Cobalt-Containing Nanoporous Nitrogen-Doped Carbon Nanocuboids from Zeolite Imidazole Frameworks for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Hybrid Electrode Materials
2.3. Assembly of Asymmetric Supercapacitors
2.4. Characterizations
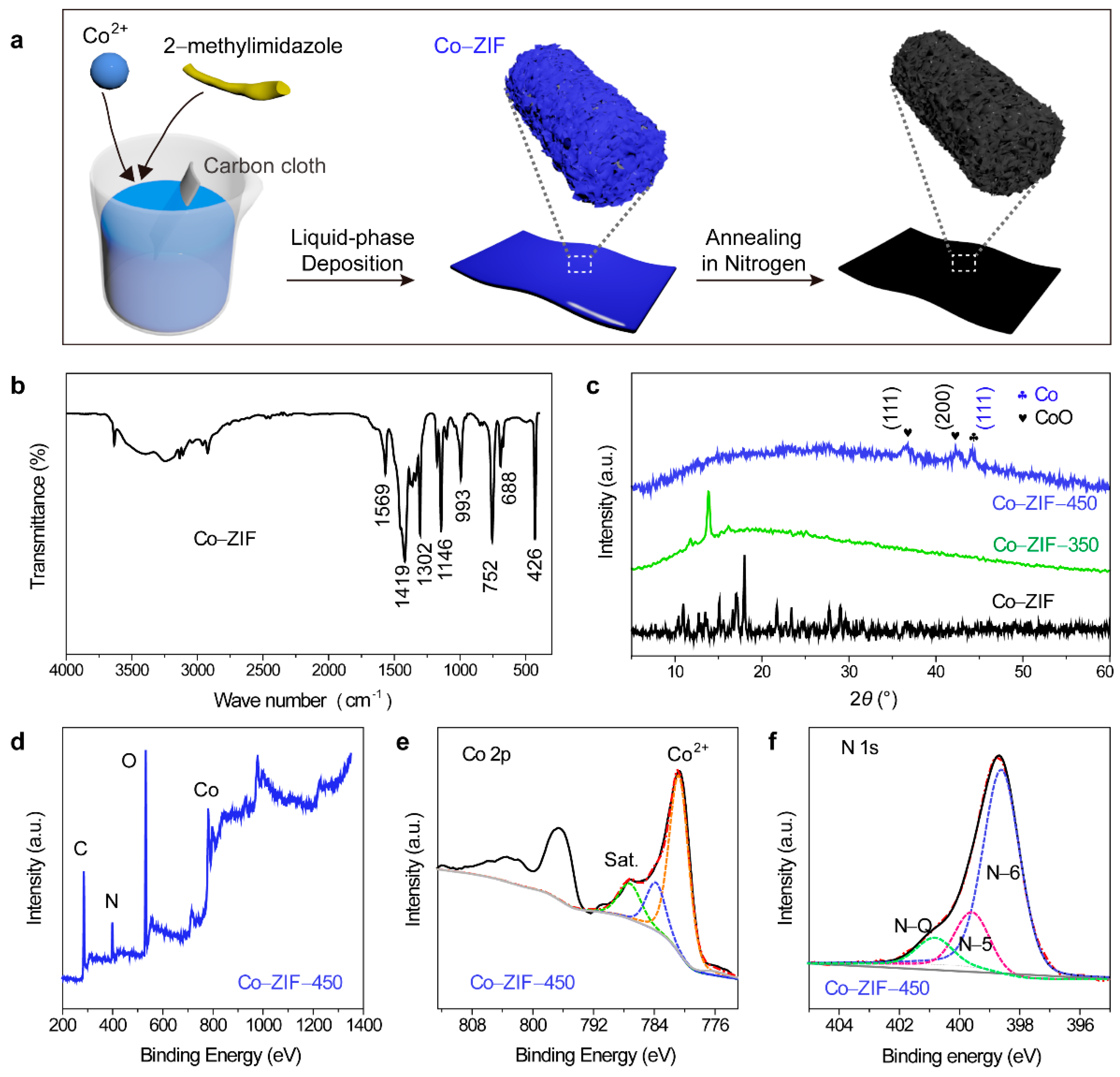
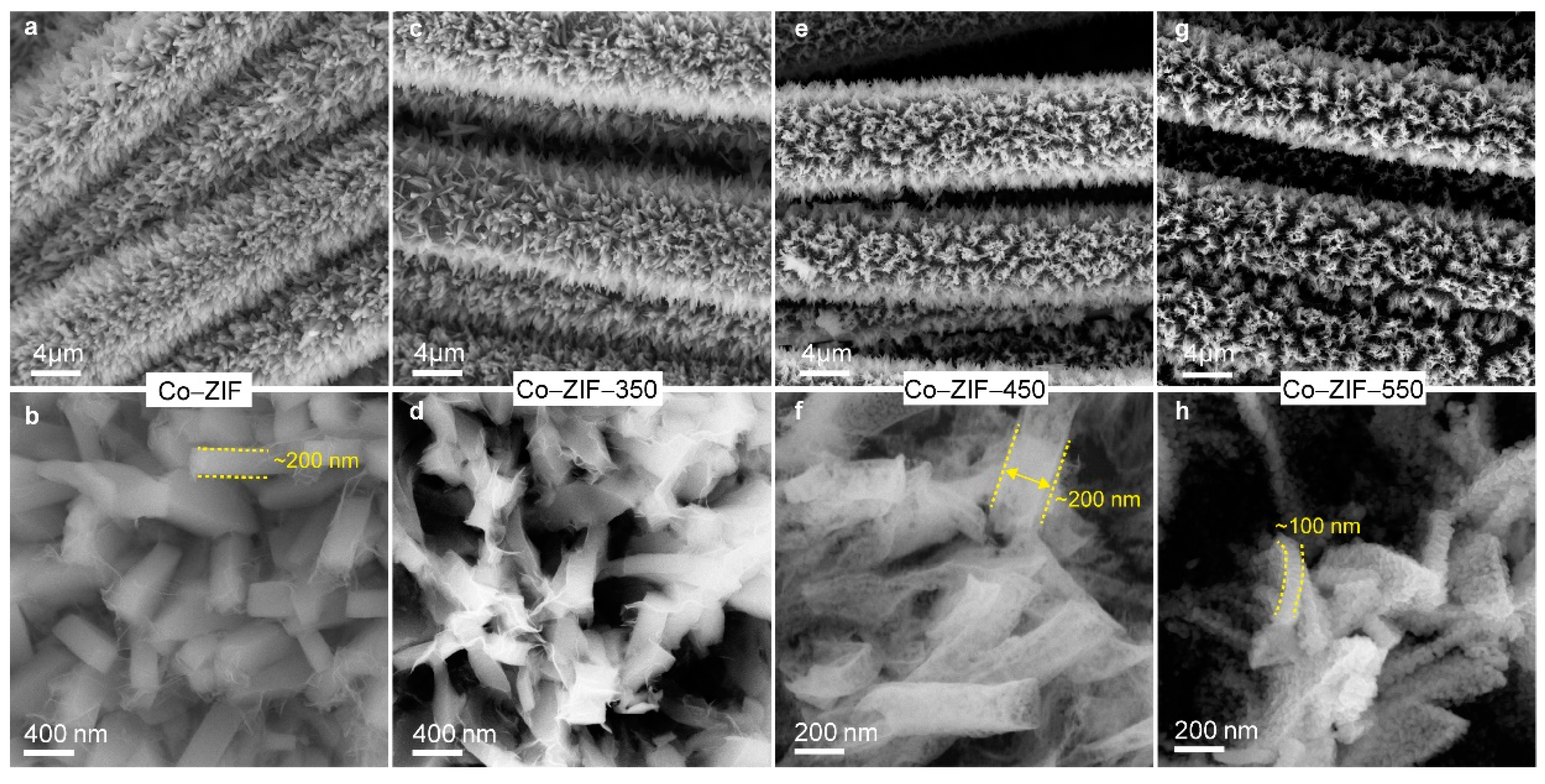
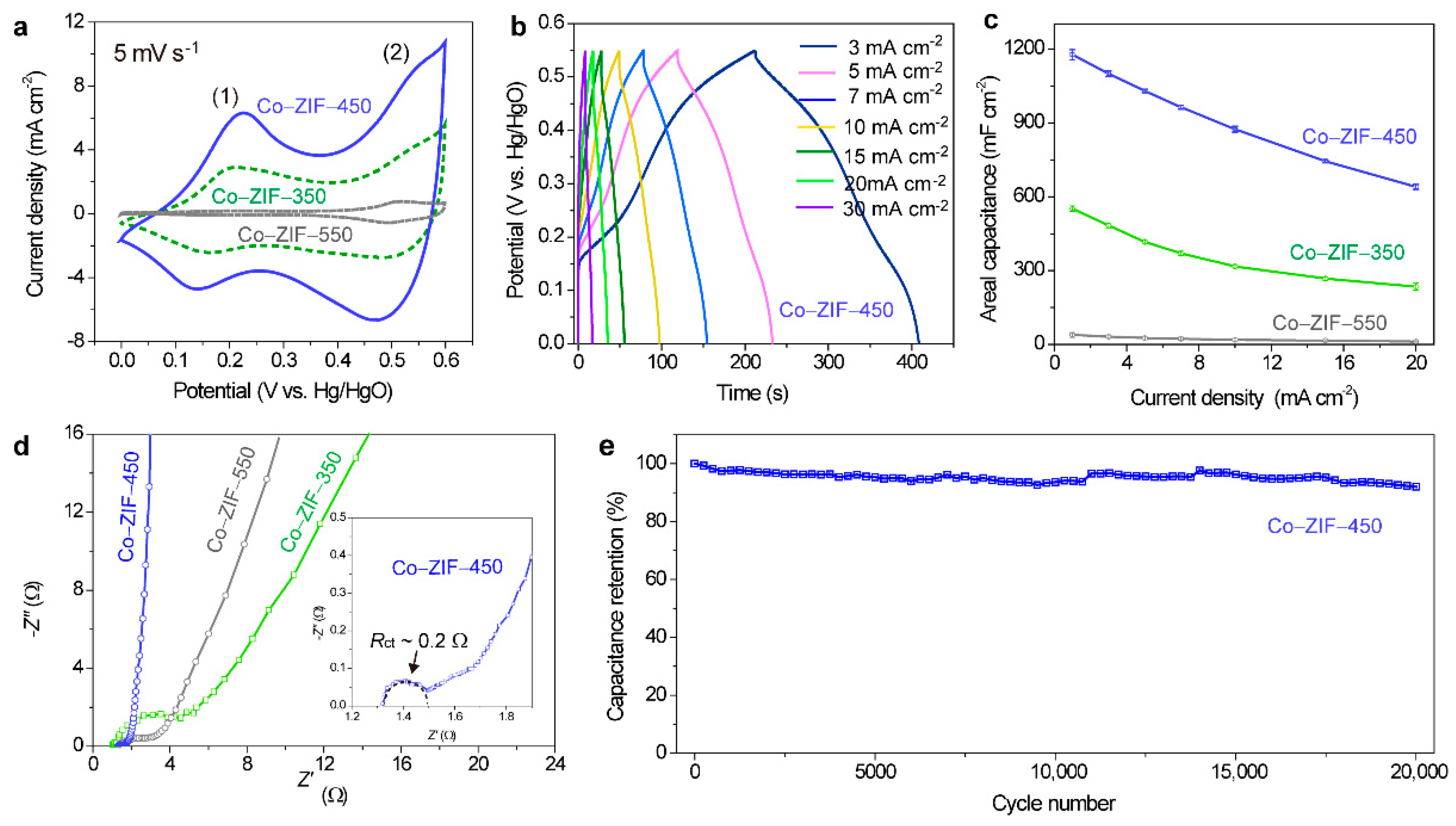
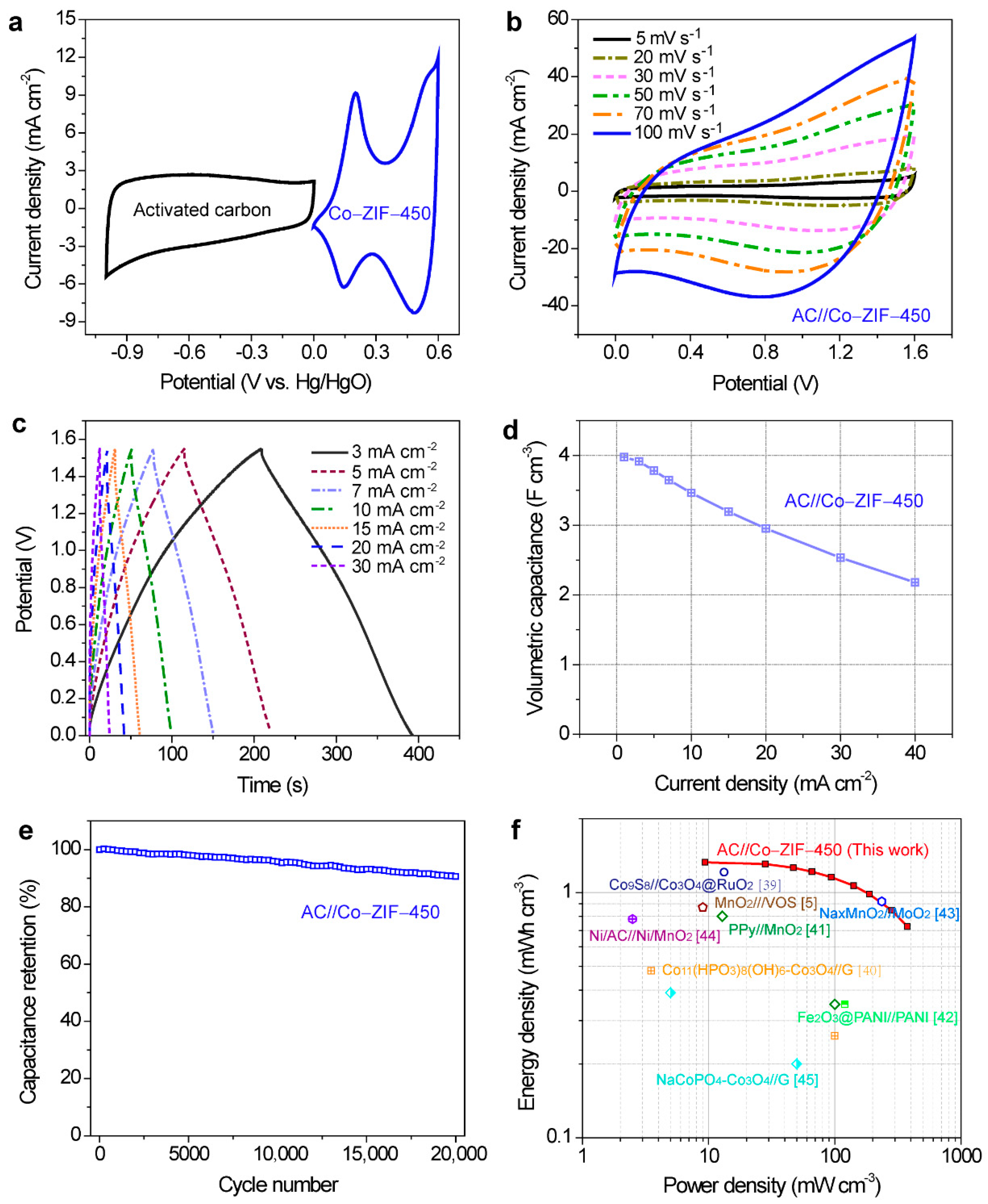
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, J.; Zhang, X.; Yang, J.; Zhou, J.; Tong, M.; Jin, Q.; Dai, F.; Li, G. Ethylenediamine-catalyzed preparation of nitrogen-doped hierarchically porous carbon aerogel under hypersaline condition for high-performance supercapacitors and organic solvent absorbents. Nanomaterials 2019, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Yan, F.; Dong, C.; An, C. Design and regulation of novel MnFe2O4@C nanowires as high performance electrode for supercapacitor. Nanomaterials 2019, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lai, Z.; Han, Y.; Zhang, H.; Xie, S.; Lu, X. Oxygen-vacancy and surface modulation of ultrathin nickel cobaltite nanosheets as a high-energy cathode for advanced Zn-ion batteries. Adv. Mater. 2018, 1802396. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lin, Z.; Wang, Z.; Wu, M.; Tong, Y.; Lu, X. In situ activation of 3D porous Bi/carbon architectures: Toward high-energy and stable nickel-bismuth batteries. Adv. Mater. 2018, 30, 1707290. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Lu, X.; Ling, Y.; Yu, M.; Wang, G.; Liu, T.; Liang, C.; Tong, Y.; Li, Y. A new benchmark capacitance for supercapacitor anodes by mixed-valence sulfur-doped V6O13-x. Adv. Mater. 2014, 26, 5869–5875. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, Z.; Guo, Y.; Guo, D.; Liu, G. Block copolymer derived uniform mesopores enable ultrafast electron and ion transport at high mass loadings. Nat. Commun. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, T.; Yao, B.; Li, M.; Kou, T.; Huang, Z.H.; Feng, D.Y.; Wang, F.; Tong, Y.; Liu, X.X.; et al. Ostwald ripening improves rate capability of high mass loading manganese oxide for supercapacitors. ACS Energy Lett. 2017, 2, 1752–1759. [Google Scholar] [CrossRef]
- Yao, B.; Chandrasekaran, S.; Zhang, J.; Xiao, W.; Qian, F.; Zhu, C.; Duoss, E.B.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Efficient 3D printed pseudocapacitive electrodes with ultrahigh MnO2 loading. Joule 2019, 3, 459–470. [Google Scholar] [CrossRef]
- Song, Y.; Liu, T.; Li, M.; Yao, B.; Kou, T.; Feng, D.; Wang, F.; Tong, Y.; Liu, X.X.; Li, Y. Engineering of mesoscale pores in balancing mass loading and rate capability of hematite films for electrochemical capacitors. Adv. Energy Mater. 2018, 8, 1801784. [Google Scholar] [CrossRef]
- Sun, S.; Zhai, T.; Liang, C.; Savilov, S.V.; Xia, H. Boosted crystalline/amorphous Fe2O3-δ core/shell heterostructure for flexible solid-state pseudocapacitors in large scale. Nano Energy 2018, 45, 390–397. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.S.; Tong, Y. Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 2014, 26, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Huang, L.; Zhang, J.; Gao, X.; Wu, J.; Cheng, Y.; Xiao, X.; Wang, B.; Li, Y.; Zhou, J. Flexible transparent molybdenum trioxide nanopaper for energy storage. Adv. Mater. 