Scalable Fabrication of Metallopolymeric Superstructures for Highly Efficient Removal of Methylene Blue
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Synthesis of Amphiphilic Polyarylene Ether Nitrile Block Copolymer (amPEN)
2.3. Preparation of Metallopolymeric Nanostructures (MPS)
2.4. Adsorption of Methylene Blue (MB) by MPS
2.5. Characterization
2.6. Kinetic Adsorption Isotherm Models
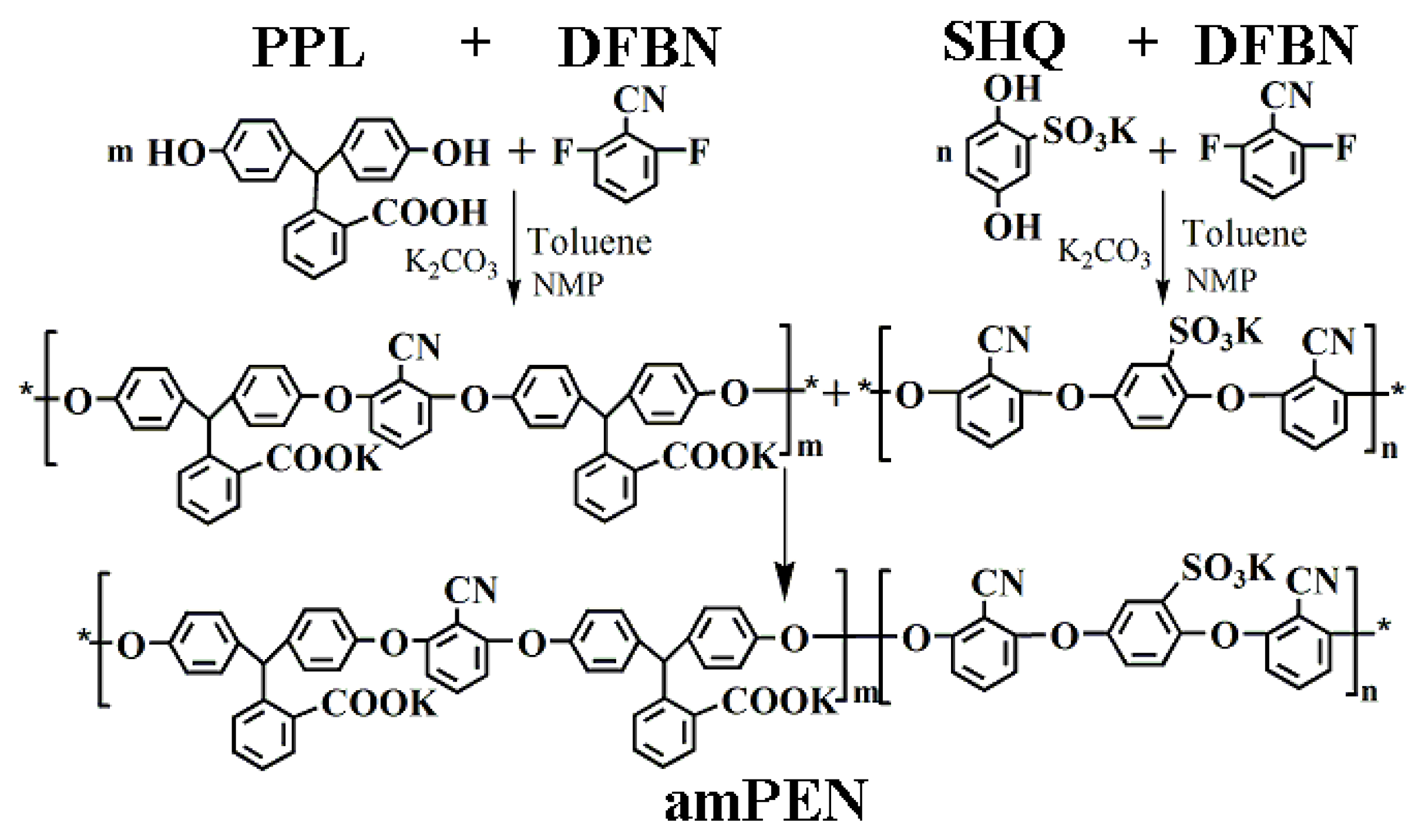
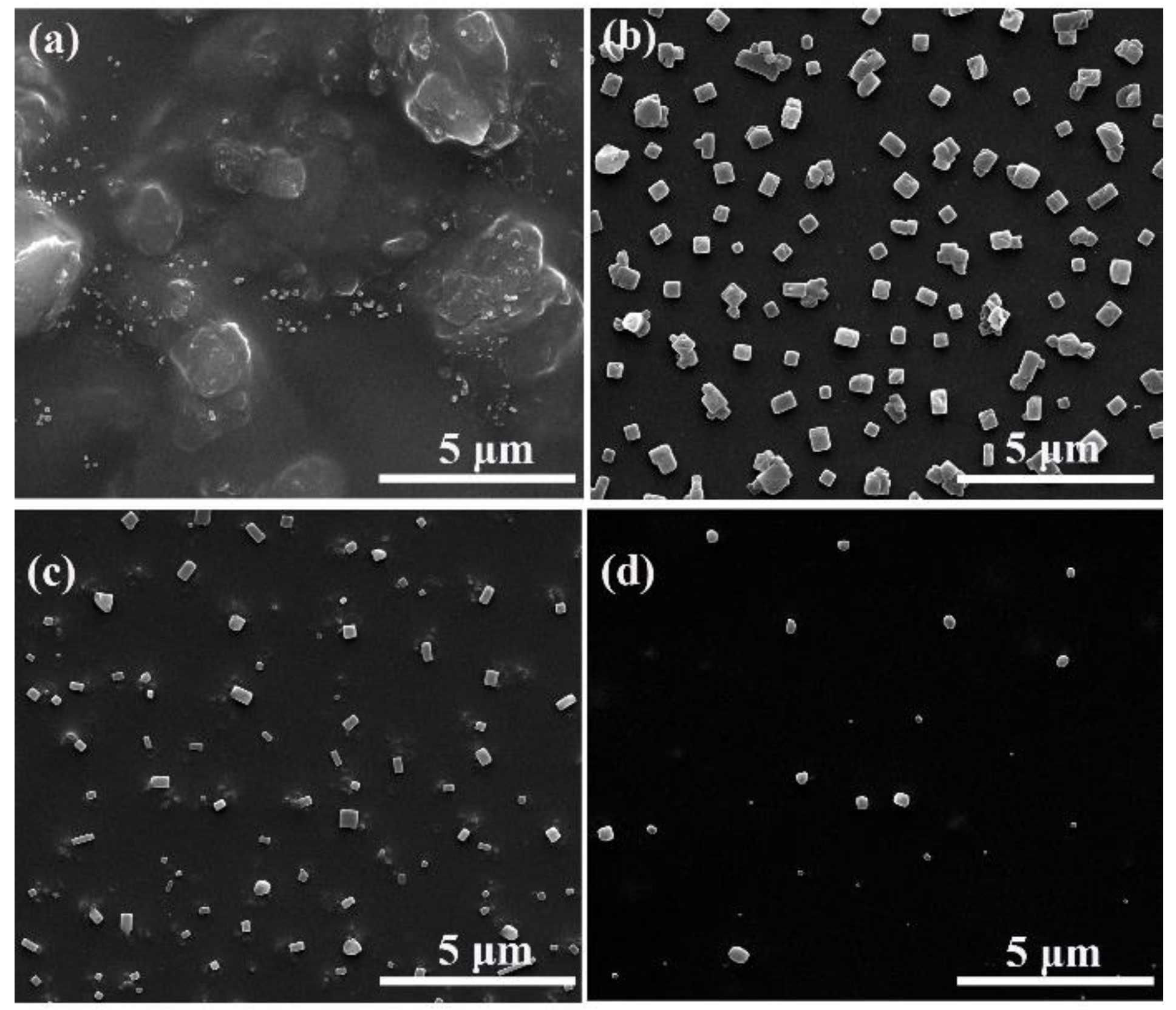
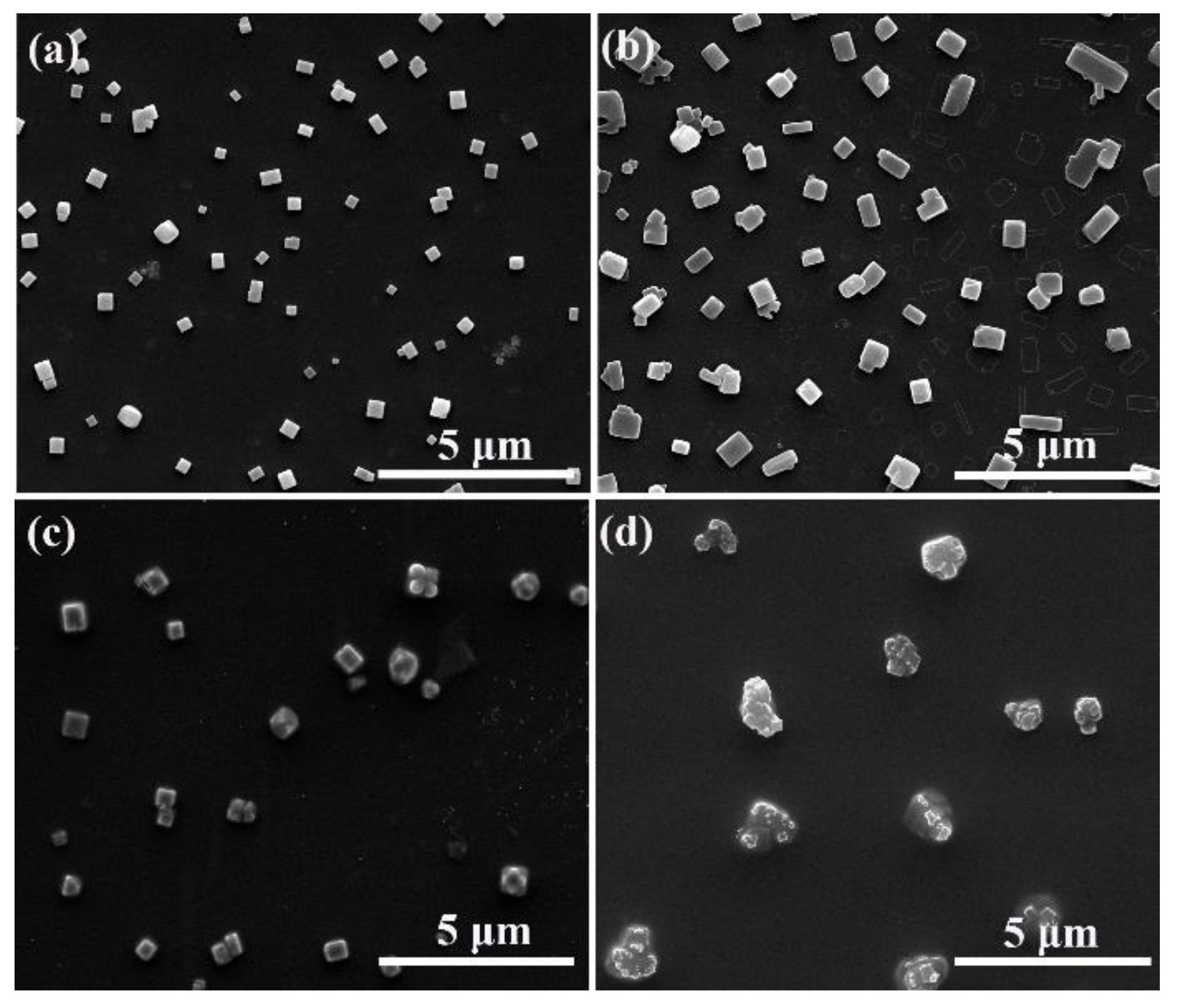
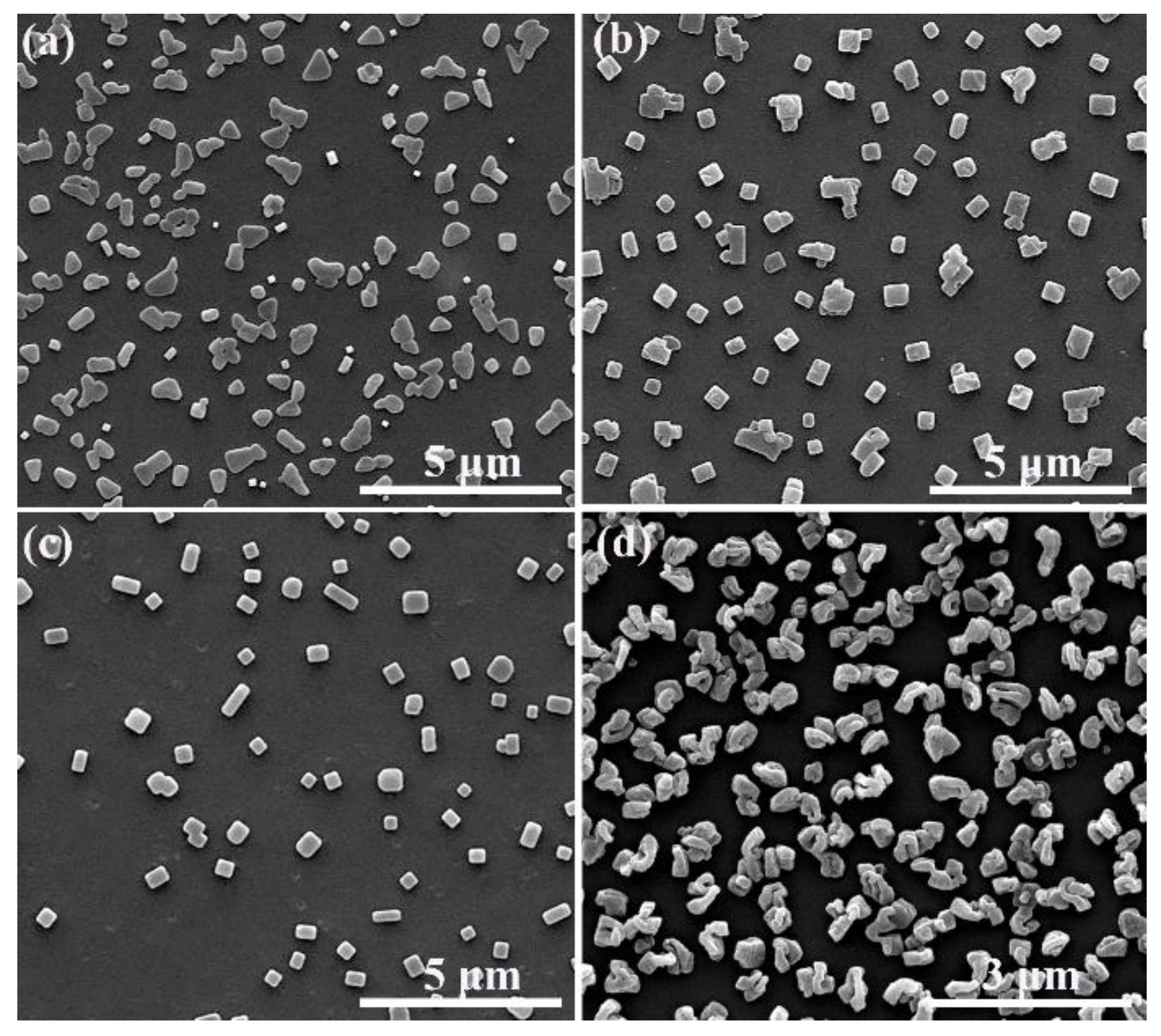
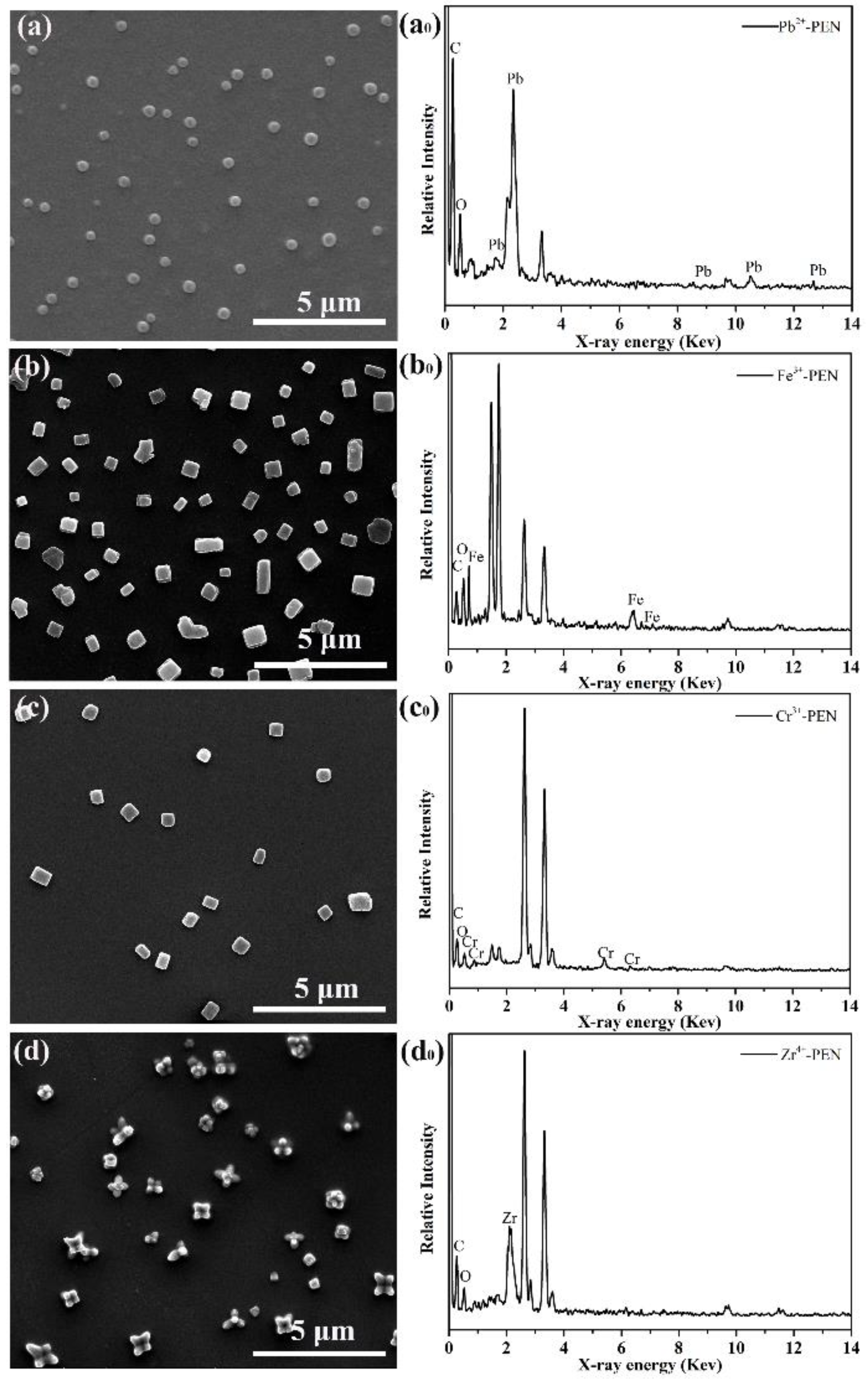
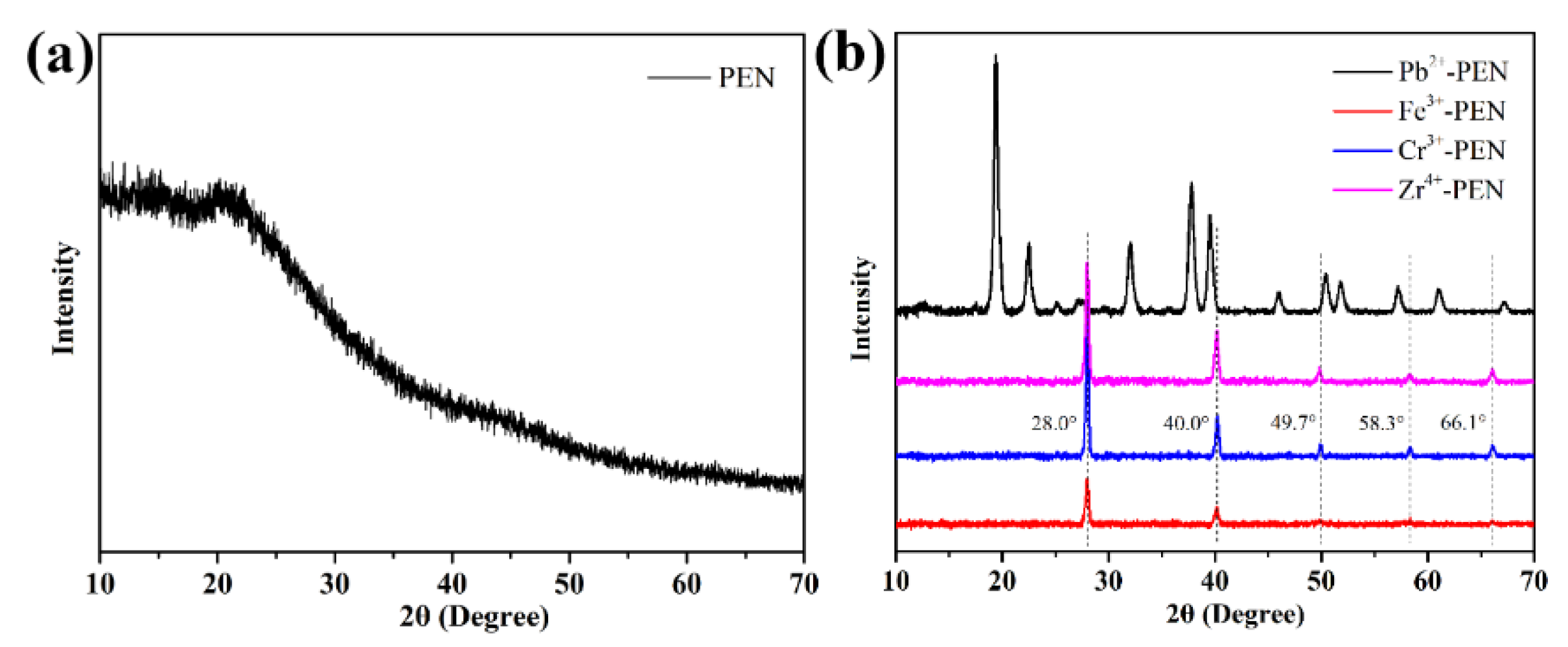
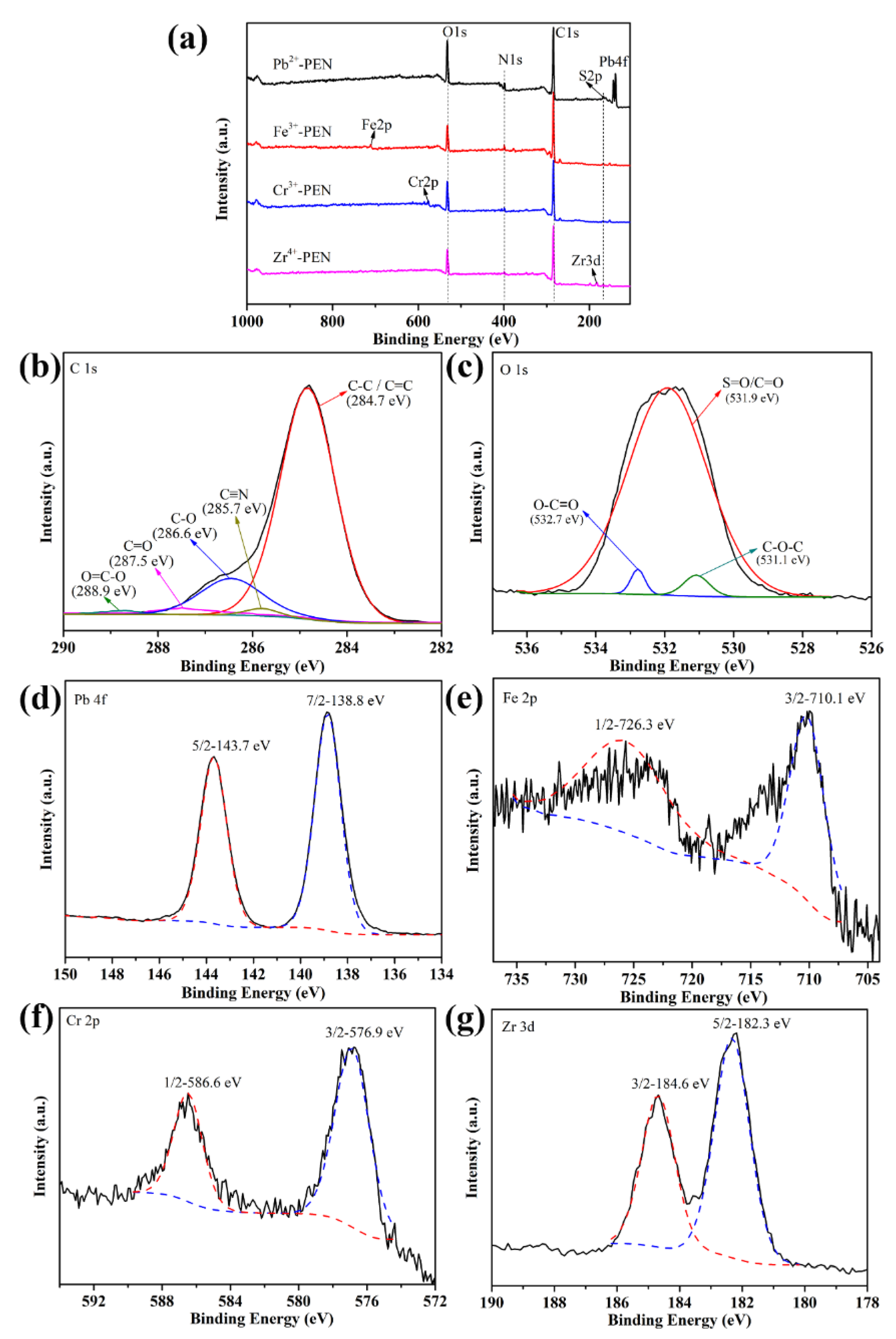
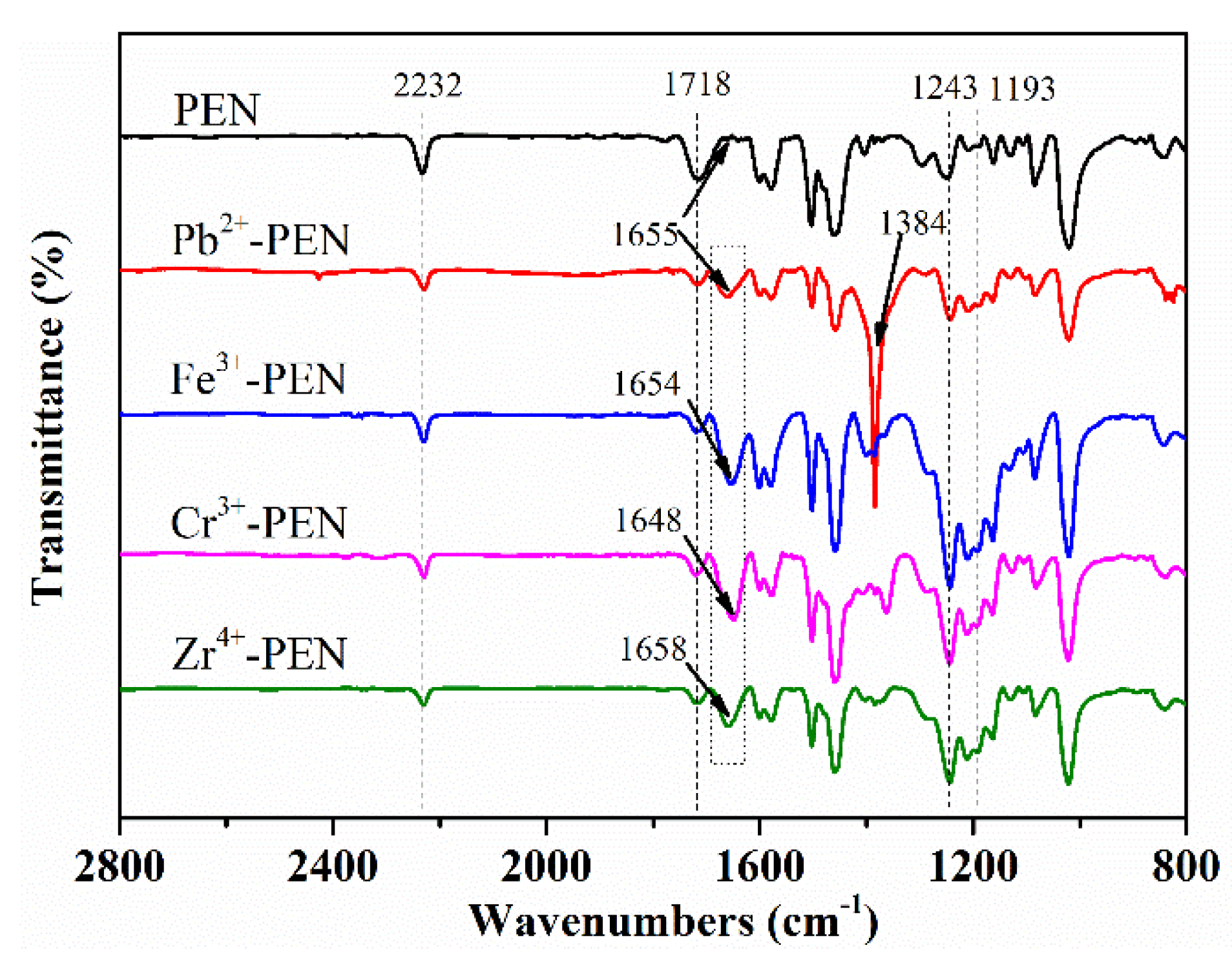
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordiation bonds: Nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cohen Stuart, M.A.; Marcelis, A.T.M.; Colomb-Delsuc, M.; Otto, S.; van der Gucht, J. Stable polymer micelles formed by metal coordination. Macromolecules 2012, 45, 7179–7185. [Google Scholar] [CrossRef]
- Pang, J.B.; Fu, F.L.; Li, W.B.; Zhu, L.J.; Tang, B. Fe-Mn binary oxide decorated diatomite for rapid decolorization of methylene blue with H2O2. Appl. Surf. Sci. 2019, 478, 54–61. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Y.F.; Sun, Y.; Hong, S.Y.; Lee, S.K.; Yoon, B.; Chen, L.; Ci, L.; Nam, J.D.; Chen, X.; et al. Hierarchical porous chitosan sponges as robust and recyclable adsorbents for anionic dye adsorption. Sci. Rep. 2017, 7, 18054. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K. Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Liu, H.; Yu, D.Q.; Sun, T.B.; Du, H.Y.; Jiang, W.T.; Muhammad, Y.; Huang, L. Fabrication of surface alkalinized g-C3N4 and TiO2 composite for the synergistic adsorption-photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2019, 473, 855–863. [Google Scholar] [CrossRef]
- Fu, J.W.; Xin, Q.Q.; Wu, X.C.; Chen, Z.H.; Yan, Y.; Liu, S.J.; Wang, M.H.; Xu, Q. Selective adsorption and separation of organic dyes from aqueous solution on polydopamine microspheres. J. Colloid Interface Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef]
