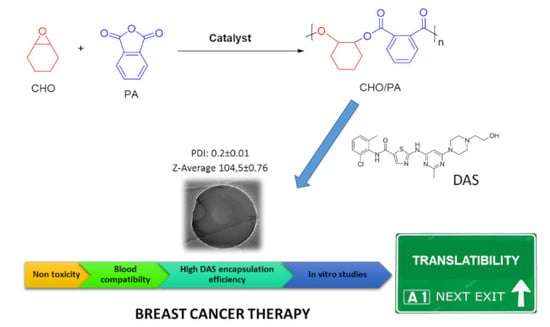
Poly(Cyclohexene Phthalate) Nanoparticles for Controlled Dasatinib Delivery in Breast Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CHO/PA NPs
2.1.1. Materials
2.1.2. Formulation
2.2. Preparation of DAS-Loaded CHO/PA NPs
2.2.1. Formulation
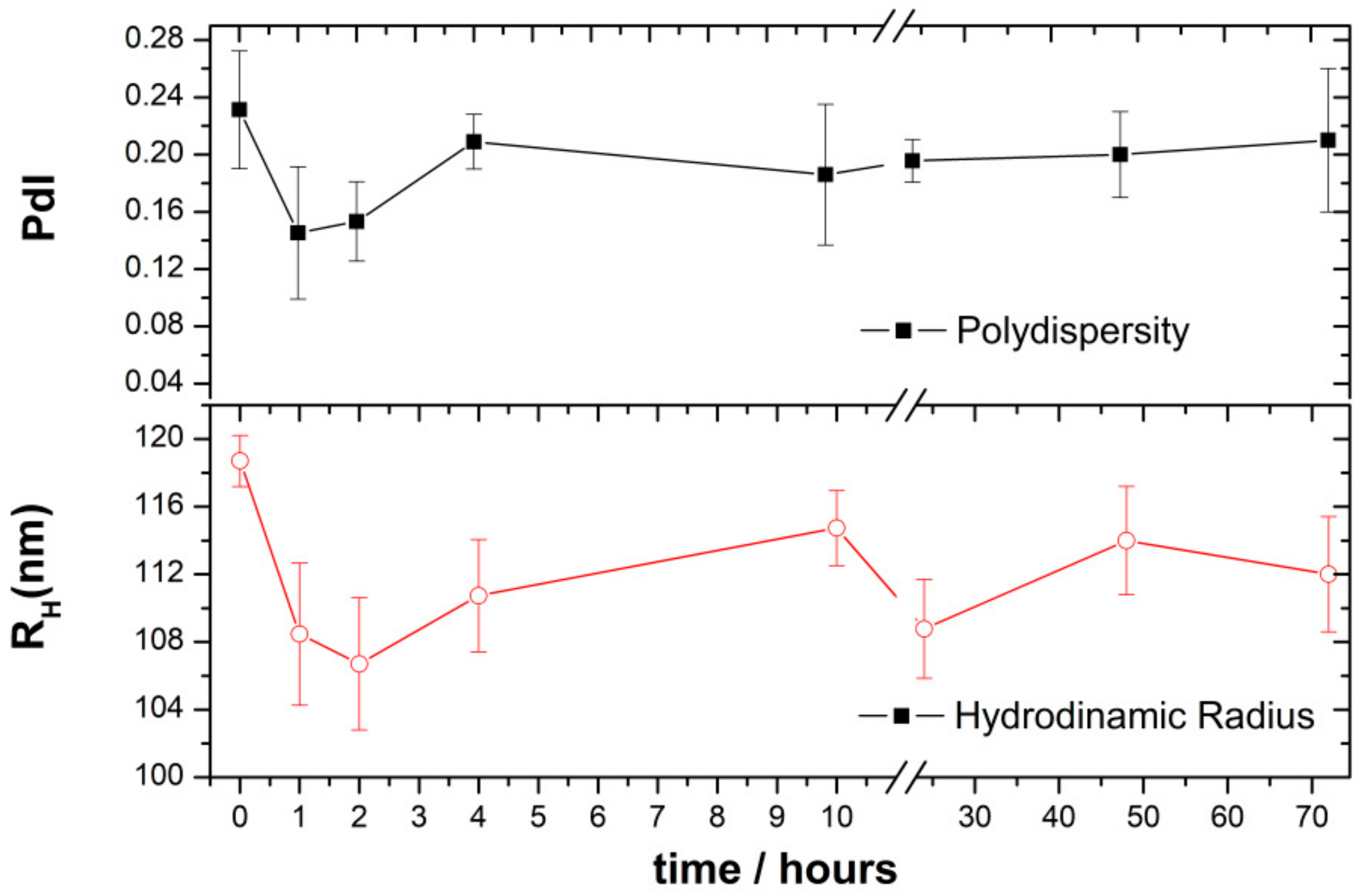
2.2.2. Stability of the CHO/PA NPs in Simulated Physiological Media
2.3. Biological Studies
2.3.1. Enzymatic Degradation Studies
2.3.2. In vitro Drug Release
2.3.3. Blood Compatibility
2.3.4. Cell Culture
2.3.5. Toxicity
2.3.6. Cell Cycle Studies
2.3.7. Apoptosis
3. Results and Discussion
3.1. Synthesis and Characterization of Raw Material
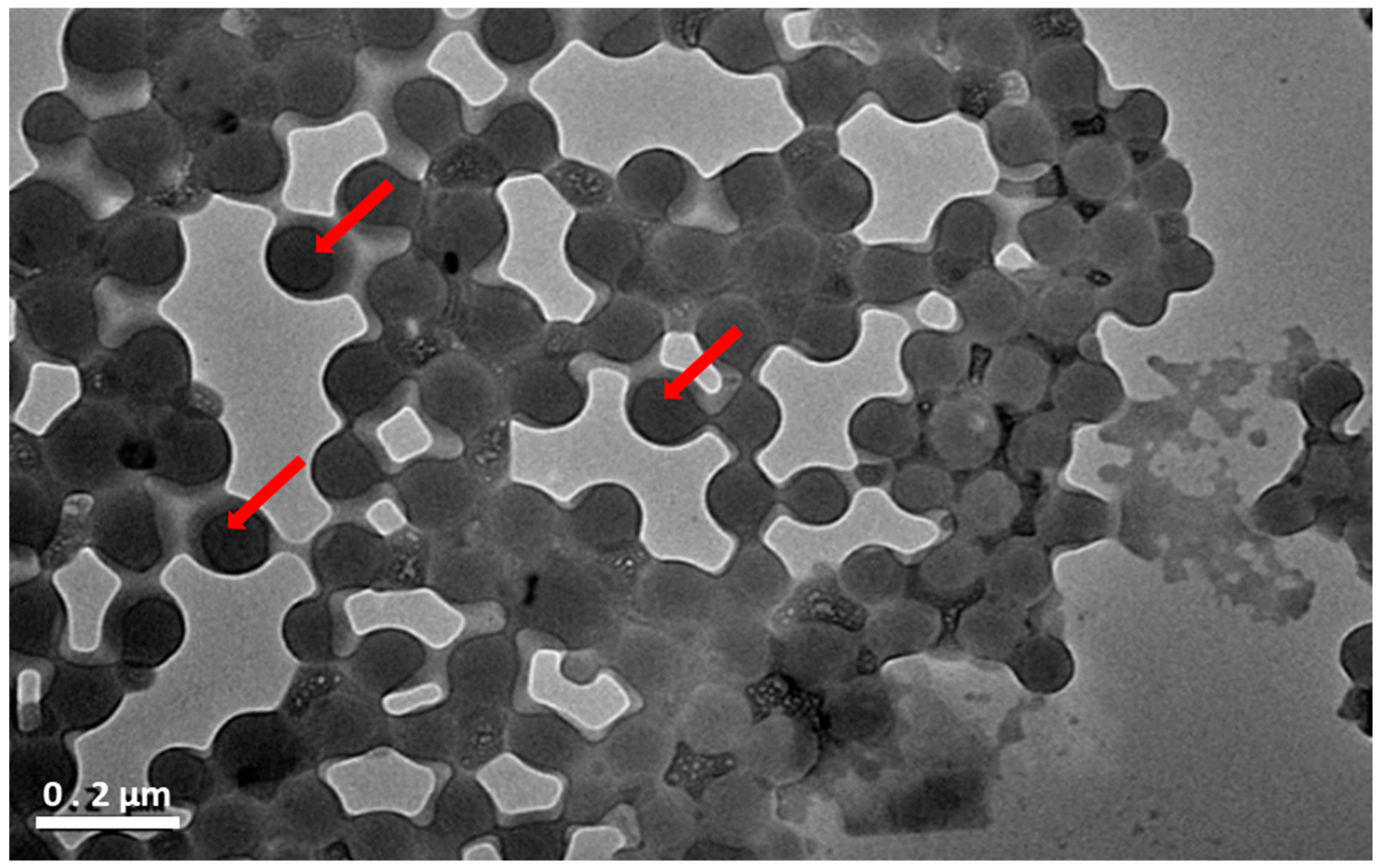
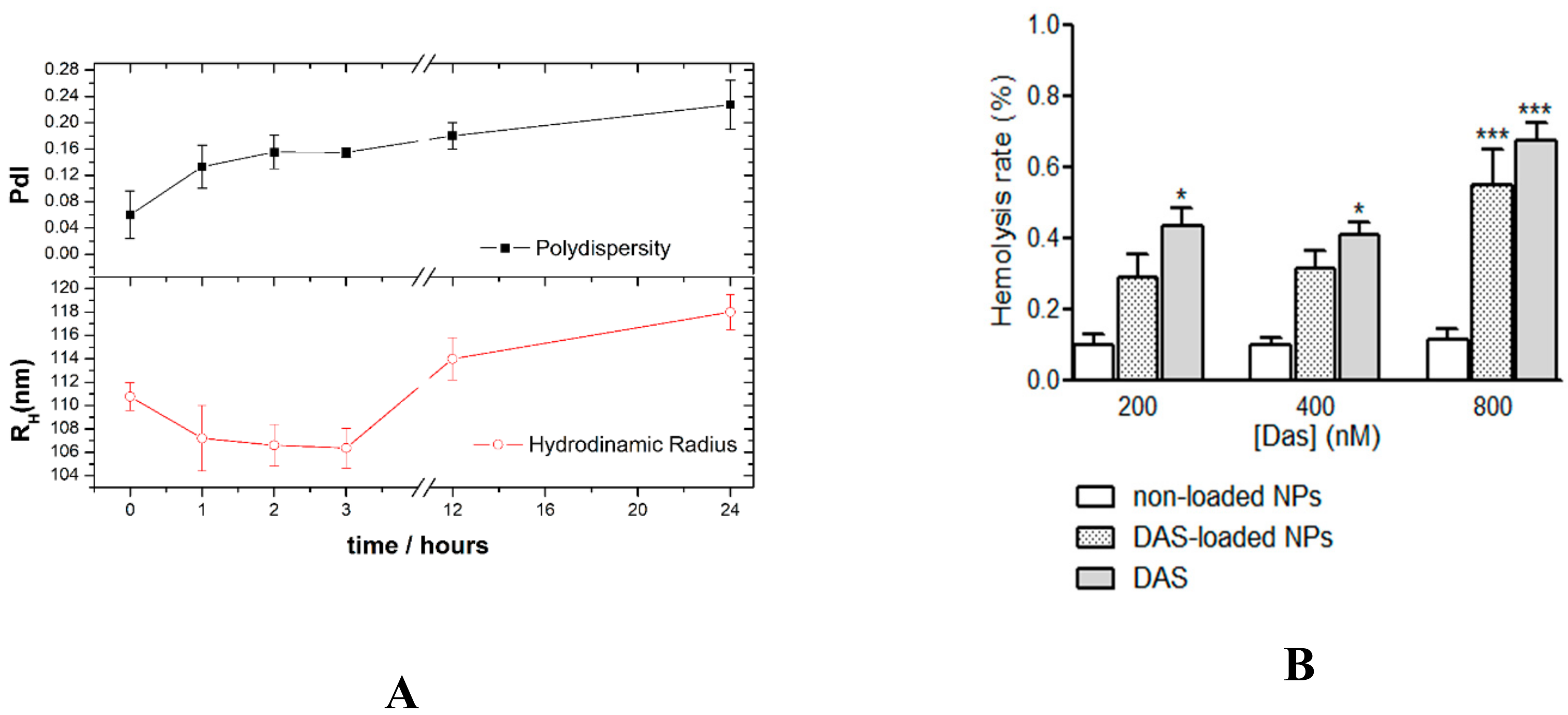
3.2. Preparation and Characterization of CHO/PA NPs
3.3. Enzymatic Degradation of CHO/PA NPs
3.4. CHO/PA NPs Toxicity in Non-Tumoral Cells
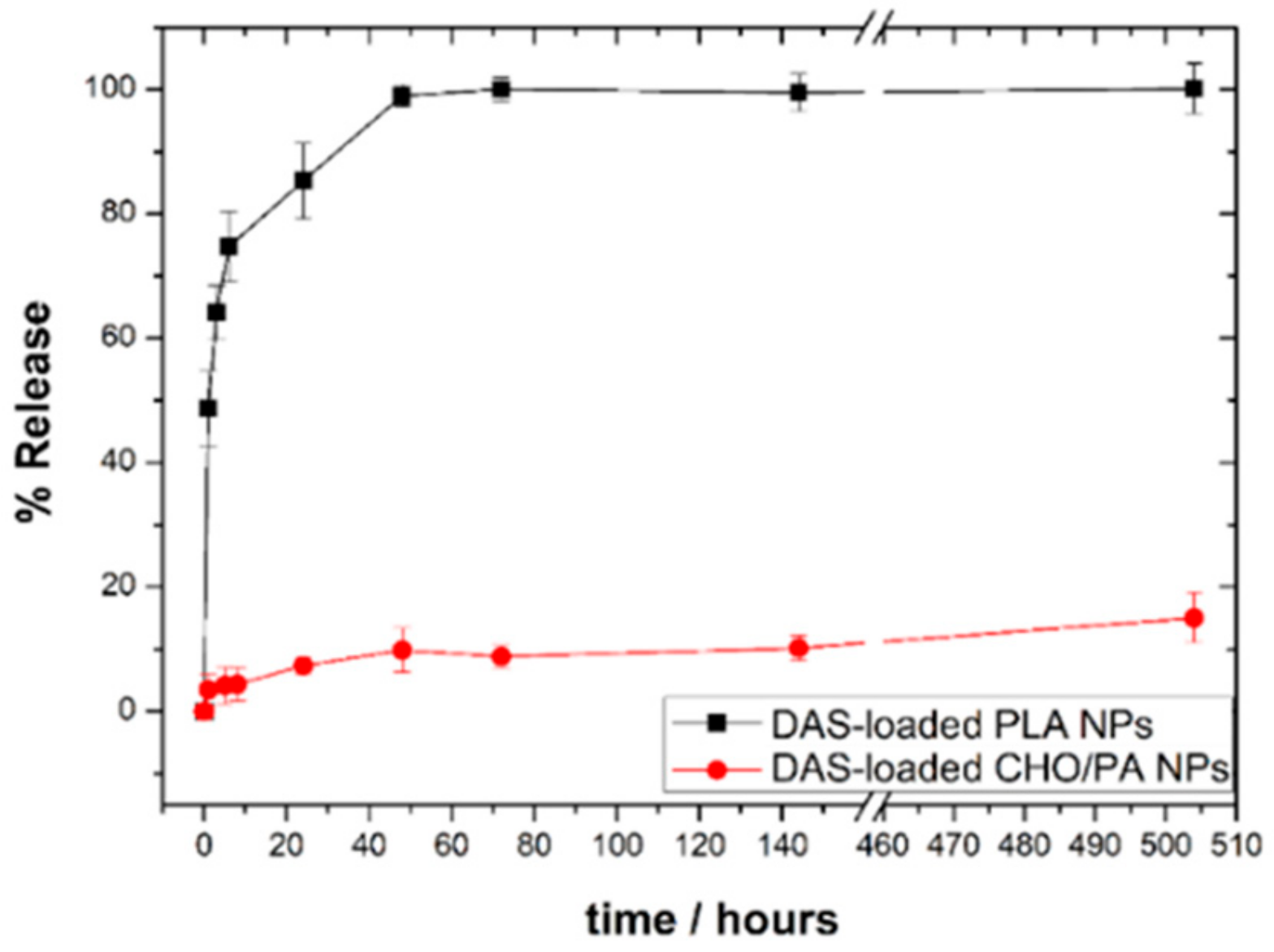
3.5. DAS Loading and Release
3.6. Stability and Compatibility in Blood of DAS-Loaded CHO/PA NPs
3.7. Antiproliferative Studies in Vitro
3.8. Cell Cycle Arrest and Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Wang, Y.; Zhao, Y.; Liu, H.; Zhao, Y.; Li, X.; Lin, Q. Biodegradable Micelles for NIR/GSH-Triggered Chemophototherapy of Cancer. Nanomaterials 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, U.; Oledzka, E.; Kamysz, W.; Białek, S.; Sobczak, M. The Effect of Polymer Microstructure on Encapsulation Efficiency and Release Kinetics of Citropin 1.1 from the Poly(ε-caprolactone) Microparticles. Nanomaterials 2018, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.R.R.; Dariya, B.; Mungamuri, S.K.; Chalikonda, G.; Kang, S.M.; Khan, I.N.; Sushma, P.S.; Nagaraju, G.P.; Pavitra, E.; Han, Y.K. Nanomaterials multifunctional behavior for enlighten cancer therapeutics. Semin. Cancer Biol. 2019, S1044-579X, 30190–30197. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2019, e1902333. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Nolasco, R.M.; Morales-Avila, E.; Ocampo-García, B.E.; Ferro-Flores, G.; Gibbens-Bandala, B.V.; Escudero-Castellanos, A.; Isaac-Olive, K. Preparation and in vitro evaluation of radiolabeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109766. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Kurd, M.; Sadegh Malvajerd, S.; Rezaee, S.; Hamidi, M.; Derakhshandeh, K. Oral delivery of indinavir using mPEG-PCL nanoparticles: preparation, optimization, cellular uptake, transport and pharmacokinetic evaluation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2123–2133. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Lee, B.K. PLA micro- and nano-particles. Adv. Drug Del. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef]
- Osorio Meléndez, D.; Castro-Osma, J.A.; Lara-Sánchez, A.; Rojas, R.S.; Otero, A. Ring-opening polymerization and copolymerization of cyclic esters catalyzed by amidinate aluminum complexes. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2397–2407. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef] [PubMed]
- Stößer, T.; Chen, T.T.D.; Zhu, Y.; Williams, C.K. Providing sustainable catalytic solutions for a rapidly changing world. Philos. Trans. A. Math. Phys. Eng. Sci. 2018, 376, 20170066. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Zhu, Y.; Ro-main, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Logothetis, C. Dasatinib: A potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat. Rev. 2010, 36, 492–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, C.; McDonald, T.; Ho, Y.W.; Liu, H.; Lin, A.; Forman, S.J.; Kuo, Y.H.; Bhatia, R. The Src and c-Kit kinase inhibitor dasatinib enhances p53-mediated targeting of human acute myeloid leukemia stem cells by chemotherapeutic agents. Blood 2013, 122, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, M.M.; Shiraga, S.; Schroeder, A.; Corbin, A.S.; Griffith, D.; Lee, F.Y.; Bokemeyer, C.; Deininger, M.W.; Druker, B.J.; Heinrich, M.C. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006, 66, 473–481. [Google Scholar] [CrossRef]
- Ceppi, P.; Papotti, M.; Monica, V.; Iacono, M.L.; Saviozzi, S.; Pautasso, M.; Novello, S.; Mussino, S.; Bracco, E.; Volante, M. Effects of Src kinase inhibition induced by dasatinib in non-small cell lung cancer cell lines treated with cisplatin. Mol. Cancer Ther. 2009, 8, 3066–3074. [Google Scholar] [CrossRef]
- Nam, S.; Kim, D.; Cheng, J.Q.; Zhang, S.; Lee, J.-H.; Buettner, R.; Mirosevich, J.; Lee, F.Y.; Jove, R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005, 65, 9185–9189. [Google Scholar] [CrossRef]
- Konecny, G.E.; Glas, R.; Dering, J.; Manivong, K.; Qi, J.; Finn, R.S.; Yang, G.R.; Hong, K.L.; Ginther, C.; Winterhoff, B.; et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br. J. Cancer 2009, 101, 1699–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.L.; Zhang, J.; Li, P.Z.; Lang, R.G.; Li, W.D.; Sun, H.; Liu, F.F.; Guo, X.J.; Gu, F.; Fu, L. Dasatinib inhibits c-src phosphorylation and prevents the proliferation of Triple-Negative Breast Cancer (TNBC) cells which overexpress Syndecan-Binding Protein (SDCBP). PLoS ONE 2017, 12, e0171169. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.C.; Seoane, S.; Ocaña, A.; Pandiella, A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: Possible combinations in solid tumors. Clin. Cancer Res. 2011, 17, 5546–5552. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Gil-Martin, M.; Antolín, S.; Atienza, M.; Montaño, Á.; Ribelles, N.; Urruticoechea, A.; Falcón, A.; Pernas, S.; Orlando, J.; et al. Efficacy and safety of dasatinib with trastuzumab and paclitaxel in first line HER2-positive metastatic breast cancer: results from the phase II GEICAM/2010-04 study. Breast Cancer Res. Treat. 2019, 174, 693–701. [Google Scholar] [CrossRef]
- Ocana, A.; Gil-Martin, M.; Martín, M.; Rojo, F.; Antolín, S.; Guerrero, Á.; Trigo, J.M.; Muñoz, M.; Pandiella, A.; Diego, N.G.; et al. A phase I study of the SRC kinase inhibitor dasatinib with trastuzumab and paclitaxel as first line therapy for patients with HER2-overexpressing advanced breast cancer. GEICAM/2010-04 study. Oncotarget 2017, 8, 73144–73153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Lai, K.L.; Chan, P.S.; Leung, S.C.; Li, H.Y.; Fang, Y.; To, K.K.W.; Choi, C.H.J.; Gao, Q.Y.; Lee, T.W.Y. Micellar delivery of dasatinib for the inhibition of pathologic cellular processes of the retinal pigment epithelium. Colloids Surf. B 2016, 140, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Choi, J.H.; Dai, Z.; Wang, J.; Kim, D.; Tang, X.; Zhu, L. Improving Tumor Specificity and Anticancer Activity of Dasatinib by Dual-Targeted Polymeric Micelles. ACS Appl. Mater. Interf. 