2016, 28, 6353–6358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Song, Y.; Guo, D.; Yang, D.; Sun, X.; Liu, X.X. Strongly coupled polypyrrole/molybdenum oxide hybrid films via electrochemical layer-by-layer assembly for pseudocapacitors. J. Mater. Chem. A 2019, 7, 9815–9821. [Google Scholar] [CrossRef]
- Geng, J.W.; Ye, Y.J.; Guo, D.; Liu, X.X. Concurrent electropolymerization of aniline and electrochemical deposition of tungsten oxide for supercapacitor. J. Power Sources 2017, 342, 980–989. [Google Scholar] [CrossRef]
- Deng, J.; Kang, L.; Bai, G.; Li, Y.; Li, P.; Liu, X.; Yang, Y.; Gao, F.; Liang, W. Solution combustion synthesis of cobalt oxides (Co3O4 and Co3O4/CoO) nanoparticles as supercapacitor electrode materials. Electrochim. Acta 2014, 132, 127–135. [Google Scholar] [CrossRef]
- Zhai, T.; Wan, L.; Sun, S.; Chen, Q.; Sun, J.; Xia, Q.; Xia, H. Phosphate ion functionalized Co3O4 ultrathin nanosheets with greatly improved surface reactivity for high performance pseudocapacitors. Adv. Mater. 2017, 29, 1604167. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Env. Sci. 2014, 7, 1597. [Google Scholar] [CrossRef]
- Liu, C.; Chen, W.; Hong, S.; Pan, M.; Jiang, M.; Wu, Q.; Mei, C. Fast microwave synthesis of hierarchical porous carbons from waste palm boosted by activated carbons for supercapacitors. Nanomaterials 2019, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yu, F.; Liu, L.; Jia, X.; Lv, Y.; Chen, L.; Xu, Y.; Shi, Y.; Guo, X. Cu-doped porous carbon derived from heavy metal-contaminated sewage sludge for high-performance supercapacitor electrode materials. Nanomaterials 2019, 9, 892. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.L.; Li, G.R.; Guo, R.; Ding, L.X.; Tong, Y.X. Design and synthesis of MnO2/Mn/MnO2 sandwich-structured nanotube arrays with high supercapacitive performance for electrochemical energy storage. Nano Lett. 2012, 12, 3803–3807. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, W.; Zhou, W.; Chen, S.; Zhang, Y. Hierarchical porous carbon derived from Sichuan pepper for high-performance symmetric supercapacitor with decent rate capability and cycling stability. Nanomaterials 2019, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yu, M.; Zhai, T.; Wang, G.; Xie, S.; Liu, T.; Liang, C.; Tong, Y.; Li, Y. High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode. Nano Lett. 2013, 13, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, T.; Li, M.; Yu, M.; Luo, Y.; Tong, Y.; Li, Y. Multiscale pore network boosts capacitance of carbon electrodes for ultrafast charging. Nano Lett. 2017, 17, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, M.; Xie, Y.; Fan, Y.; Wang, D.; Jiang, J.J.; Li, Y.; Grutzmacher, H.; Su, C.Y. Nanoparticle cookies derived from metal-organic frameworks: Controlled synthesis and application in anode materials for lithium-ion batteries. Small 2016, 12, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-organic frameworks for batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Torrisi, V.; Ruffino, F. Metal-polymer nanocomposites:(Co-)evaporation/(Co)sputtering approaches and electrical properties. Coatings 2015, 5, 378–424. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Jewell, D.; Chen, G. Carbon nanotube and conducting polymer composites for supercapacitors. Prog. Nat. Sci. 2008, 18, 777–788. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Liang, C.; Pan, A.; Zhang, C.; Tang, Y.; Tan, X.; Liu, J.; Liang, S. MOFs nanosheets derived porous metal oxide-coated three-dimensional substrates for lithium-ion battery applications. Nano Energy 2016, 26, 57–65. [Google Scholar] [CrossRef]