- Khataee, A.R.; Vafaei, F.; Jannatkhah, M. Biosorption of three textile dyes from contaminated water by filamentous green algal Spirogyra sp.: Kinetic, isotherm and thermodynamic studies. Int. Biodeterior. Biodegrad. 2013, 83, 33–40. [Google Scholar] [CrossRef]
- Faouzi, M.; Cañizares, P.; Gadri, A.; Lobato, J.; Nasr, B.; Paz, R.; Rodrigo, M.A.; Saez, C. Advanced oxidation processes for the treatment of wastes polluted with azoic dyes. Electrochim. Acta 2006, 52, 325–331. [Google Scholar] [CrossRef]
- Leong, J.; Tan, J.; Heitz, A.; Ladewig, B.P. Performance of a vibratory shear membrane filtration system during the treatment of magnetic ion exchange process concentrate. Desalination 2015, 365, 196–203. [Google Scholar] [CrossRef]
- Szygula, A.; Guibal, E.; Arino Palacin, M.; Ruiz, M.; Sastre, A.M. Removal of an anionic dye (Acid Blue 92) by coagulation-flocculation using chitosan. J. Environ. Manag. 2009, 90, 2979–2986. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M. Photocatalytic ozonation of dyes using copper ferrite nanoparticle prepared by co-precipitation method. Desalination 2011, 279, 332–337. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Liu, L.J.; Li, J.R.; Liu, Q. Efficient removal of dye MB: Through the combined action of adsorption and photodegradation from NiFe2O4/Ag3PO4. J. Alloy. Compd. 2016, 664, 169–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.B.; Zhang, J.P.; Liu, P.; Wang, A.Q. A comparative study about adsorption of natural palygorskite for methylene blue. Chem. Eng. J. 2015, 262, 390–398. [Google Scholar] [CrossRef]
- Ma, D.; Ju, W.; Tang, Y.; Chen, Y. First-principles study of the small molecule adsorption on the InSe monolayer. Appl. Surf. Sci. 2017, 426, 244–252. [Google Scholar] [CrossRef]
