The Synergistic Effect of Acidic Properties and Channel Systems of Zeolites on the Synthesis of Polyoxymethylene Dimethyl Ethers from Dimethoxymethane and Trioxymethylene
Abstract
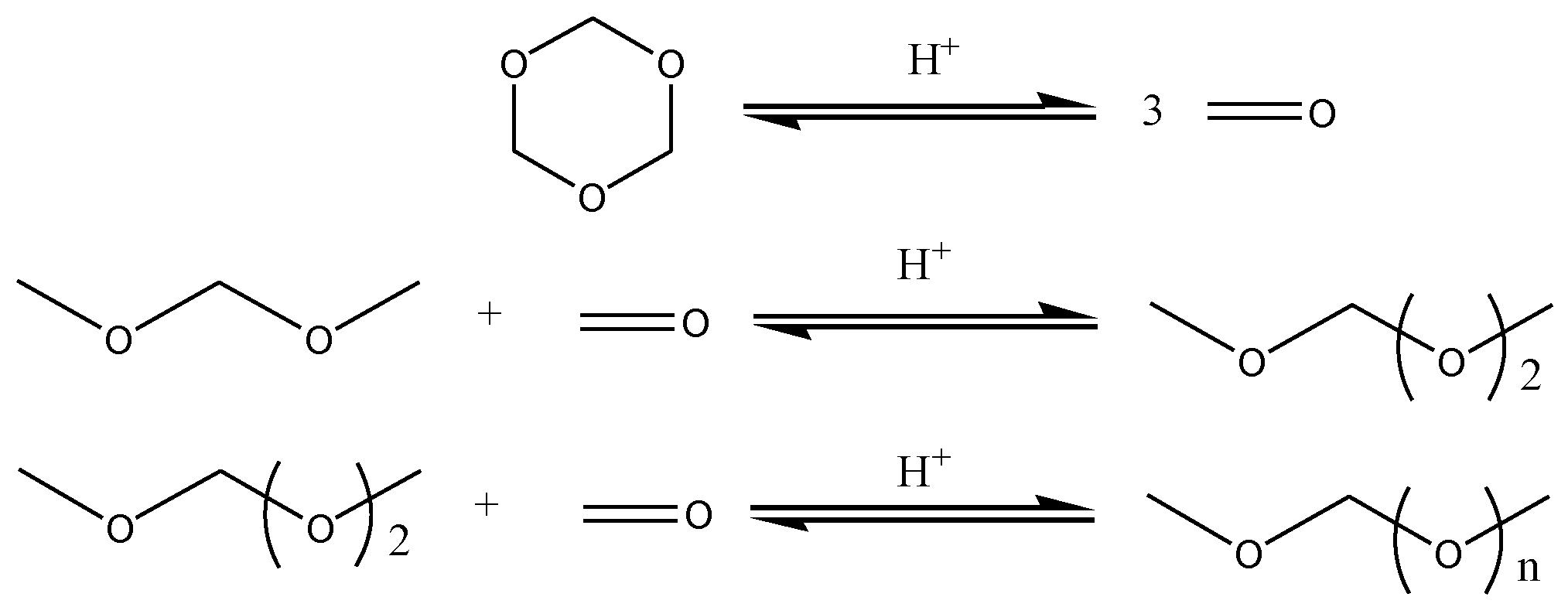
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Zhu, G.L.; Li, Z.; Xia, C.G. Polyoxymethylene dimethyl ethers as clean diesel additives: Fuel freezing and prediction. Fuel 2019, 237, 833–839. [Google Scholar] [CrossRef]
- Gao, X.J.; Wang, W.F.; Gu, Y.Y.; Zhang, Z.Z.; Zhang, J.F.; Tsubaki, N.; Zhang, Q.D.; Han, Y.Z.; Tan, Y.S. Synthesis of Polyoxymethylene Dimethyl Ethers from Dimethyl Ether Direct Oxidation over Carbon-Based Catalysts. ChemCatChem 2018, 10, 273–279. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, Z.; Wang, J.X.; He, X. Performance, combustion and emission characteristics of a diesel engine fueled with polyoxymethylene dimethyl ethers (PODE3-4)/diesel blends. Energy 2016, 97, 105–112. [Google Scholar] [CrossRef]
- Baranowski, C.J.; MBahmanpour, A.; Kröcher, O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): A review. Appl. Catal. B Environ. 2017, 217, 407–420. [Google Scholar] [CrossRef]
- Sun, R.Y.; Delidovich, I.; Palkovits, R. Dimethoxymethane as a Cleaner Synthetic Fuel: Synthetic Methods, Catalysts, and Reaction Mechanism. ACS Catal. 2019, 9, 1298–1318. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Chen, H.; Fu, Y.; Shen, J.J. Mechanism of chain propagation for the synthesis of polyoxymethylene dimethyl ethers. Energy Chem. 2013, 22, 833–836. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.Y.; Ma, X.; Wang, J.X.; Shuai, S.J.; Reitz, R.D. Homogeneous charge compression ignition (HCCI) combustion of polyoxymethylene dimethyl ethers (PODE). Fuel 2016, 183, 206–213. [Google Scholar] [CrossRef]
- Lautenschutz, L.; Oestreich, D.; Haltenort, P.; Arnold, U.; Dinjus, E.; Sauer, J. Efficient synthesis of oxymethylene dimethyl ethers (OME) from dimethoxymethane and trioxane over zeolites. Fuel Process. Technol. 2017, 165, 27–33. [Google Scholar] [CrossRef]
- Marchionna, M.; Patrini, R. A Process for the Selective Production of Dialkyl-Polyformals. Patent Number. EP1505049A1, 9 February 2005. [Google Scholar]
- Oestreich, D.; Lautenschütz, L.; Arnold, U.; Sauer, J. High Purity Oligomeric Oxymethylene Ethers as Diesel Fuels. Chem. Eng. Sci. 2017, 163, 92–104. [Google Scholar] [CrossRef]