2017, 9, 36642–36654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Ghazwani, M.; Zhao, W.; Huang, Y.; Zhang, X.; Venkataramanan, R.; Li, S. Effective co-delivery of doxorubicin and dasatinib using a PEG-Fmoc nanocarrier for combination cancer chemotherapy. Biomaterials 2015, 67, 104–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Li, B.; Li, Z.; Shetty, S.; Fu, J. Dasatinib-loaded albumin nanoparticles possess diminished endothelial cell barrier disruption and retain potent anti-leukemia cell activity. Oncotarget 2016, 7, 49699–49709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, T.L.; Grimes, S.W.; Lewis, R.L.; Alexis, F. Multilayered polymer-coated carbon nanotubes to deliver dasatinib. Mol. Pharm. 2014, 11, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Sabra, S.A.; Sheweita, S.A.; Haroun, M.; Ragab, D.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Ahmed, O.E.; Sohrab, R. Magnetically Guided Self-Assembled Protein Micelles for Enhanced Delivery of Dasatinib to Human Triple-Negative Breast Cancer Cells. Int. J. Pharm. 2019, 108. [Google Scholar] [CrossRef] [PubMed]
- Hornig, S.; Heinze, T.; Becer, C.R.; Schubert, U.S. Synthetic polymeric nanoparticles by nanoprecipitation. J. Mater. Chem. 2009, 19, 3838–3840. [Google Scholar] [CrossRef]
