Formation of ZnO/Zn0.5Cd0.5Se Alloy Quantum Dots in the Presence of High Oleylamine Contents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zn0.5Cd0.5Se Alloy QDs
2.3. HRTEM
2.4. XRD
2.5. PL
2.6. FTIR
2.7. XPS
2.8. UV-vis Spectrophotometer
3. Results and Discussion
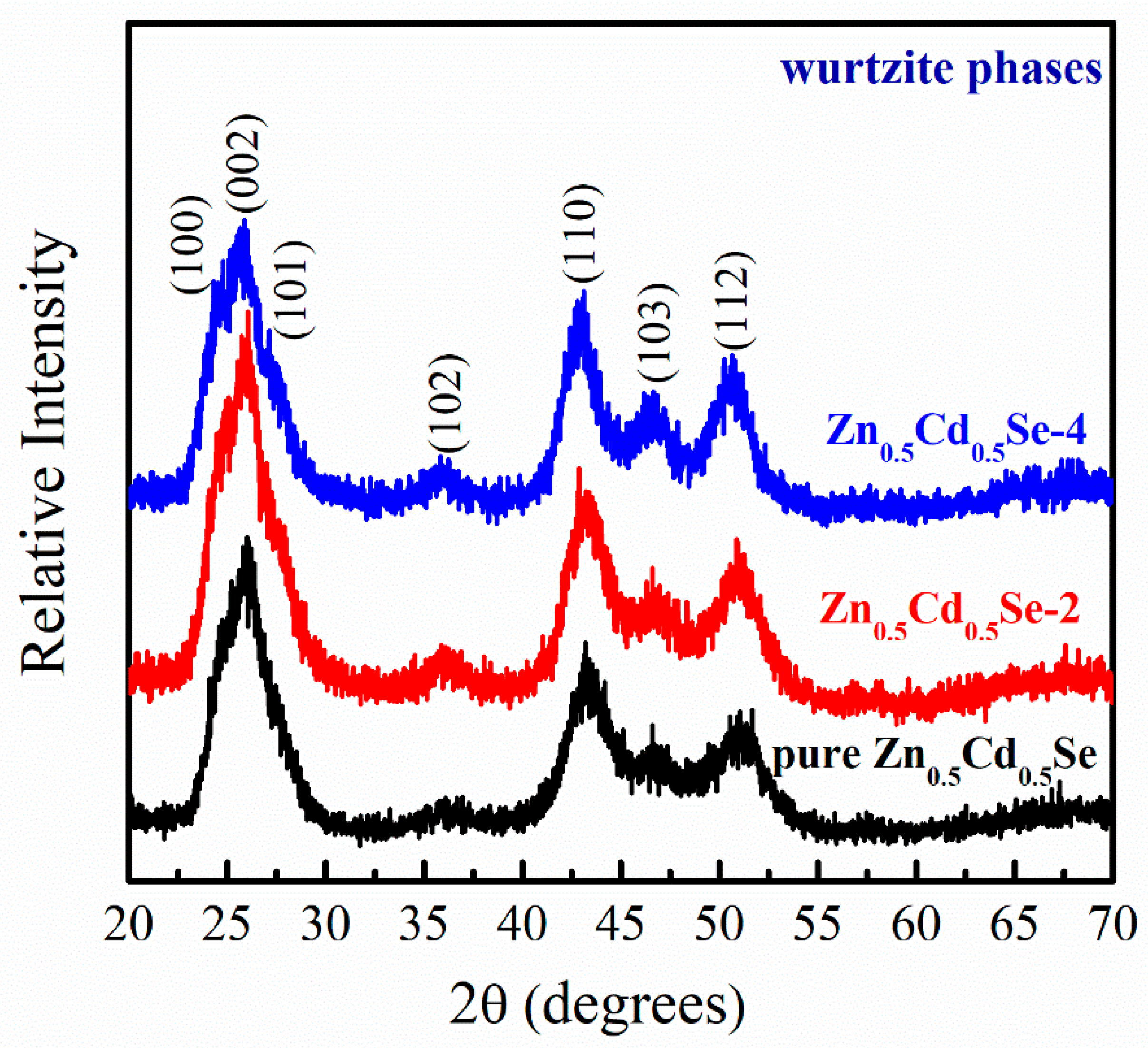
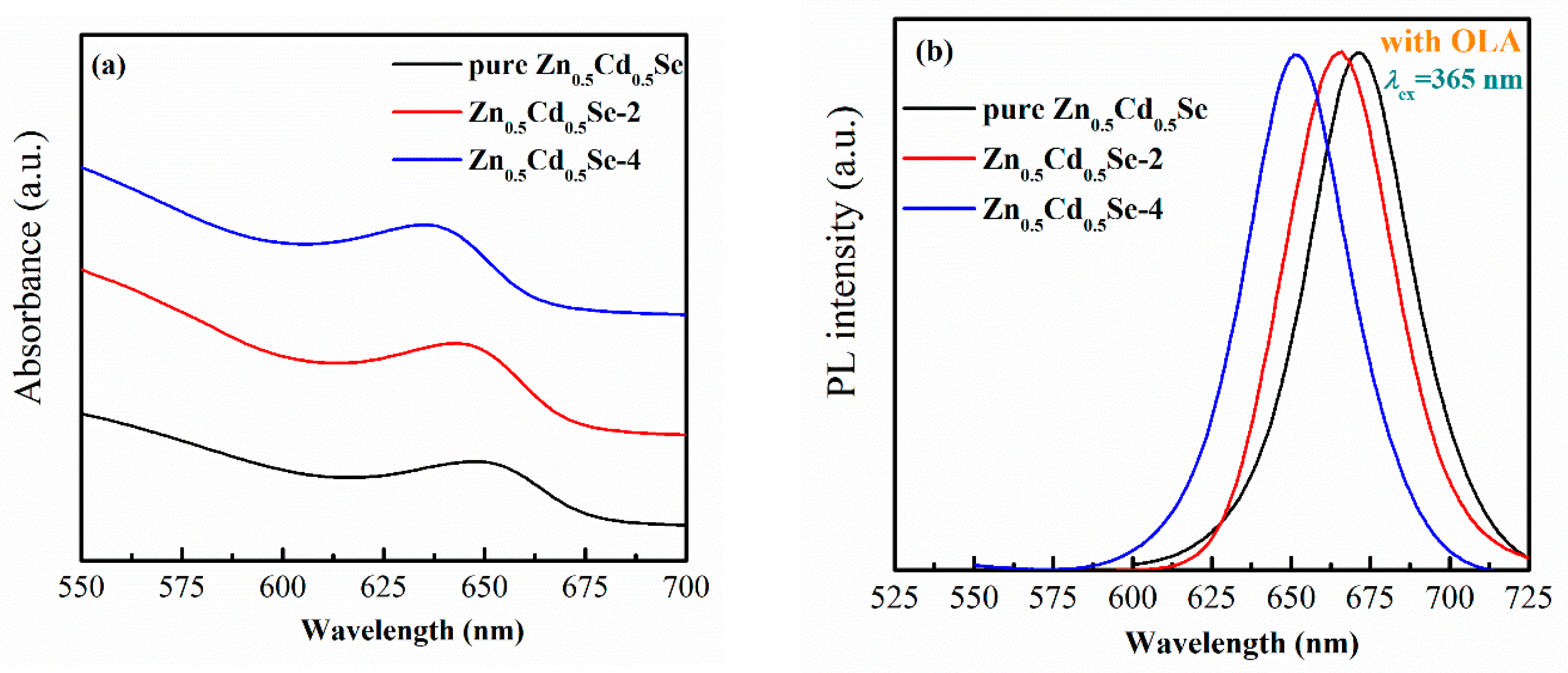
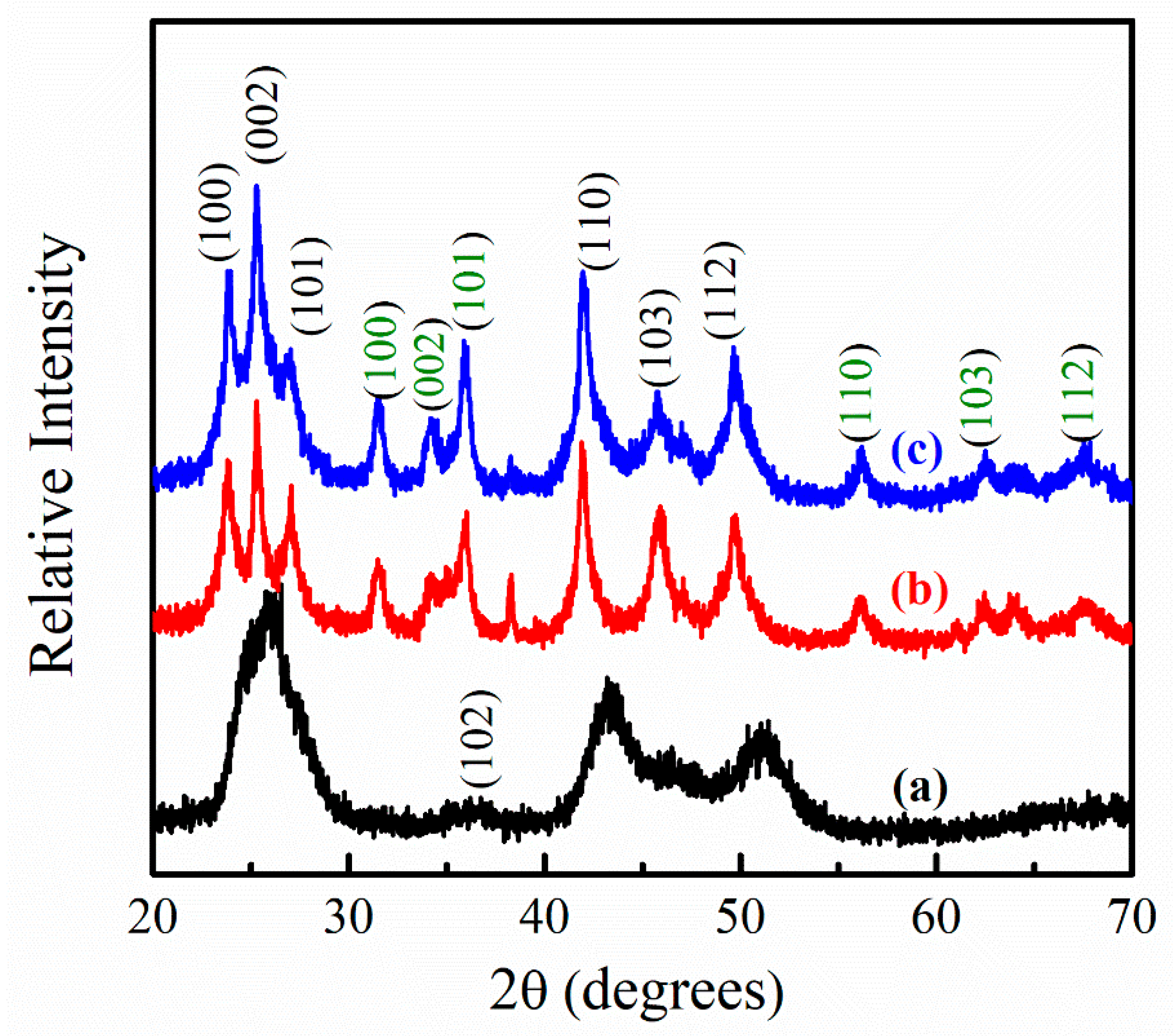
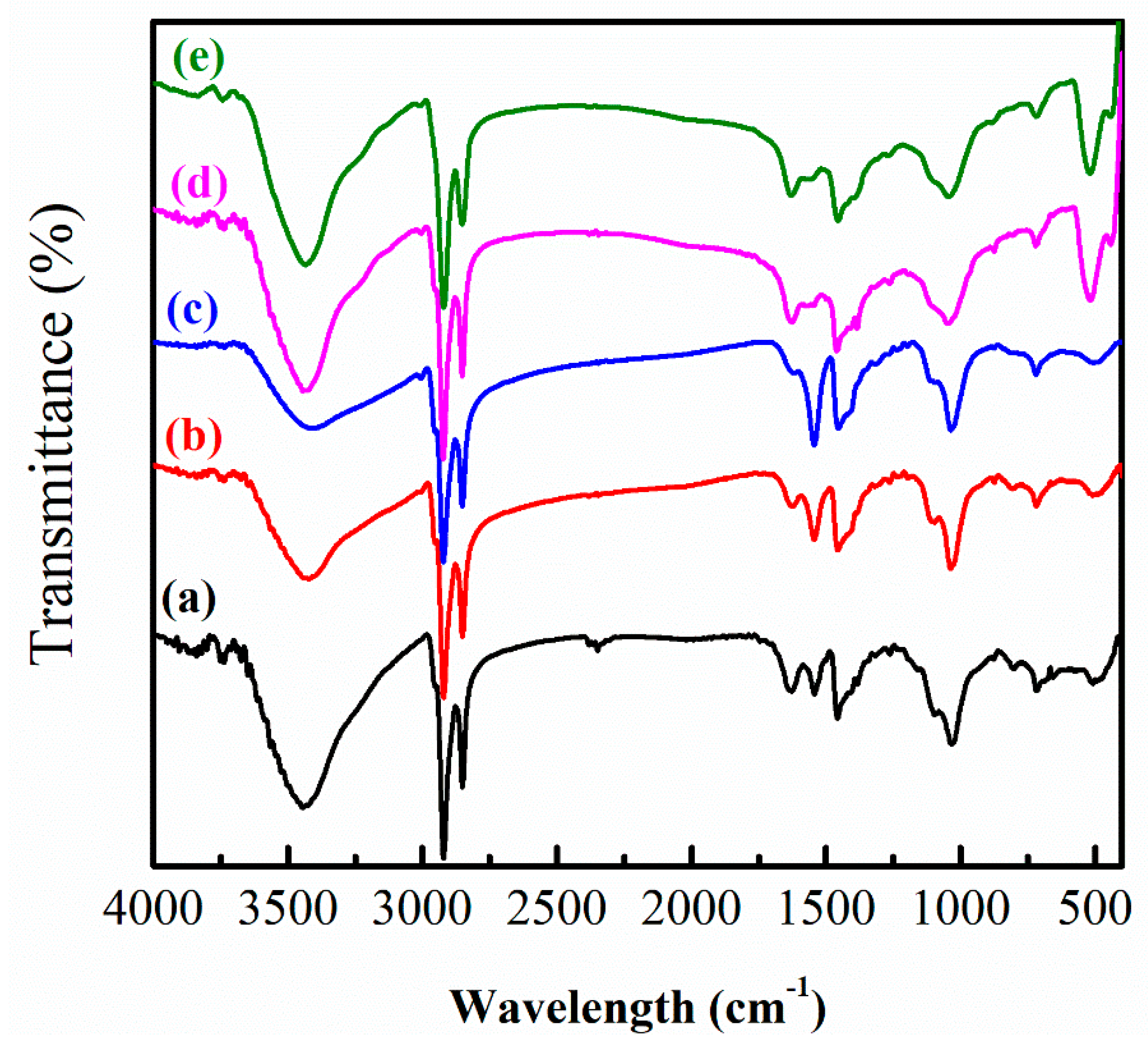
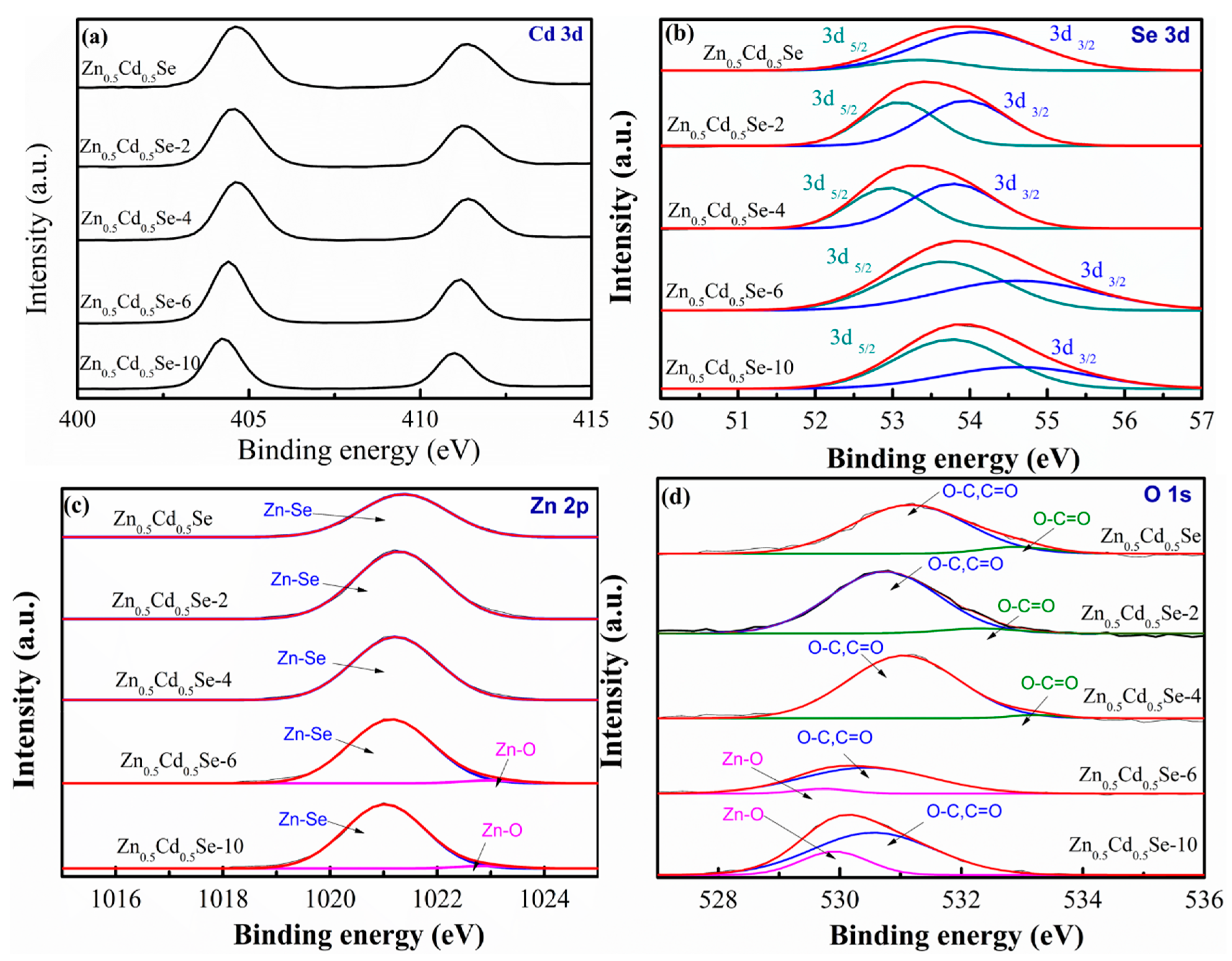
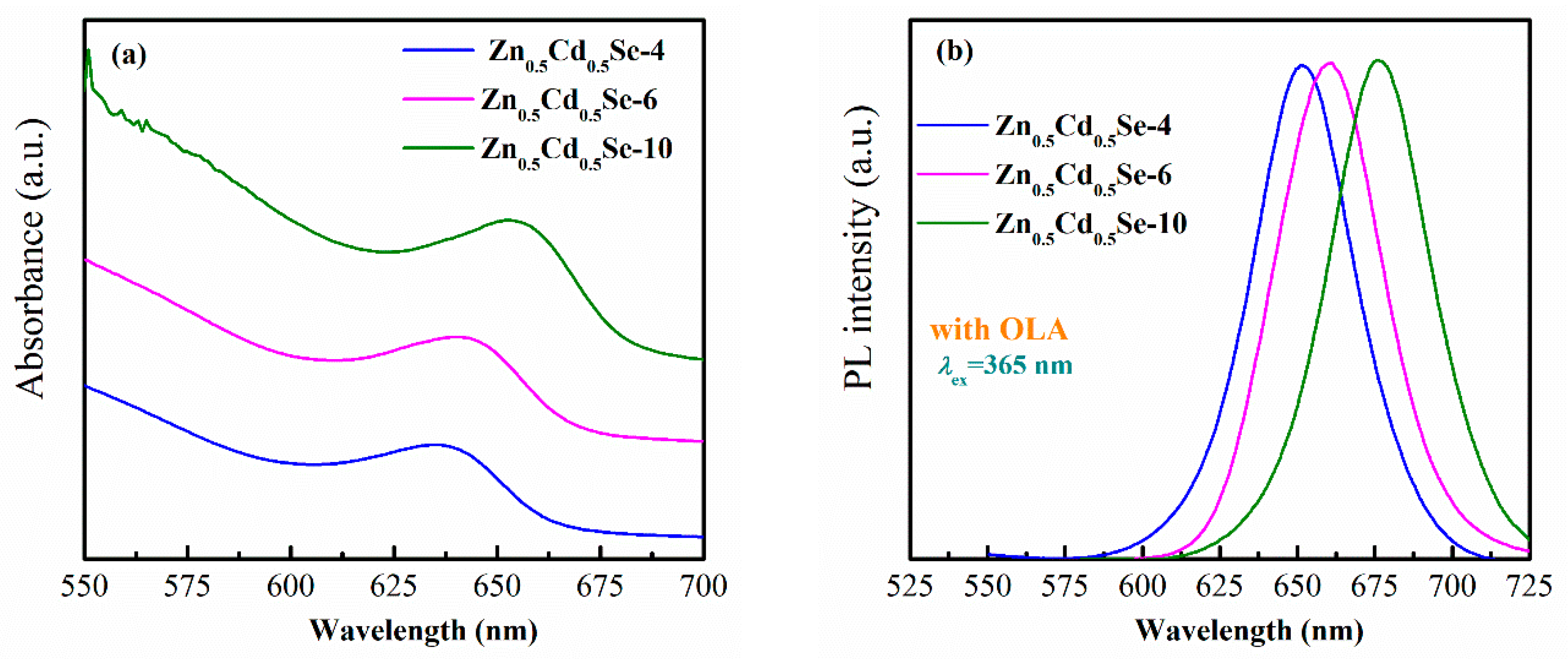
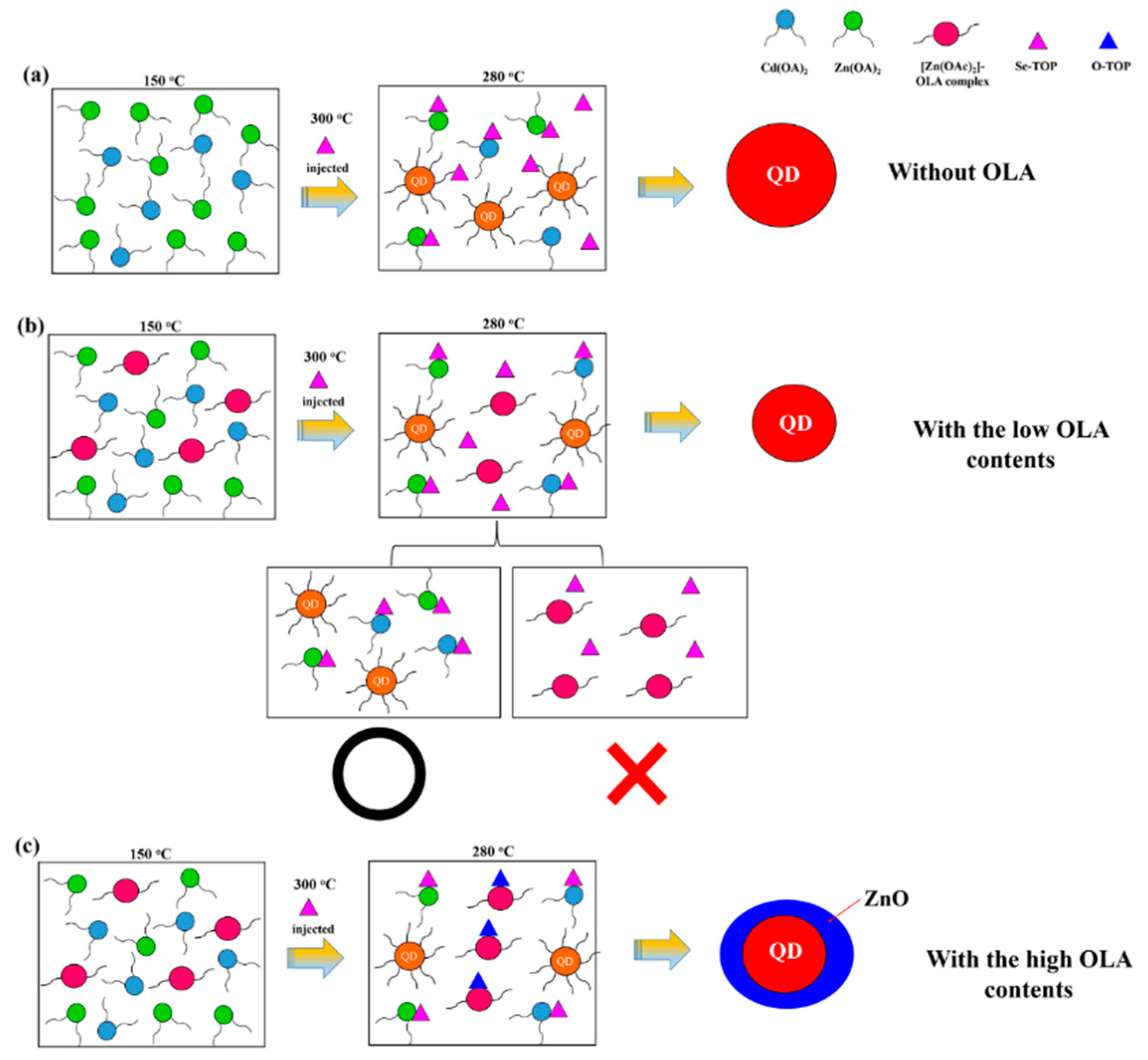
3.1. Effect of Low OLA Content
3.2. Effect of High OLA Content
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.J.; Palchik, O.; Chen, S.; Gedanken, A. Microwave assisted preparation of CdSe, PbSe, and Cu2−xSe nanoparticles. J. Phys. Chem. B 2000, 31, 7344–7347. [Google Scholar] [CrossRef]
- Xun, W.; Jing, Z.; Qing, P.; Yadong, L. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar]
- Peng, Z.A.; Peng, X.G. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Lianhua, Q.; Adam, P.Z.; Xiaogang, P. Alternative routes toward high quality CdSe nanocrystals. Nano Lett. 2001, 1, 333–337. [Google Scholar]
- Craig, R.B.; Paul, M. Nucleation and growth kinetics of CdSe nanocrystals in octadecene. Nano Lett. 2004, 4, 2303–2307. [Google Scholar]