- Lei, C.S.; Pi, M.; Xu, D.F.; Jiang, C.J.; Cheng, B. Fabrication of hierarchical porous ZnO-Al2O3 microspheres with enhanced adsorption performance. Appl. Surf. Sci. 2017, 426, 360–368. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of low-cost adsorbents for dye removal-a review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Machida, M.; Fotoohi, B.; Amamo, Y.; Mercier, L. Cadmium(II) and lead(II) adsorption onto hetero-atom functional mesoporous silica and activated carbon. Appl. Surf. Sci. 2012, 258, 7389–7394. [Google Scholar] [CrossRef]
- Li, Z.M.; Yao, Y.; Wei, G.T.; Jiang, W.Y.; Wang, Y.Z.; Zhang, L.Y. Adsorption and heat-energy-aid desorption of cationic dye on a new thermo-sensitive adsorbent: Methyl cellulose/calcium alginate beads. Polym. Eng. Sci. 2016, 56, 1382–1389. [Google Scholar] [CrossRef]
- Al-Futaisi, A.; Jamrah, A.; Al-Rawas, A.; Al-Hanai, S. Adsorption capacity and mineralogical and physico-chemical characteristics of Shuwaymiyah palygorskite (Oman). Environ. Geol. 2006, 51, 1317–1327. [Google Scholar] [CrossRef]
- Kausalya, G.; Manjubaashini, N.; Jerome, P.; Karvembu, R.; Daniel Thangadurai, T. Single crystal cupric oxide nanoflakes with {1̄11} facets for Pb2+ ion adsorption and methylene blue dye decolorization. Mater. Lett. 2016, 185, 218–221. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, C.; Wei, J.C.; Tjiu, W.; Pan, J.S.; Chen, Y.W.; Liu, T.X. Surface modifications of halloysite nanotubes with superparamagnetic Fe3O4 nanoparticles and carbonaceous layers for efficient adsorption of dyes in water treatment. Chem. Res. Chin. Univ. 2014, 30, 971–977. [Google Scholar] [CrossRef]
- Wu, X.F.; Wang, W.; Li, F.; Khaimanov, S.; Tsidaeva, N.; Lahoubi, M. PEG-assisted hydrothermal synthesis of CoFe2O4 nanoparticles with enhanced selective adsorption properties for different dyes. Appl. Surf. Sci. 2016, 389, 1003–1011. [Google Scholar] [CrossRef]
- Ghani, M.; Rezaei, B.; Ghare Aghaji, A.; Arami, M. Novel cross-linked superfine alginate-based nanofibers: Fabrication, characterization, and their use in the adsorption of cationic and anionic dyes. Adv. Polym. Technol. 2016, 35, 428–438. [Google Scholar] [CrossRef]
