3.1. Influence of Annealing Temperature on the Properties of Cu2Mg0.2Zn0.8Sn(S,Se)4 Films
As we all know, the properties of absorbers are easily affected by annealing temperature. For the sake of studying the effect of selenization temperature on the microstructure and photoelectric characteristics of CMZTSSe films during the selenization process, the CMZTSSe samples were annealed for 15 min under Se ambience at different temperatures of 500, 530, and 560 °C, hereafter named as A1, A2, and A3, respectively. In addition, the CZTSSe film annealed at a temperature of 530 °C for 15 min will be used as a reference, named as sample A.
XRD spectra were always used to evaluate the crystal quality and assess the probable impurity phase during the selenization process.
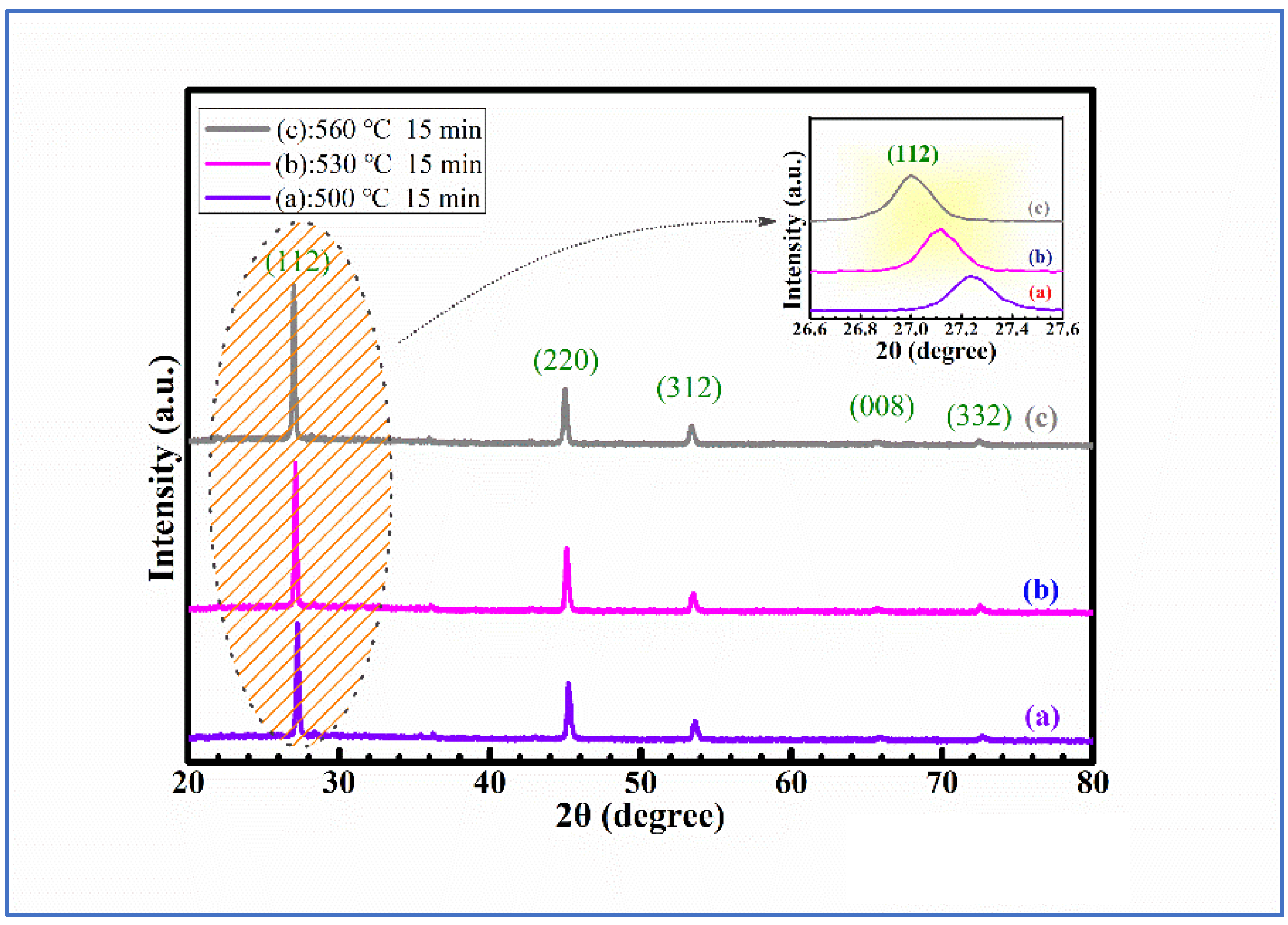
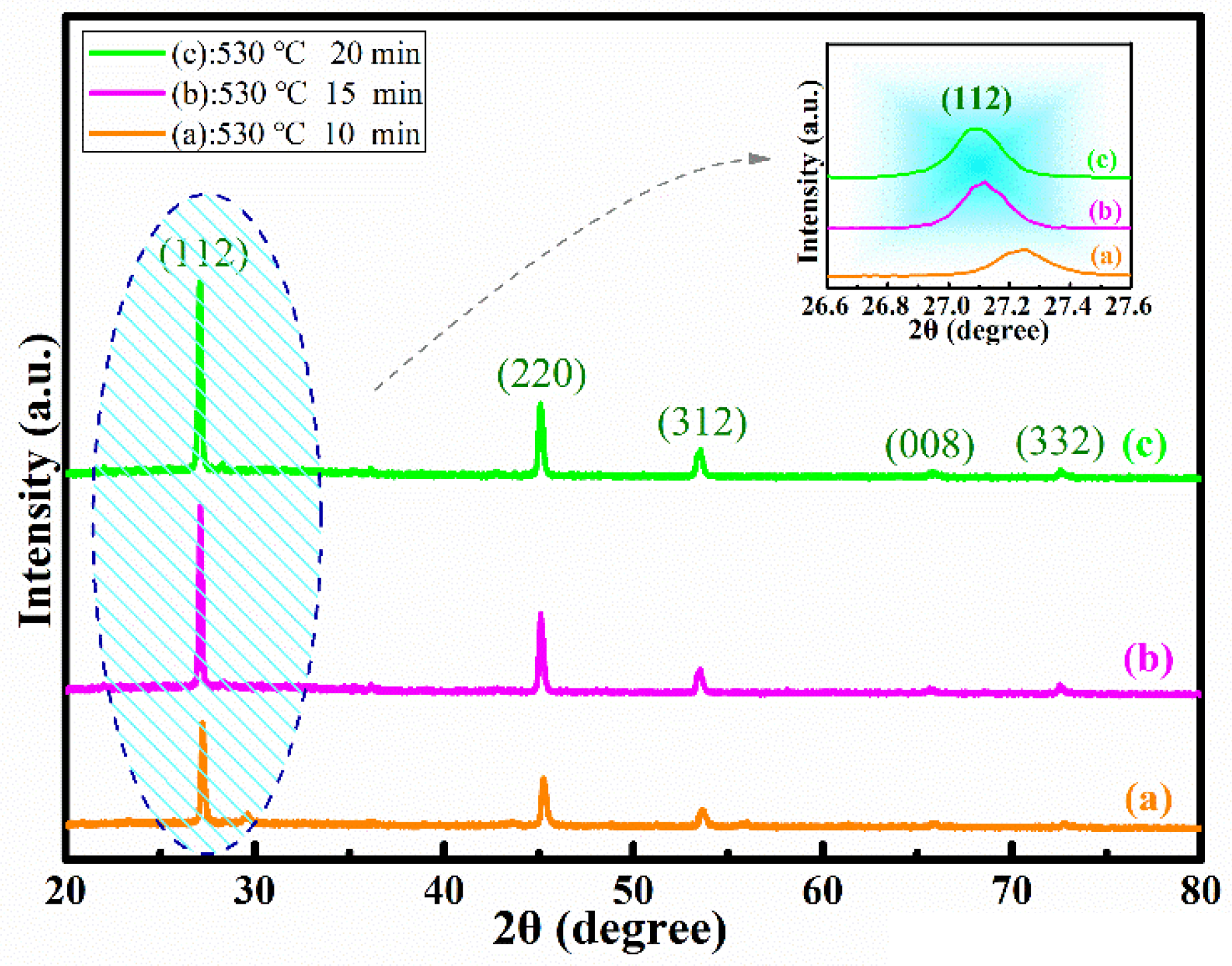
Figure 1 represents the XRD spectra of CMZTSSe samples annealed at different temperatures from 500 to 560 °C (samples A1, A2, and A3). The CMZTSSe films at all varied annealing temperatures were in the kesterite phase, with peaks corresponding to the (112), (220), (312), (008), and (332) planes of Cu
2ZnSnS
4 (CZTS) with the kesterite phase [
26,
27]. As shown in
Figure 1, the characteristic peaks of impurity phases were not found, which indicates that the single-phase CMZTSSe with kesterite structure was formed at all varied annealing temperatures.
