Preparation of TiO2 and Fe-TiO2 with an Impinging Stream-Rotating Packed Bed by the Precipitation Method for the Photodegradation of Gaseous Toluene
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
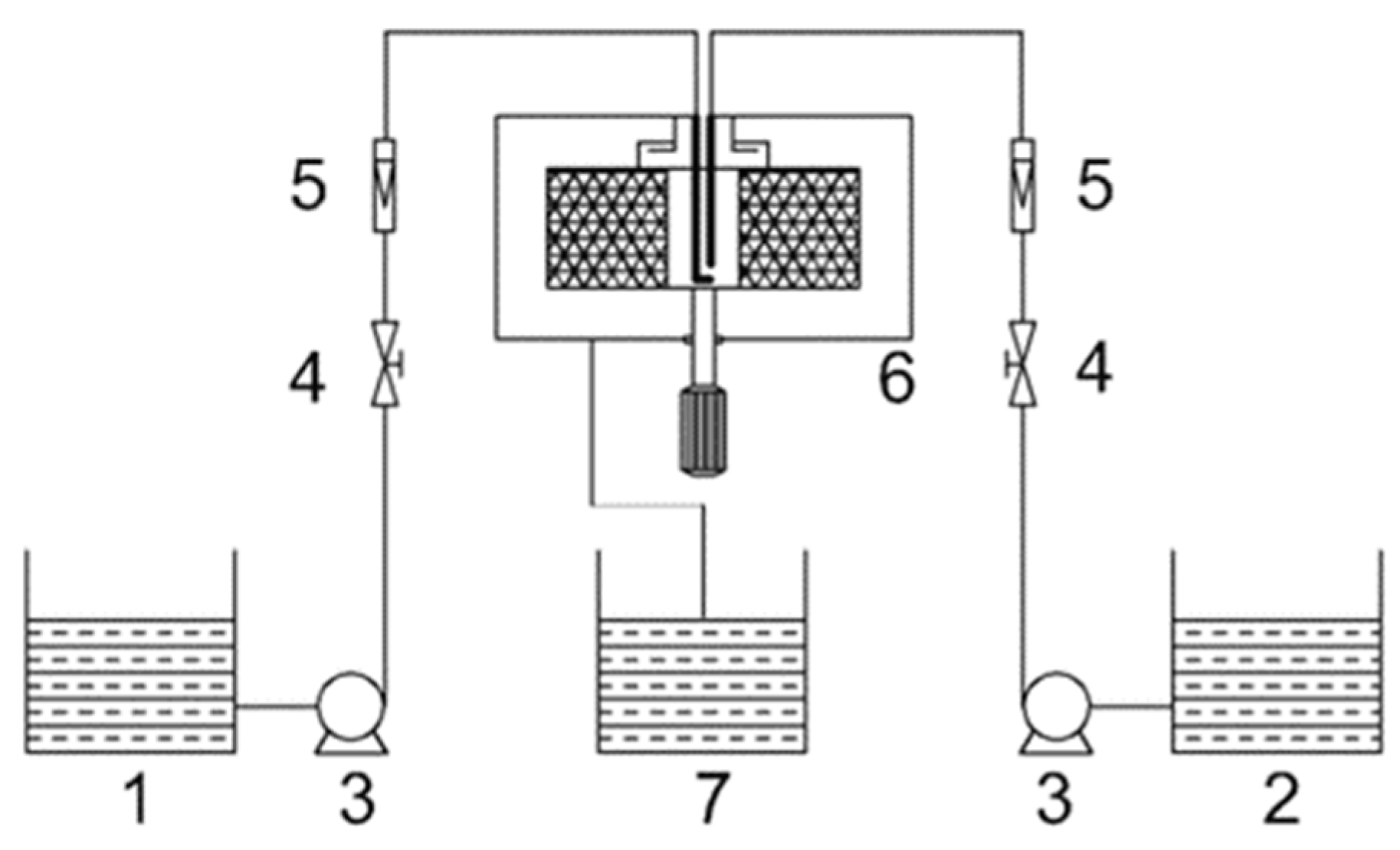
2.2. Synthesis of Nano-TiO2
2.3. Synthesis of Fe-TiO2 Nanoparticles
2.4. Characterization
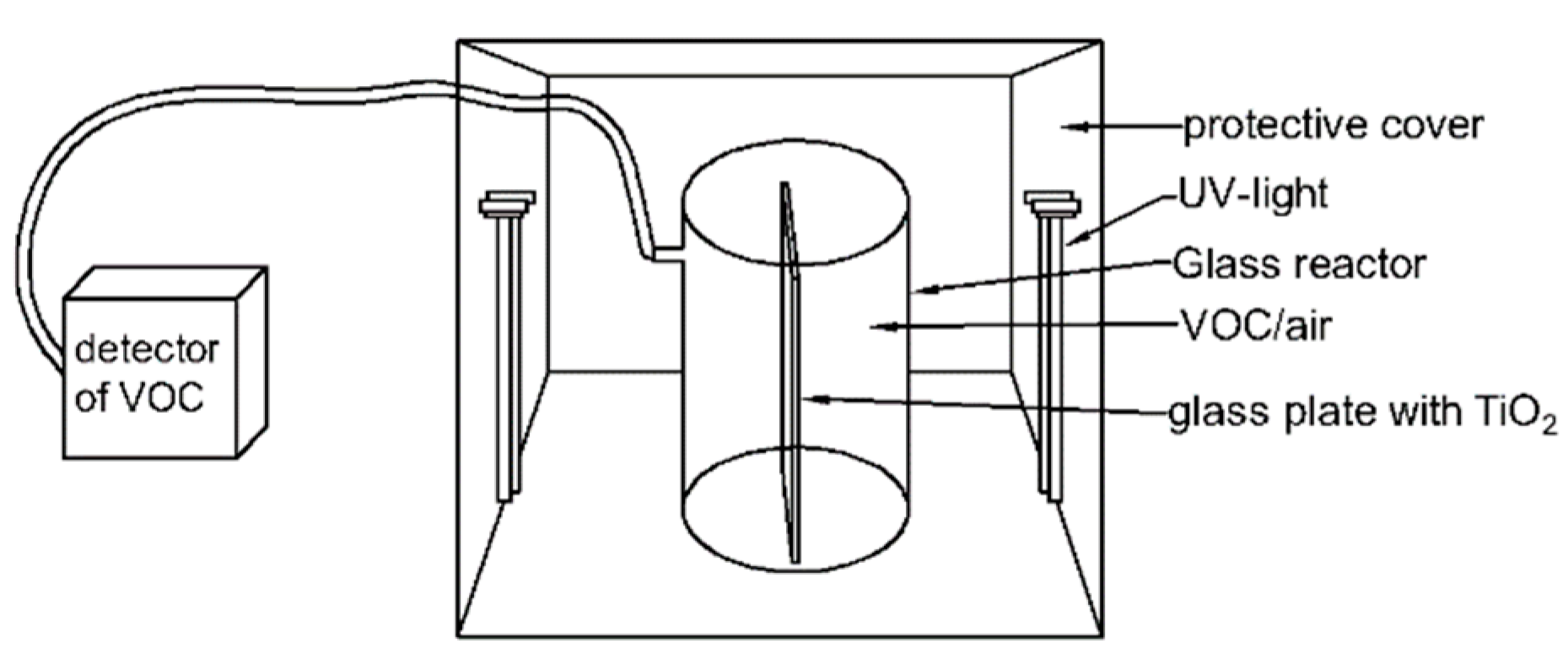
2.5. Photocatalytic Activity
3. Results and Discussion
3.1. The Effect of Different Rotating Speeds of Packing and Calcination Temperature on Nano-TiO2
3.2. Characterization of the Pure TiO2 and Fe-TiO2
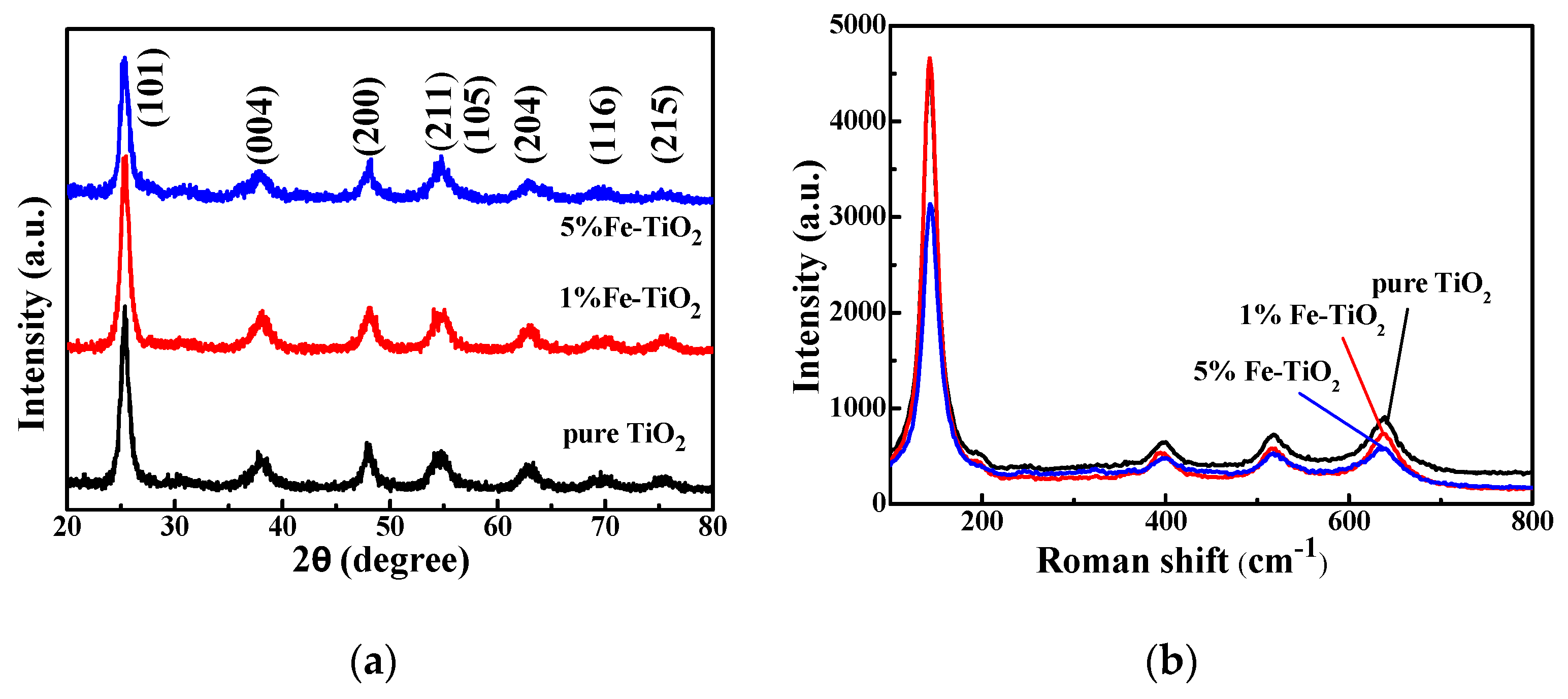
3.2.1. XRD Characterization
3.2.2. Raman Characterization
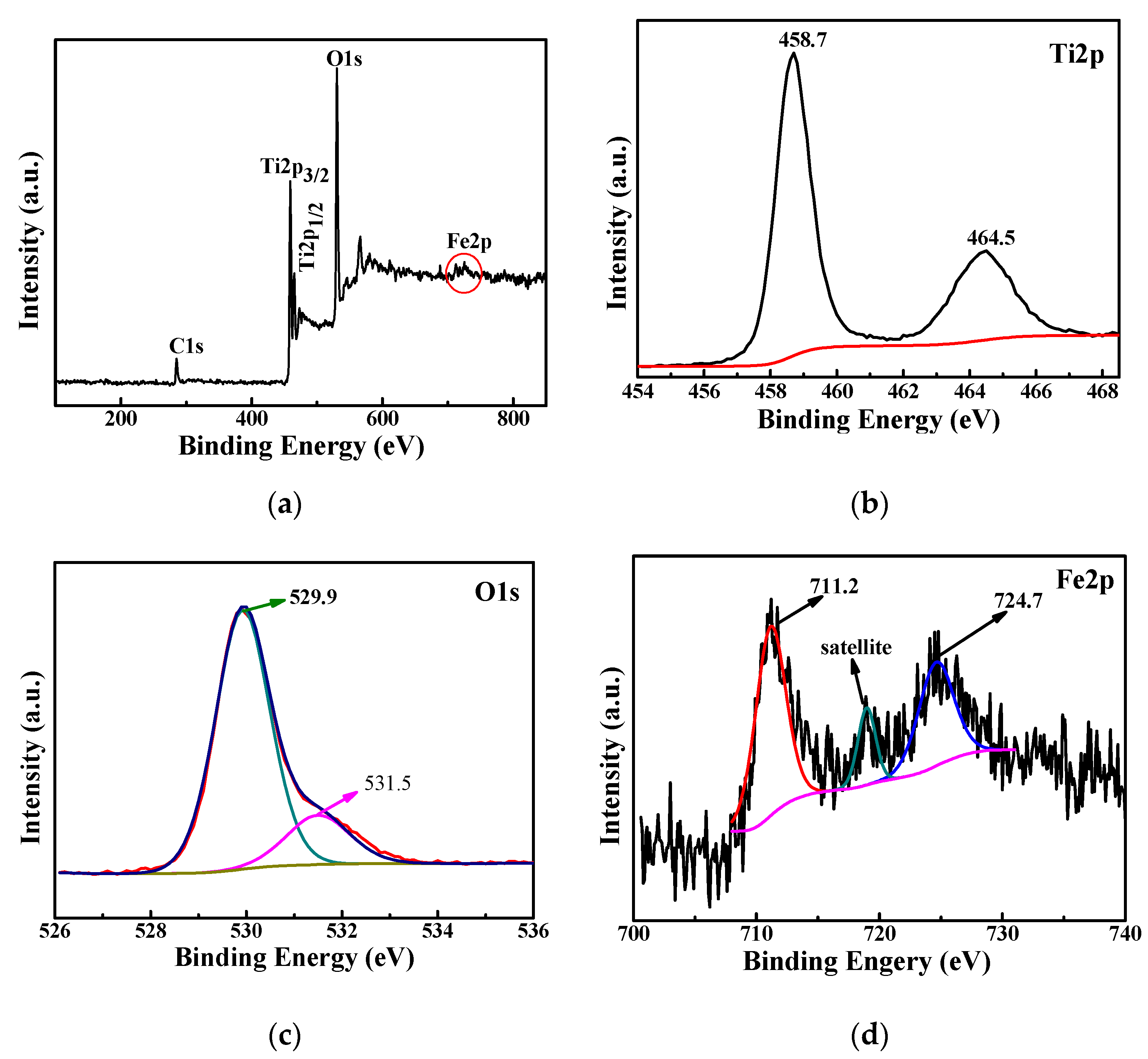
3.2.3. XPS Analysis
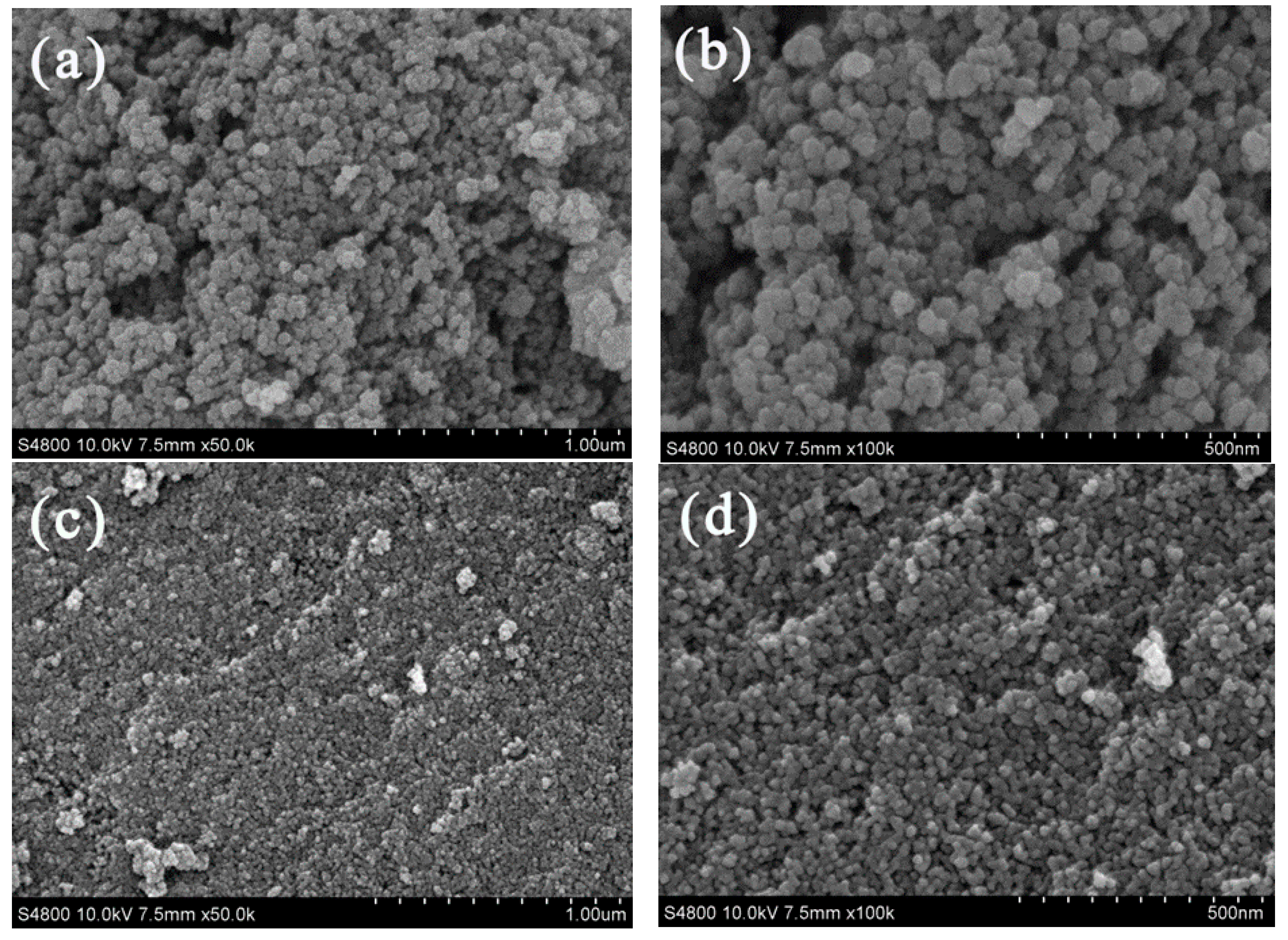
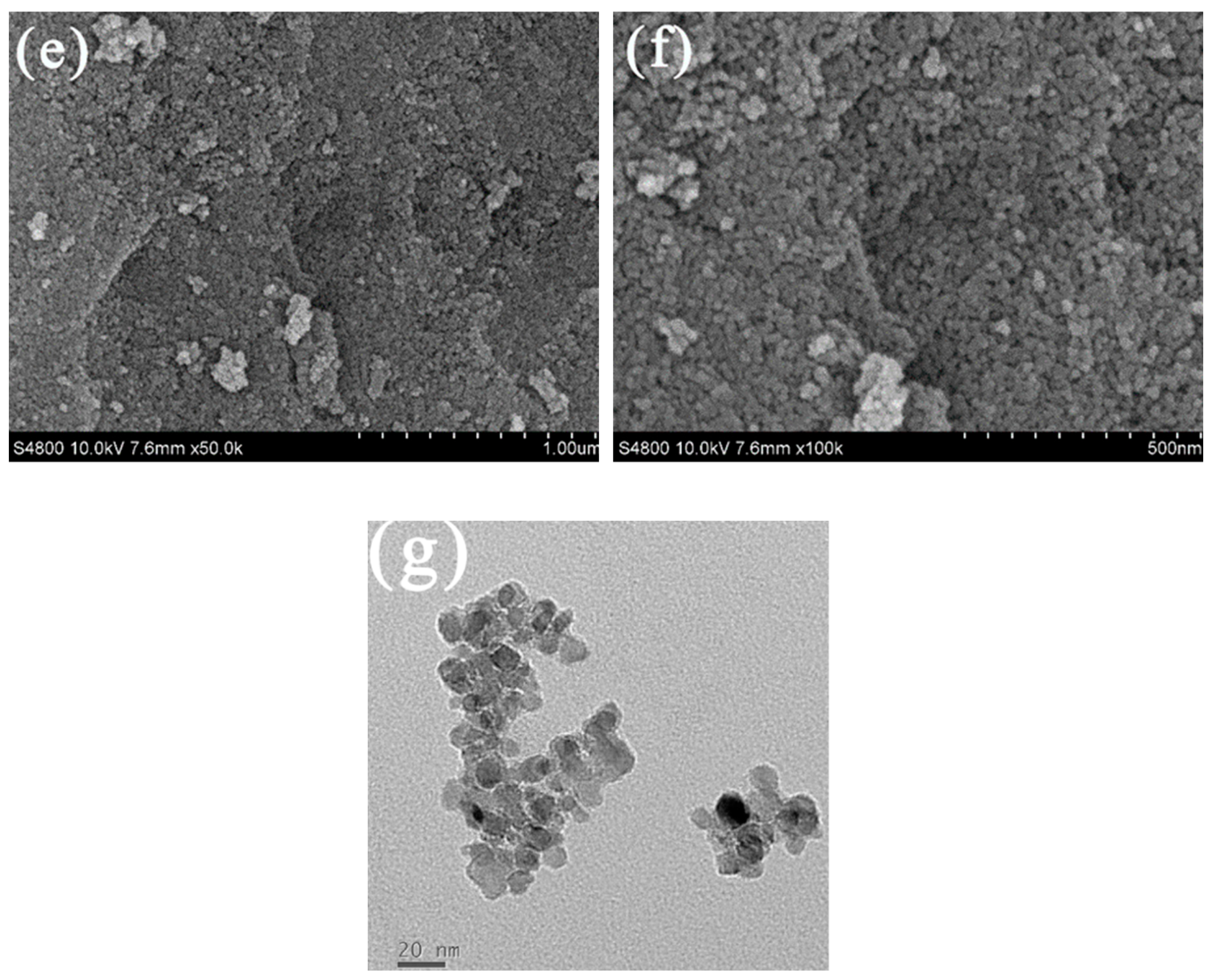
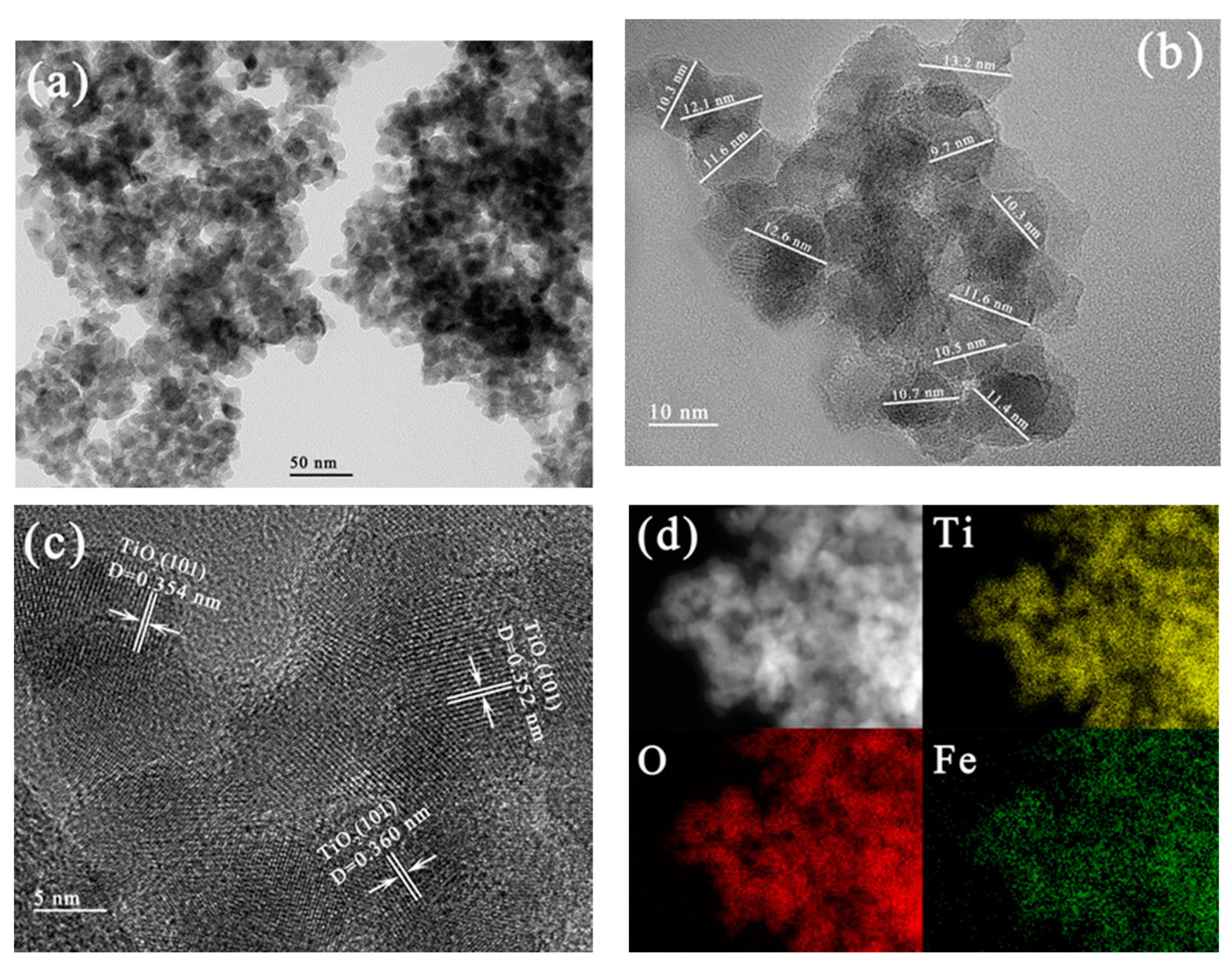
3.2.4. TEM, HRTEM and EDX Mapping
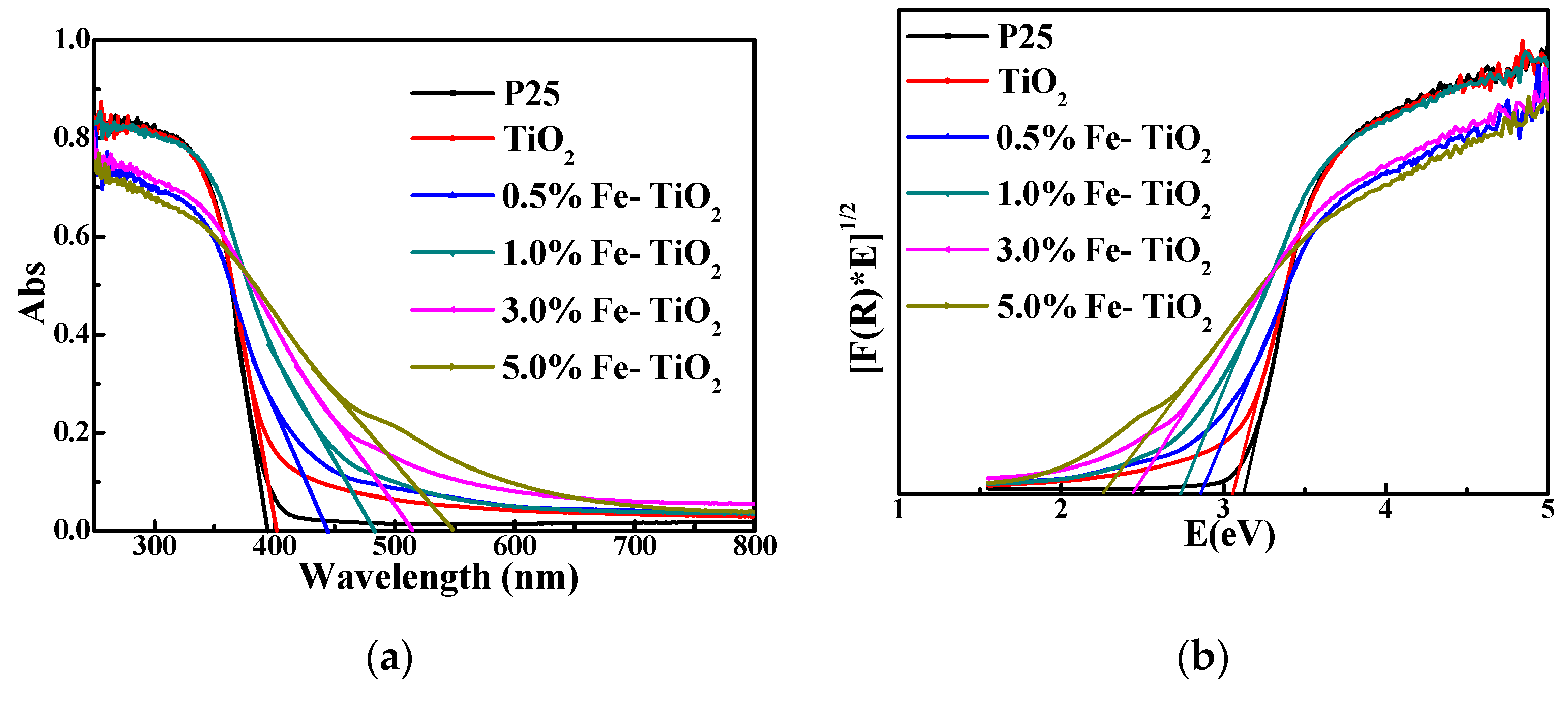
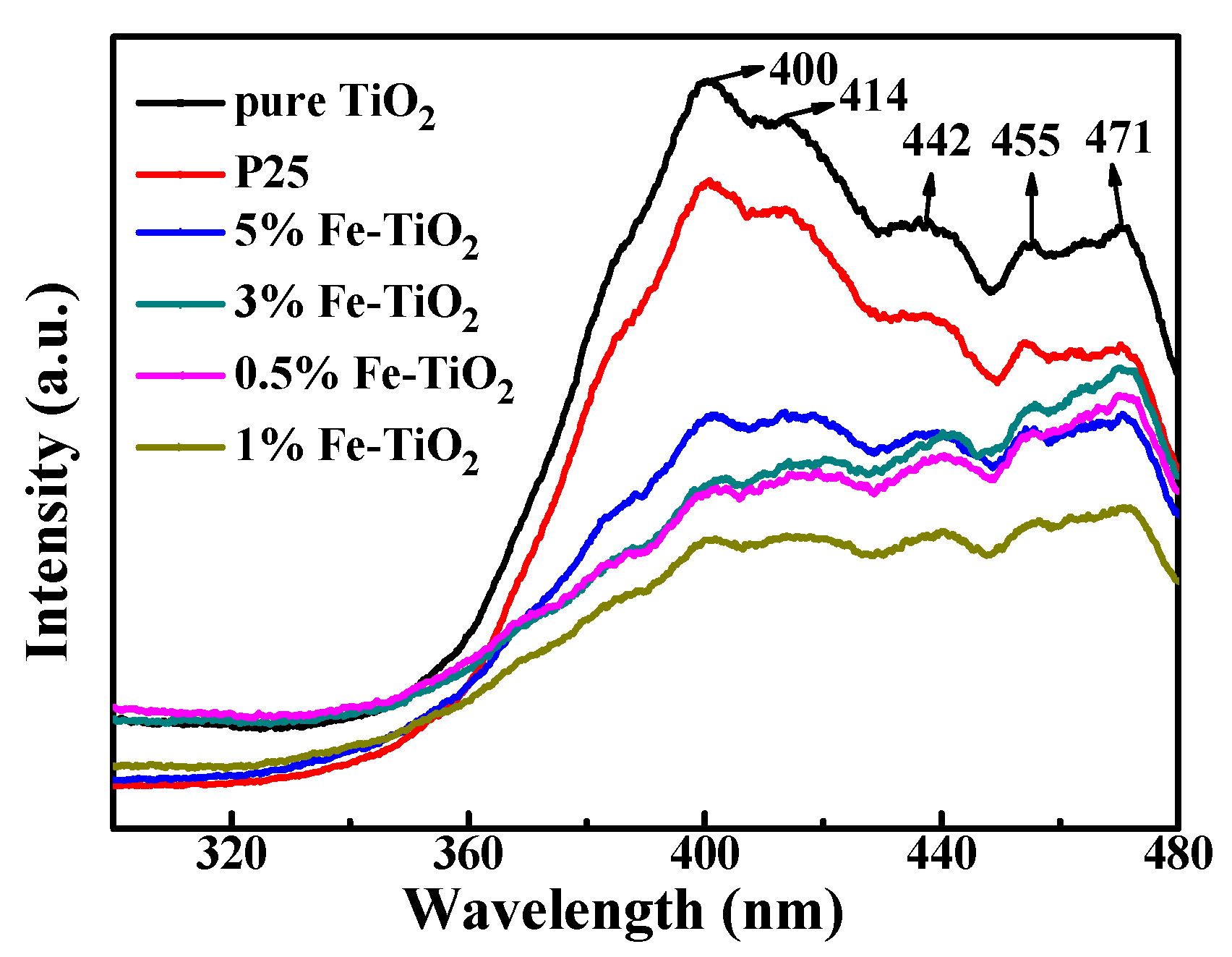
3.2.5. Photoresponse of Nano-TiO2
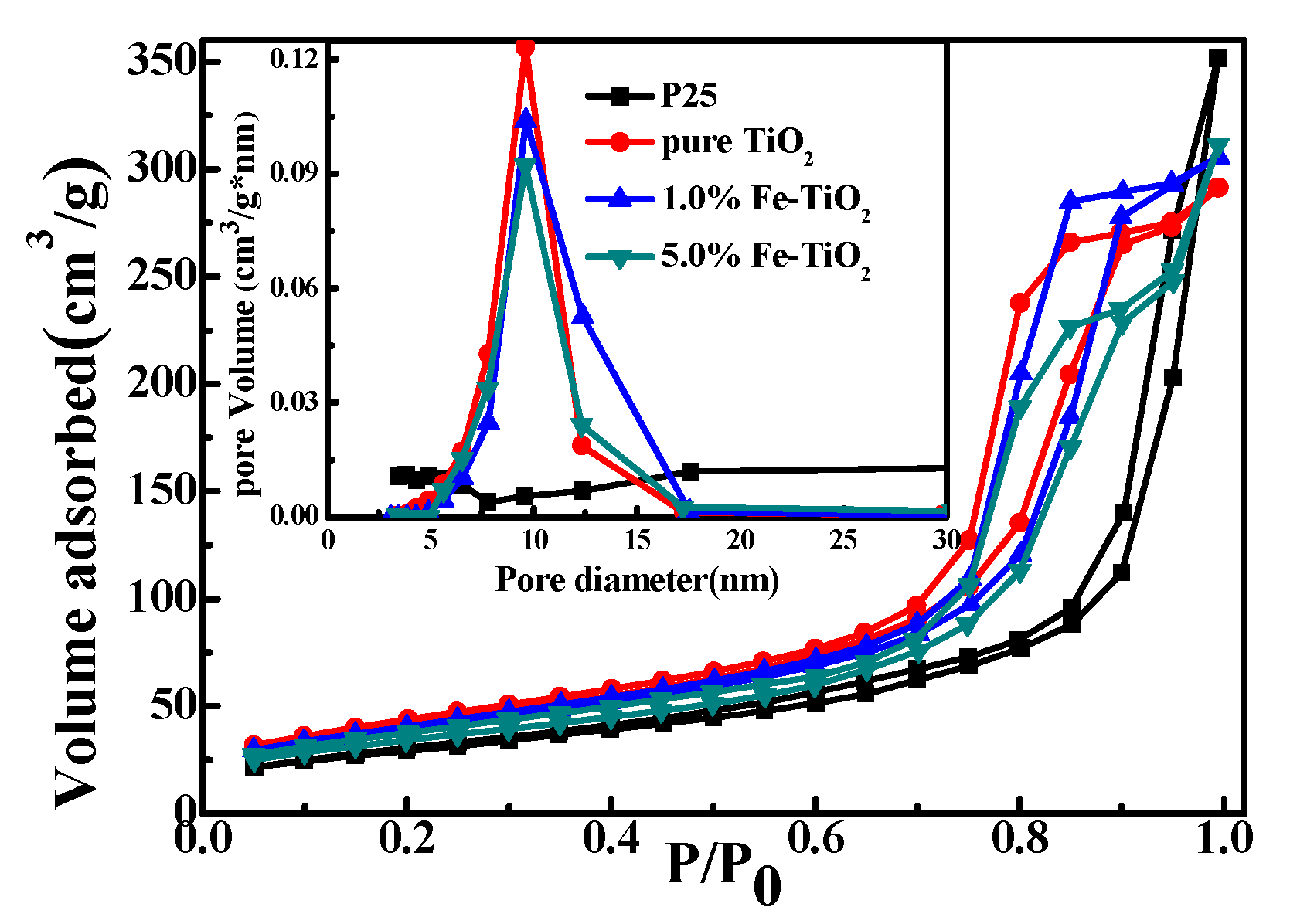
3.2.6. BET Analysis
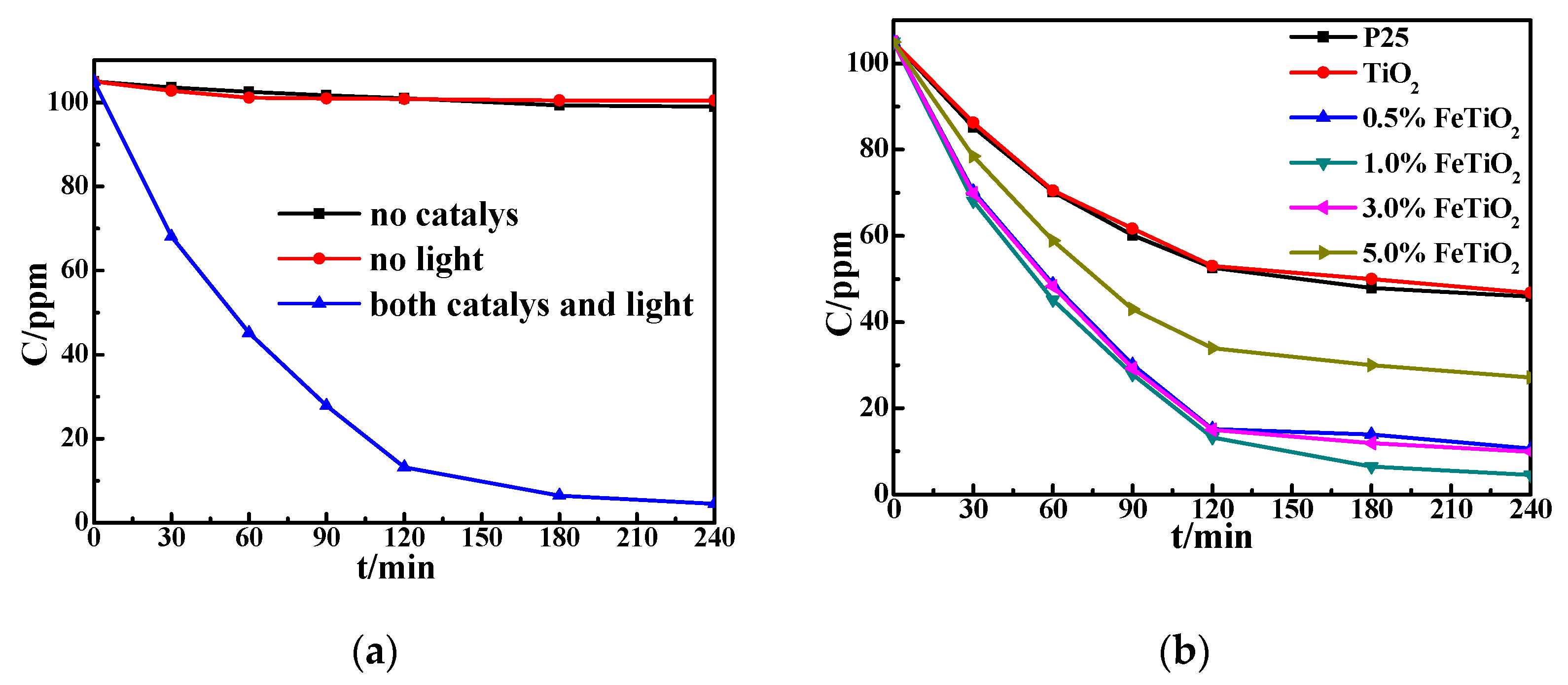
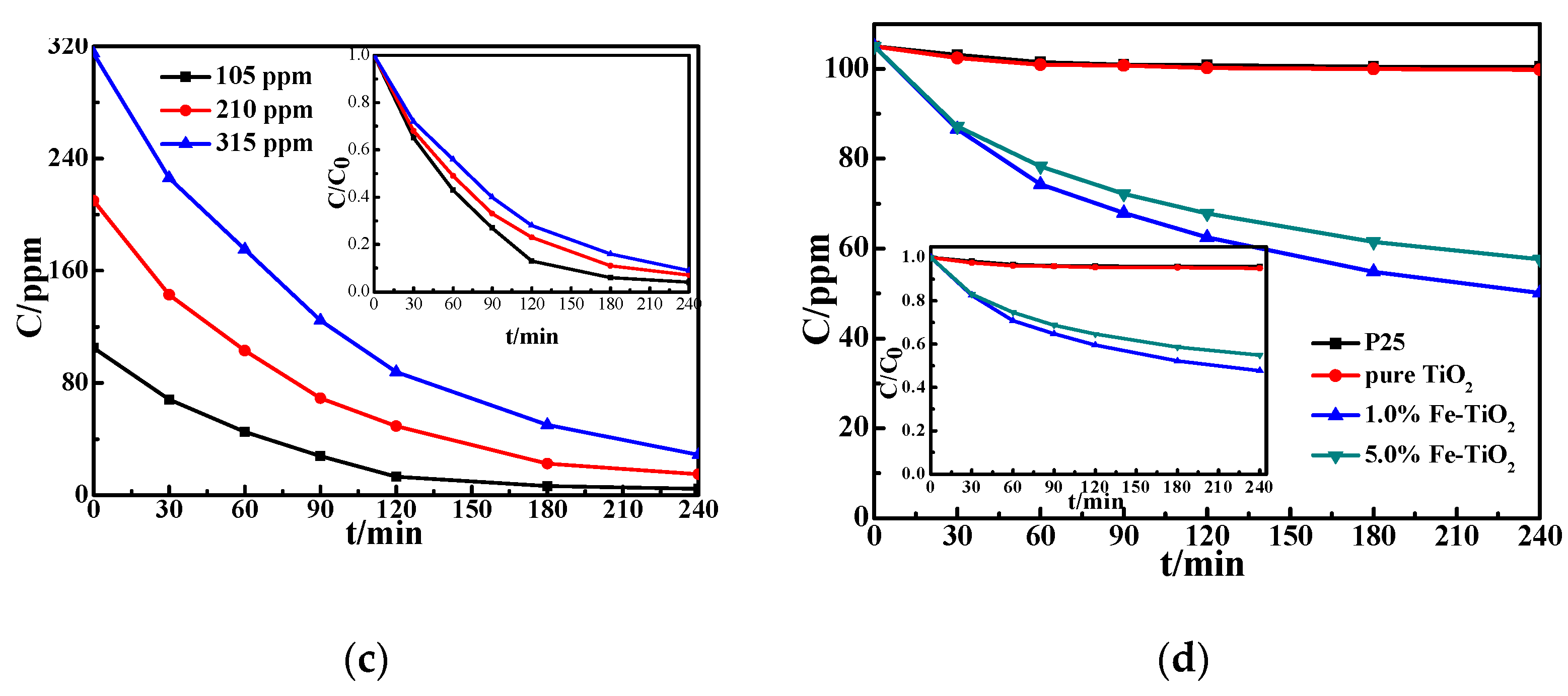
3.3. Photocatalytic Activity
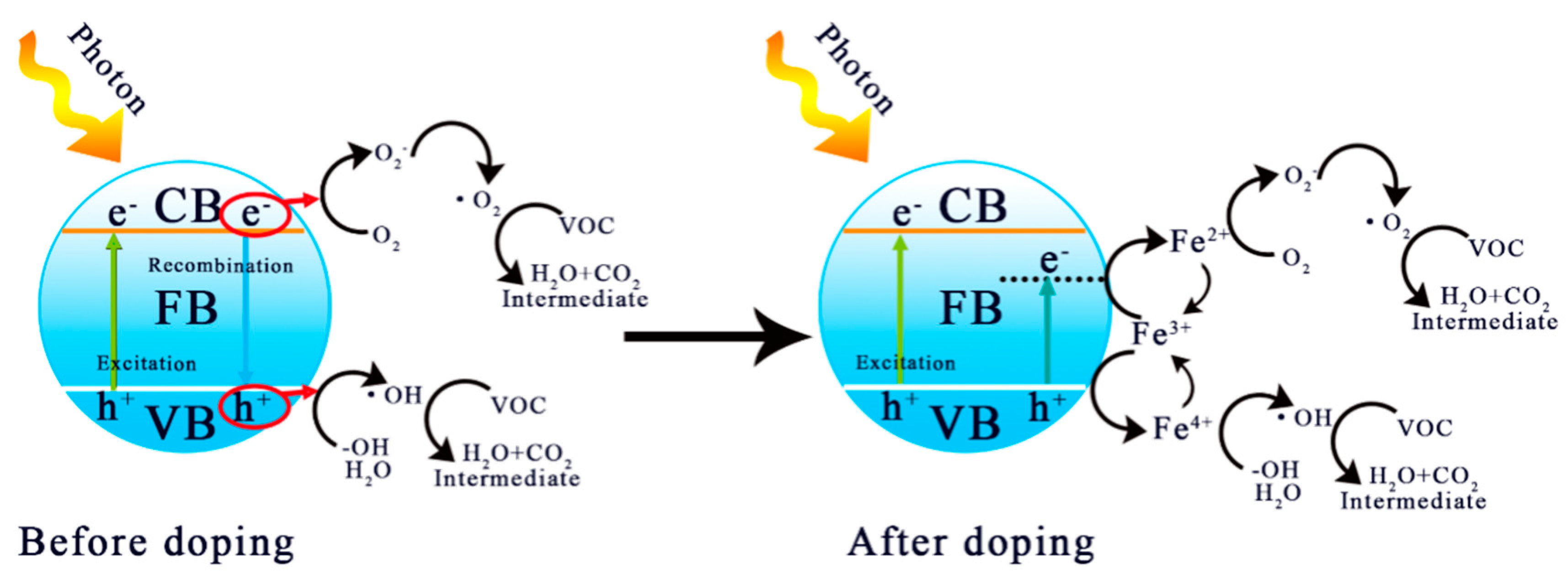
3.4. Possible Mechanism of Photocatalytic Enhancement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification Strategies of TiO2 for Potential Applications in Photocatalysis: A Critical Review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Yu, H.T.; Song, L.; Hao, Y.F.; Lu, N.; Quan, X.; Chen, S.; Zhang, Y.B.; Feng, Y.J. Fabrication of Pilot-Scale Photocatalytic Disinfection Device by Installing TiO2 Coated Helical Support into UV Annular Reactor for Strengthening Sterilization. Chem. Eng. J. 2016, 283, 1506–1513. [Google Scholar] [CrossRef]