- Zheng, Y.; Tang, Q.; Wang, T.; Liao, Y.; Wang, J. Synthesis of a Green Fuel Additive over Cation Resins. Chem. Eng. Technol. 2013, 36, 1951–1956. [Google Scholar] [CrossRef]
- Li, H.J.; Song, H.L.; Chen, L.W.; Xia, C.G. Designed SO42−/Fe2O3-SiO2 solid acids for polyoxymethylene dimethyl ethers synthesis: The acid sites control and reaction pathwaysAppl. Catal. B Environ. 2015, 165, 466–476. [Google Scholar] [CrossRef]
- Wang, R.Y.; Wu, Z.W.; Qin, Z.F.; Chen, C.M.; Zhu, H.Q.; Wu, J.B.; Chen, G.; Fan, W.B.; Wang, J.G. Graphene oxide: An effective acid catalyst for the synthesis of polyoxymethylene dimethyl ethers from methanol and trioxymethylene. Catal. Sci. Technol. 2016, 6, 993–997. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.F.; Zheng, Y.Y.; Wang, J.F. Synergistic effect of Bronsted and Lewis acid sites for the synthesis of polyoxymethylene dimethyl ethers over highly efficient SO42−/TiO2 catalysts. J. Catal. 2017, 355, 17–25. [Google Scholar] [CrossRef]
- Wu, J.B.; Zhu, H.Q.; Wu, Z.W.; Qin, Z.F.; Fan, W.B.; Wang, J.G. High Si/Al ratio HZSM-5 zeolite: An efficient catalyst for the synthesis of polyoxymethylene dimethyl ethers from dimethoxymethane and trioxymethylene. Green Chem. 2015, 17, 2353–2357. [Google Scholar] [CrossRef]
- Fu, W.H.; Liang, X.M.; Zhang, H.D.; Wang, Y.M.; He, M.Y. Shape selectivity extending to ordered supermicroporous aluminosilicates. Chem. Commun. 2015, 51, 1449–1452. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, H.; Qin, Z.F.; Wu, Z.W.; Fan, W.B.; Wang, J.G. Synthesis of polyoxymethylene dimethyl ethers from methanol and trioxymethylene with molecular sieves as catalysts. J. Fuel Chem. Technol. 2011, 39, 918–923. [Google Scholar] [CrossRef]
- Goncalves, T.J.; Arnold, U.; Plessow, P.N.; Studt, F. Theoretical Investigation of the Acid Catalyzed Formation of Oxymethylene Dimethyl Ethers from Trioxane and Dimethoxymethane. ACS Catal. 2017, 7, 3615–3621. [Google Scholar] [CrossRef]
- Grünert, A.; Losch, P.; Ochoa-Hernández, C.; Schmidt, W.; Schüth, F. Gas-phase synthesis of oxymethylene ethers over Si-rich zeolites. Green Chem. 2018, 20, 4719–4728. [Google Scholar] [CrossRef]
- Xue, Z.Z.; Shang, H.Y.; Zhang, Z.L.; Xiong, C.H.; Lu, C.B.; An, G.J. Efficient Synthesis of Polyoxymethylene Dimethyl Ethers on Al-SBA-15 Catalysts with Different Si/Al Ratios and Pore Sizes. Energy Fuels 2017, 31, 279–286. [Google Scholar] [CrossRef]