Figure 1a indicates the XRD patterns for sample A1, annealed at 500 °C under atmosphere of Se, and it was found that the peak intensity is weaker. By increasing the annealing temperature to 530 °C, as shown in
Figure 1b, the peak intensity was found to be enhanced, indicating that the crystal quality of sample A2 is enhanced at 530 °C. For sample A3, with the annealing temperature of 560 °C, the peak intensity of XRD only slightly changed, as seen from
Figure 1c. As seen from the inset, the dominant characteristic peak (112) is observed to be shifted to smaller angles, from 27.20° to 26.93°, with the annealing temperature increasing from 500 to 560 °C. This is due to the variety in atomic-lattice distance caused by elemental replacement, where the element Se will take the place of S in the CMZTSSe compound with the increase of annealing temperature. According to the results of XRD, there are no secondary phases in CMZTSSe films annealed at different selenization temperatures from 500 to 560 °C.
Table 1 shows the values of full width at half-maximum (FWHM), grain size, the a-axis lattice constant (a), and the c-axis lattice constant (c) for the CMZTSSe films annealed at different temperatures, which are obtained from the (112) peak in the XRD profile. It can be seen from
Table 1 that, as the annealing temperature increases from 500 to 560 °C, the grain size increases first and attains a maximum value at 530 °C, and then decreases when the annealing temperature is continuously increased up to 560 °C. An opposite changing trend was observed concurrently for the FWHM—when increasing the annealing temperature up to 530 °C, the FWHM has a minimum value. Meanwhile, the a-axis lattice constant gradually increases from 5.669 to 5.691 Å as the annealing temperature increases from 500 to 560 °C. The c-axis lattice constant also increases from 11.305 to 11.635 Å. The increases in the lattice constants are ascribed to the rise of the Se content in films, where the S (0.184 nm) atoms were replaced by the larger Se (0.198 nm) atoms with the increasing annealing temperature. This may be verified by the EDS results later.
It is well known that the discernment of the secondary phases in CZTSSe compounds by XRD patterns is difficult, owing to the nearly overlapped XRD patterns of the secondary ZnS(Se) and Cu
2SnS(Se)
3 with the kesterite CZTSSe [
28]. Therefore, Raman spectroscopy measurement is usually applied as an auxiliary technique to detect possible impurity phases, because it is sensitive to lattice vibrations.