- Green, M.; O’Brien, P. Recent advances in the preparation of semiconductors as isolated nanometric particles: New routes to quantum dots. Chem. Commun. 1999, 22, 2235–2241. [Google Scholar] [CrossRef]
- Cumberland, S.L.; Hanif, K.M.; Javier, A.; Khitrov, G.A.; Strouse, G.F.; Woesser, S.M.; Yun, C.S. Inorganic clusters as single-source precursors for preparation of CdSe, ZnSe, and CdSe/ZnS nanomaterials. Chem. Mater. 2002, 14, 1576–1584. [Google Scholar] [CrossRef]
- Qu, L.H.; Peng, X.G. Control of photoluminescence properties of CdSe nanocrystals in growth. J. Am. Chem. Soc. 2002, 124, 2049–2055. [Google Scholar] [CrossRef]
- Ge, J.P.; Li, Y.D.; Yang, G.Q. Mechanism of aqueous ultrasonic reaction: Controlled synthesis, luminescence properties of amorphous cluster and nanocrystalline CdSe. Chem. Commun. 2002, 17, 1826–1827. [Google Scholar] [CrossRef]
- Chung, S.R.; Siao, C.B.; Wang, K.W. Full color display fabricated by CdSe bi-color quantum dots-based white light-emitting diodes. Opt. Mater. Express 2018, 8, 2677–2686. [Google Scholar] [CrossRef]
- Jeong, D.W.; Parka, J.Y.; Seoa, H.W.; Myung, N.V.; Seong, T.Y.; Kim, B.S. One-pot synthesis of gradient interface quaternary ZnCdSSe quantum dots. Appl. Surf. Sci. 2017, 415, 19–23. [Google Scholar] [CrossRef]
- Jiaa, Y.; Wanga, H.; Xianga, L.; Liu, X.; Wei, W.; Ma, N.; Sun, D. Tunable emission properties of core-shell ZnCuInS-ZnS quantum dots with enhanced fluorescence intensity. J. Mater. Sci. Technol. 2018, 4, 942–948. [Google Scholar] [CrossRef]