- Burger, J.; Ströfer, E.; Hasse, H. Chemical equilibrium and reaction kinetics of the heterogeneously catalyzed formation of poly (oxymethylene) dimethyl ethers from methylal and trioxane. Ind. Eng. Chem. Res. 2012, 51, 12751–12761. [Google Scholar] [CrossRef]
- Baranowski, C.J.; Bahmanpour, A.M.; Héroguel, F.; Luterbacher, J.S.; Kröcher, O. Prominent role of mesopore surface area and external acid sites for the synthesis of polyoxymethylene dimethyl ethers (OME) on a hierarchical H-ZSM-5 zeolite. Catal. Sci. Technol. 2019, 9, 366–376. [Google Scholar] [CrossRef]
- Li, J.F.; Fan, W.B.; Dong, M.; He, Y.; Qin, Z.F.; Wang, J.G. Chemistry Synthesis and MTO Catalytic Performance of SAPO-34. J. Chin. Univ. 2011, 32, 765–771. [Google Scholar]
- Gao, S.; Wang, X.P.; Wang, X.Z.; Bai, Y.X. Green synthesis of SUZ-4 zeolite controllable inmorphology and SiO2/Al2O3 ratio. Microporous Mesoporous Mater. 2013, 174, 108–116. [Google Scholar] [CrossRef]
- Kim, G.J.; Ahn, W.S. Direct synthesis and characterization of high-SiO2-content mordenites. Zeolites 1991, 11, 745–750. [Google Scholar] [CrossRef]
- Xu, F.; Dong, M.; Gou, W.; Li, J.; Fan, W.; Qin, Z.; Wang, J. Rapid tuning of ZSM-5 crystal size by using polyethylene glycol or colloidal silicalite-1 seed. Microporous Mesoporous Mater. 2012, 163, 192–200. [Google Scholar] [CrossRef]
- García-Trenco, A.; Martínez, A. Direct synthesis of DME from syngas on hybrid CuZnAl/ZSM-5 catalysts: New insights into the role of zeolite acidity. Appl. Catal. A 2012, 411–412, 170–179. [Google Scholar] [CrossRef]
- Ding, X.; Geng, S.; Li, C.; Yang, C.; Wang, G. Effect of acid density of HZSM-5 on the oligomerization of ethylene in FCC dry gas. J. Nat. Gas Chem. 2009, 18, 156–160. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, H.; Dong, M.; Li, J.F.; Qin, Z.F.; Wang, J.G.; Fan, W.B. Effect of zeolite pore structure on the diffusion and catalytic behaviors in the transalkylation of toluene with 1,2,4-trimethylbenzene. RSC Adv. 2015, 5, 66301–66310. [Google Scholar] [CrossRef]
- Lukyanov, D.B.; Vazhnova, T.; Cherkasov, N.; Casci, J.L.; Birtill, J.J. Insights into Brønsted Acid Sites in the Zeolite Mordenite. J. Phys. Chem. C 2014, 118, 23918–23929. [Google Scholar] [CrossRef]
- Available online: http://www.iza-structure.org/databases (accessed on 18 October 2018).