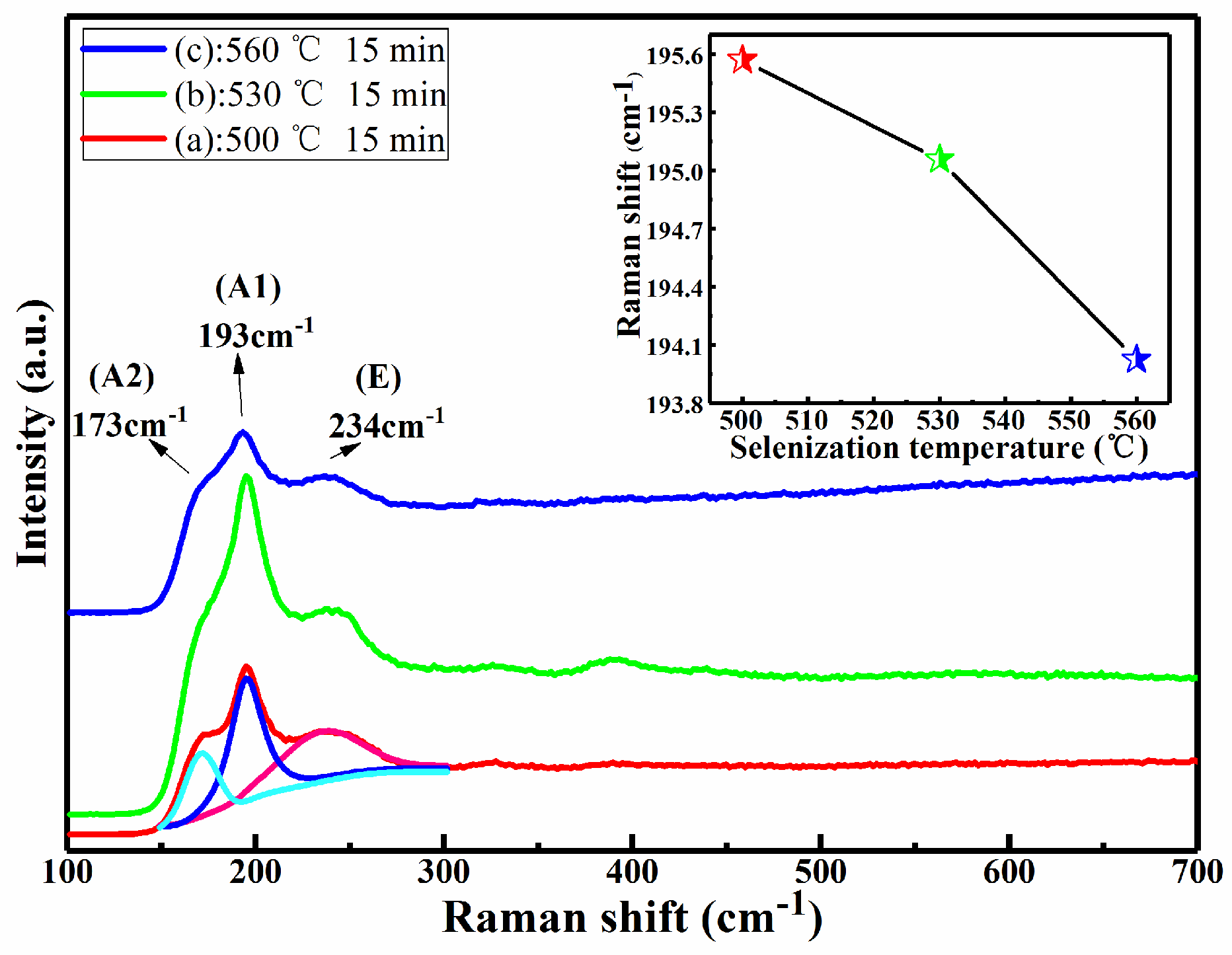
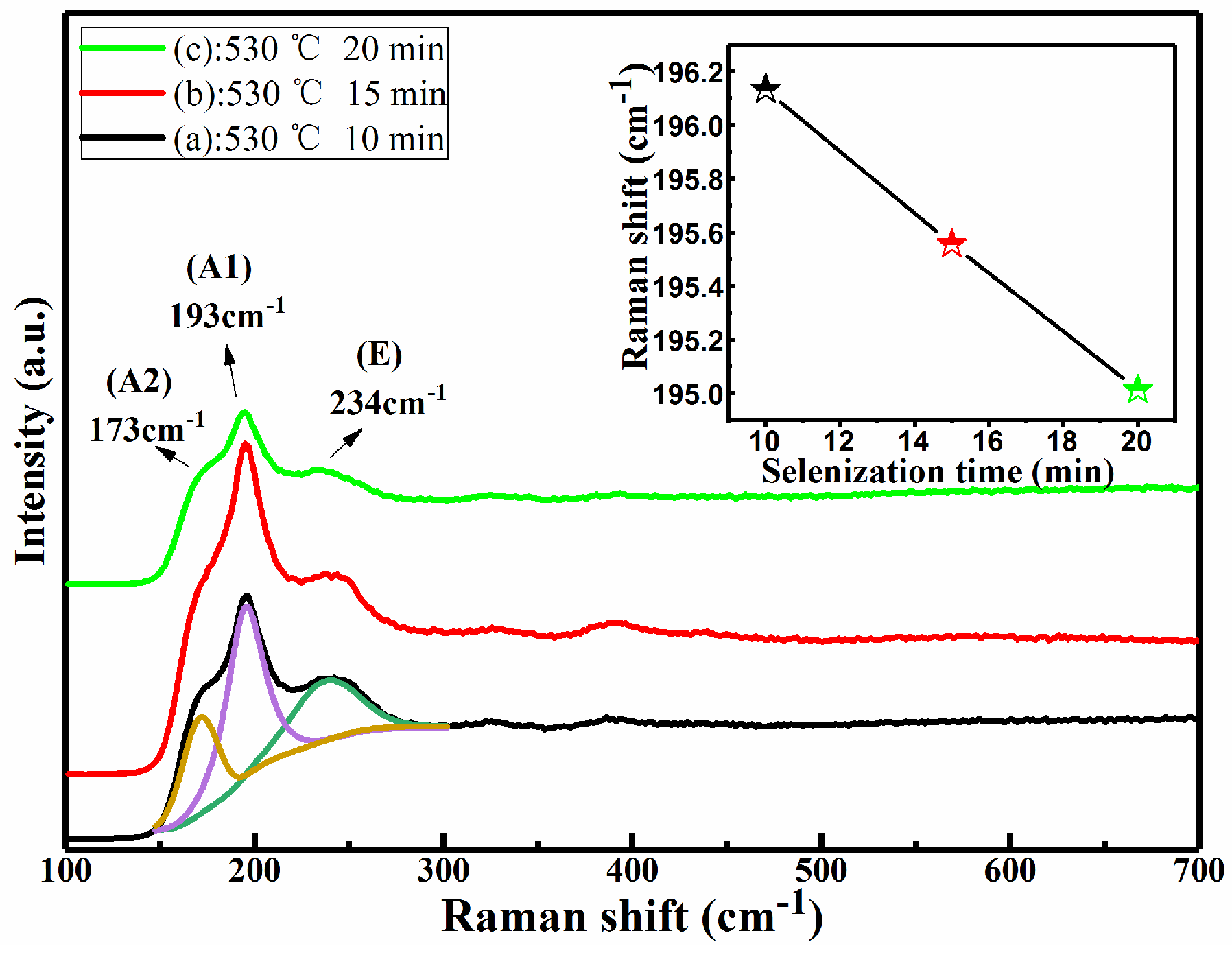
Figure 2 shows the Raman spectra of CMZTSSe films annealed at different annealing temperatures from 500 to 560 °C (samples A1, A2, and A3). The Raman spectrum of sample A1 was fitted using the Gaussian fitting method, and the peaks at 173, 193, and 234 cm
−1 can be observed. The peaks at 173 and 193 cm
−1 conform to the A (A1 and A2) mode Raman vibration peaks of the kesterite CZTSSe phase, as reported in the previous literature [
29]. The A modes are pure anion modes which correspond to vibrations of pure chalcogen (S or Se) atoms surrounded by motionless neighboring atoms. The result exhibits a broad peak located at 234 cm
−1 that corresponds to the E vibration mode in connection with Sn–Se bonding in the kesterite CZTSSe phase [
29]. In addition, it can be seen that the Raman peaks of all samples conform with the vibration peaks of the kesterite CZTSSe phase. The Raman peaks of the other secondary phases were not discovered. This indicates that all annealed samples consist of a single phase of CZTSSe with kesterite structure. Furthermore, with the variation of annealing temperature from 500 to 560 °C, it was found that all Raman peaks were shifted to the lower values, especially for the A1 mode peak. The inset of
