Robust Polymer Nanocomposite Membranes Incorporating Discrete TiO2 Nanotubes for Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
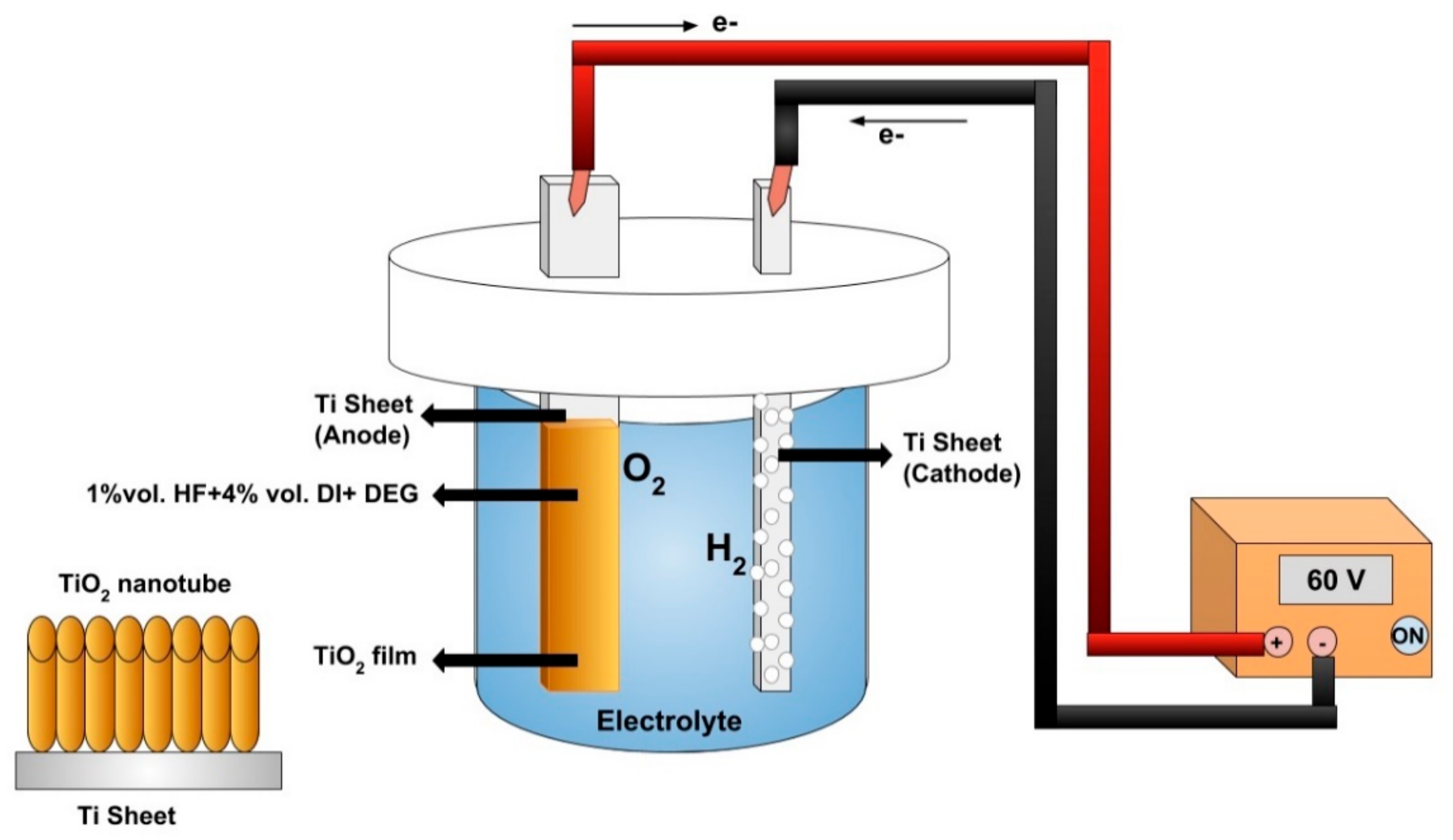
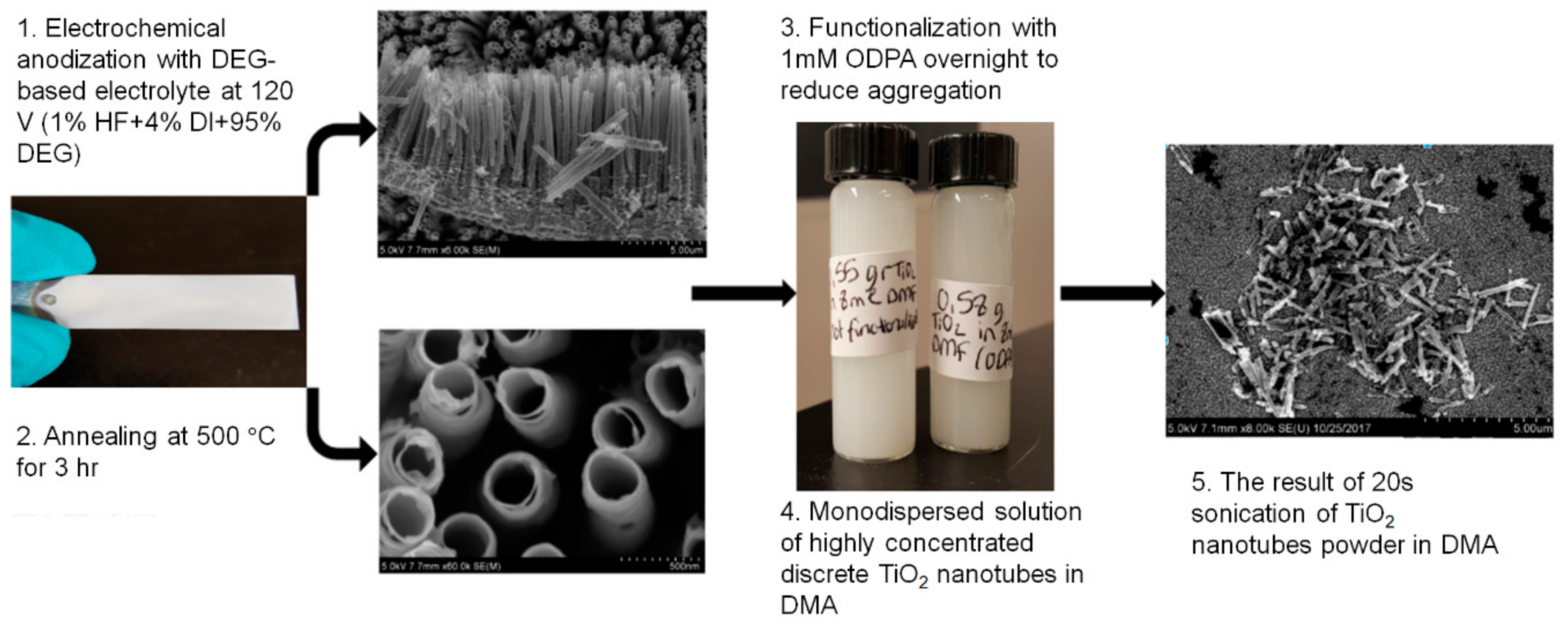
2.1. Chemical and Reagents
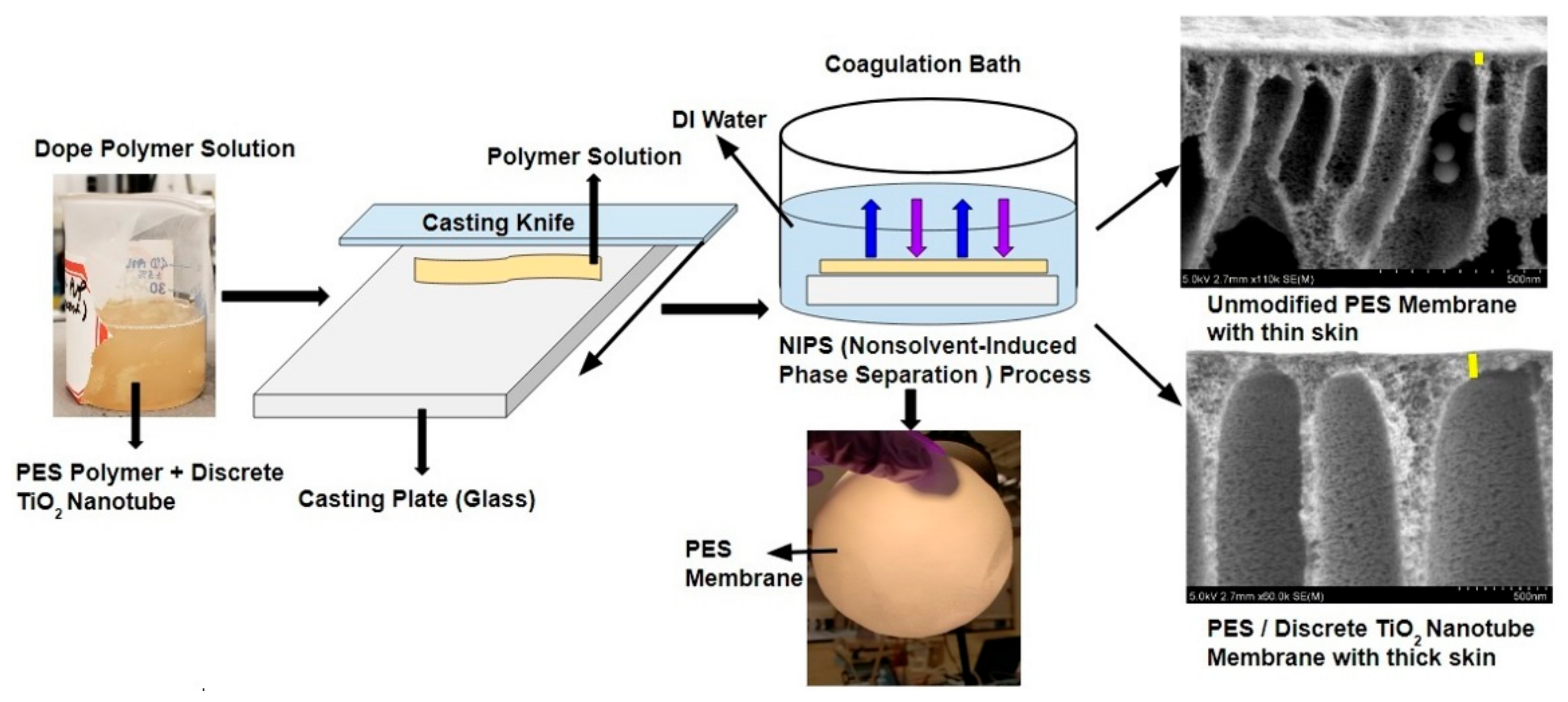
2.2. Preparation of TiO2 NTs/PES Membranes
2.3. Measurements of Porosity and Pore Size
2.4. Pure Water Flux Measurement
2.5. Fouling Tests
2.6. Total Organic Carbon Estimation
2.7. Chemical Composition Test (FTIR)
2.8. X-ray Photoelectron Spectroscopy (XPS)
2.9. Surface Properties (Wettability and Surface Charge)
2.10. Membrane Morphology Study
2.11. Atomic Force Microscopy
3. Results
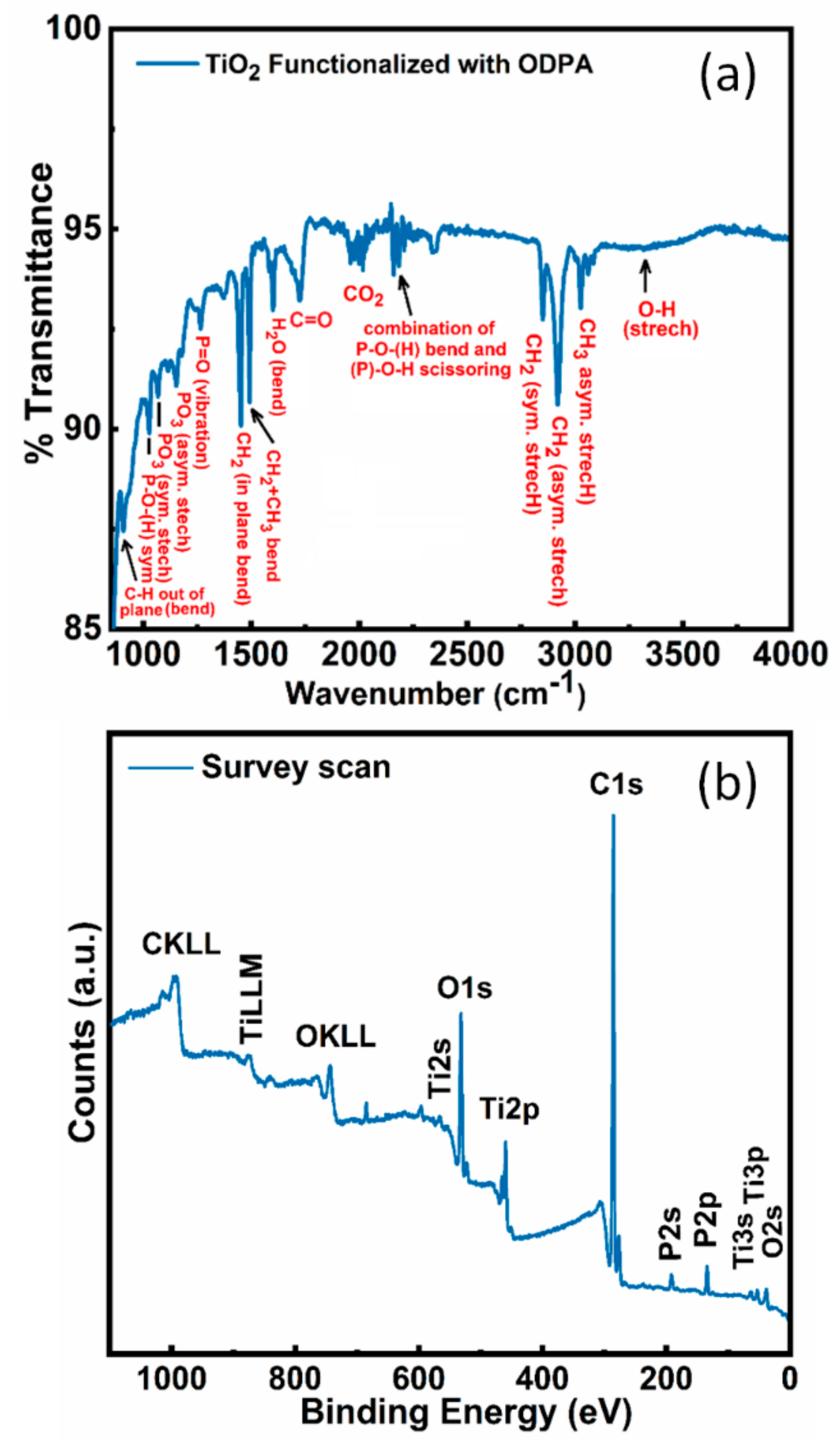
3.1. FTIR Measurement Results
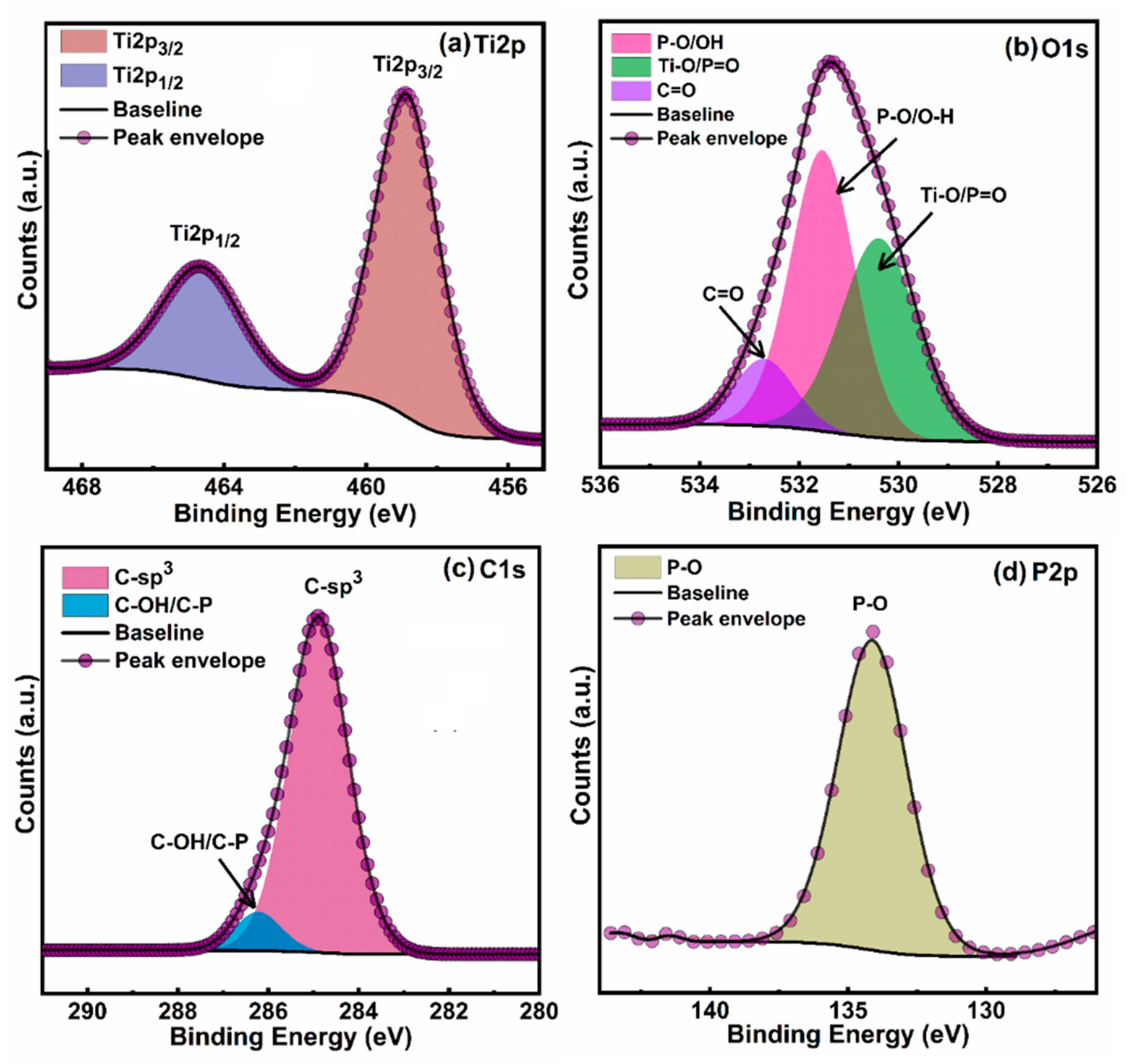
3.2. XPS Results
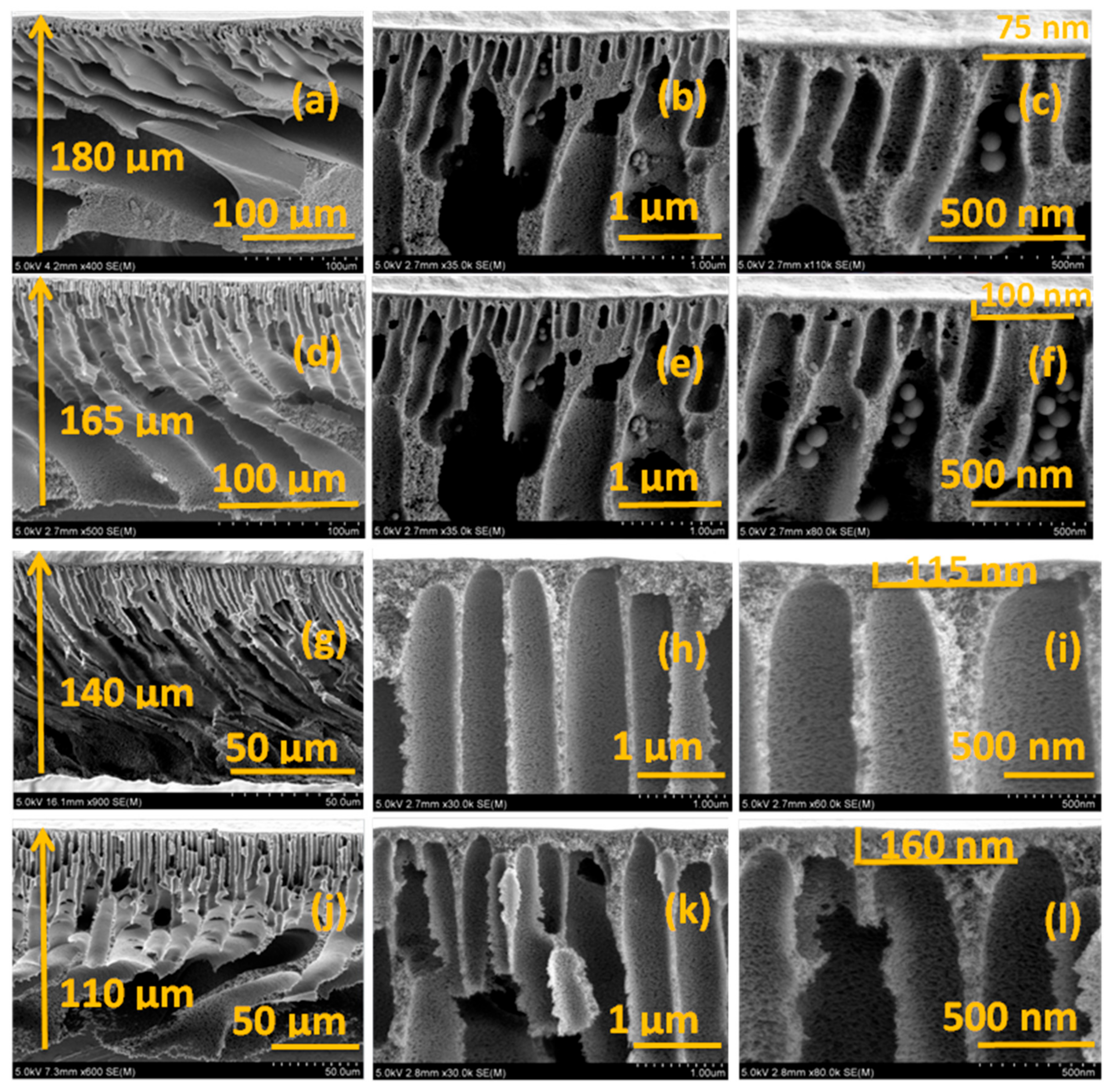
3.3. Surface and Cross-Section Morphology
3.4. Atomic Force Microscopy (AFM)
3.5. Contact Angle Measurement Results
3.6. Membrane Surface Charge Results
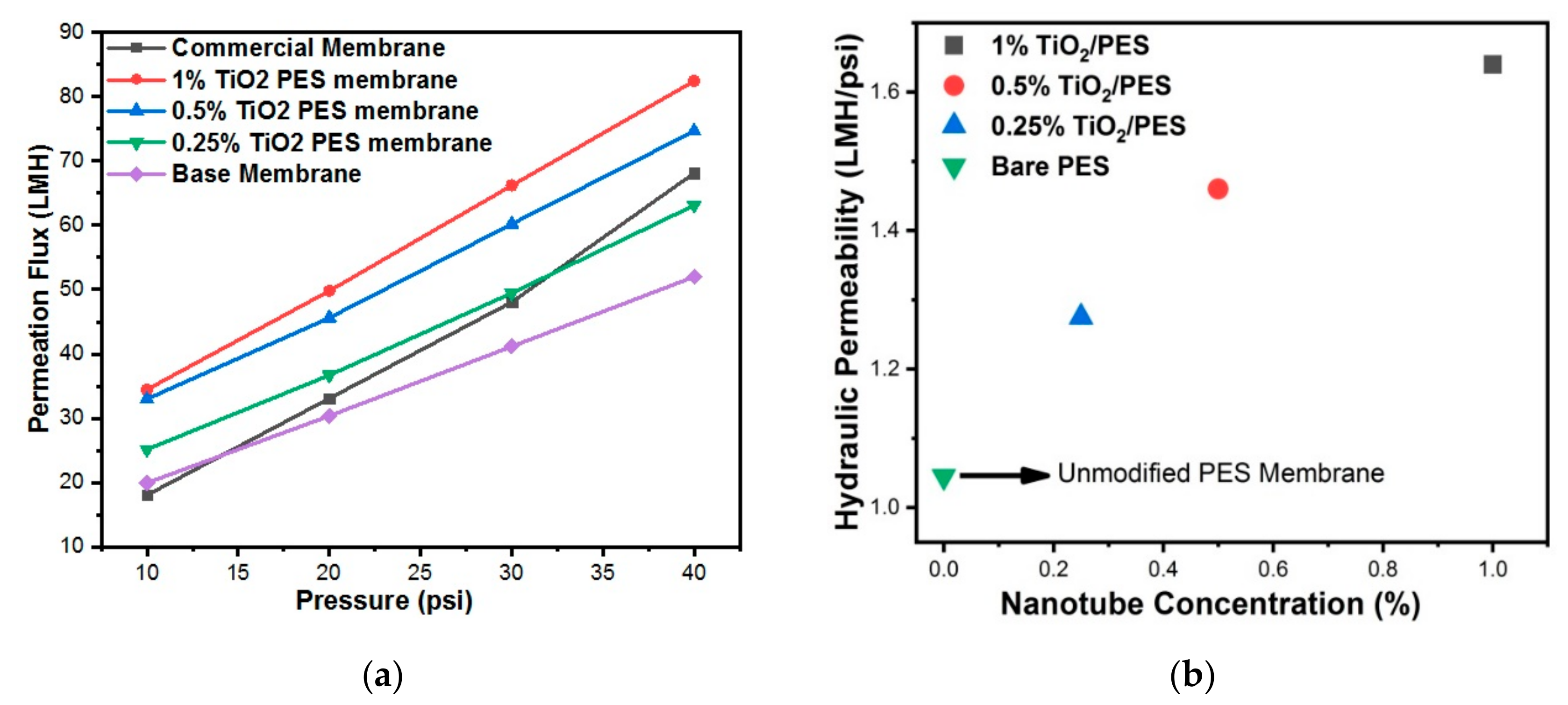
3.7. Permeability of Membranes
3.8. Porosity and Mean Pore Radius of Membrane
3.9. Separation Performance of Membranes
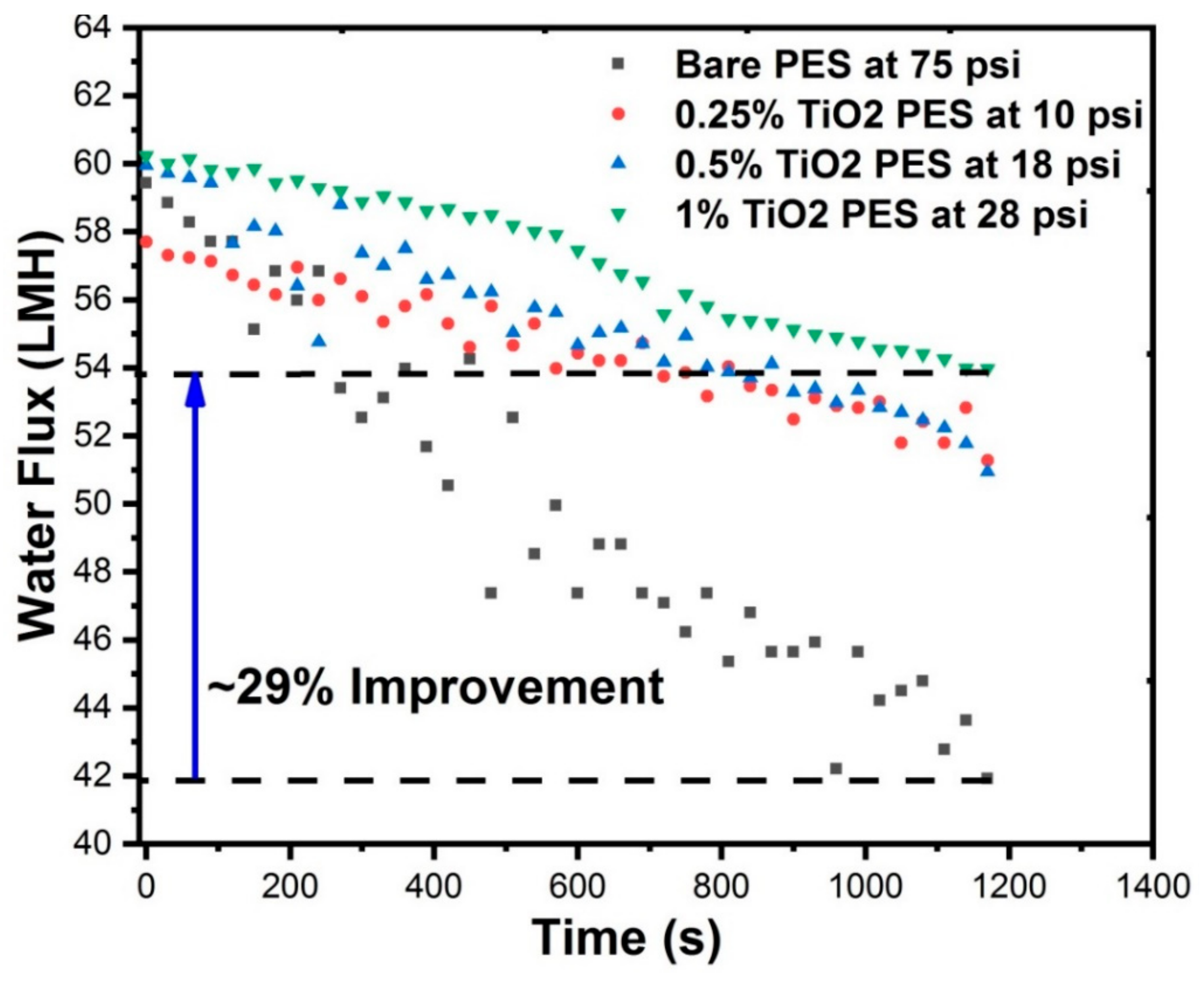
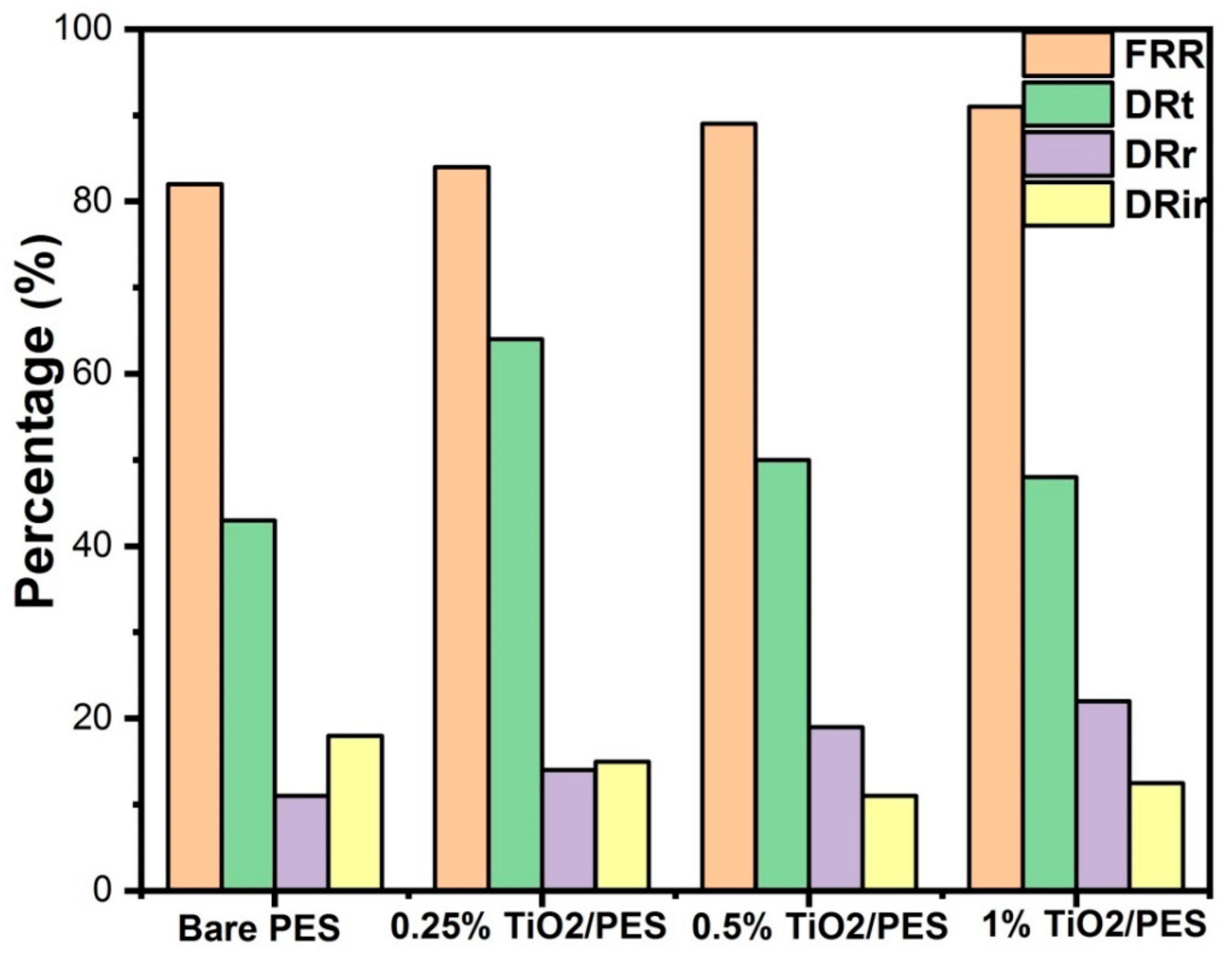
3.10. Fouling Characteristics of Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Liu, Z.; He, C.; Yue, H.; Gou, S. Water shortages raised a legitimate concern over the sustainable development of the drylands of northern China: Evidence from the water stress index. Sci. Total Environ. 2017, 590–591, 739–750. [Google Scholar] [CrossRef]