- Liu, Q.; Low, Z.X.; Feng, Y.; Leong, S.; Zhong, Z.; Yao, J.; Hapgood, K.; Wang, H. Direct conversion of two-dimensional ZIF-L film to porous ZnO nano-sheet film and its performance as photoanode in dye-sensitized solar cell. Micropor. Mesopor. Mater. 2014, 194, 1–7. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Y.X.; Liu, H.; Li, Y.; Jiang, H.L. A MOF-derived Co-CoO@N-doped porous carbon for efficient tandem catalysis: Dehydrogenation of ammonia borane and hydrogenation of nitro compounds. Chem. Commun. 2016, 52, 7719–7722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Chen, Y.Z.; Cao, L.; Lu, J.; Jiang, H.L. Conversion of a metal-organic framework to N-doped porous carbon incorporating Co and CoO nanoparticles: Direct oxidation of alcohols to esters. Chem. Commun. 2015, 51, 8292–8295. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Song, Y.; Ye, Y.J.; Zhang, M.; Sun, X.; Liu, X.X. Boosting the pseudocapacitance of nitrogen-rich carbon nanorod arrays for electrochemical capacitors. J. Mater. Chem. A 2019, 7, 12086–12094. [Google Scholar] [CrossRef]
- Yang, K.; Guo, Q.; Li, H.; Hao, X.; Ma, Y.; Yang, M.; Zhai, T.; Savilov, S.; Lunin, V.; Xia, H. Highly efficient sol-gel synthesis for ZnS@N, S co-doped carbon nanosheets with embedded heterostructure for sodium ion batteries. J. Power Sources 2018, 402, 340–344. [Google Scholar] [CrossRef]