Figure 2 displays the tracking of the peak position of the A1 vibration mode, and it is obviously noted that the A1 vibration peak shifted from 195.6 to 193.9 cm
−1 as the annealing temperature changed from 500 to 560 °C. This phenomenon can be attributed to the increment of selenization temperature from 500 to 560 °C, which easily allows larger Se to replace S in the CZTSSe, significantly increasing the lattice constant. It was found from the Raman results that the secondary phases were not discovered, which is consistent with the XRD results. In addition, the peak shift as observed from the XRD and Raman spectra is considered to be due to larger Se taking the site of smaller S in CMZTSSe films with the increase of selenization temperature from 500 to 560 °C, which will be better understood from the compositional and morphological studies later.
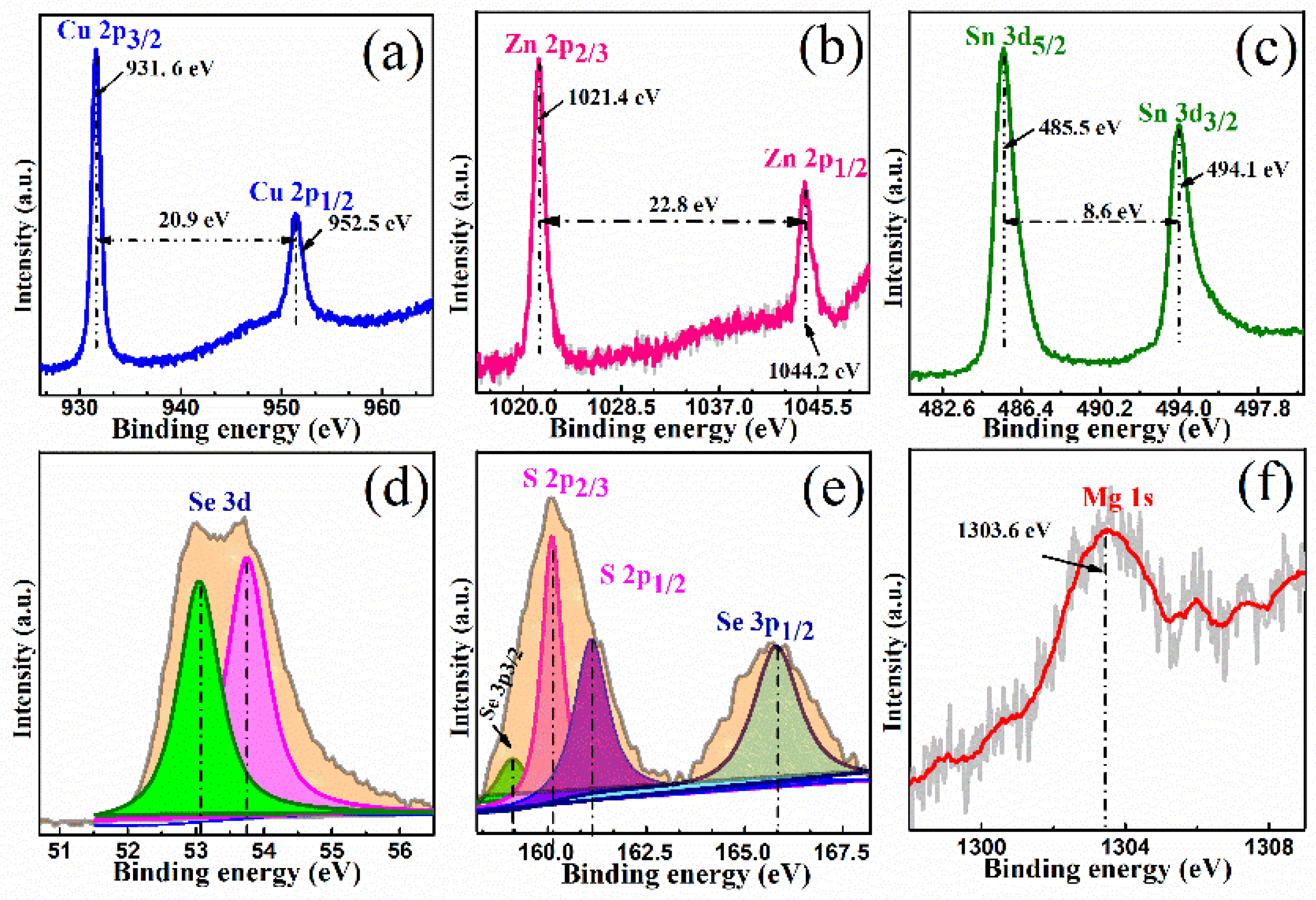
XPS is sensitive to information about the chemical bonding state and element content. As shown in
