Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer
Abstract
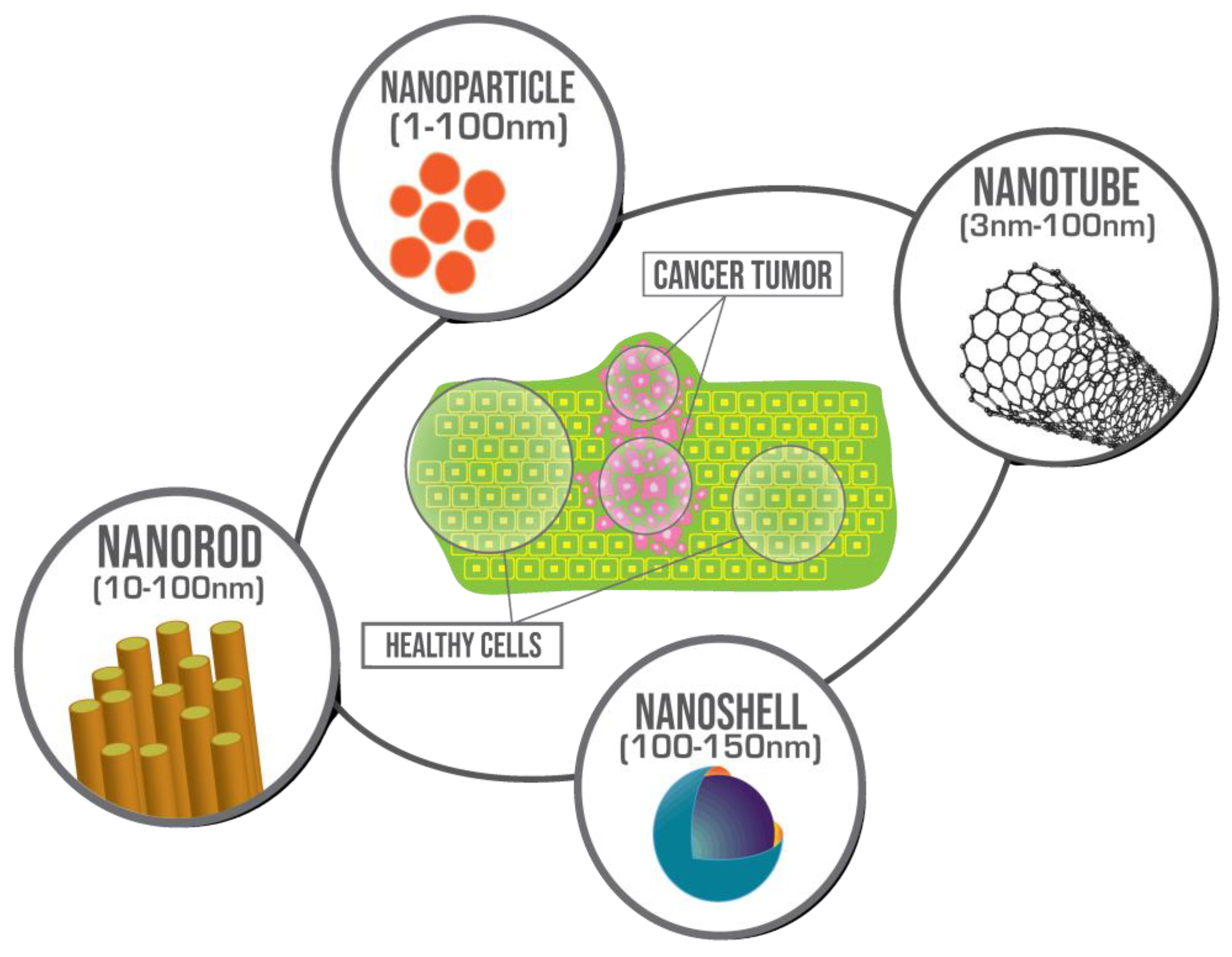
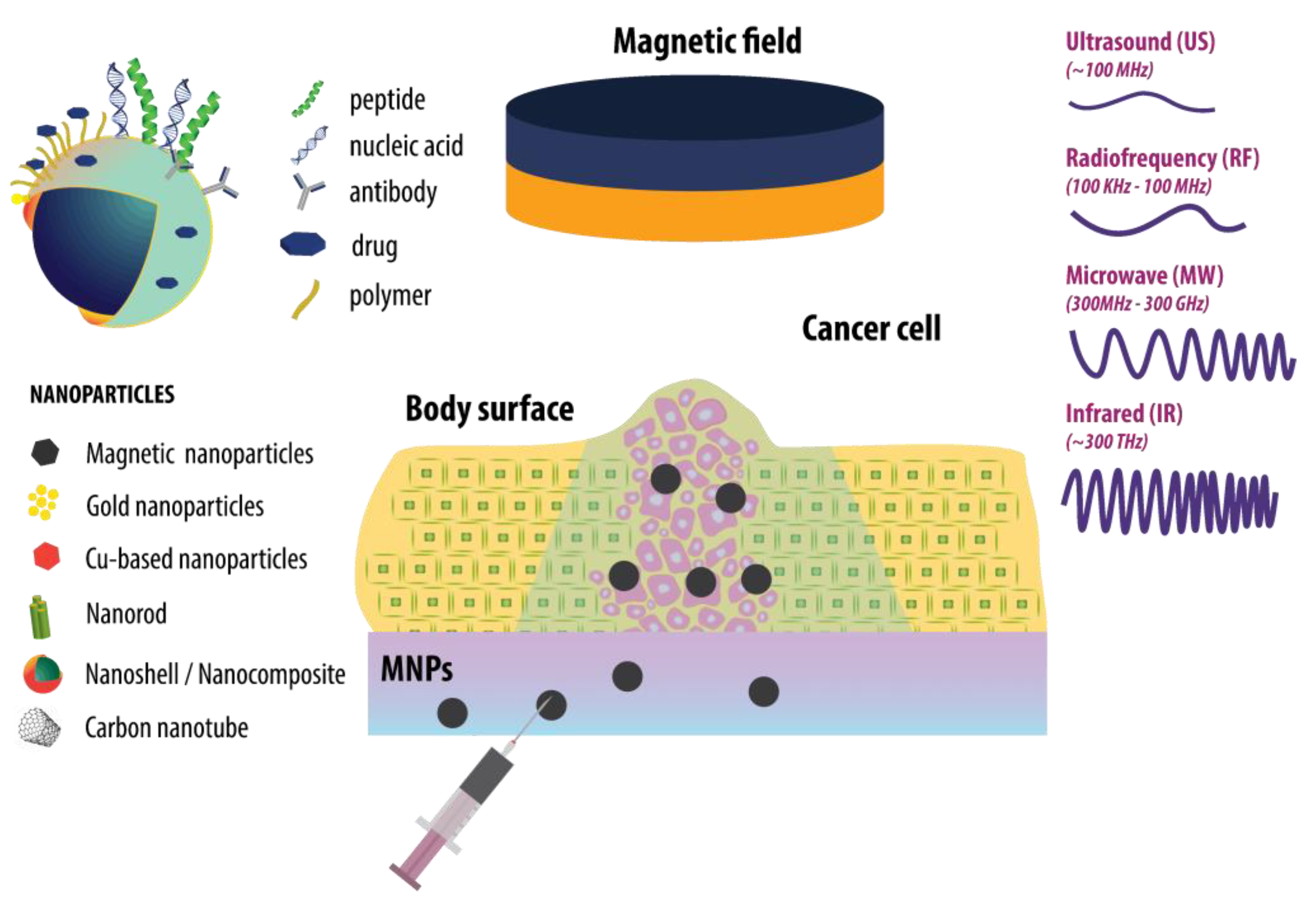
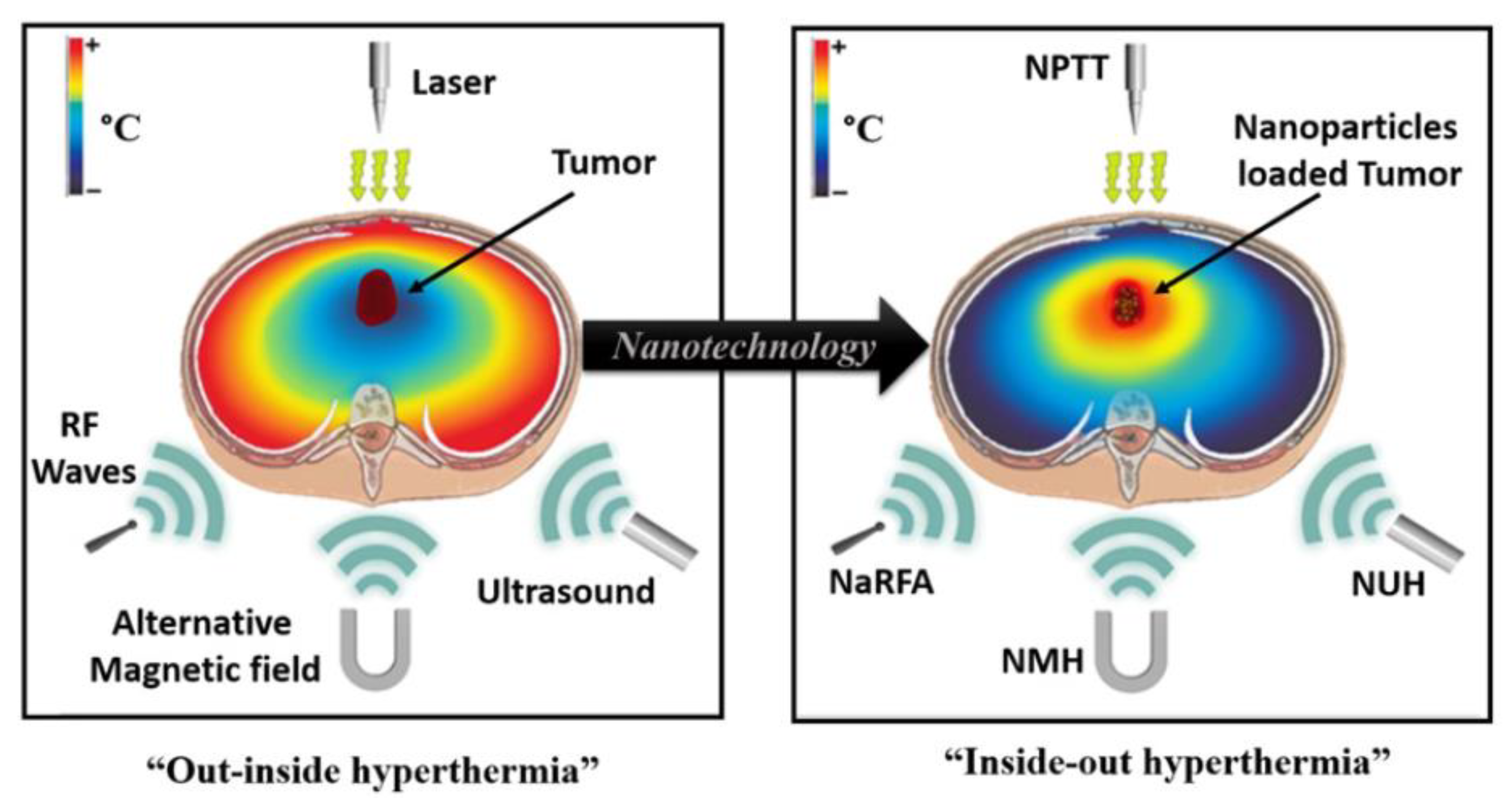
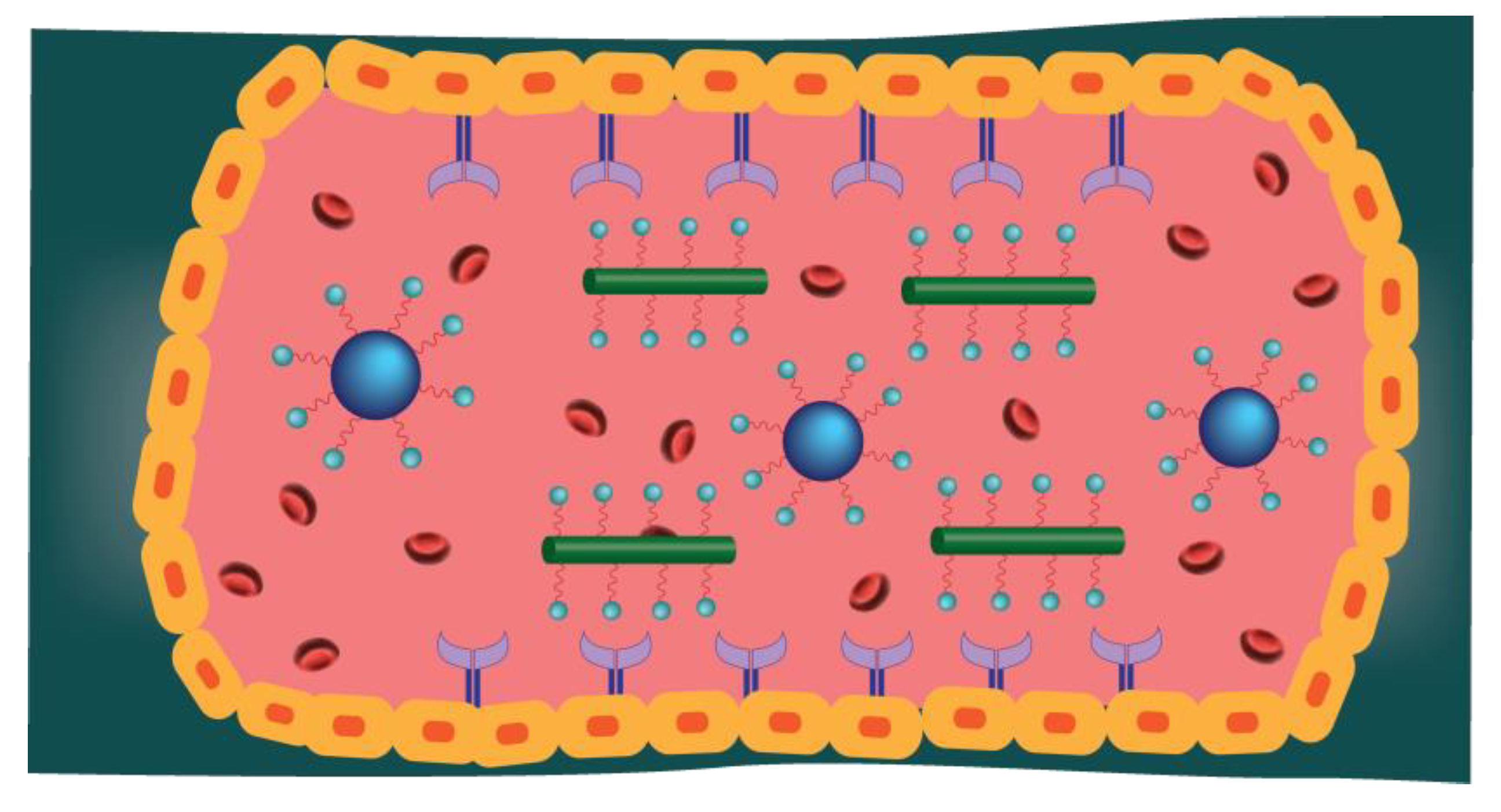
1. Introduction
2. Magnetic Nanoparticles (MNP)
3. Gold Nanoparticles (AuNP)
4. CuS Nanoparticles
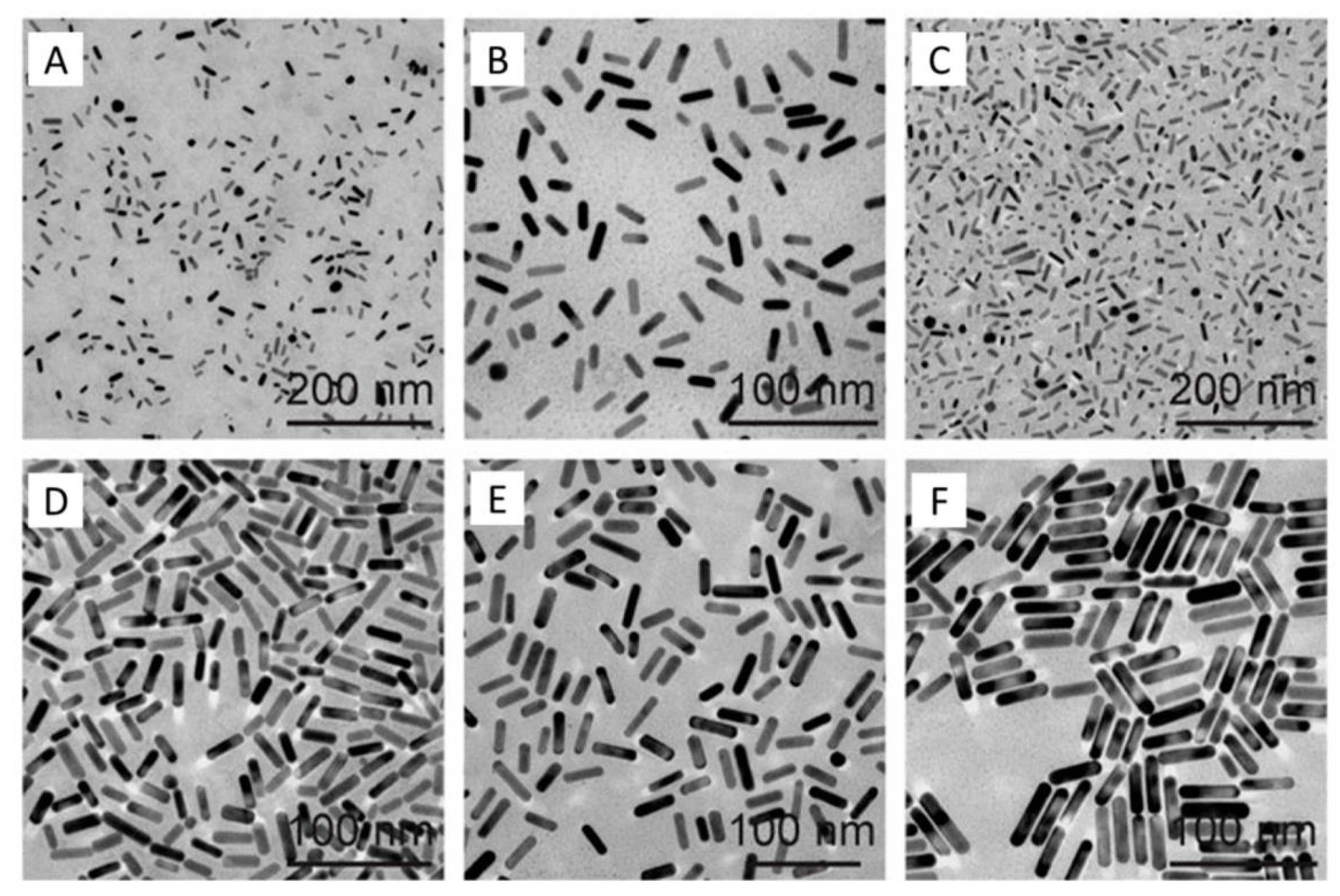
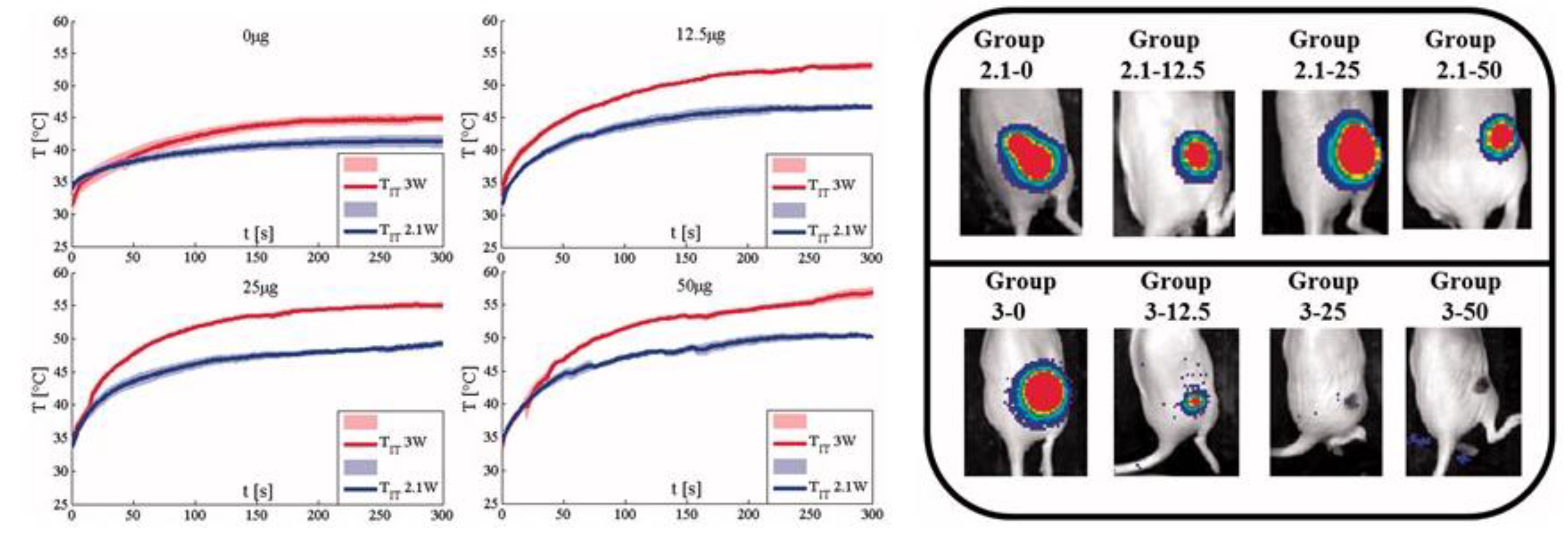
5. Nanorods
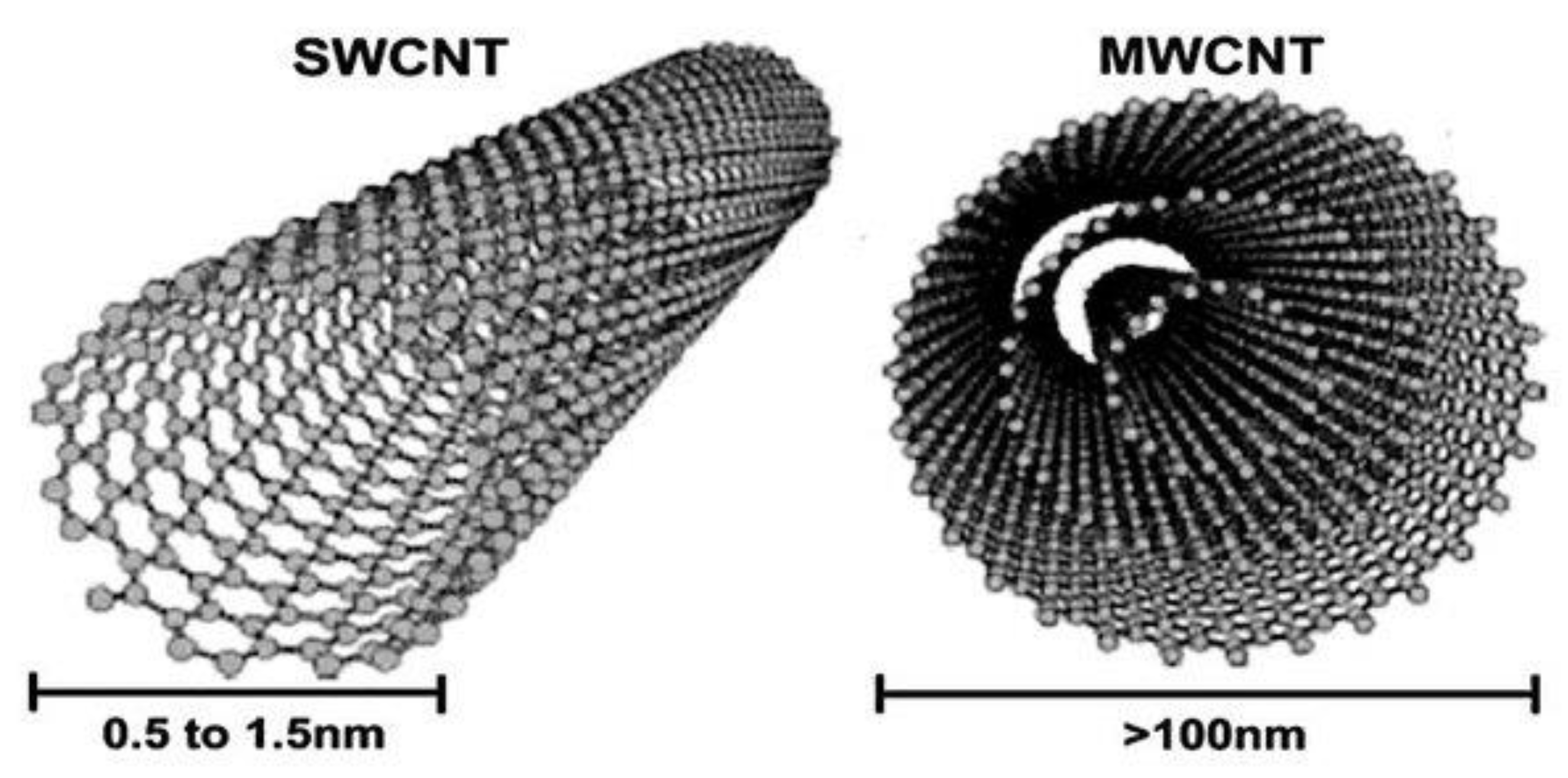
6. Carbon Nanotubes (CNTs)
7. Nanoshells/Nanocomposites
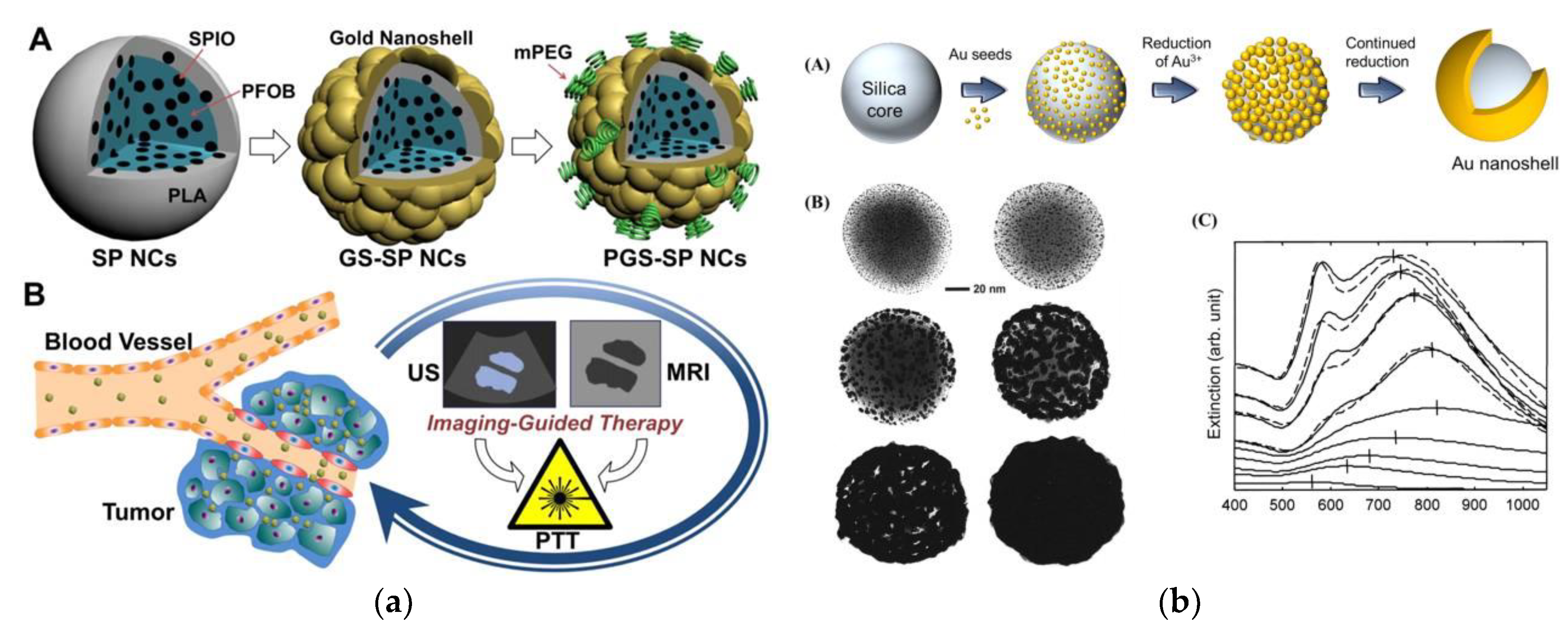
7.1. Nanoshells
7.2. Nanocomposites
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research in Cancer. Latest Global Cancer Data, 2018; Press Release #263; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Ren, Y.; Qi, H.; Chen, Q.; Ruan, L. Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int. J. Heat Mass Transf. 2017, 106, 212–221. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Brace, C.L.; Lee, F.T.; Goldberg, S.N. Principles of and Advances in Percutaneous Ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Saccomandi, P.; Fong, Y. Laser Ablation for Cancer: Past, Present and Future. J. Funct. Biomater. 2017, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Carrafiello, G.; Laganà, D.; Mangini, M.; Fontana, F.; Dionigi, G.; Boni, L.; Rovera, F.; Cuffari, S.; Fugazzola, C. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int. J. Surg. 2008, 6 (Suppl. 1), 65–69. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for Thermal Cancer Therapy. J. Biomech. Eng. 2009, 131, 074001. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, G.A.; Mohazzabi, P.; McCormack, P.; Jabbari, S. Nanotechnology in cancer prevention, detection and treatment: Bright future lies ahead. World Rev. Sci. Technol. Sustain. Dev. 2007, 4, 226. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef]
- Lu, B.Q.; Zhu, Y.J.; Ao, H.Y.; Qi, C.; Chen, F. Synthesis and characterization of magnetic iron oxide/calcium silicate mesoporous nanocomposites as a promising vehicle for drug delivery. ACS Appl. Mater. Interfaces 2012, 4, 6969–6974. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Naghdi, N.; Nokhbatolfoghahaei, H.; Bagheri, H. The combination of laser therapy and metal nanoparticles in cancer treatment originated from epithelial tissues: A literature review. J. Lasers Med. Sci. 2016, 7, 62–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manthe, R.L.; Foy, S.P.; Krishnamurthy, N.; Sharma, B.; Labhasetwar, V. Tumor ablation and nanotechnology. Mol. Pharm. 2010, 7, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Wust, P.; Fähling, H.; John, W.; Hinz, A.; Felix, R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int. J. Hyperth. 2009, 25, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Letfullin, R.R.; Iversen, C.B.; George, T.F. Modeling nanophotothermal therapy: Kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Irudayaraj, J. Multifunctional magnetic-optical nanoparticle probes for simultaneous detection, separation, and thermal ablation of multiple pathogens. Small 2010, 6, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Burford, C.D.; Bhattacharyya, K.D.; Boriraksantikul, N.; Whiteside, P.J.D.; Robertson, B.P.; Peth, S.M.; Islam, N.E.; Viator, J.A. Nanoparticle mediated thermal ablation of breast cancer cells using a nanosecond pulsed electric field. IEEE Trans. Nanobiosci. 2013, 12, 112–118. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Hilger, I.; Hiergeist, R.; Hergt, R.; Winnefeld, K.; Schubert, H.; Kaiser, W.A. Thermal ablation of tumors using magnetic nanoparticles: An in vivo feasibility study. Investig. Radiol. 2002, 37, 580–586. [Google Scholar] [CrossRef]
- Attar, M.M.; Haghpanahi, M.; Amanpour, S.; Mohaqeq, M. Analysis of bioheat transfer equation for hyperthermia cancer treatment. J. Mech. Sci. Technol. 2014, 28, 763–771. [Google Scholar] [CrossRef]