- Lim, D.-I.; Jung, S.W.; Choi, M.S.; Kang, S.M.; Jung, H.S.; Choi, J.Y. Historical record of metal accumulation and lead source in the southeastern coastal region of Korea. Mar. Pollut. Bull. 2013, 74, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124, 030901. [Google Scholar] [CrossRef]
- Liu, J.; Tian, C.; Xiong, J.; Wang, L. Polypyrrole blending modification for PVDF conductive membrane preparing and fouling mitigation. J. Colloid Interface Sci. 2017, 494, 124–129. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Genné, I.; Kuypers, S.; Leysen, R. Effect of the addition of ZrO2 to polysulfone based UF membranes. J. Membr. Sci. 1996, 113, 343–350. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Jafari, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J. Environ. Health Sci. Eng. 2015, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Richards, H.L.; Baker, P.G.; Iwuoha, E. Metal nanoparticle modified polysulfone membranes for use in wastewater treatment: A critical review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 183. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdulkarim, A.; Ooi, B.; Ismail, S. Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem. Eng. J. 2013, 223, 246–267. [Google Scholar] [CrossRef]
- Shen, J.-N.; Ruan, H.-M.; Wu, L.-G.; Gao, C.-J. Preparation and characterization of PES–SiO2 organic–inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Khulbe, K.; Feng, C.; Matsuura, T. The art of surface modification of synthetic polymeric membranes. J. Appl. Polym. Sci. 2010, 115, 855–895. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Burtovyy, O.; Klep, V.; Turel, T.; Gowayed, Y.; Luzinov, I. Polymeric Membranes: Surface Modification by Grafting to Method and Fabrication of Multilayered Assemblies. In Nanoscience and Nanotechnology for Chemical and Biological Defense; ACS symposium series; American Chemical Society: Washington, DC, USA, 2009; pp. 289–305. [Google Scholar]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M. Preparation, characterization and fouling analysis of in-air hydrophilic/underwater oleophobic bio-inspired polydopamine coated PES membranes for oily wastewater treatment. J. Membr. Sci. 2019, 582, 402–413. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Modification of polysulfone ultrafiltration membranes by CO2 plasma treatment. Desalination 2005, 172, 189–205. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Zhang, D.; Karkooti, A.; Liu, L.; Sadrzadeh, M.; Thundat, T.; Liu, Y.; Narain, R. Fabrication of antifouling and antibacterial polyethersulfone (PES)/cellulose nanocrystals (CNC) nanocomposite membranes. J. Membr. Sci. 2018, 549, 350–356. [Google Scholar] [CrossRef]
- María Arsuaga, J.; Sotto, A.; del Rosario, G.; Martínez, A.; Molina, S.; Teli, S.B.; de Abajo, J. Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes. J. Membr. Sci. 2013, 428, 131–141. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.-S.; Cao, C.; Kulprathipanja, S. The effects of polymer chain rigidification, zeolite pore size and pore blockage on polyethersulfone (PES)-zeolite A mixed matrix membranes. J. Membr. Sci. 2005, 260, 45–55. [Google Scholar] [CrossRef]