- Cho, J.; Jung, Y.K.; Lee, J.K.; Jung, H.S. Highly efficient blue-emitting CdSe-derived core/shell gradient alloy quantum dots with improved photoluminescent quantum yield and enhanced photostability. Langmuir 2016, 33, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Leea, H.; Yang, H.; Holloway, P.H. Single-step growth of colloidal ternary ZnCdSe nanocrystals. J. Lumin. 2007, 126, 314–318. [Google Scholar] [CrossRef]
- Ouyang, J.; Vincent, M.; Kingston, D.; Descours, P.; Boivineau, T.; Zaman, M.B.; Wu, X.; Yu, K. Noninjection, one-pot synthesis of photoluminescent colloidal homogeneously alloyed CdSeS quantum dots. J. Phys. Chem. C 2009, 113, 5193–5200. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, B.H.; Kim, S.H.; Lee, J.W.; Lee, S.W.; Kim, S.W.; Gopalan, S.A.; Kwon, J.B.; Bae, J.H.; Kim, E.S.; et al. All-solution-processed high-brightness hybrid white quantum-dot light-emitting devices utilizing polymer modified quantum dots. Org. Electron. 2017, 42, 393–398. [Google Scholar] [CrossRef]
- Coe, S.; Woo, W.K.; Bawendi, M.; Bulović, V. Electroluminescence from single monolayers of nanocrystals in molecular organic devices. Nature 2002, 420, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Z. Synthesis of ZnxCd1−xSe@ZnO hollow spheres in