- Toy, R. The Effect of Particle Size and Shape on the In Vivo Journey of Nanoparticles. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2014. [Google Scholar]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Gui, C.; Cui, D.-X. Functionalized gold nanorods for tumor imaging and targeted therapy. Cancer Biol. Med. 2012, 9, 221–233. [Google Scholar] [PubMed]
- Gajbhiye, K.R.; Gajbhiye, J.M. EPR effect based nanocarriers targeting for treatment of cancer. Int. J. Drug Deliv. 2017, 8, 117–124. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- García-Jimeno, S.; Ortega-Palacios, R.; Cepeda-Rubio, M.; Vera, A.; Leija, L.; Estelrich, J. Improved Thermal Ablation Efficacy Using Magnetic Nanoparticles: A Study in Tumor Phantoms. Prog. Electromagn. Res. 2012, 128, 229–248. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Original Contribution Thermal dose Determination in Cancer Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Haemmerich, D.; Wood, B.J. Hepatic radiofrequency ablation at low frequencies preferentially heats tumour tissue. Int. J. Hyperth. 2006, 22, 563–574. [Google Scholar] [CrossRef]
- Gassino, R.; Liu, Y.; Konstantaki, M.; Vallan, A.; Pissadakis, S.; Perrone, G. A fiber optic probe for tumor laser ablation with integrated temperature measurement capability. J. Light. Technol. 2017, 35, 3447–3454. [Google Scholar] [CrossRef]
- Jacques, S.L. Erratum: Optical properties of biological tissues: A review (Physics in Medicine and Biology (2013) 58). Phys. Med. Biol. 2013, 58, 5007–5008. [Google Scholar] [CrossRef]
- Mathew, M.; Mikhail, A.S.; Wood, B.J.; Partanen, A.; Graham, C.; Negussie, A.H. Evaluation of a tissue-mimicking thermochromic phantom for radiofrequency ablation. Med. Phys. 2016, 43, 4304–4311. [Google Scholar]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, H. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Vedernikova, I.A. Magnetic nanoparticles: Advantages of using, methods for preparation, characterization, application in pharmacy. Rev. J. Chem. 2015, 5, 256–280. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef] [PubMed]
- Abenojar, E.C.; Wickramasinghe, S.; Bas-Concepcion, J.; Samia, A.C.S. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Prog. Nat. Sci. Mater. Int. 2016, 26, 440–448. [Google Scholar] [CrossRef]
- Nedelcu, G. Magnetic nanoparticles impact on tumoral cells in the treatment by magnetic fluid hyperthermia. Dig. J. Nanomater. Biostruct. 2008, 3, 103–107. [Google Scholar]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Particles, F.; Hilger, I.; Hergt, R.; Andr, W.; Ambly, C.G.; Hilger, I. Physical Limits of Hyperthermia Using Magnetite Physical Limits of Hyperthermia Using Magnetite Fine Particles. IEEE Trans. Magn. 1998, 34, 3745–3754. [Google Scholar]
- Salloum, M.; Ma, R.H.; Weeks, D.; Zhu, L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int. J. Hyperth. 2008, 24, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S.; Zeisberger, M. Validity limits of the Néel relaxation model of magnetic nanoparticles for hyperthermia. Nanotechnology 2010, 21, 015706. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.D.S.R.K. Effects of Electromagetic Heating on Internal Viscera: A Preliminary to the Treatment of Human Tumors. Ann. Surg. 1965, 161, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Dürr, S.; Janko, C.; Lyer, S.; Tripal, P.; Schwarz, M.; Zaloga, J.; Tietze, R.; Alexiou, C. Magnetic nanoparticles for cancer therapy. Nanotechnol. Rev. 2013, 2, 395–409. [Google Scholar]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment (Review). Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Kettering, M.; Winter, J.; Zeisberger, M.; Bremer-Streck, S.; Oehring, H.; Bergemann, C.; Alexiou, C.; Hergt, R.; Halbhuber, K.J.; Kaiser, W.A.; et al. Magnetic nanoparticles as bimodal tools in magnetically induced labelling and magnetic heating of tumour cells: An in vitro study. Nanotechnology 2007, 18, 175101. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Hilger, I.; Andrä, W.; Hergt, R.; Hiergeist, R.; Schubert, H.; Kaiser, W.A. Electromagnetic Heating of Breast Tumors in Interventional Radiology: In Vitro and in Vivo Studies in Human Cadavers and Mice. Radiology 2001, 218, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Towards breast cancer treatment by magnetic heating. J. Magn. Magn. Mater. 2005, 293, 314–319. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauf, K.; Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldofner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of Prostate Cancer Using Magnetic Nanoparticles: Feasibility, Imaging, and Three-Dimensional Temperature Distribution. Eur. Urol. 2007, 52, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Thiesen, B.; Jordan, A.; Taymoorian, K.; Gneveckow, U.; Waldofner, N.; Scholz, R.; Koch, M.; Lein, M.; Jung, K.; et al. Magnetic fluid hyperthermia (MFH) reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate 2005, 64, 283–292. [Google Scholar] [CrossRef]
- Rich, J.N.; Robinson, J.W.; Rowitch, D.H.; Sampson, J.H.; Taylor, M.D.; Workman, P.; Gilbertson, R.J. Statement brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar]
- Bredlau, A.L.; McCrackin, M.A.; Motamarry, A.; Helke, K.; Chen, C.; Broomee, A.-M.; Haemmerich, D. Thermal Therapy Approaches for Treatment of Brain Tumors in Animals and Humans. Crit Rev Biomed Eng. 2016, 44, 443–457. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Bruners, P.; Braunschweig, T.; Hodenius, M.; Pietsch, H.; Penzkofer, T.; Baumann, M.; Gunter, R.W.; Schmitz-Rode, T.; Mahnken, A.H. Thermoablation of malignant kidney tumors using magnetic nanoparticles: An in vivo feasibility study in a rabbit model. Cardiovasc. Intervent. Radiol. 2010, 33, 127–134. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldöfner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int. J. Hyperth. 2007, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Wiekhorst, F.; Steinhoff, U.; Trahmz, L. Localization and quantification of magnetic nanoparticles by multichannel magnetorelaxometry for in vivo hyperthermia studies in carcinoma models. IFMBE Proc. 2009, 25, 302–305. [Google Scholar]
- Andrä, W.; D’Ambly, C.G.; Hergt, R.; Hilger, I.; Kaiser, W.A. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J. Magn. Magn. Mater. 1999, 194, 197–203. [Google Scholar] [CrossRef]
- Versiani, A.F.; Andrade, L.M.; Martins, E.M.N.; Scalzo, S.; Geraldo, J.M.; Chaves, C.R.; Ferreira, D.C.; Ladeira, M.; Guatimosim, S.; Ladeira, L.O.; et al. Gold nanoparticles and their applications in biomedicine. Futur Med. Virol. 2016, 11, 293–309. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Cardinal, J.; Klune, J.R.; Chory, E.; Jeyabalan, G.; Kanzius, J.S.; Nalesnik, M.; Geller, D.A. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery 2008, 144, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gannon, C.J.; Patra, C.R.; Bhattacharya, R.; Mukherjee, P.; Curley, S.A. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J. Nanobiotechnol. 2008, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Zhu, C.; Massey, K.L.; Thompson, C.S.; Kaluarachchi, W.D.; Hamir, A.N.; Curley, S.A. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin. Cancer Res. 2010, 16, 5712–5721. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Curley, S.A. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer 2010, 116, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; de Palma, R.; Orditura, M.; de Vita, F.; Ciardiello, F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin. Exp. Immunol. 2009, 158, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the Minimum Temperature Required for Selective Photothermal Destruction of Cancer Cells with the Use of Immunotargeted Gold Nanoparticles. Photochem. Photobiol. 