Catalytic Reduction of Hexavalent Chromium Using Iron/Palladium Bimetallic Nanoparticle-Assembled Filter Paper
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
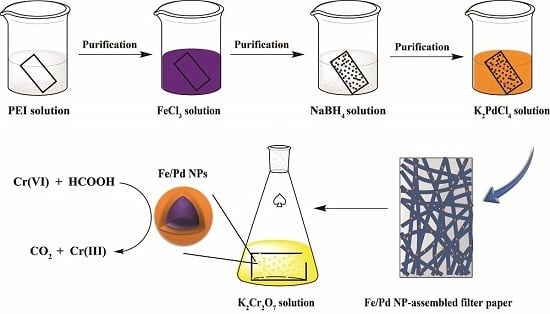
2.2. Preparation of Fe/Pd NP-Assembled Filter Paper
2.3. Characterization Techniques
2.4. Catalytic Activity Evaluation
3. Results and Discussion
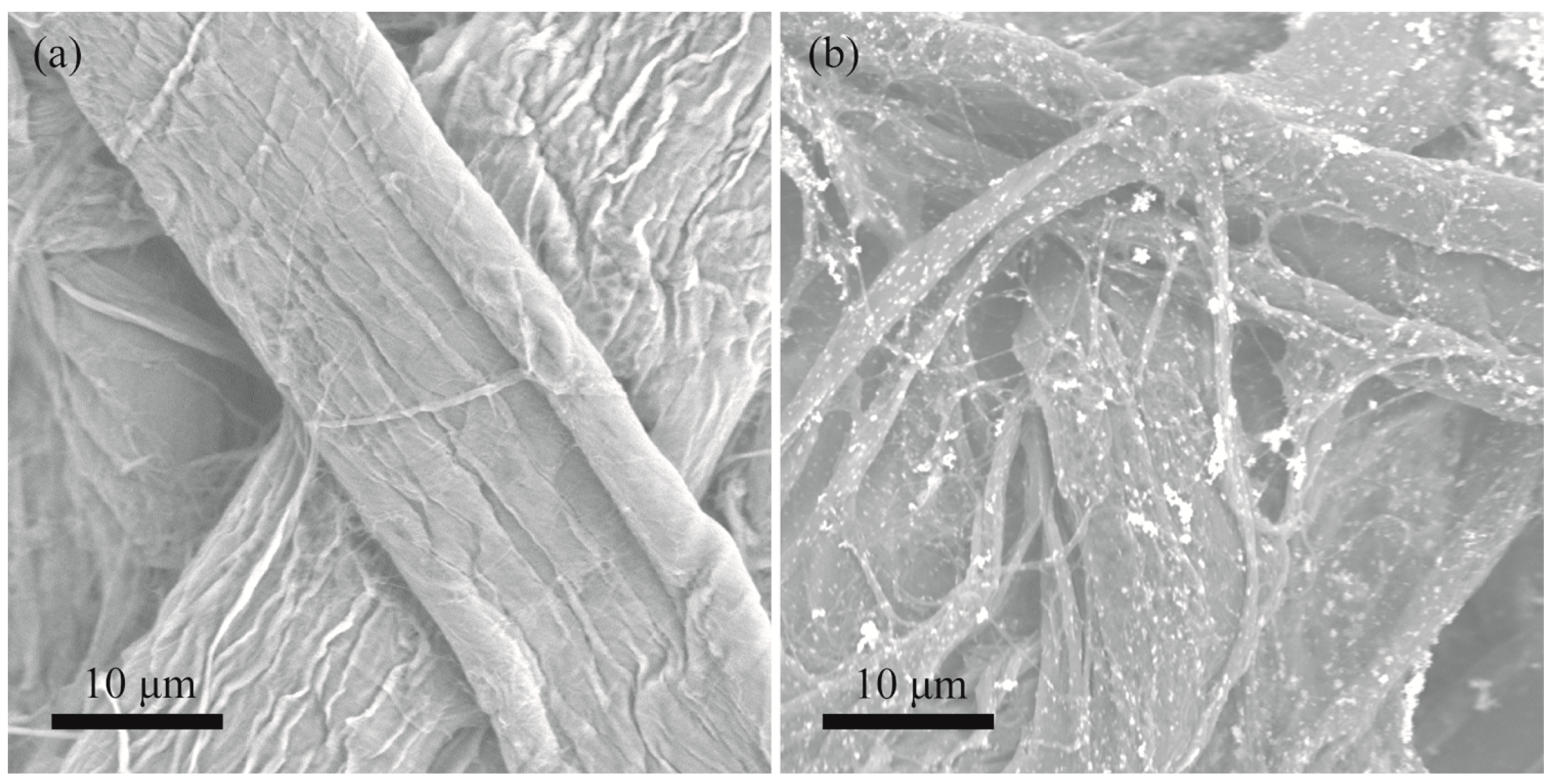
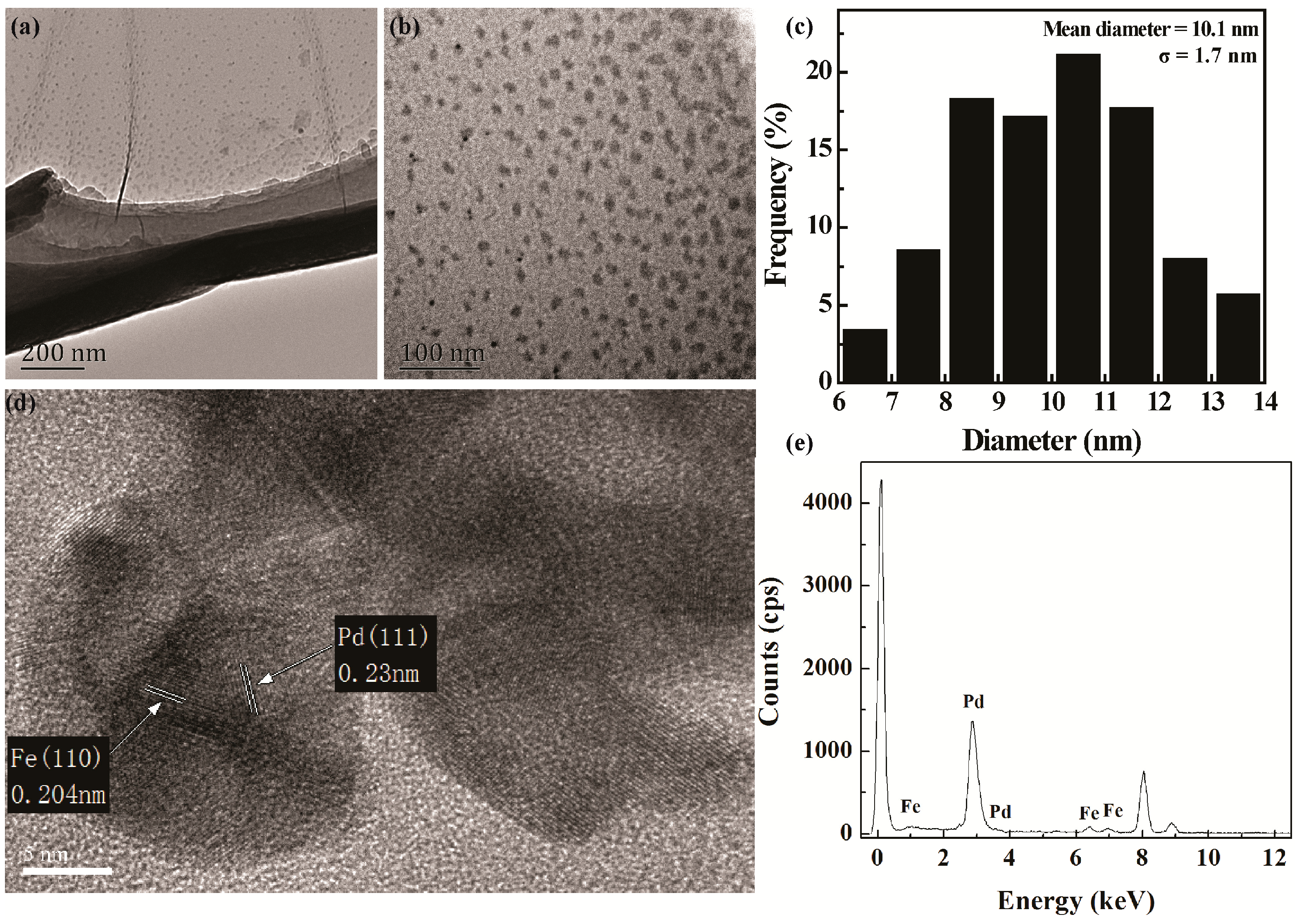
3.1. Preparation and Characterization of Fe/Pd NP-Assembled Filter Paper
3.2. Catalytic Reduction of Cr(VI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao, Y.J.; Zhang, J.; Chen, H.; Yu, M.J.; Gao, M.X.; Hu, Y.; Wang, S.B. Ni-0 encapsulated in N-doped carbon nanotubes for catalytic reduction of highly toxic hexavalent chromium. Appl. Surf. Sci. 2018, 440, 421–431. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium Cr(VI): A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Aslani, H.; Kosari, T.E.; Naseri, S.; Nabizadeh, R.; Khazaei, M. Hexavalent chromium removal from aqueous solution using functionalized chitosan as a novel nano-adsorbent: modeling and optimization, kinetic, isotherm, and thermodynamic studies, and toxicity testing. Environ. Sci. Pollut. Res. 2018, 25, 20154–20168. [Google Scholar] [CrossRef] [PubMed]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Narayani, M.; Shetty, K.V. Chromium-resistant bacteria and their environmental condition for hexavalent chromium removal: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 955–1009. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent Chromium Reduction with Zero-Valent Iron (ZVI) in Aquatic Systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Guo, X.M.; Zhao, Y.L.; Qiu, Y.P.; Shi, X.Y. Zero-valent iron nanoparticle-supported composite materials for environmental remediation applications. Curr. Nanosci. 2015, 11, 748–759. [Google Scholar] [CrossRef]