- Nordin, N.; Zakaria, Z.A.; Ahmad, W.A. Utilisation of rubber wood shavings for the removal of Cu(II) and Ni(II) from aqueous solution. Water. Air. Soil Poll. 2011, 223, 1649–1659. [Google Scholar] [CrossRef]
- Wang, S.B.; Wu, H.W. Environmental-benign utilisation of fly ash as low-cost adsorbents. J. Hazard. Mater. 2006, 136, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bell, G.E.; Penn, C.J.; Moss, J.Q.; Payton, M.E. Phosphorus reduction in turfgrass runoff using a steel slag trench filter system. Crop Sci. 2014, 54, 1859–1867. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Gupta, G.S.; Shukla, S.P.; Prasad, G.; Singh, V.N. China clay as an adsorbent for dye house wastewaters. Environ. Tech. 1992, 13, 925–936. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Banat, F.; Abu-Aitah, L. Adsorption of phenol using different types of activated bentonites. Sep. Purif. Technol. 2003, 33, 1–10. [Google Scholar] [CrossRef]
- Qu, J.H. Research progress of novel adsorption processes in water purification: A review. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar] [CrossRef]
- Jin, L.; Qian, X.; Wang, J.; Aslan, H.; Dong, M. MIL-68 (In) nano-rods for the removal of Congo red dye from aqueous solution. J. Colloid. Interface Sci. 2015, 453, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiong, Y.; Tian, S.; He, C.; Su, Q.; Wen, Z. One-dimensional coordiation supramolecular polymer [Cu (bipy)(SO4)]n as an adsorbent for adsorption and kinetic separation of anionic dyes. Chem. Eng. J. 2015, 265, 157–163. [Google Scholar] [CrossRef]
- Pan, B.J.; Pan, B.C.; Zhang, W.M.; Lv, L.; Zhang, Q.X.; Zheng, S.R. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009, 151, 19–29. [Google Scholar] [CrossRef]
- Jia, K.; He, X.H.; Zhou, X.F.; Zhang, D.W.; Wang, P.; Huang, Y.M.; Liu, X.B. Solid state effective luminescent probe based on CdSe@CdS/amphiphilic co-polyarylene ether nitrile core-shell superparticles for Ag+ detection and optical strain sensing. Sens. Actuators B Chem. 2018, 257, 442–450. [Google Scholar] [CrossRef]