- Ganesh, B.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K.; Wang, K.; Xie, Z.; Ladewig, B.; Wang, H. Fabrication of polyethersulfone-mesoporous silica nanocomposite ultrafiltration membranes with antifouling properties. J. Membr. Sci. 2012, 423–424, 362–370. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, K.; Davies, C.H.J.; Wang, H.T. Carbon Nanotube/Alumina/Polyethersulfone Hybrid Hollow Fiber Membranes with Enhanced Mechanical and Anti-Fouling Properties. Nanomaterials 2015, 5, 1366–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.-C.; Waldman, R.Z.; Chen, Z.; Darling, S.B. Atomic layer deposition for membrane interface engineering. Nanoscale 2018, 10, 20505–20513. [Google Scholar] [CrossRef]
- Yang, H.-C.; Xie, Y.; Chan, H.; Narayanan, B.; Chen, L.; Waldman, R.Z.; Sankaranarayanan, S.K.; Elam, J.W.; Darling, S.B. Crude-Oil-Repellent Membranes by Atomic Layer Deposition: Oxide Interface Engineering. ACS Nano 2018, 12, 8678–8685. [Google Scholar] [CrossRef]
- Li, J.-F.; Xu, Z.-L.; Yang, H.; Yu, L.-Y.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Botcha, N.K.; Zarie, E.S.; Elbahri, M. Ups and Downs of Water Photodecolorization by Nanocomposite Polymer Nanofibers. Nanomaterials 2019, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Zhang, Y.; Farsinezhad, S.; Mohammadpour, A.; Wiltshire, B.D.; Sharma, H.; Shankar, K. Rutile phase n- and p-type anodic titania nanotube arrays with square-shaped pore morphologies. Chem. Commun. 