- Beltrán-Partidaab, E.; Valdez-Salasb, B.; Escamillab, A.; Curielb, M.; Valdez-Salasc, E.; Nedevb, N.; Bastidasd, J.M. Disinfection of Titanium Dioxide Nanotubes using Super-Oxidized Water Decrease Bacterial Viability without Disrupting Osteoblast Behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, H.; Liu, Y.; He, G.A.; Jiang, C.C. Comparative Activity of TiO2 Microspheres and P25 Powder for Organic Degradation: Implicative Importance of Structural Defects and Organic Adsorption. Appl. Surf. Sci. 2014, 319, 2–7. [Google Scholar] [CrossRef]
- Lyu, J.; Zhu, L.Z.; Burda, C. Optimizing Nanoscale TiO2 for Adsorption-Enhanced Photocatalytic Degradation of Low-Concentration Air Pollutants. ChemCatChem 2013, 5, 3114–3123. [Google Scholar] [CrossRef]
- Cha, B.J.; Saqlaina, S.; Seob, H.O.; Kim, Y.D. Hydrophilic Surface Modification of TiO2 to Produce a Highly Sustainable Photocatalyst for Outdoor Air Purification. Appl. Surf. Sci. 2019, 479, 31–38. [Google Scholar] [CrossRef]
- Kovalevskiyab, N.S.; Lyulyukinab, M.N.; Selishchevabet, D.S.; Kozlovabal, D.V. Analysis of Air Photocatalytic Purification using a Total Hazard Index: Effect of the Composite TiO2/Zeolite Photocatalyst. J. Hazard. Mater. 2018, 358, 302–309. [Google Scholar] [CrossRef]
- Khalilzadeh, H.; Fatemi, S. Spouted Bed Reactor for VOC Removal by Modified Nano-TiO2 Photocatalytic Particles. Chem. Eng. Res. Des. 2016, 115, 241–250. [Google Scholar] [CrossRef]
- Cui, M.F.; Pan, S.; Tang, Z.; Chen, X.; Qiao, X.; Xu, Q. Physiochemical Properties of N-N Heterostructured TiO2/Mo-TiO2 Composites and Their Photocatalytic Degradation of Gaseous Toluene. Chem. Speciat. Bioavailab. 2017, 29, 60–69. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic Technologies for Environmental Applications. J. Photochem. Photobiol. A. 2012, 238, 41–52. [Google Scholar] [CrossRef]