- Jäger, A.; Jäger, E.; Syrová, Z.; Mazel, T.; Kováčik, L.; Raška, I.; Höcherl, A.; Kučka, J.; Konefal, R.; Humajov, J.; et al. Poly(ethylene oxide monomethyl ether)- block-poly(propylene succinate) Nanoparticles: Synthesis and Characterization, Enzymatic and Cellular Degradation, Micellar Solubilization of Paclitaxel, and in Vitro and in Vivo Evaluation. Biomacromolecules 2018, 19, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Castro-Osma, J.A.; Posadas, I.; Alonso-Moreno, C.; Bravo, I.; Garzón, A.; Canales-Vázquez, J.; Ceña, V.; Lara-Sánchez, A.; Albaladejo, J.; et al. Assessment of doxorubicin delivery devices based on tailored bare polycaprolactone against glioblastoma. Int. J. Pharm. 2019, 558, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Martí-nez, J.; Alonso-Moreno, C.; Fernández-Baeza, J.; Tejeda, J.; Niza, E.; Castro-Osma, J.A.; Otero, A.; Lara-Sánchez, A. Alternating Copolymerization of Epoxides and Anhydrides Catalyzed by Aluminum Complexes. ACS Omega 2019, 3, 17581–17589. [Google Scholar] [CrossRef]
- Nachtergael, A.; Coulembier, O.; Dubois, P.; Helvenstein, M.; Duez, P.; Blankert, B.; Mespouille, L. Organocatalysis paradigm revisited: are metal-free catalysts really harmless? Biomacromolecules 2015, 16, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Higashi, M.; Kaneko, T.; Kida, T.; Akashi, M. Hydrolytic and enzymatic degradation of nanoparticles based on amphiphilic poly(gamma-glutamic acid)-graft-L-phenylalanine copolymers. Biomacromolecules 2006, 7, 297–303. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, W.; Choi, S.H. American Phamaceutical Rev. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/188841-FDA-s-Regulatory-Science-Program-for-Generic-PLA-PLGA-Based-Drug-Products/ (accessed on 15 June 2016).