2015, 51, 7816–7819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadpour, A.; Shankar, K. Anodic TiO2 nanotube arrays with optical wavelength-sized apertures. J. Mater. Chem. 2010, 20, 8474–8477. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Waghmare, P.R.; Wiltshire, B.D.; Sharma, H.; Amiri, S.; Mitra, S.K.; Shankar, K. Amphiphobic surfaces from functionalized TiO2 nanotube arrays. RSC Adv. 2014, 4, 33587–33598. [Google Scholar] [CrossRef]
- Hua, W.; Kar, P.; Roy, P.; Bu, L.; Shoute, L.C.T.; Kumar, P.; Shankar, K. Resistance of Superhydrophobic Surface-Functionalized TiO2 Nanotubes to Corrosion and Intense Cavitation. Nanomaterials 2018, 8, 783. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, M. Microstructure and anti-adhesion properties of PES/TAP/Ag hybrid ultrafiltration membrane. Desalination 2012, 287, 71–77. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A novel approach toward fabrication of high performance thin film composite polyamide membranes. Sci. Rep. 2016, 6, 22069. [Google Scholar] [CrossRef]
- Kar, P.; Pandey, A.; Greer, J.J.; Shankar, K. Ultrahigh sensitivity assays for human cardiac troponin I using TiO2 nanotube arrays. Lab Chip 2012, 12, 821–828. [Google Scholar] [CrossRef]
- Cattani-Scholz, A. Functional Organophosphonate Interfaces for Nanotechnology: A Review. ACS Appl. Mater. Interfaces 2017, 9, 25643–25655. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Sharma, H.; Shankar, K. Interfacial band alignment for photocatalytic charge separation in TiO2 nanotube arrays coated with CuPt nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 29723–29733. [Google Scholar] [CrossRef]