- Xiang, J.; Yin, Q.; Zhang, K.; Guo, X.; Wang, X. Fabrication, Structural Morphology and Photocatalytic Activity of Mesoporous TiO2 Prepared by Fast Sol-Gel Method. Mater. Technol. 2014, 30, 213–217. [Google Scholar] [CrossRef]
- Jang, I.; Leong, H.J.; Noh, H.; Kang, T.; Kong, S.; Oh, S.G. Preparation of N-Functionalized TiO2 Particles using One-step Sol-Gel Method and Their Photocatalytic Activity. J. Ind. Eng. Chem. 2016, 37, 380–389. [Google Scholar] [CrossRef]
- Xiang, Y.; Wen, L.X.; Chu, G.W.; Shao, L.; Xiao, G.T.; Chen, J.F. Modeling of the Precipitation Process in a Rotating Packed Bed and Its Experimental Validation. Chin. J. Chem. Eng. 2010, 18, 249–257. [Google Scholar] [CrossRef]
- Yang, H.J.; Chu, G.W.; Xiang, Y.; Chen, J.F. Characterization of Micromixing Efficiency in Rotating Packed Beds by Chemical Methods. Chem. Eng. J. 2006, 121, 147–152. [Google Scholar] [CrossRef]
- He, X.L.; Wang, Z.; Pu, Y.; Wang, D.; Tang, R.J.; Cui, S.M.; Wang, J.X.; Chen, J.F. High-Gravity-Assisted Scalable Synthesis of Zirconia Nanodispersion for Light Emitting Diodes Encapsulation with Enhanced Light Extraction Efficiency. Chem. Eng. Sci. 2019, 195, 1–10. [Google Scholar] [CrossRef]
- Fan, H.L.; Zhou, S.F.; Qi, G.S.; Liu, Y.Z. Continuous Preparation of Fe3O4 Nanoparticles using Impinging Stream-Rotating Packed Bed Reactor and Magnetic Property Thereof. J. Alloy. Compd. 2015, 662, 497–504. [Google Scholar] [CrossRef]