- Foster, M.D.; Rivin, I.; Treacy MM, J.; Friedrichs, O.D. A geometric solution to the largest-free-sphere problem in zeolite frameworks. Microporous Mesoporous Mater. 2006, 90, 32–38. [Google Scholar] [CrossRef]
- Celik, F.E.; Kim, T.; Bell, A.T. Effect of zeolite framework type and Si/Al ratio on dimethoxymethane carbonylation. J. Catal. 2010, 270, 185–195. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.Y.; Zhang, L.; Qin, Z.F.; Chen, Y.Y.; Dong, M.; Li, J.F.; Fan, W.B.; Wang, J.G. Mechanistic insights into the catalytic role of various acid sites on ZSM-5 zeolite in the carbonylation of methanol and dimethyl ether. Catal. Sci. Technol. 2018, 8, 3193–3204. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.Y.; Qin, Z.F.; Zhao, T.S.; Fan, S.B.; Dong, M.; Li, J.F.; Fan, W.B.; Wang, J.G. Origin and evolution of the initial hydrocarbon pool intermediates in the transition period for the conversion of methanol to olefins over H-ZSM-5 zeolite. J. Catal. 2019, 369, 382–395. [Google Scholar] [CrossRef]
 : USY;
: USY;  : Beta;
: Beta;  : ZSM-5; ■: SUZ-4; ×: SAPO-34;
: ZSM-5; ■: SUZ-4; ×: SAPO-34;  : MOR; (a): TOM conversion, (b): PODE2-8 selectivity, (c): DMM conversion, (d): PODE2-8 yield, (e): MeOH selectivity; (f): FA selectivity; (g): MF selectivity; (h): DME selectivity.
: MOR; (a): TOM conversion, (b): PODE2-8 selectivity, (c): DMM conversion, (d): PODE2-8 yield, (e): MeOH selectivity; (f): FA selectivity; (g): MF selectivity; (h): DME selectivity.
 : USY;
: USY;  : Beta;
: Beta;  : ZSM-5; ■: SUZ-4; ×: SAPO-34;
: ZSM-5; ■: SUZ-4; ×: SAPO-34;  : MOR; (a): TOM conversion, (b): PODE2-8 selectivity, (c): DMM conversion, (d): PODE2-8 yield, (e): MeOH selectivity; (f): FA selectivity; (g): MF selectivity; (h): DME selectivity.
: MOR; (a): TOM conversion, (b): PODE2-8 selectivity, (c): DMM conversion, (d): PODE2-8 yield, (e): MeOH selectivity; (f): FA selectivity; (g): MF selectivity; (h): DME selectivity.
 : ZSM-5 (Si/Al = 267), ○: USY (Si/Al = 10), black bar: PODE2, red bar: PODE3, sky-blue bar: PODE4, magenta bar: PODE5, olive bar: PODE6, blue bar: PODE7, violet bar: PODE8, orange bar: PODE9, wine bar: PODE10, dark yellow bar: PODE11.
: ZSM-5 (Si/Al = 267), ○: USY (Si/Al = 10), black bar: PODE2, red bar: PODE3, sky-blue bar: PODE4, magenta bar: PODE5, olive bar: PODE6, blue bar: PODE7, violet bar: PODE8, orange bar: PODE9, wine bar: PODE10, dark yellow bar: PODE11.
 : ZSM-5 (Si/Al = 267), ○: USY (Si/Al = 10), black bar: PODE2, red bar: PODE3, sky-blue bar: PODE4, magenta bar: PODE5, olive bar: PODE6, blue bar: PODE7, violet bar: PODE8, orange bar: PODE9, wine bar: PODE10, dark yellow bar: PODE11.