different sizes for quantum dots sensitized solar cells application. Nanomaterials 2019, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-stable allinorganic nanocrystal solar cells processed from solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, Y.A.; Yao, N.; Norris, D.J. Synthesis of photonic crystals for optical wavelengths from semiconductor quantum dots. Adv. Mater. 1999, 11, 165–169. [Google Scholar] [CrossRef]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Willner, I. Optical molecular sensing with semiconductor quantum dots (QDs). Chem. Soc. Rev. 2012, 41, 4067–4085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Xia, L.; Xie, H.Y.; Zhang, Z.L.; Pang, D.W. Quantum dot based biotracking and biodetection. Anal. Chem. 2019, 91, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Han, M.; Dong, Z.; White, T.J.; Knoll, W. Composition-tunable ZnxCd1−xSe nanocrystals with high luminescence and stability. J. Am. Chem. Soc. 2003, 125, 8589–8594. [Google Scholar] [CrossRef]
- Deng, Z.; Lie, F.L.; Shen, S.; Ghosh, I.; Mansuripur, M.; Muscat, A.J. Water-based route to ligand-selective synthesis of ZnSe and Cd-doped ZnSe quantum dots with tunable ultraviolet A to blue photoluminescence. Langmuir 2009, 25, 434–442. [Google Scholar] [CrossRef]