2006, 82, 412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Panderi, I.; Yan, D.D.; Szulak, K.; Li, Y.; Chen, Y.; Ma, H.; Niesen, D.B.; Seeram, N.; Ahmed, A.; et al. A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano 2013, 7, 8780–8793. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, Y.; Shi, S.; Hao, S.; Wei, J.; Chen, X. Copper sulfide nanoparticles with phospholipid-PEG coating for in vivo near-infrared photothermal cancer therapy. Chem. Asian J. 2015, 10, 370–376. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Y.; Adachi, M.; Wen, X.; Erwin, B.; Mawlawi, O.; Lai, S.Y.; Li, C. Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials 2015, 57, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: A potential cancer diagnostic marker. Nano Lett. 2007, 7, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhou, L.; Liu, Y.; Meng, L.; Zhang, K.; Wu, X.; Zhang, L.; Li, B.; Chen, C. Characterization of gold nanorods in vivo by integrated analytical techniques: Their uptake, retention, and chemical forms. Anal. Bioanal. Chem. 2010, 396, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine 2007, 2, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, Y.; Wang, L.; Xu, L.; Bai, R.; Ji, Y.; Wu, X.; Zhao, Y.; Li, Y.; Chen, C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 2010, 31, 7606–7619. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, Y.; Tian, Q.; Yang, S. Small gold nanorods: Recent advances in synthesis, biological imaging, and cancer therapy. Materials 2017, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Fang, C.; Zhu, X.M.; Ruan, Q.; Wang, Y.-X.J.; Wang, J. Synthesis of Absorption-Dominant Small Gold Nanorods and Their Plasmonic Properties. Langmuir 2015, 31, 7418–7426. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- von Maltzahn, G.; Park, J.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef]
- Huang, H.C.; Rege, K.; Heys, J.J. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano 2010, 4, 2892–2900. [Google Scholar] [CrossRef]
- Soni, S.; Tyagi, H.; Taylor, R.A.; Kumar, A. Investigation on nanoparticle distribution for thermal ablation of a tumour subjected to nanoparticle assisted thermal therapy. J. Therm. Biol. 2014, 43, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Emoto, K.; Su, L.J.; Yang, X.; Flaig, T.W.; Park, W. Functionalized gold nanorods for thermal ablation treatment of bladder cancer. J. Biomed. Nanotechnol. 2014, 10, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Schena, E.; Zhumkhawala, A.; Aboody, K.S.; Berlin, J.M. Internal temperature increase during photothermal tumour ablation in mice using gold nanorods. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 2563–2566. [Google Scholar]
- Mooney, R.; Schena, E.; Saccomandi, P.; Zhumkhawala, A.; Aboody, K.; Berlin, J.M. Gold nanorod-mediated near-infrared laser ablation: In vivo experiments on mice and theoretical analysis at different settings. Int. J. Hyperth. 2017, 33, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Kim, Y.S.; Choi, Y. Effects of gold Nanorod concentration on the depth-related temperature increase during hyperthermic ablation. Small 2011, 7, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mceuen, P.L.; Fuhrer, M.S.; Park, H. Single-walled carbon nanotube electronics. IEEE Trans. Nanotechnol. 2002, 1, 78–85. [Google Scholar] [CrossRef]
- Elhissi, A.; Ahmed, W.; Dhanak, V.R.; Subramani, K. Carbon Nanotubes in Cancer Therapy and Drug Delivery, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef]
- Ding, R.G.; Lu, G.Q.; Yan, Z.F.; Wilson, M.A. Recent Advances in the Preparation and Utilization of Carbon Nanotubes for Hydrogen Storage. J. Nanosci. Nanotechnol. 2001, 1, 7–29. [Google Scholar] [CrossRef]
- Zhou, F.; Xing, D.; Ou, Z.; Wu, B.; Resasco, D.E.; Chen, W.R. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J. Biomed. Opt. 2009, 14, 021009. [Google Scholar] [CrossRef]