Figure 3, we identify the elemental composition and valence states of the constituent elements (Cu, Zn, Sn, Se, S, and Mg) in CMZTSSe films annealed at 530 °C for 15 min by XPS measurement, and all peaks were corrected by the C1s binding energy (284.8 eV). As seen from
Figure 3a, the Cu 2p XPS spectrum consists of a Cu 2p
3/2 peak and a Cu 2p
1/2 peak at 931.6 and 952.5 eV, respectively, with a peak separation of 20.9 eV, which indicates that Cu is in the state of +1 [
30].
Figure 3b displays a Zn 2p XPS spectrum, and the peaks presented at 1021.4 and 1044.2 eV are ascribed to Zn 2p
3/2 and Zn 2p
1/2, respectively. The binding energy interval between the Zn 2p
3/2 peak and the Zn 2p
1/2 peak is 22.8 eV, indicating the existence of divalent Zn ions [
31].
Figure 3c shows the XPS spectrum of Sn 3d, and two peaks attributed to Sn 3d
5/2 and Sn 3d
3/2 can be observed at 485.5 and 494.1 eV. The energy difference of the two Sn 3d peaks is 8.6 eV, suggesting the presence of the Sn
4+ state [
32].
Figure 3d displays the Se 3d XPS spectrum, and the S 2p high-resolution spectrum is shown in