- Cao, J.; Xue, B.; Li, H.; Deng, D.; Gu, Y. Facile synthesis of high-quality water-soluble N-acetyl-L-cysteine-capped Zn1−xCdxSe/ZnS core/shell quantum dots emitting in the violet–green spectral range. J. Colloid Interface Sci. 2010, 348, 369–376. [Google Scholar] [CrossRef]
- Miao, S.; Hickey, S.G.; Rellinghaus, B.; Waurisch, C.; Eychmüller, A. Synthesis and characterization of cadmium phosphide quantum dots emitting in the visible red to near-infrared. J. Am. Chem. Soc. 2010, 132, 5613–5615. [Google Scholar] [CrossRef]
- Yang, P.; Wang, S.; Ando, M.; Murase, N. CdSe/Cd1−xZnxS core/shell quantum dots with tunable emission: Growth and morphology evolution. J. Mater. Sci. 2013, 48, 651–658. [Google Scholar] [CrossRef]
- Bailey, R.E.; Nie, S.M. Alloyed semiconductor quantum dots: Tuning the optical properties without changing the particle size. J. Am. Chem. Soc. 2003, 125, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.G.; Yang, Z.C.; Ying, J.Y. Aqueous synthesis of glutathione-capped ZnSe and Zn1−xCdxSe alloyed quantum dots. Adv. Mater. 2007, 19, 1475–1479. [Google Scholar] [CrossRef]
- Huang, C.H.; Yang, C.H.; Shieh, Y.T.; Wang, T.L. Synthesis and properties of alloyed CdxZn1−xSe core and manganese doped CdxZn1−xSe/ZnS core/shell nanocrystals. J. Alloy. Compd. 2018, 748, 265–272. [Google Scholar] [CrossRef]