- Iancu, C.; Mocan, L. Advances in cancer therapy through the use of carbon nanotube-mediated targeted hyperthermia. Int. J. Nanomed. 2011, 6, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Nakatsuji, H.; Inada, M.; Matoba, Y.; Umeyama, T.; Tsujimoto, M.; Isoda, S.; Hashida, M.; Imahori, H. Photodynamic and photothermal effects of semiconducting and metallic-enriched single-walled carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 17862–17865. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H. Selective Cancer Cell Destruction Linked references are available on JSTOR for this article: Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Pnas 2005, 102, 1600–11605. [Google Scholar]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011, 6, 2963–2979. [Google Scholar]
- Ji, S.R.; Liu, C.; Zhang, B.; Yang, F.; Xu, J.; Long, J.; Jin, C.; Fu, D.; Ni, Q.; Yu, X. Carbon nanotubes in cancer diagnosis and therapy. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.; Botelho, E.C.; Costa, M.L.; Bandeira, C.F. Carbon nanotube buckypaper reinforced polymer composites: A review. Polímeros 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Mayr, E. American Association for the Advancement of Science Handbook. Science 1998, 266, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Munge, B.; Patel, V.; Jensen, G.; Bhirde, A.; Gong, J.D.; Kim, S.N.; Gillespie, J.; Gutkind, J.S.; Papadimitrakopoulos, F.; et al. Carbon nanotube amplification strategies for highly sensitive immunodetection of cancer biomarkers. J. Am. Chem. Soc. 2006, 128, 11199–11205. [Google Scholar] [CrossRef]
- Park, D.-W.; Kim, Y.-H.; Kim, B.S.; So, H.-M.; Won, K.; Lee, J.-O.; Kong, K.-J.; Chang, H. Detection of Tumor Markers Using Single-Walled Carbon Nanotube Field Effect Transistors. J. Nanosci. Nanotechnol. 2006, 6, 3499–3502. [Google Scholar] [CrossRef]
- Liu, X.; Tao, H.; Yang, K.; Zhang, S.; Lee, S.T.; Liu, Z. Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials 2011, 32, 144–151. [Google Scholar] [CrossRef]
- Gannon, C.J.; Cherukuri, P.; Yakobson, B.I.; Cognet, L.; Kanzius, J.S.; Kittrell, C.; Weisman, R.B.; Pasquali, M.; Schmidt, H.K.; Smalley, R.E.; et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 2007, 110, 2654–2665. [Google Scholar] [CrossRef]
- Marches, R.; Mikoryak, C.; Wang, R.H.; Pantano, P.; Draper, R.K.; Vitetta, E.S. The importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cells. Nanotechnology 2011, 22, 095101. [Google Scholar] [CrossRef]
- Hashida, Y.; Tanaka, H.; Zhou, S.; Kawakami, S.; Yamashita, F.; Murakami, T.; Umeyama, T.; Imahori, H.; Hashida, M. Photothermal ablation of tumor cells using a single-walled carbon nanotube-peptide composite. J. Control. Release 2014, 173, 58–66. [Google Scholar] [CrossRef]
- Fisher, J.W.; Sarkar, S.; Buchanan, C.F.; Szot, C.S.; Whitney, J.; Hatcher, H.C.; Torti, S.V.; Rylander, C.G.; Rylander, M.N. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010, 70, 9855–9864. [Google Scholar] [CrossRef]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Bele, C.; Orza, A.I.; Lucan, C.; Stiufiuc, R.; Manaila, I.; Iulia, F.; Dana, I.; et al. Selective ex-vivo photothermal ablation of human pancreatic cancer with albumin functionalized multiwalled carbon nanotubes. Int. J. Nanomed. 2011, 6, 915–928. [Google Scholar]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef]
- Lal, S.; Clare, S.E.; Halas, N.J. Photothermal Therapy: Impending Clinical Impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Liu, A.; Wang, G.; Wang, F.; Zhang, Y. Gold nanostructures with near-infrared plasmonic resonance: Synthesis and surface functionalization. Coord. Chem. Rev. 2017, 336, 28–42. [Google Scholar] [CrossRef]