- Kar, P.; Zeng, S.; Zhang, Y.; Vahidzadeh, E.; Manuel, A.; Kisslinger, R.; Alam, K.M.; Thakur, U.K.; Mahdi, N.; Kumar, P.; et al. High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Appl. Catal. B Environ. 2019, 243, 522–536. [Google Scholar] [CrossRef]
- Ai, G.; Mo, R.; Li, H.; Zhong, J. Cobalt phosphate modified TiO2 nanowire arrays as co-catalysts for solar water splitting. Nanoscale 2015, 7, 6722–6728. [Google Scholar] [CrossRef]
- Yang, D.-S.; Bhattacharjya, D.; Inamdar, S.; Park, J.; Yu, J.-S. Phosphorus-Doped Ordered Mesoporous Carbons with Different Lengths as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media. J. Am. Chem. Soc. 2012, 134, 16127–16130. [Google Scholar] [CrossRef]
- Ma, T.Y.; Qiao, S.Z. Acid–Base Bifunctional Periodic Mesoporous Metal Phosphonates for Synergistically and Heterogeneously Catalyzing CO2 Conversion. ACS Catal. 2014, 4, 3847–3855. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Bhattacharjee, S. Rational design of phase inversion membranes by tailoring thermodynamics and kinetics of casting solution using polymer additives. J. Membr. Sci. 2013, 441, 31–44. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.; Sadrzadeh, M.; Pernitsky, D.; Bhattacharjee, S.; Hajinasiri, J. Treatment of an in situ oil sands produced water by polymeric membranes. Desalin. Water Treat. 2016, 57, 14869–14887. [Google Scholar] [CrossRef]