- Lin, C.C.; Ho, J.M.; Wu, M.S. Continuous Preparation of Fe3O4 Nanoparticles using a Rotating Packed Bed: Dependence of Size and Magnetic Property on Temperature. Powder Technol. 2015, 274, 441–445. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, B.; Wu, X.; Wang, M.; Zhang, C.; Zenf, X.F.; Wang, J.X.; Chen, J.F. Preparation of Transparent Suspension of Lamellar Magnesium Hydroxide Nanocrystals Using a High-Gravity Reactive Precipitation Combined with Surface Modification. Ind. Eng. Chem. Res. 2015, 54, 666–671. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Wang, Z.Y.; Wang, D.; Wang, J.X.; Fosterac, N.R.; Pu, Y.; Chen, J.F. Preparation of 3D Graphene/Iron Oxides Aerogels Based on High-Gravity Intensified Reactive Precipitation and Their Applications for Photo-Fenton Reaction. Chem. Eng. Process. 2018, 129, 77–83. [Google Scholar] [CrossRef]
- Fan, H.L.; Ma, X.Z.; Zhou, S.F.; Huang, J.; Liu, Y.Q.; Liu, Y.Z. Highly Efficient Removal of Heavy Metal Ions by Carboxymethyl Cellulose-Immobilized Fe3O4 Nanoparticles Prepared via High-Gravity Technology. Carbohydr. Polym. 2019, 213, 39–49. [Google Scholar] [CrossRef]
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-Metal Modified TiO2: A Step towards Visible Light Photocatalysis. J. Mater. Sci. Mater. Electron. 2019, 30, 3186–3207. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 Photocatalyst for Removal of Volatile Organic Compounds in Gas Phase–A Review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillaiab, S.C. Photocatalytic Hydrogen Production using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B Environ. 2018, 224, 1021–1064. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of Doped Photocatalysts for Organic Pollutant Degradation-A Review. J. Environ. Manage. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Umar, K.; Ibrahim, M.N.M.; Ahmad, A.; Rafatullah, M. Synthesis of Mn-Doped TiO2 by Novel Route and Photocatalytic Mineralization/Intermediate Studies of Organic Pollutants. Res. Chem. Intermed. 2019, 45, 2927–2945. [Google Scholar] [CrossRef]