- Wang, P.; Jia, K.; Zhou, X.F.; Guan, X.T.; Wang, L.H.; Tian, Y.; Wu, C.H.; Liu, X.B. Ca2+ induced crosslinking of AIE-active polyarylene ether nitrile into fluorescent polymeric nanoparticles for cellular bioimaging. Macromol. Rapid Commun. 2017, 38, 1700360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.R.; Ji, Y.; Li, F.; Hu, H.H.; Wang, P.; Jia, K.; Liu, X.B. Fe3+ mediated self-assembling of polyarylene ether nitrile block copolymer into cationic dye adsorptive sub-micrometer spheres. Mater. Lett. 2018, 222, 183–186. [Google Scholar] [CrossRef]
- Wei, R.B.; Tu, L.; You, Y.; Zhan, C.H.; Wang, Y.J.; Liu, X.B. Fabrication of crosslinked single-component polyarylene ether nitrile composite with enhanced dielectric properties. Polymer 2019, 161, 162–169. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.Y.; Chen, Y.M.; Zhou, J.X.; Suo, Z.G. Correction to strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Interfaces 2013, 5, 13484. [Google Scholar] [CrossRef]
- Xie, J.N.; He, X.H.; Hu, W.B.; Zhou, M.R.; Jia, K.; Liu, X.B. Pb2+ coordination-driven self-assembling of amorphous amphiphilic aromatic block copolymer into semi-crystallized nanostructures with enhanced fluorescence emission. J. Mater. Chem. C 2019, 7, 1057–1064. [Google Scholar] [CrossRef]
- Peng, G.; Zhu, Z.J.; Tian, Y.; Long, Y.L.; Cui, T.T.; Wang, C.F.; Chen, S. Dendrimer-induced colloids towards robust fluorescent photonic crystal films and high performance WLEDs. J. Mater. Chem. C 2018, 6, 8187–8193. [Google Scholar] [CrossRef]
- Velo-Gala, I.; López-Peñalver, J.J.; Sánchez-Polo, M.; Rivera-Utrilla, J. Role of activated carbon on micropollutans degradation by ionizing radiation. Carbon 2014, 67, 288–299. [Google Scholar] [CrossRef]
- Wang, J.; Deng, B.L.; Wang, X.R.; Zheng, J.Z. Adsorption of aqueous Hg by sulfur-impregnated actived carbon. Environ. Eng. Sci. 2009, 26, 1693–1699. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lo, S.L.; Kuo, J. Pb(II) adsorption capacity and behavior of titanate nanotubes made by microwave hydrothermal method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 126–131. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W.; Xiong, L.; Xu, N.; Ni, J.R. Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes. Chem. Eng. J. 2013, 215, 366–374. [Google Scholar] [CrossRef]