: ZSM-5 (Si/Al = 267), ○: USY (Si/Al = 10), black bar: PODE2, red bar: PODE3, sky-blue bar: PODE4, magenta bar: PODE5, olive bar: PODE6, blue bar: PODE7, violet bar: PODE8, orange bar: PODE9, wine bar: PODE10, dark yellow bar: PODE11.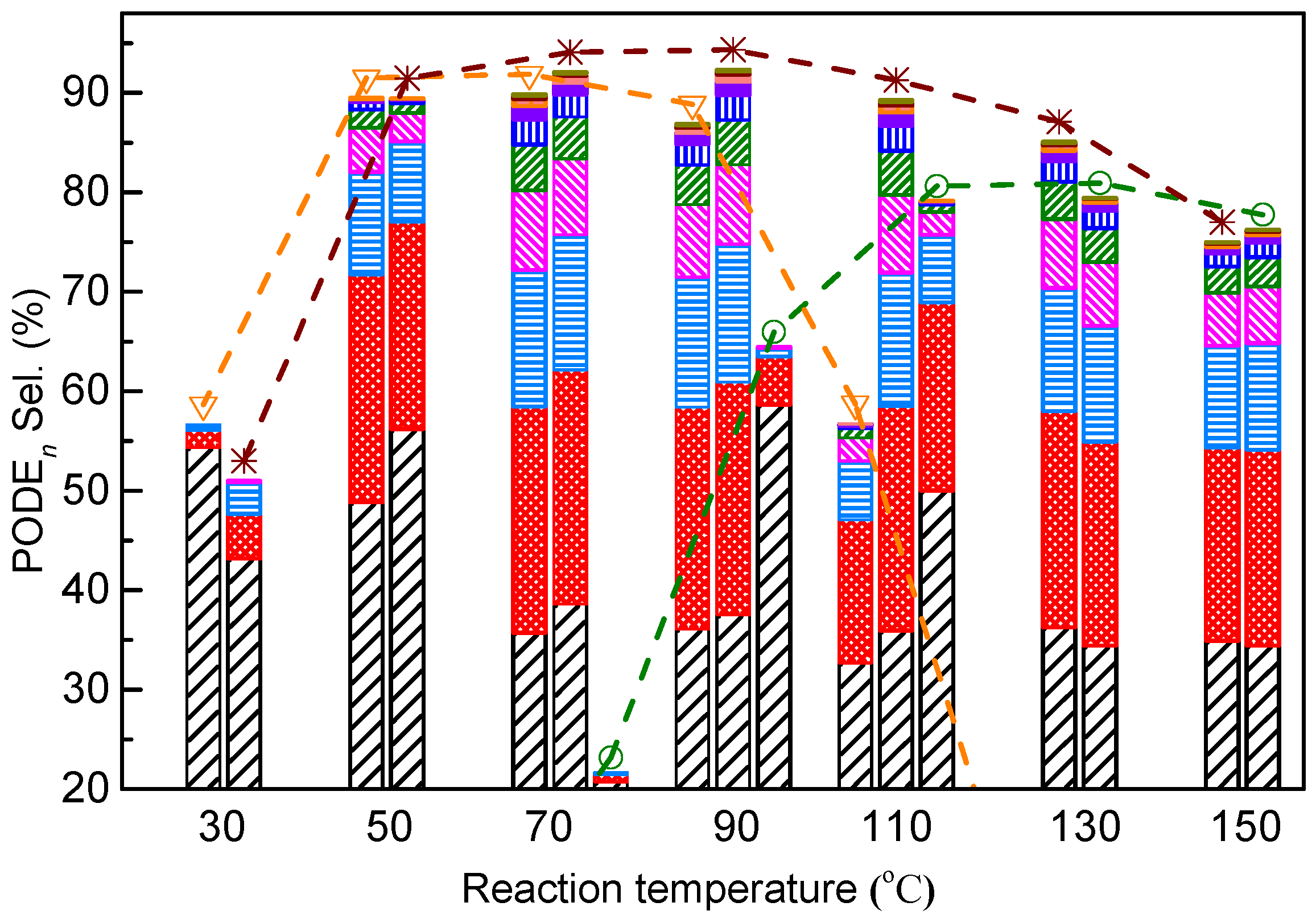

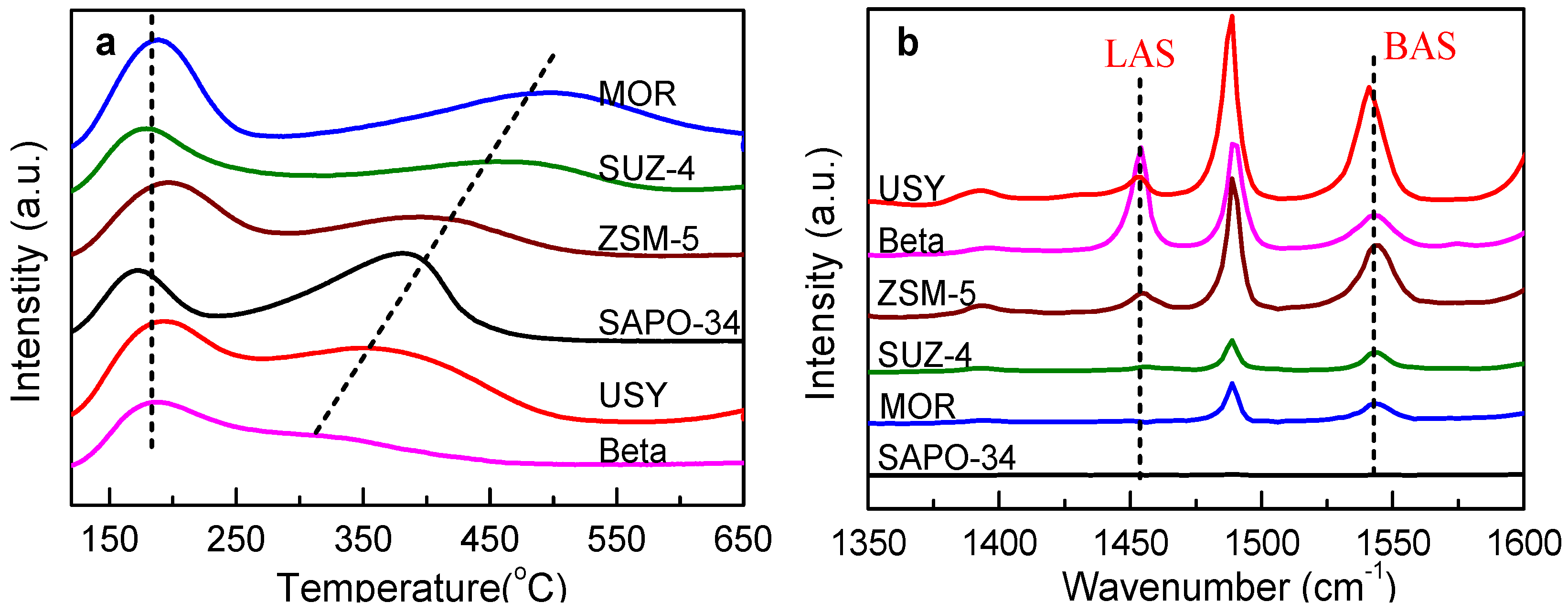
| Samples | Physicochemical Properties | Acidity by Strength d (mmol g−1) | Acidity by Type e (mmol g−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Si/Al Ratio a | SBET (m2 g−1) c | Vpore (cm3 g−1) | Weak | Medium—Strong | Total | Brønsted | Lewis | |
| USY | 10 | 641 | 0.372 | 0.40 | 0.24 | 0.64 | 0.50 | 0.092 |
| Beta | 12 | 500 | 0.346 | 0.53 | 0.23 | 0.76 | 0.25 | 0.40 |
| ZSM-5 | 17 | 368 | 0.231 | 0.52 | 0.47 | 0.99 | 0.47 | 0.086 |
| SUZ-4 | 6 | 348 | 0.337 | 0.40 | 0.44 | 0.84 | 0.073 | 0.013 |