- Jiao, W.Z.; Liu, Y.Z.; Qi, G.S. A New Impinging Stream-Rotating Packed Bed Reactor for Improvement of Micromixing Iodide and Iodate. Chem. Eng. J. 2010, 157, 168–173. [Google Scholar] [CrossRef]
- Jiao, W.Z.; Wang, Y.; Li, X.; Xu, C.; Liu, Y.Z.; Zhang, Q.L. Stabilization Performance of Methanol-Diesel Emulsified Fuel Prepared using an Impinging Stream-Rotating Packed Bed. Renew. Energy 2016, 85, 573–579. [Google Scholar] [CrossRef]
- Shin, S.H.; Jo, W.K. Volatile Organic Compound Concentrations in Newly Built Apartment Buildings during Pre- and Post-Occupancy Stages. Int. J. Environ. Anal. Chem. 2014, 94, 356–369. [Google Scholar] [CrossRef]
- Jo, W.K.; Choi, S.J. Vehicle Occupants’ Exposure to Aromatic Volatile Organic Compounds while Commuting on an Urban-suburban Route in Korea. J. Air Waste Manag. 1996, 46, 749–754. [Google Scholar] [CrossRef]
- Jo, W.K. Purification of Aromatic Hydrocarbons via Fibrous Activated Carbon/Photocatalytic Composite Coupled with UV Light-Emitting Diodes. Environ. Technol. 2013, 34, 1175–1181. [Google Scholar] [CrossRef]
- Jo, W.K.; Kang, H.J.; Chun, H.H. Degradation of Gas-Phase Organic Contaminants via Nitrogen-Embedded One-Dimensional Rod-Shaped Titania in a Plug-Flow Reactor. Environ. Technol. 2014, 35, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K. Coupling of Titania with Multiwall Carbon Nanotubes for Decomposition of Gas-Phase Pollutants under Simulated Indoor Conditions. J. Air Waste Manag. 2013, 63, 963–970. [Google Scholar] [CrossRef]