- Leveneur, J.; Waterhouse, G.I.N.; Kennedy, J.; Metson, J.B.; Mitchell, D.R.G. Nucleation and growth of Fe nanoparticles in SiO2: A TEM, XPS, and Fe L-Edge XANES investigation. J. Phys. Chem. C 2011, 115, 20978–20985. [Google Scholar] [CrossRef]
- Calderon, V.S.; Cavaleiro, A.; Carvalho, S. Chemical and structural characterization of ZrCNAg coatings: XPS, XRD and Raman spectroscopy. Appl. Surf. Sci. 2015, 346, 240–247. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Q.; Zhang, N.; Hu, H.Q.; Li, B.; Kang, X.J. Characterization of the surface film on Zr-based bulk metallic glass using X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM). J. Alloy. Compd. 2011, 509, 5926–5930. [Google Scholar] [CrossRef]
- Fu, J.W.; Chen, Z.H.; Wu, X.C.; Wang, M.H.; Wang, X.Z.; Zhang, J.Z.; Zhang, J.H.; Xu, Q. Hollow poly (cyclotriphosphazene-co-phloroglucinol) microspheres: An effective and selective adsorbent for the removal of cationic dyes from aqueous solution. Chem. Eng. J. 2015, 281, 42–52. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xie, Y.; Zhang, Y.K.; Tang, S.Y.; Guo, C.C.; Wu, J.S.; Lau, R. Anionic and cationic dyes adsorption on porous poly-melamine-formaldehyde polymer. Chem. Eng. Res. Des. 2016, 114, 258–267. [Google Scholar] [CrossRef]
- Fu, J.W.; Chen, Z.H.; Wang, M.H.; Liu, S.J.; Zhang, J.H.; Zhang, J.N.; Han, R.P.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Murugesan, A.; Divakaran, M.; Raveendran, P.; Nitin Nikamanth, A.B.; Thelly, K.J. An Eco-friendly Porous Poly (imide-ether) s for the Efficient Removal of Methylene Blue: Adsorption Kinetics, Isotherm, Thermodynamics and Reuse Performances. J. Polym. Environ. 2019, 27, 1007–1024. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhang, Q.; Xiong, G.; Ding, F.; He, Y.K.; Ren, B.Y.; You, L.X.; Fan, X.L.; Hardacre, C.; Sun, Y.G. Bakelite-type anionic microporous organic polymers with high capacity for selective adsorption of cationic dyes from water. Chem. Eng. J. 2019, 366, 404–414. [Google Scholar] [CrossRef]