- Pang, H.; Gao, F.; Chen, Q.; Liu, R.; Lu, Q. Dendrite-like Co3O4 nanostructure and its applications in sensors, supercapacitors and catalysis. Dalton Trans. 2012, 41, 5862–5868. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Shinde, D.V.; Kim, E.K.; Lee, W.; Oh, I.W.; Shrestha, N.K.; Lee, J.K.; Han, S.H. Supercapacitive property of metal–organic-frameworks with different pore dimensions and morphology. Micropor. Mesopor. Mater. 2013, 171, 53–57. [Google Scholar] [CrossRef]
- Meng, F.; Fang, Z.; Li, Z.; Xu, W.; Wang, M.; Liu, Y.; Zhang, J.; Wang, W.; Zhao, D.; Guo, X. Porous Co3O4 materials prepared by solid-state thermolysis of a novel Co-MOF crystal and their superior energy storage performances for supercapacitors. J. Mater. Chem. A 2013, 1, 7235. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Lu, X.; Ling, Y.; Yu, M.; Zhai, T.; Tong, Y.; Li, Y. Solid-state supercapacitor based on activated carbon cloths exhibits excellent rate capability. Adv. Mater. 2014, 26, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Q.; Wang, X.; Xiang, Q.; Liang, B.; Chen, D.; Shen, G. Flexible asymmetric supercapacitors based upon Co9S8 nanorod//Co3O4@RuO2 nanosheet arrays on carbon cloth. Acs Nano 2013, 7, 5453–5462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, M.; Qu, M.; Sun, M.; Pang, H. Core–shell Co11(HPO3)8(OH)6–Co3O4 hybrids for high-performance flexible all-solid-state asymmetric supercapacitors. J. Alloy. Compd. 2015, 651, 214–221. [Google Scholar] [CrossRef]
- Feng, D.Y.; Song, Y.; Huang, Z.H.; Xu, X.X.; Liu, X.X. Rate capability improvement of polypyrrole via integration with functionalized commercial carbon cloth for pseudocapacitor. J. Power Sources 2016, 324, 788–797. [Google Scholar] [CrossRef]