- Jiao, W.Z.; Qin, Y.; Luo, S.; He, Z.; Feng, Z.; Liu, Y.Z. Simultaneous Formation of Nanoscale Zero-Valent Iron and Degradation of Nitrobenzene in Wastewater in an Impinging Stream-Rotating Packed Bed Reactor. Chem. Eng. J. 2017, 321, 564–571. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Tala-Ighilaet, R.; Tounanea, M.; Iratnia, A.; Souierd, T.; Liu, S.; Cai, W. Optical, Structural and Photocatalysis Properties of Cu-Doped TiO2 Thin Films. Appl. Surf. Sci. 2017, 395, 110–116. [Google Scholar] [CrossRef]
- Sathyaseelan, B.; Manikandan, E.; Lakshmanan, V.; Baskaranf, I.; Sivakumarb, K.; Ladchumananan- dasivamg, R.; Kennedydh, J.; Maazad, M. Structural, Optical and Morphological Properties of Post-Growth Calcined TiO2 Nanopowder for Opto-Electronic Device Application: Ex-Situ Studies. J. Alloy. Compd. 2016, 671, 486–492. [Google Scholar] [CrossRef]
- Majeed Khana, M.A.; Siwachb, R.; Kumarb, S.; Alhazaaac, A.N. Role of Fe Doping in Tuning Photocatalytic and Photoelectrochemical Properties of TiO2 for Photodegradation of Methylene Blue. Opt. Laser Technol. 2019, 11, 170–178. [Google Scholar] [CrossRef]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic Degradation of Aqueous Nitirobenzene by Nanocrystalline TiO2. Ind. Eng. Chem. Res. 2006, 45, 922–927. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lee, C.Y.; Yang, M.Y.; Chiu, H.T. The Effect of Calcination Temperature on the Crystallinity of TiO2 Nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Surolia, P.K.; Tayade, R.J.; Jasra, R.V. Effect of Anions on the Photocatalytic Activity of Fe(III) Salts Impregnated TiO2. Ind. Eng. Chem. Res. 2007, 46, 6196–6203. [Google Scholar] [CrossRef]