- Huang, D.L.; Chen, G.M.; Zeng, G.M.; Xu, P.; Yan, M.; Lai, C.; Zhang, C.; Li, N.J.; Cheng, M.; He, X.X.; et al. Synthesis and application of modified zero-valent iron nanoparticles for removal of hexavalent chromium from wastewater. Water Air Soil Pollut. 2015, 226, 375. [Google Scholar] [CrossRef]
- Rivero-Huguet, M.; Marshall, W.D. Reduction of hexavalent chromium mediated by micro- and nano-sized mixed metallic particles. J. Hazard. Mater. 2009, 169, 1081–1087. [Google Scholar] [CrossRef]
- Kadu, B.S.; Sathe, Y.D.; Ingle, A.B.; Chikate, R.C.; Patil, K.R.; Rode, C.V. Efficiency and recycling capability of montmorillonite supported Fe-Ni bimetallic nanocomposites towards hexavalent chromium remediation. Appl. Catal. B 2011, 104, 407–414. [Google Scholar] [CrossRef]
- Yang, L.; Lv, L.; Zhang, S.J.; Pan, B.C.; Zhang, W.M. Catalytic dechlorination of monochlorobenzene by Pd/Fe nanoparticles immobilized within a polymeric anion exchanger. Chem. Eng. J. 2011, 178, 161–167. [Google Scholar] [CrossRef]
- Choi, H.; Al-Abed, S.R.; Agarwal, S.; Dionysiou, D.D. Synthesis of reactive nano-Fe/Pd bimetallic system-impregnated activated carbon for the simultaneous adsorption and dechlorination of PCBs. Chem. Mater. 2015, 20, 3649–3655. [Google Scholar] [CrossRef]
- Meng, Z.H.; Liu, Y.; Liu, H.L. Synthesis of nanoscale Pd/Fe bimetallic particles on PVDF membrane matrix for dechlorination of monochloroacetic acid. Adv. Mater. Res. 2011, 154–155, 381–385. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Huang, Y.P.; Shen, M.W.; Guo, R.; Cao, X.Y.; Shi, X.Y. Enhanced dechlorination of trichloroethylene using electrospun polymer nanofibrous mats immobilized with iron/palladium bimetallic nanoparticles. J. Hazard. Mater. 2012, 211–212, 349–356. [Google Scholar] [CrossRef] [PubMed]
- D’Halluin, M.; Rullbarrull, J.; Bretel, G.; Labrugère, C.; Grognec, E.L.; Felpin, F. Chemically modified cellulose filter paper for heavy metal remediation in water. ACS Sustainable Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Jia, D.L.; Wang, K.; Huang, J.G. Filter paper derived nanofibrous silica-carbon composite as anodic material with enhanced lithium storage performance. Chem. Eng. J. 2017, 317, 673–686. [Google Scholar] [CrossRef]
- Nava-Ortiz, C.A.B.; Alvarez-Lorenzo, C.; Bucio, E.; Concheiro, A.; Burillo, G. Cyclodextrin-functionalized polyethylene and polypropylene as biocompatible materials for diclofenac delivery. Int. J. Pharm. 2009, 382, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, Y.L.; Chen, Q.; Shi, X.Y.; Shen, M.W. The assembly of polyethyleneimine-entrapped gold nanoparticles onto filter paper for catalytic applications. RSC Adv. 2015, 5, 104324–104329. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Liu, L.; Li, C.Y.; Ye, B.X.; Xiong, J.; Shi, X.Y. Immobilization of polyethyleneimine-templated silver nanoparticles onto filter paper for catalytic applications. Colloids Surf. A 2019, 571, 44–49. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Liu, L.; Shi, D.; Shi, X.Y.; Shen, M.W. Performing a catalysis reaction on filter paper: development of a metal palladium nanoparticle-based catalyst. Nanoscale Adv. 2019, 1, 342–346. [Google Scholar] [CrossRef]