- Chen, Y.F.; He, F.B.; Ren, Y.; Peng, H.; Huang, K.X. Fabrication of chitosan/PAA multilayer onto magnetic microspheres by LbL method for removal of dyes. Chem. Eng. J. 2014, 249, 79–92. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Hu, Y.H.; Huang, W.W.; Cheng, G.; Cui, C.Z.; Lu, J. A novel amphoteric β-cyclodextrin-based adsorbent for simultaneous removal of cationic/anionic dyes and bisphenol A. Chem. Eng. J. 2018, 341, 47–57. [Google Scholar] [CrossRef]

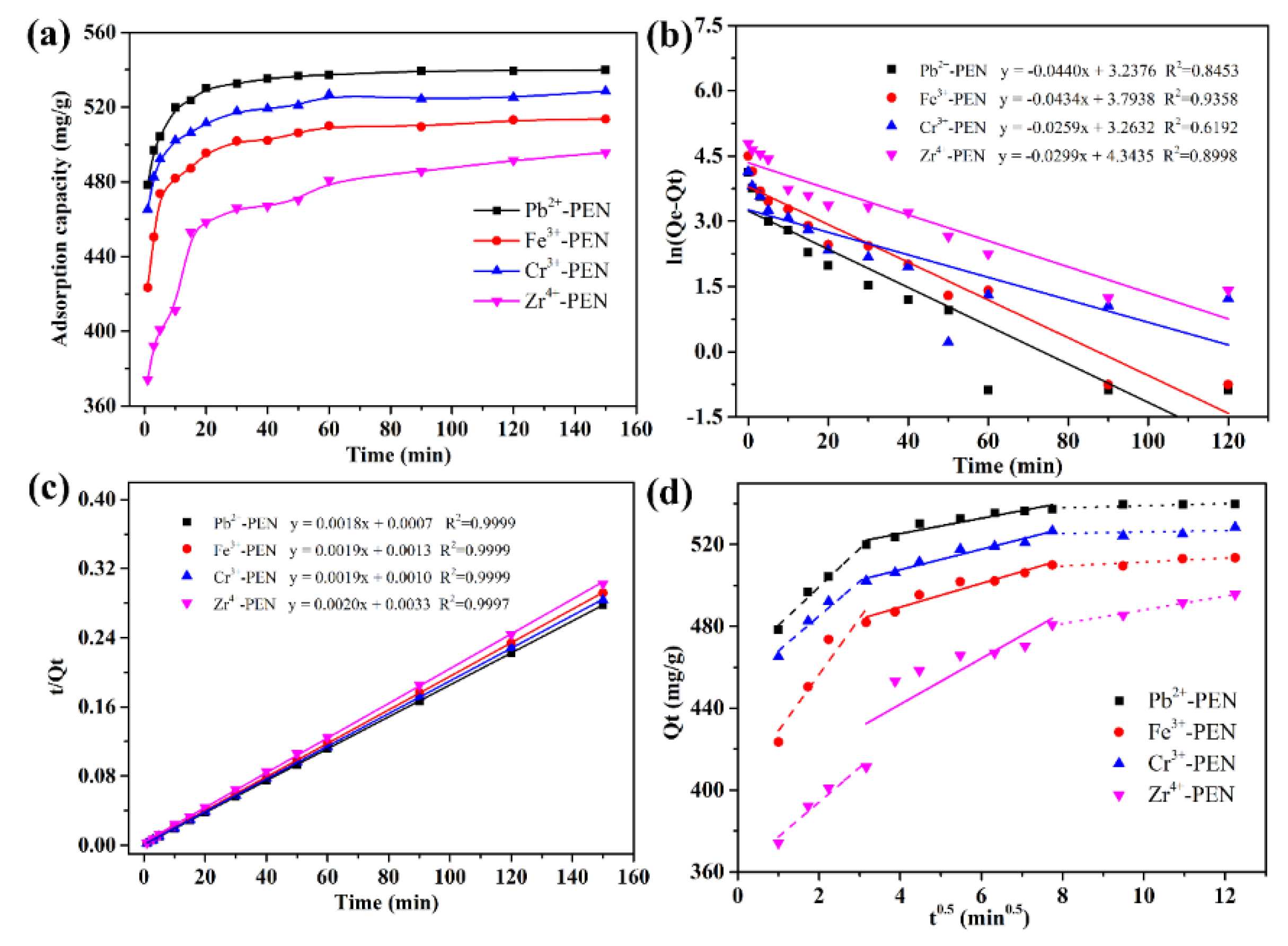
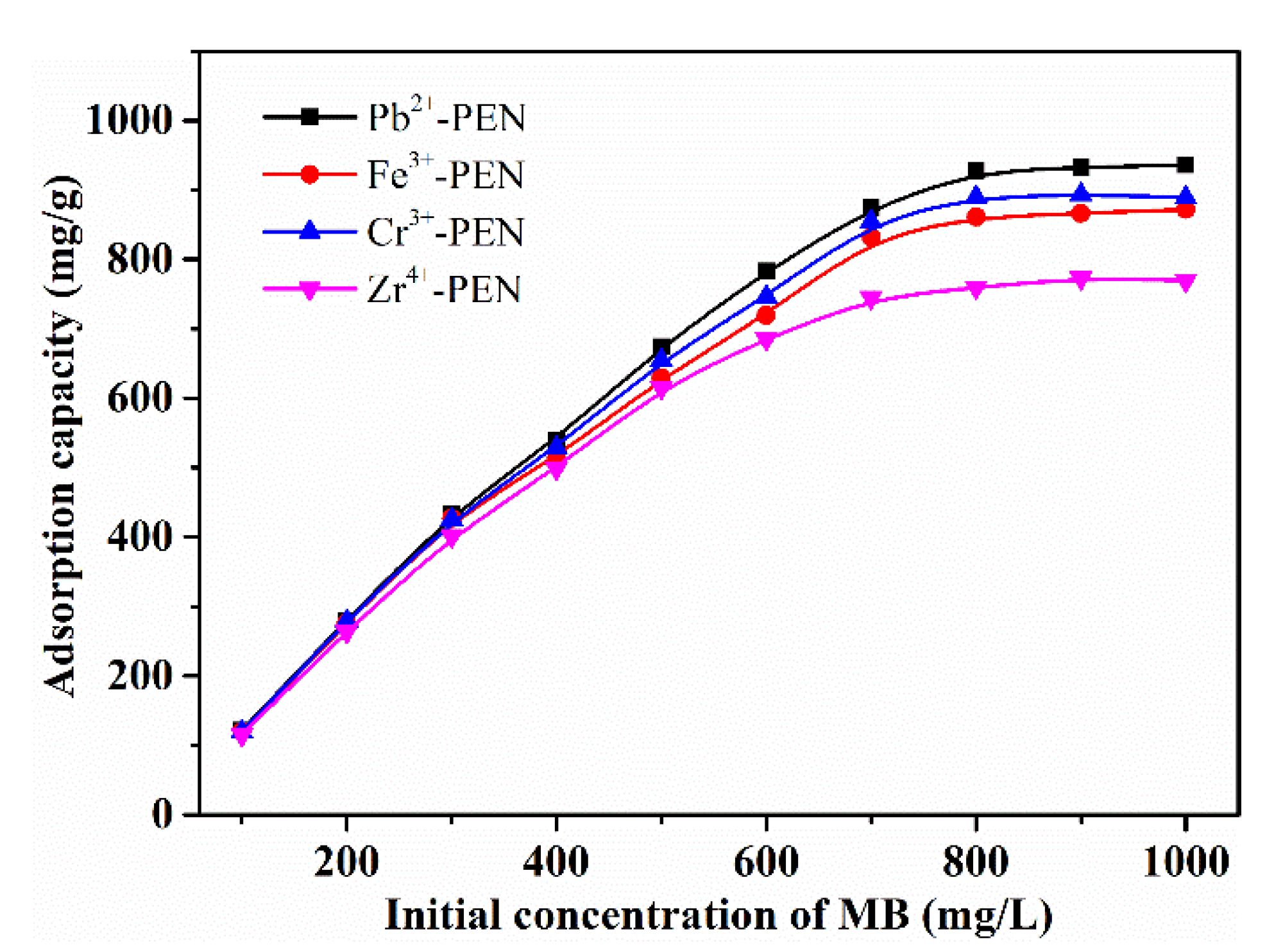
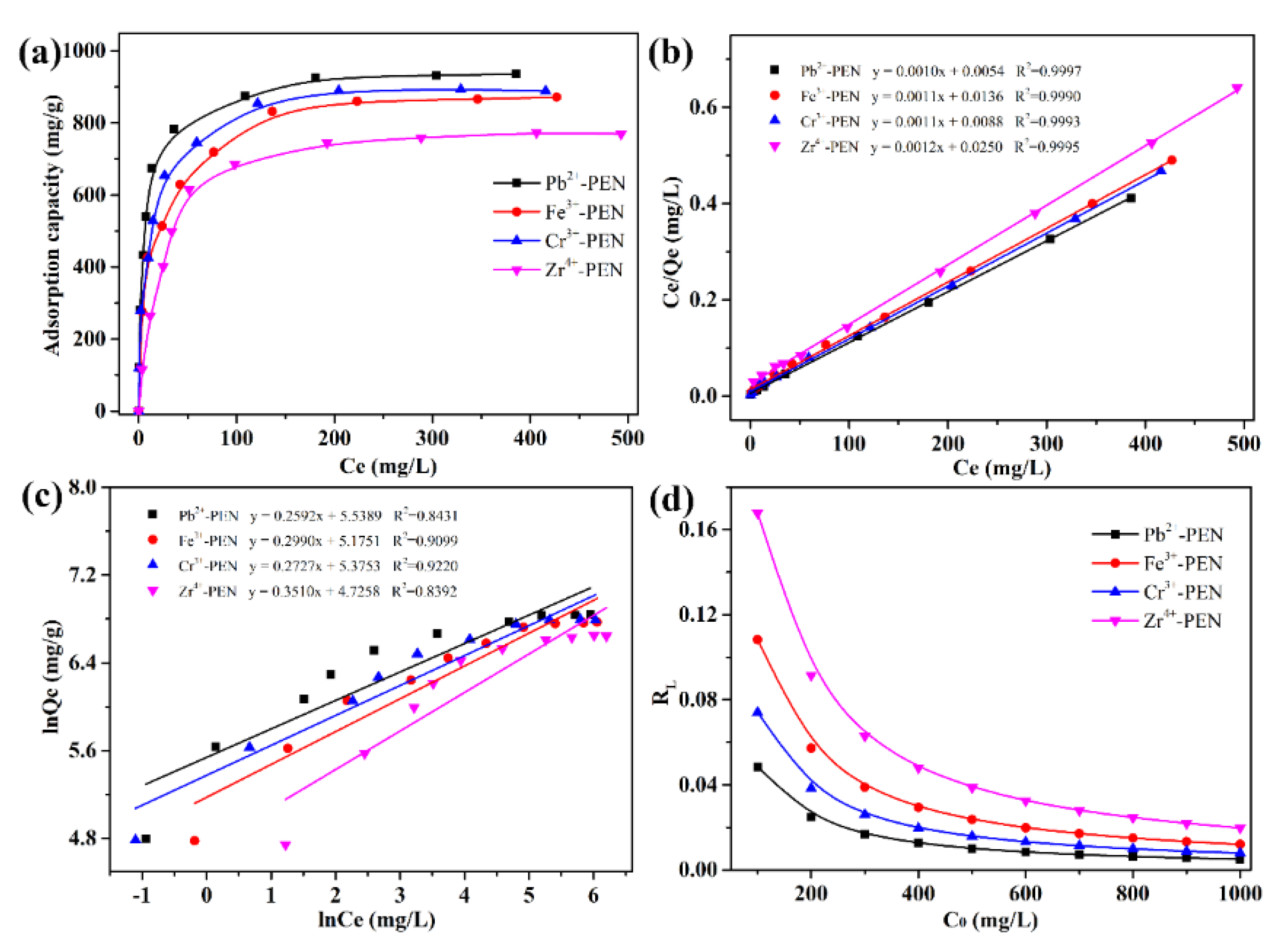
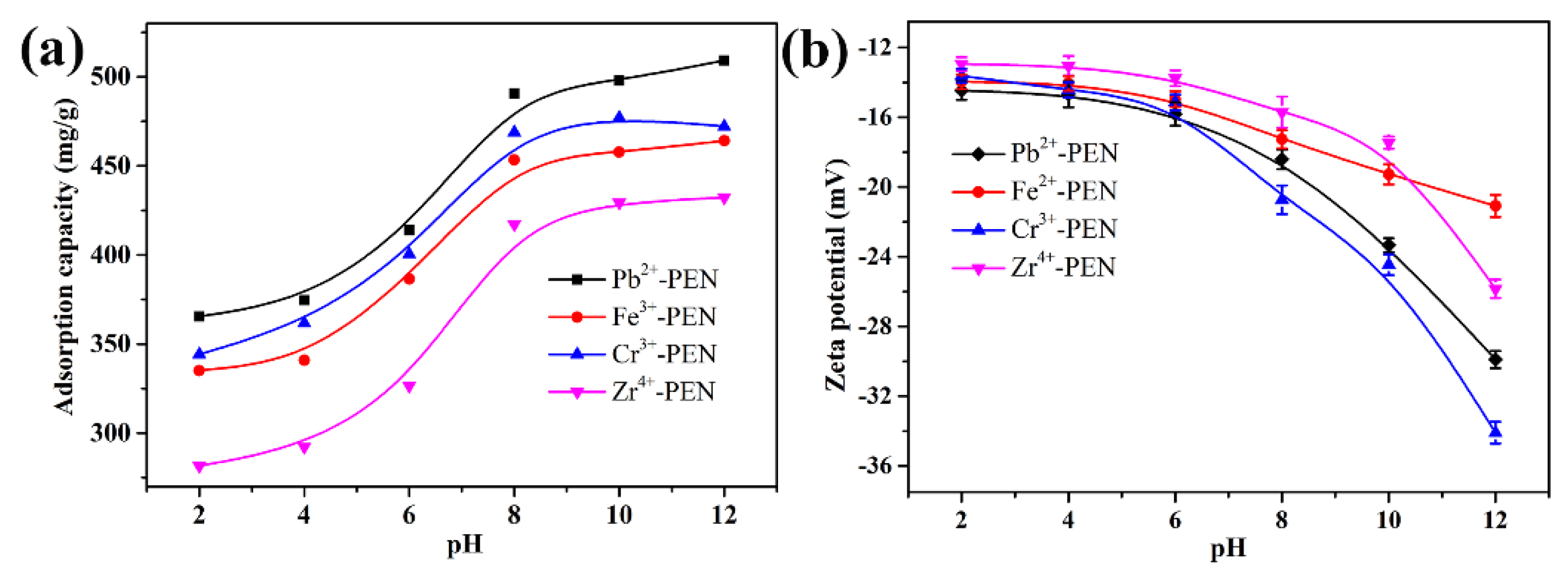
| Entry | Qe,exp | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| K1 | qe,cal | R2 | K2 | qe,cal | R2 | ||
| (mg/g) | (min−1) | (mg/g) | (g/(mg·min)) | (mg/g) | |||
| Pb2+-PEN | 539.89 | 0.0440 | 25.473 | 0.8453 | 0.0046 | 555.56 | 0.9999 |
| Fe3+-PEN | 513.57 | 0.0434 | 44.425 | 0.9358 | 0.0028 | 526.32 | 0.9999 |
| Cr3+-PEN | 528.51 | 0.0259 | 26.133 | 0.6192 | 0.0036 | 526.32 | 0.9999 |
| Zr4+-PEN | 495.67 | 0.0299 | 76.976 | 0.8998 | 0.0012 | 500.00 | 0.9997 |
| Entry | 0~10 min | 10~60 min | 60~150 min | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K1 | ci | R | K2 | ci | R2 | Kid | ci | R2 | |
| Pb2+-PEN | 18.76 | 461.69 | 0.9974 | 3.77 | 510.24 | 0.8880 | 0.54 | 533.61 | 0.9918 |
| Fe3+-PEN | 27.56 | 401.35 | 0.8625 | 5.82 | 466.12 | 0.9262 | 0.95 | 501.88 | 0.9940 |
| Cr3+-PEN | 16.98 | 451.06 | 0.9417 | 5.01 | 487.66 | 0.9579 | 0.35 | 522.67 | 0.9915 |
| Zr4+-PEN | 16.96 | 360.15 | 0.9372 | 11.23 | 396.96 | 0.6665 | 3.36 | 454.40 | 0.9904 |
| Kinds of Adsorbents | Adsorption Capacity | Temperatures | Reference |
|---|---|---|---|
| Poly(cyclotriphosphazene-co-phloroglucinol) (PCCP) microspheres | 50.7 mg/g | 298 K | [51] |
| Porous poly-melamine-formaldehyde (PMF) | 82.5 mg/g | 298 K | [52] |