- Karkooti, A.; Yazdi, A.Z.; Chen, P.; McGregor, M.; Nazemifard, N.; Sadrzadeh, M. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Membr. Sci. 2018, 560, 97–107. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Bilad, M.R.; Mansoor, B.; Arafat, H.A. A comparative study of image analysis and porometry techniques for characterization of porous membranes. J. Mater. Sci. 2016, 51, 2017–2032. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, J.; Wang, Z.; Wen, G.; Jiang, J.; Shi, F.; Sheng, L. Hyperbranched-polymer functionalized multi-walled carbon nanotubes for poly (vinylidene fluoride) membranes: From dispersion to blended fouling-control membrane. Desalination 2012, 303, 29–38. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Hajinasiri, J.; Bhattacharjee, S.; Pernitsky, D. Nanofiltration of oil sands boiler feed water: Effect of pH on water flux and organic and dissolved solid rejection. Sep. Purif. Technol. 2015, 141, 339–353. [Google Scholar] [CrossRef]
- Weis, A.; Bird, M.R.; Nyström, M.; Wright, C. The influence of morphology, hydrophobicity and charge upon the long-term performance of ultrafiltration membranes fouled with spent sulphite liquor. Desalination 2005, 175, 73–85. [Google Scholar] [CrossRef]


| Membranes | Surface Roughness (nm) | Contact Angle | Zeta Potential (mV) at pH 5 | Zeta Potential (mV) at pH 6 | Zeta Potential (mV) at pH 7 | Zeta Potential (mV) at pH 8 |
|---|---|---|---|---|---|---|
| Unmodified PES | 57.1 | 65.1 ± 2 ° | −24.2 ± 0.1 | −26.6 ± 0.7 | −27.5 ± 0.1 | −28.0 ± 0.9 |
| Unmodified TiO2 NT membrane | – | 8.0 ± 2 ° | −8.3 ± 0.5 | −8.8 ± 0.4 | −9.2 ± 0.2 | −10.1 ± 0.8 |
| PES with 0.25 wt% TiO2 NTs | 83.3 | 49.1 ± 1 ° | −25.2 ± 0.8 | −27.0 ± 0.6 | −27.8 ± 0.4 | −28.4 ± 0.5 |