- Huang, Y.P.; Ma, H.; Wang, S.G.; Shen, M.W.; Guo, R.; Cao, X.Y.; Zhu, M.F.; Shi, X.Y. Efficient catalytic reduction of hexavalent chromium using palladium nanoparticle-immobilized electrospun polymer nanofibers. ACS Appl. Mater. Interfaces 2012, 4, 3054–3061. [Google Scholar] [CrossRef]
- Liu, M.Y.; Huan, R.L.; Li, C.X.; Che, M.; Su, R.X.; Li, S.Z.; Yu, J.; Qi, W.; He, Z.M. Continuous rapid dechlorination of p-chlorophenol by Fe-Pd nanoparticles promoted by procyanidin. Chem. Eng. Sci. 2019, 201, 121–131. [Google Scholar] [CrossRef]
- Mhlongo, G.H.; Motaung, D.E.; Cummings, F.R.; Swart, H.C.; Ray, S.S. A highly responsive NH3 sensor based on Pd-loaded ZnO nanoparticles prepared via a chemical precipitation approach. Sci. Rep. 2019, 9, 9881. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Ouyang, Z.; Zhao, Y.; Xiong, J.; Shi, X. Catalytic Reduction of Hexavalent Chromium Using Iron/Palladium Bimetallic Nanoparticle-Assembled Filter Paper. Nanomaterials 2019, 9, 1183. https://doi.org/10.3390/nano9081183
Shi D, Ouyang Z, Zhao Y, Xiong J, Shi X. Catalytic Reduction of Hexavalent Chromium Using Iron/Palladium Bimetallic Nanoparticle-Assembled Filter Paper. Nanomaterials. 2019; 9(8):1183. https://doi.org/10.3390/nano9081183
Chicago/Turabian StyleShi, Daniel, Zhijun Ouyang, Yili Zhao, Jie Xiong, and Xiangyang Shi. 2019. "Catalytic Reduction of Hexavalent Chromium Using Iron/Palladium Bimetallic Nanoparticle-Assembled Filter Paper" Nanomaterials 9, no. 8: 1183. https://doi.org/10.3390/nano9081183
APA StyleShi, D., Ouyang, Z., Zhao, Y., Xiong, J., & Shi, X. (2019). Catalytic Reduction of Hexavalent Chromium Using Iron/Palladium Bimetallic Nanoparticle-Assembled Filter Paper. Nanomaterials, 9(8), 1183. https://doi.org/10.3390/nano9081183