| Polydopamine (PDA) microspheres | 90.7 mg/g | 298 K | [53] |
| Porous Poly(imide-ether)s (PIEs) | 166.8 mg/g | 303 K | [54] |
| Bakelite-type anionic microporous organic polymers (MOPs) | 712.2 mg/g | 298 K | [55] |
| Magnetic adsorbent (Na-(CS/PAA)n/MPC) | 305.8 mg/g | 298 K | [56] |
| amphoteric β-cyclodextrin-based adsorbent | 335.5 mg/g | 298 K | [57] |
| Pb2+-PEN | 936.13 mg/g | 298 K | This work |
| Fe3+-PEN | 871.67 mg/g | 298 K | This work |
| Cr3+-PEN | 889.08 mg/g | 298 K | This work |
| Zr4+-PEN | 769.44 mg/g | 298 K | This work |
| Entry | qm,exp | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| (mg/g) | KL (L/mg) | qm (mg/g) | R2 | KF (L/g) | n | R2 | |
| Pb2+-PEN | 936.13 | 0.1963 | 943.40 | 0.9997 | 254.40 | 3.8580 | 0.8431 |
| Fe3+-PEN | 871.67 | 0.0824 | 892.86 | 0.9990 | 176.81 | 3.3444 | 0.9099 |
| Cr3+-PEN | 889.08 | 0.1250 | 909.10 | 0.9993 | 216.00 | 3.6670 | 0.9220 |
| Zr4+-PEN | 769.44 | 0.0496 | 806.45 | 0.9995 | 112.82 | 5.6252 | 0.8392 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Yang, T.; Hu, W.; He, X.; Xie, J.; Wang, P.; Jia, K.; Liu, X. Scalable Fabrication of Metallopolymeric Superstructures for Highly Efficient Removal of Methylene Blue. Nanomaterials 2019, 9, 1001. https://doi.org/10.3390/nano9071001
Zhou M, Yang T, Hu W, He X, Xie J, Wang P, Jia K, Liu X. Scalable Fabrication of Metallopolymeric Superstructures for Highly Efficient Removal of Methylene Blue. Nanomaterials. 2019; 9(7):1001. https://doi.org/10.3390/nano9071001
Chicago/Turabian StyleZhou, Meirong, Tianyu Yang, Weibin Hu, Xiaohong He, Junni Xie, Pan Wang, Kun Jia, and Xiaobo Liu. 2019. "Scalable Fabrication of Metallopolymeric Superstructures for Highly Efficient Removal of Methylene Blue" Nanomaterials 9, no. 7: 1001. https://doi.org/10.3390/nano9071001
APA StyleZhou, M., Yang, T., Hu, W., He, X., Xie, J., Wang, P., Jia, K., & Liu, X. (2019). Scalable Fabrication of Metallopolymeric Superstructures for Highly Efficient Removal of Methylene Blue. Nanomaterials, 9(7), 1001. https://doi.org/10.3390/nano9071001