| PES with 0.5 wt% TiO2 NTs | 121 | 40.5 ± 2 ° | −26.4 ± 0.7 | −27.3 ± 0.4 | −28.6 ± 0.6 | −29.7 ± 0.8 |
| PES with 1 wt% TiO2 NTs | 133 | 37.4 ± 1 ° | −28.2 ± 1 | −29.4 ± 0.4 | −31.5 ± 0.3 | −32.0 ± 0.6 |
| Membrane | Mass of Dry Membrane (mg) | Mass of Wet Membrane (mg) |
|---|---|---|
| Unmodified PES | 14.6 ± 1 | 502.1 ± 2 |
| PES with 0.25 wt% TiO2 NTs | 25.0 ± 2 | 557.5 ± 3 |
| PES with 0.5 wt% TiO2 NTs | 27.2 ± 2 | 597.2 ± 1 |
| PES with 1 wt% TiO2 NTs | 24.2 ± 2 | 639.2 ± 2 |
| Permeate TOC (mg/L) | Permeate TC (mg/L) | Permeate IC (mg/L) | WLS Feed Water Rejection (%) | |
|---|---|---|---|---|
| Bare PES | 441.28 | 468.74 | 27.46 | 11.7 |
| 1 wt% TiO2/PES | 230.70 | 253.80 | 23.10 | 53.9 |
| 0.5 wt% TiO2/PES | 413.51 | 447.40 | 33.90 | 17.3 |
| 0.25 wt% TiO2/PES | 424.23 | 451.20 | 26.97 | 15.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdi, N.; Kumar, P.; Goswami, A.; Perdicakis, B.; Shankar, K.; Sadrzadeh, M. Robust Polymer Nanocomposite Membranes Incorporating Discrete TiO2 Nanotubes for Water Treatment. Nanomaterials 2019, 9, 1186. https://doi.org/10.3390/nano9091186
Mahdi N, Kumar P, Goswami A, Perdicakis B, Shankar K, Sadrzadeh M. Robust Polymer Nanocomposite Membranes Incorporating Discrete TiO2 Nanotubes for Water Treatment. Nanomaterials. 2019; 9(9):1186. https://doi.org/10.3390/nano9091186
Chicago/Turabian StyleMahdi, Najia, Pawan Kumar, Ankur Goswami, Basil Perdicakis, Karthik Shankar, and Mohtada Sadrzadeh. 2019. "Robust Polymer Nanocomposite Membranes Incorporating Discrete TiO2 Nanotubes for Water Treatment" Nanomaterials 9, no. 9: 1186. https://doi.org/10.3390/nano9091186