- Ke, H.; Wang, J.; Tong, S.; Jin, Y.; Wang, S.; Qu, E.; Bao, G.; Dai, Z. Gold nanoshelled liquid perfluorocarbon magnetic nanocapsules: A nanotheranostic platform for bimodal ultrasound/magnetic resonance imaging guided photothermal tumor ablation. Theranostics 2014, 4, 12–23. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Bernardi, R.J.; Lowery, A.R.; Thompson, P.A.; Blaney, S.M.; West, J.L. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines. J. Neurooncol. 2008, 86, 165–172. [Google Scholar] [CrossRef]
- Stern, J.M.; Stanfield, J.; Kabbani, W.; Hsieh, J.T.; Cadeddu, J.A. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J. Urol. 2008, 179, 748–753. [Google Scholar] [CrossRef]
- Frémeaux, S.; Noël, C. Une analyse philosophique du management de la RSE: De la difficile conciliation entre l’ordre économique, l’ordre juridique et l’ordre moral. Manag. Avenir 2014, 73, 107. [Google Scholar] [CrossRef]
- Schwartz, J.A.; Price, R.E.; Gill-Sharp, K.L.; Sang, K.L.; Khorchani, J.; Goodwin, B.S.; Payne, J.D. Selective nanoparticle-directed ablation of the canine prostate. Lasers Surg. Med. 2011, 43, 213–220. [Google Scholar] [CrossRef]
- Xu, Y.; Mahmood, M.; Li, Z.; Dervishi, E.; Trigwell, S.; Zharov, V.P.; Ali, N.; Saini, V.; Biris, A.R.; Lupu, D.; et al. Cobalt nanoparticles coated with graphitic shells as localized radio frequency absorbers for cancer therapy. Nanotechnology 2008, 19, 435102. [Google Scholar] [CrossRef]
- Gyergyek, S.; Huskić, M.; Makovec, D.; Drofenik, M. Superparamagnetic nanocomposites of iron oxide in a polymethyl methacrylate matrix synthesized by in situ polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 49–55. [Google Scholar] [CrossRef]
- Xu, Y.; Karmakar, A.; Wang, D.; Mahmood, M.W.; Watanabe, F.; Zhang, Y.; Fejleh, A.; Fejleh, P.; Li, Z.; Kannarpady, G.; et al. Multifunctional Fe3O4 cored magnetic-quantum dot fluorescent nanocomposites for RF nanohyperthermia of cancer cells. J. Phys. Chem. C 2010, 114, 5020–5026. [Google Scholar] [CrossRef]
- Shi, X.; Gong, H.; Li, Y.; Wang, C.; Cheng, L.; Liu, Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 2013, 34, 4786–4793. [Google Scholar] [CrossRef]
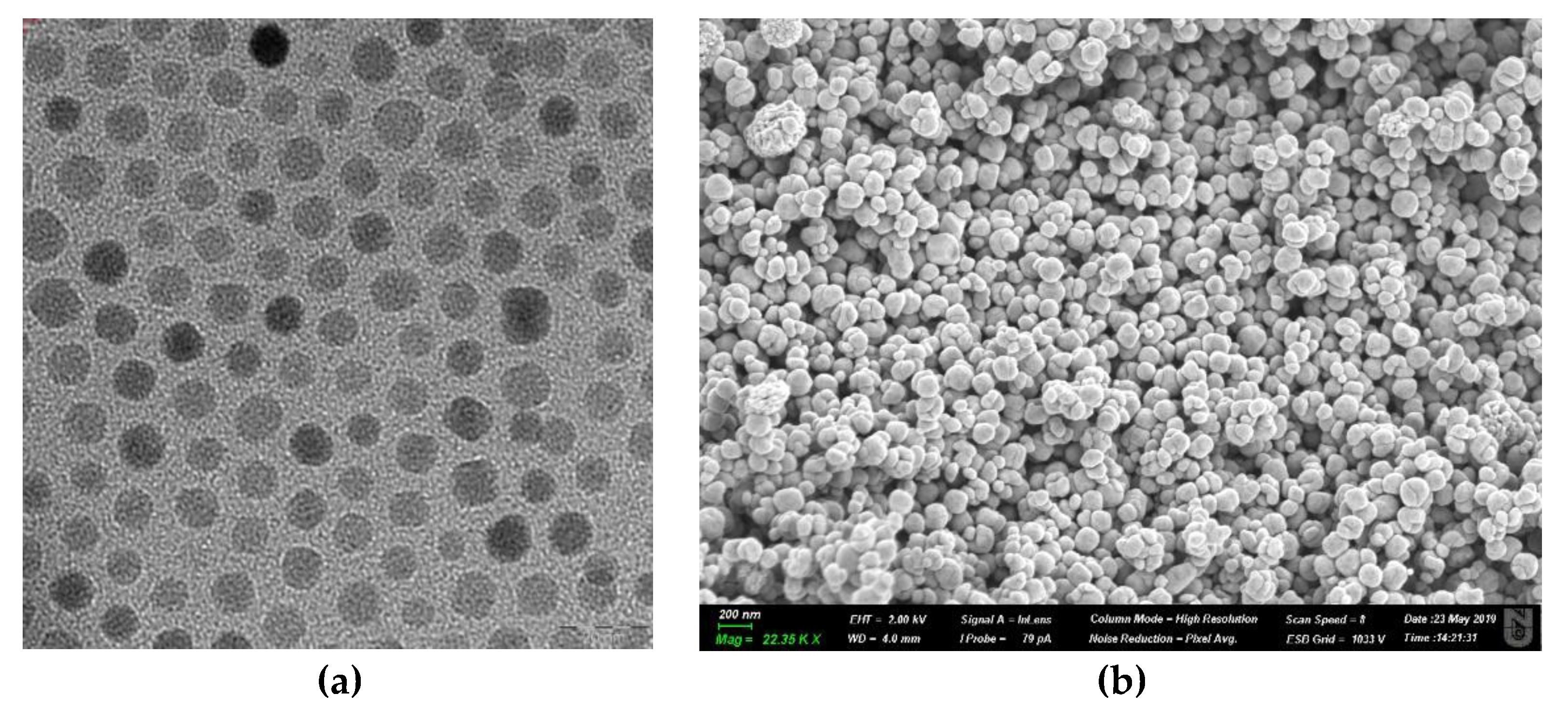
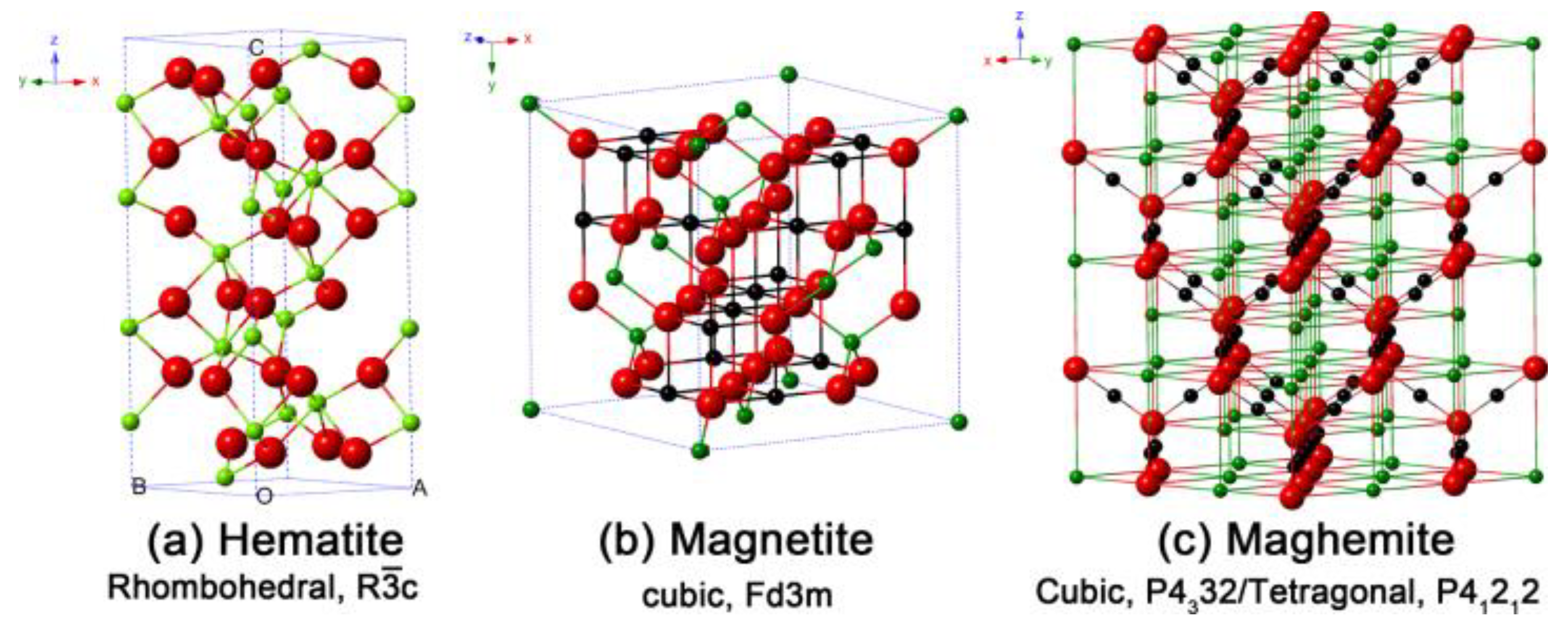
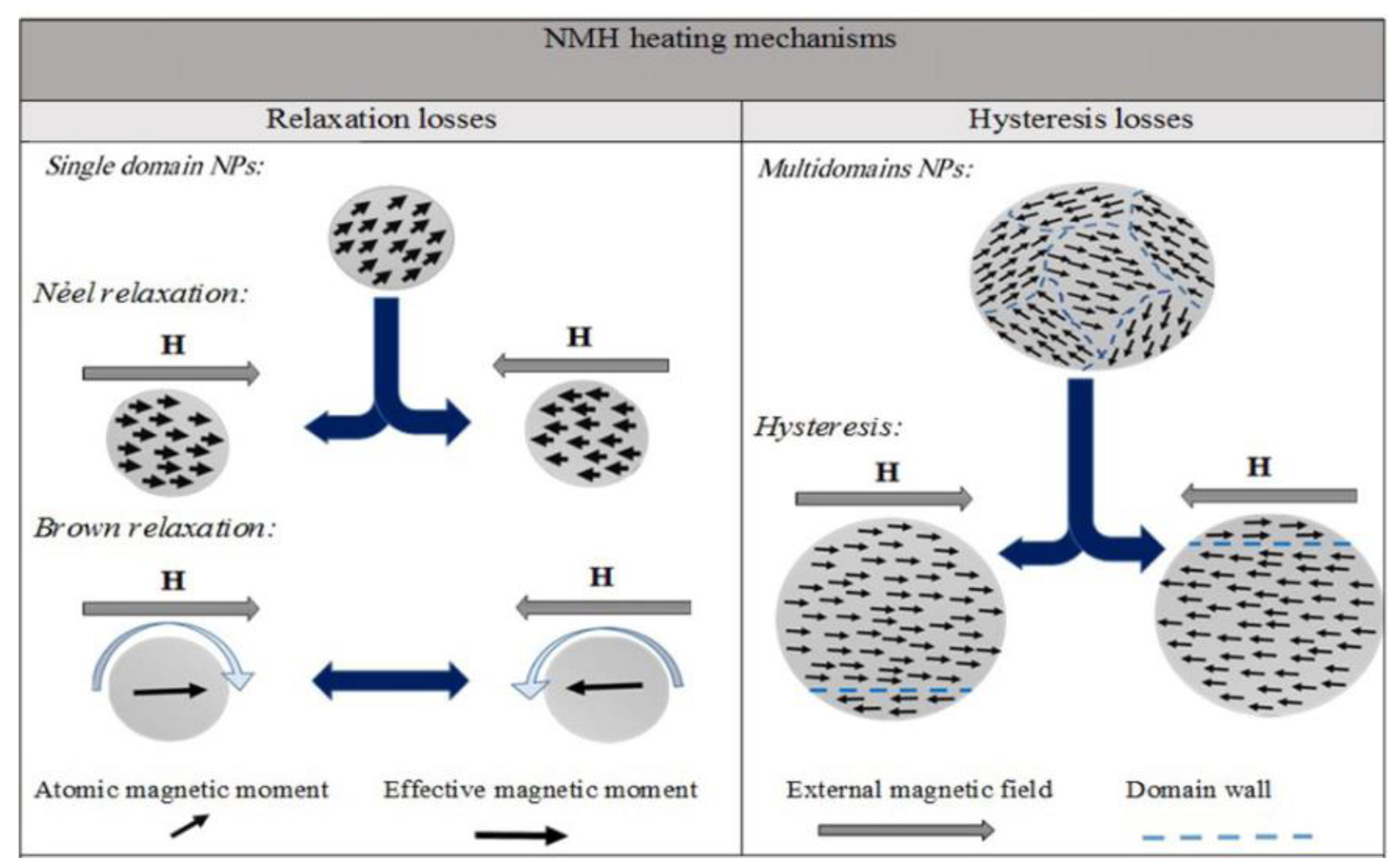
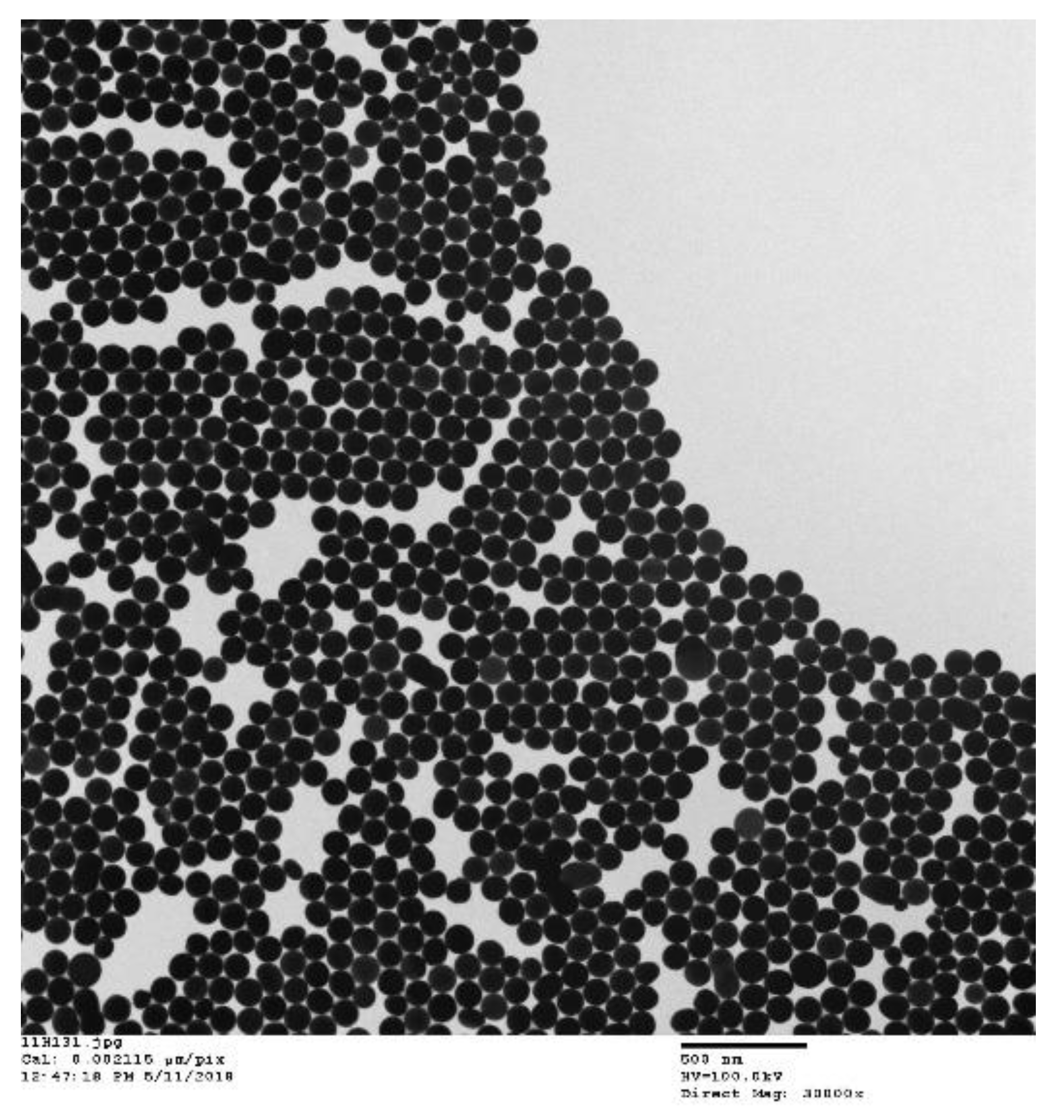
| Type of MNP/MNP with a Surface Coating | Nanoparticle Size | Injection Dose/Nanoparticle Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Temperature, °C | Cell Death | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 | 20–100 nm | 5 mg of Fe2O3 per gram of tissue | intratumoral | 200 to 240 oersteds, 3 min | RF ablation | dog’s lymph nodes | max 50 °C | N/A | [45] |
| Fe3O4 | 50–100 nm | 5 mg of Fe2O3 per gram of tissue | intratumoral | 55,000 cycles/second, 500 oesterds, 30 min | RF ablation | lymph node metastases by | max 50 °C | N/A | [46] |
| Fe3O4 | 10–20 nm | 21 mg ± 9 of magnetite per 299 mm3 of tumor tissue | intratumoral | 1.2–6.5 kA/m; 400 kHz, 242 s | AMF | human breast tissue | max 79 °C | N/A | [53] |
| Fe3O4 | 10 nm | 5 ± 0.3 mg magnetite per 100 mg of tumor tissue | intratumoral | 400 kHz, 6.5 kA/m, 4 min | AMF | human breast adenocarcinoma cells | max 73 °C at tumor center and 12 °C at tumor periphery | N/A | [20] |
| Fe3O4—dextran coated | 10–20 nm | 107 pg/cells | intratumoral | 410 kHz, 10 kA/m, 242 s | AMF | human breast adenocarcinoma cells | max 71 °C at tumor center | N/A | [54] |
| Fe3O4—starch coated | 11.4 nm ± 0.38 | 0.32 mg Fe mL−1 culture medium | intratumoral | 400 kHz, 24.6 kA m−1 | AMF | breast carcinoma cell line BT474 | to 28.2 ± 0.4 °C | N/A | [51] |
| Fe3O4—amino-silane coated | 15 nm | N/A | intratumoral | 100 kHz, 0–18 kA/m | AMF | RG-2 glioma cells | max 43–47 °C | N/A | [55] |
| Fe3O4 | 20 nm | 11.4 mL per 100 mg of tumor tissue | interstitial | 100 kHz, 2.5–15 kA/m | AMF | prostate cancer | max 55 °C | N/A | [57] |
| Fe3O4—amino-silane coated | 15 nm | 112 mg/mL | transperineally | 100 kHz, 2.5–18.0 kA/m, 60 min | AMF | prostate cancer | max 50 °C | N/A | [56] |