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Ocana, A.; Pandiella, A.; Siu, L.L.; Tannock, I.F. Preclinical development of molecular-targeted agents for cancer. Nat. Rev. Clin. Oncol. 2010, 8, 200–209. [Google Scholar] [CrossRef]
- Giacomelli, F.C.; Stepánek, P.; Schmidt, V.; Jäger, E.; Jäger, A.; Giacomelli, C. Light scattering evidence of selective protein fouling on biocompatible block copolymer micelles. Nanoscale 2012, 4, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Jäger, E.; Jäger, A.; Chytil, P.; Etrych, T.; Říhová, B.; Giacomellic, F.C.; Štěpánek, P.; Ulbricha, K. Combination chemotherapy using core-shell nanoparticles through the self-assembly of HPMA-based copolymers and degradable polyester. J. Control. Release 2013, 165, 153–161. [Google Scholar] [CrossRef] [PubMed]
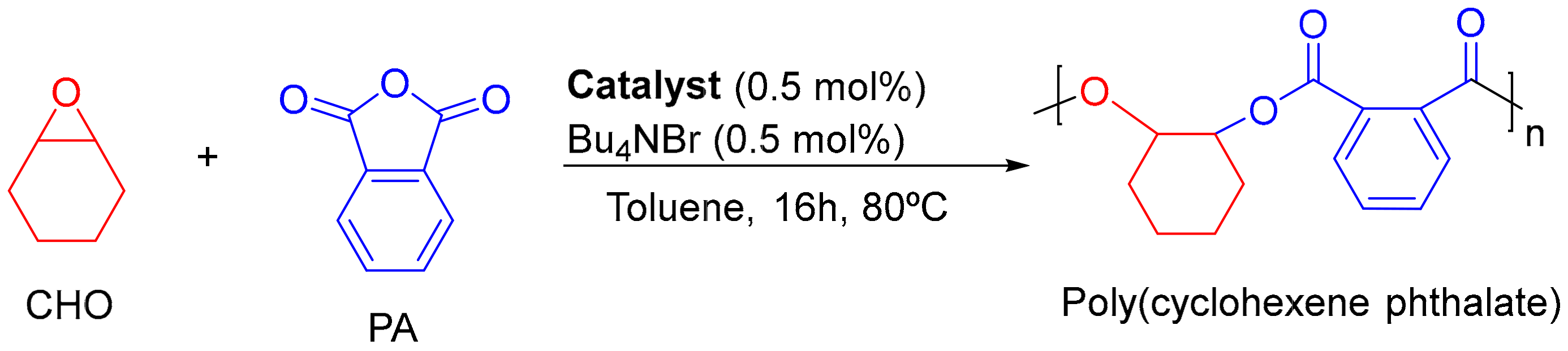



| Entry | Conv. (%) 2 | Polyester (%) 2 | Mnexp 3 | PdI 3 |
|---|---|---|---|---|
| 1 1 | 100 | 95 | 3572 | 1.11 |
| 2 4 | 100 | 94 | 3114 | 1.16 |
| 3 5 | 100 | 95 | 2533 | 1.21 |
| 4 6 | 100 | 98 | 2088 | 1.09 |
| Entry | Mnexp | Average Size (nm) | PdI | Z-Potential (mV) |
|---|---|---|---|---|
| 1 | 2088 | 100.0 ± 1.0 | 0.125 ± 0.02 | −23.6 ± 1.74 |
| 2 | 2533 | 100.1 ± 0.7 | 0.123 ± 0.01 | −21.9 ± 1.0 |
| 3 | 3114 | 113.7 ± 1.5 | 0.120 ± 0.03 | −26.9 + 0.54 |
| 4 | 3572 | 107.3 ± 1.5 | 0.142 ± 0.04 | −26.3 ± 0.8 |
| Formulation | Average Size (nm) | PdI | Z-value (mV) | RE (%) (72 h) | EE (%) | LE (%) |
|---|---|---|---|---|---|---|
| CHO/PA NPs | 104.5 ± 0.8 | 0.20 ± 0.01 | 32.1 ± 1.8 | 99.9 ± 2.0 | 96.7 ± 0.8 | 10.1 ± 0.4 |