- Isaria, A.A.; Payana, A.; Fattahia, M.; Jorfibc, S.; Kakavandide, B. Photocatalytic Degradation of Rhodamine B and Real Textile Wastewater using Fe-Doped TiO2 Anchored on Reduced Graphene Oxide (Fe-TiO2/rGO): Characterization and Feasibility, Mechanism and Pathway Studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Lim, J.H.; Bae, D.; Fong, A. Titanium Dioxide in Food Products: Quantitative Analysis using ICP-MS and Raman Spectroscopy. J. Agric. Food. Chem. 2018, 66, 13533–13540. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Ma, J.; He, H.; Liu, F. Effect of Fe on the Photocatalytic Removal of NOx over Visible Light Responsive Fe/TiO2 Catalysts. Appl. Catal. B Environ. 2015, 179, 21–28. [Google Scholar] [CrossRef]
- Hemraj, M.; Yadav, T.V.; Kolekar, S.H.; Pawar, J.S.K. Enhanced Photocatalytic Inactivation of Bacteria on Fe-Containing TiO2 Nanoparticles under Fluorescent Fight. J. Mater. Sci. Mater. Med. 2016, 27, 57. [Google Scholar]
- Sun, S.; Ding, J.; Bao, J.; Gao, C.; Qi, Z.; Yang, X.; He, B.; Li, C. Photocatalytic degradation of gaseous toluene on Fe-TiO2 under visible light irradiation: A study on the structure, activity and deactivation mechanism. Appl. Surf. Sci. 2012, 258, 5031–5037. [Google Scholar] [CrossRef]
- Meng, H.; Wang, B.; Liu, S.; Jiang, R.; Long, H. Hydrothermal Preparation, Characterization and Photocatalytic Activity of TiO2/Fe–TiO2 Composite Catalysts. Ceram. Int. 2013, 39, 5785–5793. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Mao, M.; Lan, L.; Lao, X.; Zhao, X. Significant Improvement in Photocatalytic Activity by Forming Homojunction between Anatase TiO2 Nanosheets and Anatase TiO2 Nanoparticles. Appl. Surf. Sci. 2019, 490, 283–292. [Google Scholar] [CrossRef]

| Temperature (°C) | FWHM (°) | β’ (rad) | XRD Size (nm) | Anatase (%) | Rutile (%) | Crystallinity (%) |