| Fe3O4—amino-silane coated | 15 nm | 200–400 µl of MNP per 0.5 mL/cm3 tumor volume and 120 mg/mL | intratumoral | 100 kHz, 18.0 kA/m | AMF | prostate cancer | max 54.88 °C centrally and 41.28 °C—peripherally | N/A | [58] |
| Type of NP/NP with a Surface Coating | Nanoparticle Size | Injection Dose/Nanoparticle Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Temperature, °C | Cell Death | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AuNP—anti-EGFR antibody coated | 40 nm | N/A | intratumoral | 57 W/cm2, 514 nm, 4 min | Laser photothermal therapy | Epithelial carcinoma HaCaT cells | N/A | 100% | [72] |
| AuNP—citrate coated | 13 nm | N/A | intratumoral | 10–100 W, 7 min | Radiowave ablation | HepG2 cancerous cells | >50 °C | 80% | [73] |
| AuNP | 5 nm | 67 µM/L | intratumoral | 13.56 MHz, 5 min | RF ablation | Hepatocellular (Hep3B) and Pancreatic cancerous cells (Panc-1) | N/A | 99.8 ± 3.1 Hep3B 96.5 ± 8.4 Panc-1 | [74] |
| AuNP—anti-EGFR coated | N/A | N/A | intratumoral | 200 nW | Laser photothermal therapy | Cancerous cell | 70–80 °C | N/A | [69] |
| AuNP—anti-EGFR coated | 20 nm | N/A | intratumoral | 13.56 MHz, 200 S, 10−15 kV/m | RF ablation | Pancreatic cancerous cell line | N/A | Panc-1 61% | [76] |
| AuNP—PAM4 hemi-antibody coated and AuNP—C225 antibody-coated | 36.9 ± 1.5 nm 32.6 ± 0.7 nm | 100 µg/mL | invivo | 600 W, 10 min | RF ablation | Panc-1 and Capan-1 pancreatic carcinoma cell lines | N/A | N/A | [75] |
| Type of Nanoparticle/Nanoparticle with a Surface Coating | Nanoparticle Size | Injection Dose/Nanoparticle Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Cell Death | Temperature, °C | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Thioglycolic acid-stabilized CuS NPs | 3 nm | 4.6 g/cm3, 770 µM | intratumoral | 24 W/cm2 for 5 min | photothermal ablation | HeLa cells | 55.6 ± 5.8% | Increased to 12.7 °C | [79] |
| Chitosan-coated HCuSNPs | 10 × 12 nm | intratumoral | photothermal ablation | [81] | |||||
| Phospholipid-PEG coated– CuS NPs | 3.8 nm | 400 mg/mL−1 | intratumoral | 1.0 W cm2, 8 min | Photothermal ablation | HeLa cells | >80% | Max 59.2 °C | [82] |
| PEG coated—CuS NP | N/A | 400 mg/mL, 0.1 mm | intratumoral | 2.5 W/cm2 | Photothermal ablation | Anaplastic thyroid carcinoma | N/A | Max 98 °C | [83] |
| Type of Nanorods/Nanorods with a Surface Coating | Nanorod Size/Concentration | Injection Dose/Nanorods Concentration | Injection Rote | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Cell Death | Temperature, °C | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AuNP | 5 nm | 0.001% volume fraction | intratumoral | 1.25 W/cm2, 300 s | photothermal therapy | Skin tumor | Total cell death | 75 °C at tumor surface, 43–48 °C at tumor depth | [93] |
| PEGylated gold nanorods– C225 antibody | 3 mm | 4 mg/mL | intratumoral | 20 W/cm2 to 90 W/cm2, from 30 s to 3 min | photothermal laser ablation | HTB-9 cells | N/A | N/A | [94] |
| PEGylated gold nanorods | dimensions 12 nm in width and 50 nm in length | N/A | intravenous | 3 min | photothermal therapy | HSC-3 human squamous carcinoma cells | >90% | N/A | [97] |
| PEG-coated gold nanorods | N/A | N/A | intratumoral | 2 W cm–2, 5 min. | photothermal ablation | Cancer tumor | N/A | 50–52 °C | [98] |
| gold nanorods | N/A | N/A | intratumoral | 30 J/cm2, 30 s | photothermal laser ablation | human KB cells | N/A | Increased by 5 °C | [86] |
| PEG-coated gold nanorods | N/A | 20 mg Au/kg in PBS | intravenous | 2 W cm–2, 5 min | photothermal ablation | MDA-MB-435 human cancerouscells | Within 10 days all the irradiated, PEG-NR-targeted tumors completely disappeared | over 70 °C | [91] |
| gold nanorods | N/A | N/A | intratumoral | 20 W/cm2, 4 to 20 min | photothermal ablation | prostate cancerouscells | N/A | N/A | [92] |
| gold nanorods | N/A | N/A | intratumoral | 1.4 and 2 W/cm2, and 0.5, 1, 2, and 5 min | photothermal ablation | MDA-MB-231 human breast cancerous cells | N/A | About 55 °C | [95,96] |