| PLA NPs | 179.3 ± 3.8 | 0.20 ± 0.03 | 26.9 ± 1.74 | 8.8 ± 1.9 | 83.3 ± 2.5 | 11.7 ± 3.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niza, E.; Nieto-Jiménez, C.; Noblejas-López, M.d.M.; Bravo, I.; Castro-Osma, J.A.; de la Cruz-Martínez, F.; Martínez de Sarasa Buchaca, M.; Posadas, I.; Canales-Vázquez, J.; Lara-Sanchez, A.; et al. Poly(Cyclohexene Phthalate) Nanoparticles for Controlled Dasatinib Delivery in Breast Cancer Therapy. Nanomaterials 2019, 9, 1208. https://doi.org/10.3390/nano9091208
Niza E, Nieto-Jiménez C, Noblejas-López MdM, Bravo I, Castro-Osma JA, de la Cruz-Martínez F, Martínez de Sarasa Buchaca M, Posadas I, Canales-Vázquez J, Lara-Sanchez A, et al. Poly(Cyclohexene Phthalate) Nanoparticles for Controlled Dasatinib Delivery in Breast Cancer Therapy. Nanomaterials. 2019; 9(9):1208. https://doi.org/10.3390/nano9091208
Chicago/Turabian StyleNiza, Enrique, Cristina Nieto-Jiménez, María del Mar Noblejas-López, Iván Bravo, José Antonio Castro-Osma, Felipe de la Cruz-Martínez, Marc Martínez de Sarasa Buchaca, Inmaculada Posadas, Jesús Canales-Vázquez, Agustín Lara-Sanchez, and et al. 2019. "Poly(Cyclohexene Phthalate) Nanoparticles for Controlled Dasatinib Delivery in Breast Cancer Therapy" Nanomaterials 9, no. 9: 1208. https://doi.org/10.3390/nano9091208
APA StyleNiza, E., Nieto-Jiménez, C., Noblejas-López, M. d. M., Bravo, I., Castro-Osma, J. A., de la Cruz-Martínez, F., Martínez de Sarasa Buchaca, M., Posadas, I., Canales-Vázquez, J., Lara-Sanchez, A., Hermida-Merino, D., Solano, E., Ocaña, A., & Alonso-Moreno, C. (2019). Poly(Cyclohexene Phthalate) Nanoparticles for Controlled Dasatinib Delivery in Breast Cancer Therapy. Nanomaterials, 9(9), 1208. https://doi.org/10.3390/nano9091208