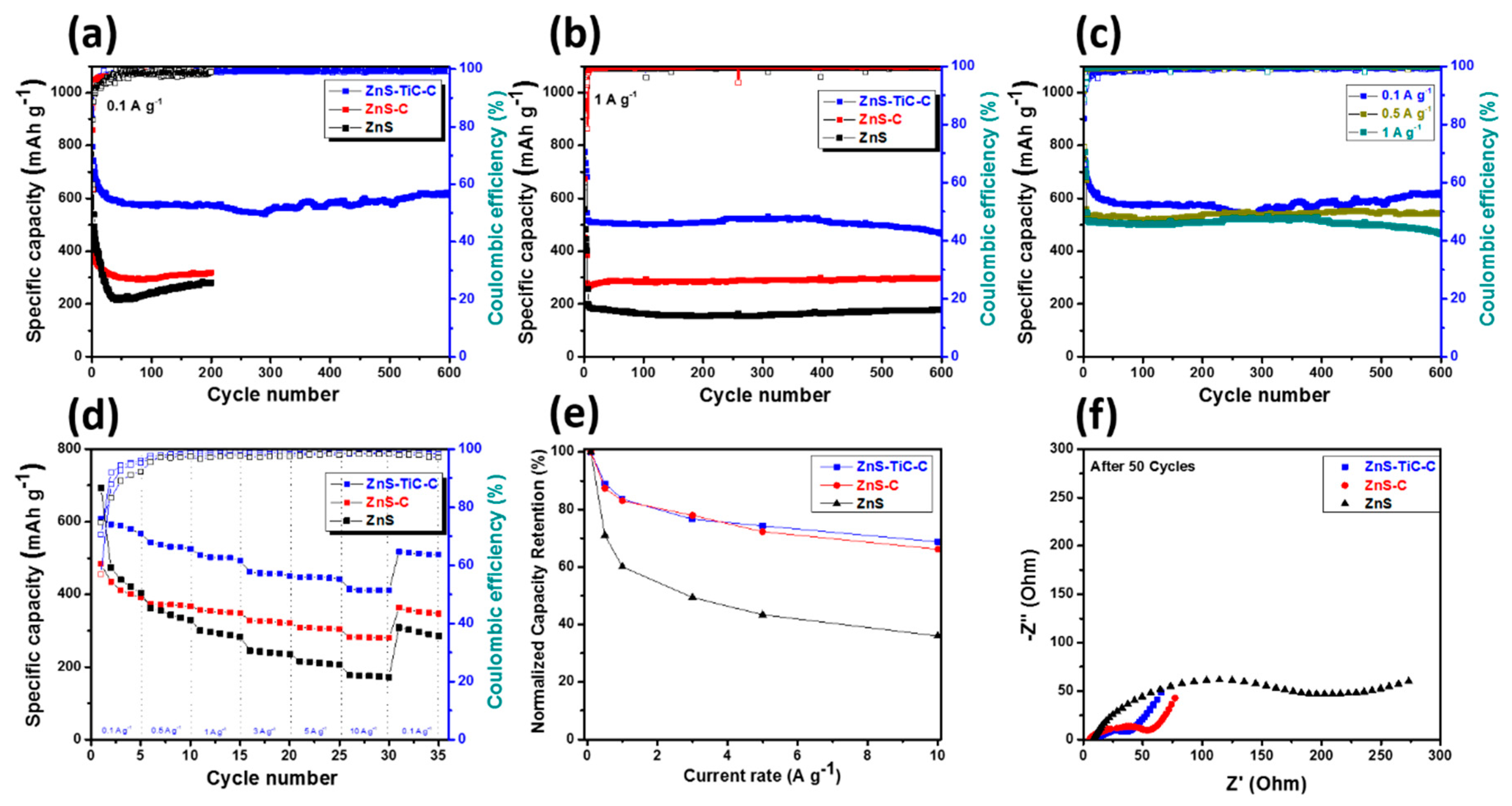
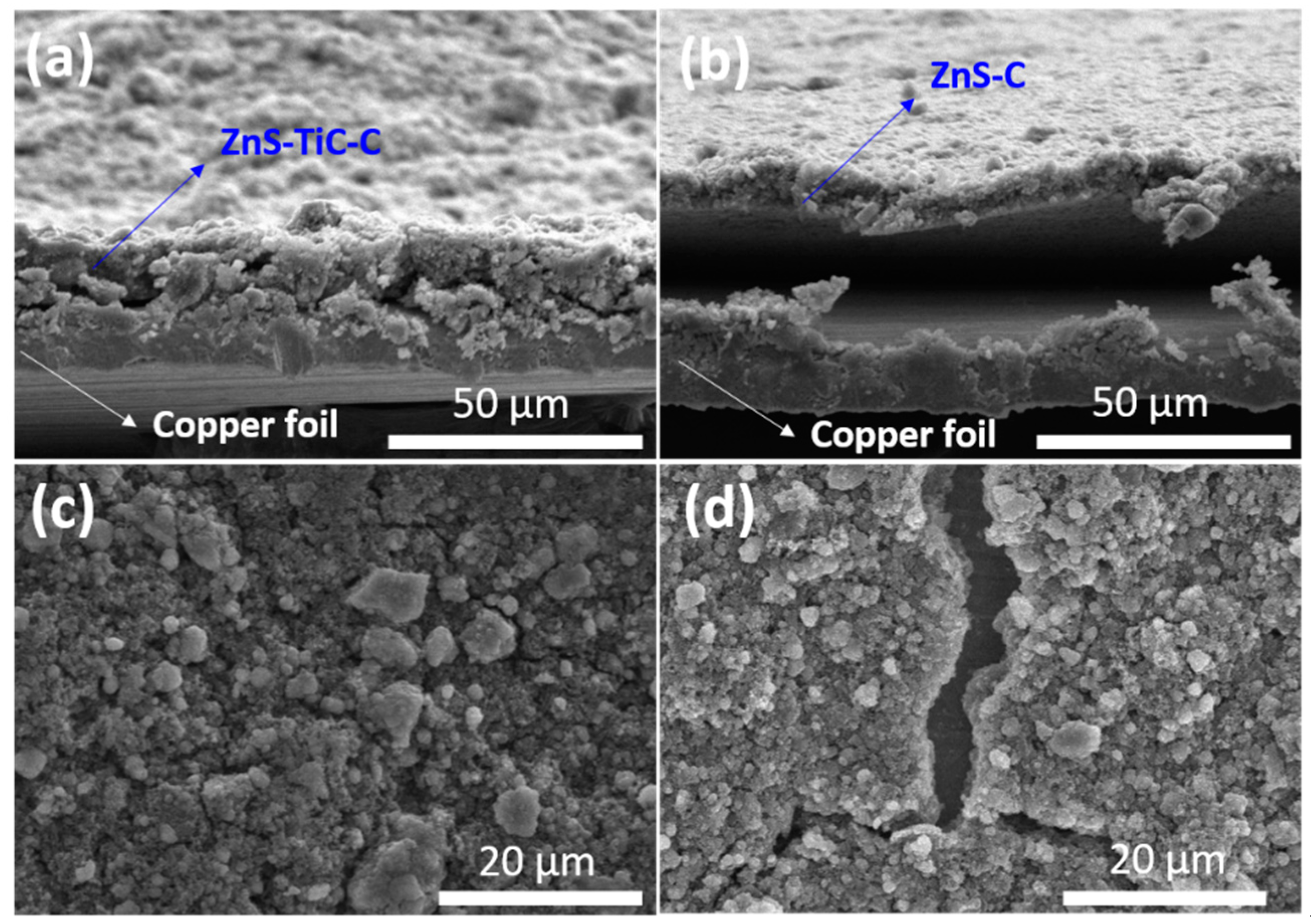
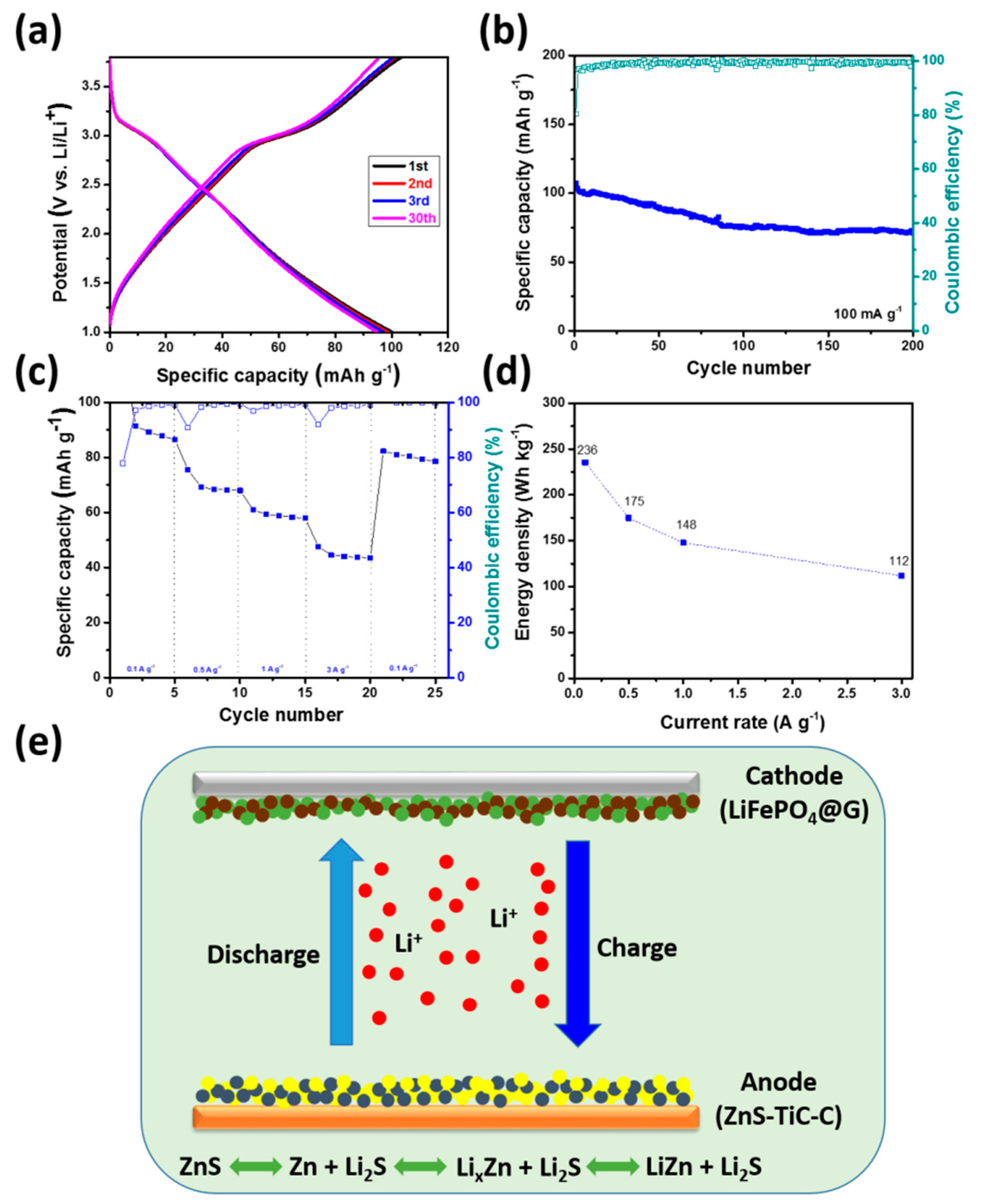
| Electrode | 1st Discharge Capacity (mA h g−1) | 1st Charge Capacity (mA h g−1) | 1st Coulombic Efficiency (%) | Capacity Retention (%) (nth/2nd Discharge Capacity) |
|---|---|---|---|---|
| ZnS-TiC–C | 903 | 594 | 65.8 | 77.2 (n = 600) |
| ZnS-C | 859 | 501 | 58.3 | 49.4 (n = 200) |
| ZnS | 1014 | 609 | 60.1 | 44.0 (n = 200) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.H.; Park, T.; Hur, J. Enhanced Cycle Stability of Zinc Sulfide Anode for High-Performance Lithium-Ion Storage: Effect of Conductive Hybrid Matrix on Active ZnS. Nanomaterials 2019, 9, 1221. https://doi.org/10.3390/nano9091221
Nguyen QH, Park T, Hur J. Enhanced Cycle Stability of Zinc Sulfide Anode for High-Performance Lithium-Ion Storage: Effect of Conductive Hybrid Matrix on Active ZnS. Nanomaterials. 2019; 9(9):1221. https://doi.org/10.3390/nano9091221
Chicago/Turabian StyleNguyen, Quoc Hanh, Taehyun Park, and Jaehyun Hur. 2019. "Enhanced Cycle Stability of Zinc Sulfide Anode for High-Performance Lithium-Ion Storage: Effect of Conductive Hybrid Matrix on Active ZnS" Nanomaterials 9, no. 9: 1221. https://doi.org/10.3390/nano9091221
APA StyleNguyen, Q. H., Park, T., & Hur, J. (2019). Enhanced Cycle Stability of Zinc Sulfide Anode for High-Performance Lithium-Ion Storage: Effect of Conductive Hybrid Matrix on Active ZnS. Nanomaterials, 9(9), 1221. https://doi.org/10.3390/nano9091221