| Type of Carbon Nanotubes/Carbon Nanotubes with a Surface Coating | Carbon Nanotube Size | Injection Dose/Carbon Nanotube Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Cell Death | Temperature, °C | Reference |
|---|---|---|---|---|---|---|---|---|---|
| SWNT–polymer coated | N/A | 50 mg/mL | intratumoral | 600 W, 13.56 MHz | RF ablation | human cancer cell lines (HepG2, Hep3B and Panc-1 | 100% | Increase by 1.6 °C per second | [114] |
| Carbon nanotube—anti-Her2+ antibody coated | N/A | N/A | intratumoral | 9.5 W cm−2, 4 min | laser ablation | Her2+ human breast carcinoma cancer cells | 90% | N/A | [115] |
| Carbon nanotube—(KFKA)7 –peptide coated | N/A | 0.75 μg/mL for colon26 cells 2.5 μg/mL for HepG2 cells | intratumoral | 30 s | photothermal therapy | Colon and HepG2 cells | N/A | 43 °C | [116] |
| Multi-walled carbon nanotube | 900 nm | N/A | intratumoral | 15.3 W/cm2, 5 min | laser ablation | prostate cancer cell line (PC3) and murine renal cancer cell line (RENCA) | N/A | 43 °C | [117] |
| Carbon nanotube— human albumin protein coated | N/A | N/A | Ex vivo | 5 W/cm2, 20 min | laser-mediated ablation | pancreatic cancer Panc-1 cells | N/A | 29.3 °C at tumor centre | [118] |
| Type of Nanoshells/Nanocomposites (Core/Shell) | Size and/or Core/Shell Thickness | Injection Dose/Concertation | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Temperature, °C | Cell Death | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Gold nanoshells (silica/gold) | 110 nm/10 nm | N/A | intratumoral | 35 W/cm2, 7 min | photothermal therapy | breast cancer SK-BR-3 cells | >37.4 °C | N/A | [119] |
| Gold nanoshells (gold/PEG) | 110 nm/8–10 nm | 100 µL of 2.4 × 1011 nanoshells/mL solution | intratumoral | 10 days | laser ablation | CT26.WT murine colon carcinoma tumor cells | 50 °C | 100% | [121] |
| Gold nanoshells (silica/gold) | 110 ± 11 nm/10 nm | 8.5 µL/gm body weight | intratumoral | 21 days | NIR laser ablation | prostate cancer tumor | 65.4 °C | 93% | [126] |
| anti-HER2—silica core nanoshell anti-IL13Ra2 antibody—silica core nanoshell (silica/gold) | 100 nm and 10 nm | 32.61 and 48.52 mg/g | intratumoral | N/A | photothermal ablation | medulloblastoma and glioma cell lines | N/A | 100% | [126] |
| Gold nanoshells (silica/gold) | 150 nm | N/A | intratumoral | 3.5 W for 3 min | laser ablation | canine prostate cancer | N/A | 100% | [128] |
| Graphitic carbon coated C–Co-NPs | 7 nm | 20 μg mL−1 | intratumoral | 350 kHz, 5 kW, 10 min | RF ablation | HeLa cells | N/A | 98% | [129] |
| Fe3O4 nanoparticles– silica shell (silica/Fe3O4) | N/A | 1.66 µg/mL | intratumoral | 350 kHz, 10 min | RF ablation | Panc-1 cell line | N/A | 98.7– 99.2% | [131] |
| GO-IONP-Au-PEG | N/A | 50 mg/mL | intratumoral | 0.75 W/cm2, 5 min | Laser ablation | 4T1 tumor cells | Max 55 °C | N/A | [132] |
| Type of Nanoparticles/Source of Ablation | RF | MW | Laser Ablation | Photothermal Ablation |
|---|---|---|---|---|
| Magnetic nanoparticle | ||||
| Gold nanoparticle | ||||
| Cu-based nanoparticle | ||||
| Nanorod | ||||
| Carbon nanotubes | ||||
| Nanoshell/Nanocomposite |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials 2019, 9, 1195. https://doi.org/10.3390/nano9091195
Ashikbayeva Z, Tosi D, Balmassov D, Schena E, Saccomandi P, Inglezakis V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials. 2019; 9(9):1195. https://doi.org/10.3390/nano9091195
Chicago/Turabian StyleAshikbayeva, Zhannat, Daniele Tosi, Damir Balmassov, Emiliano Schena, Paola Saccomandi, and Vassilis Inglezakis. 2019. "Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer" Nanomaterials 9, no. 9: 1195. https://doi.org/10.3390/nano9091195
APA StyleAshikbayeva, Z., Tosi, D., Balmassov, D., Schena, E., Saccomandi, P., & Inglezakis, V. (2019). Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials, 9(9), 1195. https://doi.org/10.3390/nano9091195