- Wei, H.; Su, Y.; Han, Z.; Li, T.; Ren, X.; Yang, Z.; Wei, L.; Cong, F.; Zhang, Y. ZnxCd1−xSe nanomultipods with tunable band gaps: Synthesis and first-principles calculations. Nanotechnology 2013, 24, 235706. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Michael, M.; Mulvaney, P. Synthesis of Highly Crystalline CdSe@ZnO Nanocrystals via Monolayer-by-Monolayer Epitaxial Shell Deposition. Chem. Mater. 2014, 26, 4274–4279. [Google Scholar] [CrossRef]
- An, L.; Chen, X.; Han, X.; Yi, J.; Liu, C.; An, W.; Qu, Y.; Chi, J.; Wei, H.; Wen, Y.; et al. CdSe/ZnO core/shell semiconductor nanocrystals: Synthesis and Characterization. Appl. Mech. Mater. 2013, 268, 207–210. [Google Scholar] [CrossRef]
- Lu, Q.; Yubai Bai, G.S. Synthesis and characterization of CdSe/ZnO core/shell nanocrystals. Int. J. Nanosci. 2006, 5, 299–306. [Google Scholar] [CrossRef]
- Aldeek, F.; Mustin, C.; Balan, L.; Medjahdi, G.; Roques-Carmes, T.; Arnoux, P.; Schneider, R. Enhanced photostability from CdSe(S)/ZnO core/shell quantum dots and their use in biolabeling. Eur. J. Inorg. Chem. 2011, 2011, 794–801. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, T.; Li, C.; Xie, A.; Li, S.; Zhang, M.; Shen, Y. ZnxCd1−xSe nanoparticles decorated ordered mesoporous ZnO inverse opal with binder-free heterojunction interfaces for highly efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 245, 469–476. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 24962–24972. [Google Scholar] [CrossRef]