| SAPO-34 | 0.085 b | 531 | 0.302 | 0.27 | 0.80 | 1.07 | -- | -- |
| MOR | 15 | 500 | 0.259 | 0.63 | 1.00 | 1.63 | 0.12 | 0 |
| Samples | Channel System | ||
|---|---|---|---|
| Pore Diameter (nm) | Channel Structure | Maximum Included Sphere Diameter (Å) | |
| USY | 0.74 × 0.74 | 3D, 12-ring | 11.18 |
| Beta | 0.66 × 0.67 | 3D, 12-ring | 6.62 |
| ZSM-5 | 0.53 × 0.56 | 3D, 10-ring | 6.30 |
| SUZ-4 | 0.41 × 0.52 | 3D, 10-ring | 6.27 |
| SAPO-34 | 0.38 × 0.38 | 3D, 8-ring | 7.31 |
| MOR | 0.65 × 0.70 | 1D, 12-ring | 6.64 |
| TOM Chair Conformation | TOM Boat Conformation | ||
|---|---|---|---|
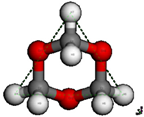 |  | ||
| H4-H6 | 4.033 Å | H4-H9 | 4.004 Å |
| H4-H7 | 4.033 Å | H8-H5 | 4.004 Å |
| H6-H7 | 4.033 Å | H8-H9 | 4.038 Å |
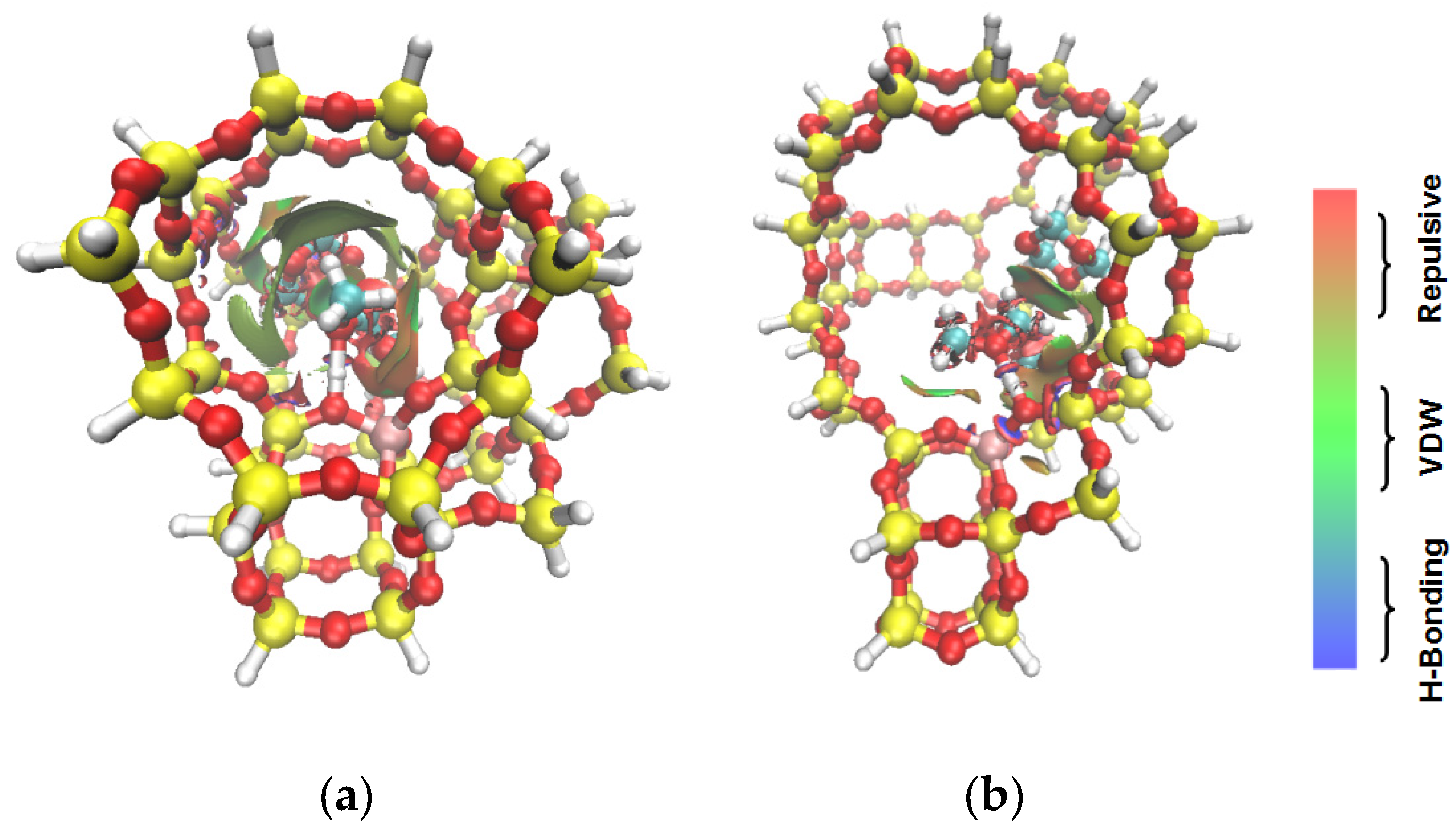