|---|---|---|---|---|---|---|
| P25 | - | - | - | 77% | 23% | 100% |
| 400 | 0.951 | 0.0166 | 8.4 | 100% | 0% | 70% |
| 500 | 0.837 | 0.0143 | 9.6 | 100% | 0% | 82% |
| 600 | 0.488 | 0.0085 | 16.5 | 100% | 0% | 110% |
| 700 | 0.308 | 0.0054 | 26.0 | 95% | 5% | 97% |
| 800 | 0.163 | 0.0028 | 50.2 | 67% | 33% | 81% |
| Photocatalysts | Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| P25 | 105.96 | 0.5438 | 30.56 |
| TiO2 | 148.47 | 0.4508 | 9.58 |
| 1.0%Fe-TiO2 | 140.18 | 0.4643 | 9.60 |
| 5.0%Fe-TiO2 | 123.50 | 0.4813 | 9.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; Zhang, Q.; Liu, Y.; Zhang, S.; Guo, J. Preparation of TiO2 and Fe-TiO2 with an Impinging Stream-Rotating Packed Bed by the Precipitation Method for the Photodegradation of Gaseous Toluene. Nanomaterials 2019, 9, 1173. https://doi.org/10.3390/nano9081173
Zeng G, Zhang Q, Liu Y, Zhang S, Guo J. Preparation of TiO2 and Fe-TiO2 with an Impinging Stream-Rotating Packed Bed by the Precipitation Method for the Photodegradation of Gaseous Toluene. Nanomaterials. 2019; 9(8):1173. https://doi.org/10.3390/nano9081173
Chicago/Turabian StyleZeng, Guangping, Qiaoling Zhang, Youzhi Liu, Shaochuang Zhang, and Jing Guo. 2019. "Preparation of TiO2 and Fe-TiO2 with an Impinging Stream-Rotating Packed Bed by the Precipitation Method for the Photodegradation of Gaseous Toluene" Nanomaterials 9, no. 8: 1173. https://doi.org/10.3390/nano9081173
APA StyleZeng, G., Zhang, Q., Liu, Y., Zhang, S., & Guo, J. (2019). Preparation of TiO2 and Fe-TiO2 with an Impinging Stream-Rotating Packed Bed by the Precipitation Method for the Photodegradation of Gaseous Toluene. Nanomaterials, 9(8), 1173. https://doi.org/10.3390/nano9081173