- Khalaf, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef] [PubMed]
- Gakhar, R.; Merwin, A.; Summers, K.; Pilli, S.K.; Chidambaram, D. Application of ZnxCd1−xSe-sensitized TiO2 nanotube arrays as photoanodes for solar cells. J. Mater. Chem. A 2014, 2, 10116–10125. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Meléndez, A.M.; Tirado, J.; Escobar, M.A.M.; Jaramillo, F.; Niño-Gómez, M.E. Hidden energy levels? Carrier transport ability of CdS/CdS1−xSex quantum dot solar cells impacted by Cd–Cd level formation. Nanoscale 2019, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Xu, Y.F.; Chen, H.Y.; Wang, X.D.; Kuang, D.B. Porous ZnO@ZnSe nanosheet array for photoelectrochemical reduction of CO2. Electrochim. Acta 2018, 274, 298–305. [Google Scholar] [CrossRef]
- Mittal, V.; Sessions, N.P.; Wilkinson, J.S.; Murugan, G.S. Optical quality ZnSe films and low loss waveguides on Si substrates for mid-infrared applications. Opt. Mater. Express 2017, 3, 712–725. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Masoud, S.N.; Davar, F.; Mazaheri, M. Preparation of ZnO nanoparticles from [bis(acetylacetonato)zinc(II)]–oleylamine complex by thermal decomposition. Mater. Lett. 2008, 62, 1890–1892. [Google Scholar]
- Musić, S.; Šarić, A.; Popović, S. Formation of nanosize ZnO particles by thermal decomposition of zinc acetylacetonate monohydrate. Ceram. Int. 2010, 36, 1117–1123. [Google Scholar] [CrossRef]