- Lu, X.F.; Chen, X.Y.; Zhou, W.; Tong, Y.X.; Li, G.R. α-Fe2O3@PANI core-shell nanowire arrays as negative electrodes for asymmetric supercapacitors. ACS Appl. Mater. Interf. 2015, 7, 14843–14850. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Huang, Z.X.; Tong, Y.X.; Li, G.R. Asymmetric supercapacitors with high energy density based on helical hierarchical porous NaxMnO2 and MoO2. Chem. Sci. 2016, 7, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, P.; Zhou, F.; Zeng, W.; Su, H.; Li, G.; Gao, J.; Sun, R.; Wong, C.P. Flexible asymmetrical solid-state supercapacitors based on laboratory filter paper. ACS Nano 2016, 10, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Cheng, C.; Zhou, B.; Yuan, X.; Cui, T.; Wang, S.; Zheng, M.; Pang, H. Hierarchically porous NaCoPO4-Co3O4 hollow microspheres for flexible asymmetric solid-state supercapacitors. Part. Part. Syst. Char. 2015, 32, 831–839. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zhang, M.; Liu, T.; Li, T.; Guo, D.; Liu, X.-X. Cobalt-Containing Nanoporous Nitrogen-Doped Carbon Nanocuboids from Zeolite Imidazole Frameworks for Supercapacitors. Nanomaterials 2019, 9, 1110. https://doi.org/10.3390/nano9081110
Song Y, Zhang M, Liu T, Li T, Guo D, Liu X-X. Cobalt-Containing Nanoporous Nitrogen-Doped Carbon Nanocuboids from Zeolite Imidazole Frameworks for Supercapacitors. Nanomaterials. 2019; 9(8):1110. https://doi.org/10.3390/nano9081110
Chicago/Turabian StyleSong, Yu, Mingyue Zhang, Tianyu Liu, Tianjiao Li, Di Guo, and Xiao-Xia Liu. 2019. "Cobalt-Containing Nanoporous Nitrogen-Doped Carbon Nanocuboids from Zeolite Imidazole Frameworks for Supercapacitors" Nanomaterials 9, no. 8: 1110. https://doi.org/10.3390/nano9081110