| Samples | DMM | TOM | DMM + TOM |
|---|---|---|---|
| ZSM-5 |  | 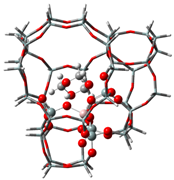 | 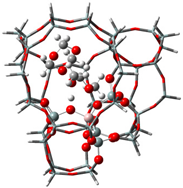 |
| −90 kJ/mol | −65 kJ/mol | −117 kJ/mol | |
| USY | 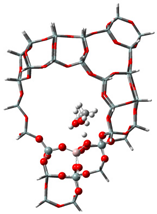 | 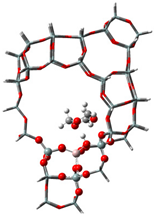 |  |
| −60 kJ/mol | −53 kJ/mol | −107 kJ/mol |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, S.; Li, H.; Zhang, Y.; Shi, R.; Zhao, Y. The Synergistic Effect of Acidic Properties and Channel Systems of Zeolites on the Synthesis of Polyoxymethylene Dimethyl Ethers from Dimethoxymethane and Trioxymethylene. Nanomaterials 2019, 9, 1192. https://doi.org/10.3390/nano9091192
Wu J, Wang S, Li H, Zhang Y, Shi R, Zhao Y. The Synergistic Effect of Acidic Properties and Channel Systems of Zeolites on the Synthesis of Polyoxymethylene Dimethyl Ethers from Dimethoxymethane and Trioxymethylene. Nanomaterials. 2019; 9(9):1192. https://doi.org/10.3390/nano9091192
Chicago/Turabian StyleWu, Jianbing, Sen Wang, Haitao Li, Yin Zhang, Ruiping Shi, and Yongxiang Zhao. 2019. "The Synergistic Effect of Acidic Properties and Channel Systems of Zeolites on the Synthesis of Polyoxymethylene Dimethyl Ethers from Dimethoxymethane and Trioxymethylene" Nanomaterials 9, no. 9: 1192. https://doi.org/10.3390/nano9091192
APA StyleWu, J., Wang, S., Li, H., Zhang, Y., Shi, R., & Zhao, Y. (2019). The Synergistic Effect of Acidic Properties and Channel Systems of Zeolites on the Synthesis of Polyoxymethylene Dimethyl Ethers from Dimethoxymethane and Trioxymethylene. Nanomaterials, 9(9), 1192. https://doi.org/10.3390/nano9091192