- Wood, A.; Giersig, M.; Hilgendorff, M.; Vilas-Campos, A.; Liz-Marzán, L.M.; Mulvaney, P. Size effects in ZnO: The cluster to quantum dot transition. Aust. J. Chem. 2003, 56, 1051–1057. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-A.; Chou, K.-H.; Kuo, Y.-Y.; Wu, C.-Y.; Hsiao, P.-W.; Chen, P.-W.; Yuan, S.-H.; Wuu, D.-S. Formation of ZnO/Zn0.5Cd0.5Se Alloy Quantum Dots in the Presence of High Oleylamine Contents. Nanomaterials 2019, 9, 999. https://doi.org/10.3390/nano9070999
Chen Y-A, Chou K-H, Kuo Y-Y, Wu C-Y, Hsiao P-W, Chen P-W, Yuan S-H, Wuu D-S. Formation of ZnO/Zn0.5Cd0.5Se Alloy Quantum Dots in the Presence of High Oleylamine Contents. Nanomaterials. 2019; 9(7):999. https://doi.org/10.3390/nano9070999
Chicago/Turabian StyleChen, Yi-An, Kuo-Hsien Chou, Yi-Yang Kuo, Cheng-Ye Wu, Po-Wen Hsiao, Po-Wei Chen, Shuo-Huang Yuan, and Dong-Sing Wuu. 2019. "Formation of ZnO/Zn0.5Cd0.5Se Alloy Quantum Dots in the Presence of High Oleylamine Contents" Nanomaterials 9, no. 7: 999. https://doi.org/10.3390/nano9070999
APA StyleChen, Y.-A., Chou, K.-H., Kuo, Y.-Y., Wu, C.-Y., Hsiao, P.-W., Chen, P.-W., Yuan, S.-H., & Wuu, D.-S. (2019). Formation of ZnO/Zn0.5Cd0.5Se Alloy Quantum Dots in the Presence of High Oleylamine Contents. Nanomaterials, 9(7), 999. https://doi.org/10.3390/nano9070999