Figure 3e. The Se 3d XPS spectra can be fitted into two sub-peaks located at 53.3 and 53.8 eV, which can be ascribed to Se 3d
3/2 (green area) and Se 3d
1/2 (purple area), respectively. The above results indicate that Se in the films is likely to exist in the Se
2− state [
33]. It is well known that the S 2p core level and Se 3p core level are almost overlapping, and we used the Gaussian fitting method to fit the XPS spectra into four sub-peaks presented at 159.1, 160.2, 161.3, and 165.8 eV, which are ascribed to Se 2p
3/2 (light green area), S 2p
3/2 (pink area), S 2p
1/2 (deep purple area), and Se 3p
1/2 (grey green area), respectively. The S 2p
3/2 and S 2p
1/2 peaks located at 160.2 and 161.3 eV, respectively, are in the standard reference value range (160–164 eV) [
33], which means that S exists in the form of S
2−.
Figure 3f displays the XPS high-resolution spectrum of Mg 1s, and the peak can be observed at 1303.6 eV, which indicates that divalent Mg
2+ exists in our study [
19]. The results of the XPS analysis show that the constituent elements (Cu, Zn, Sn, Se, S, and Mg) exist in the forms of Cu
1+, Zn
2+, Mg
2+, Sn
4+, Se
2−, and S
2− in CMZTSSe.
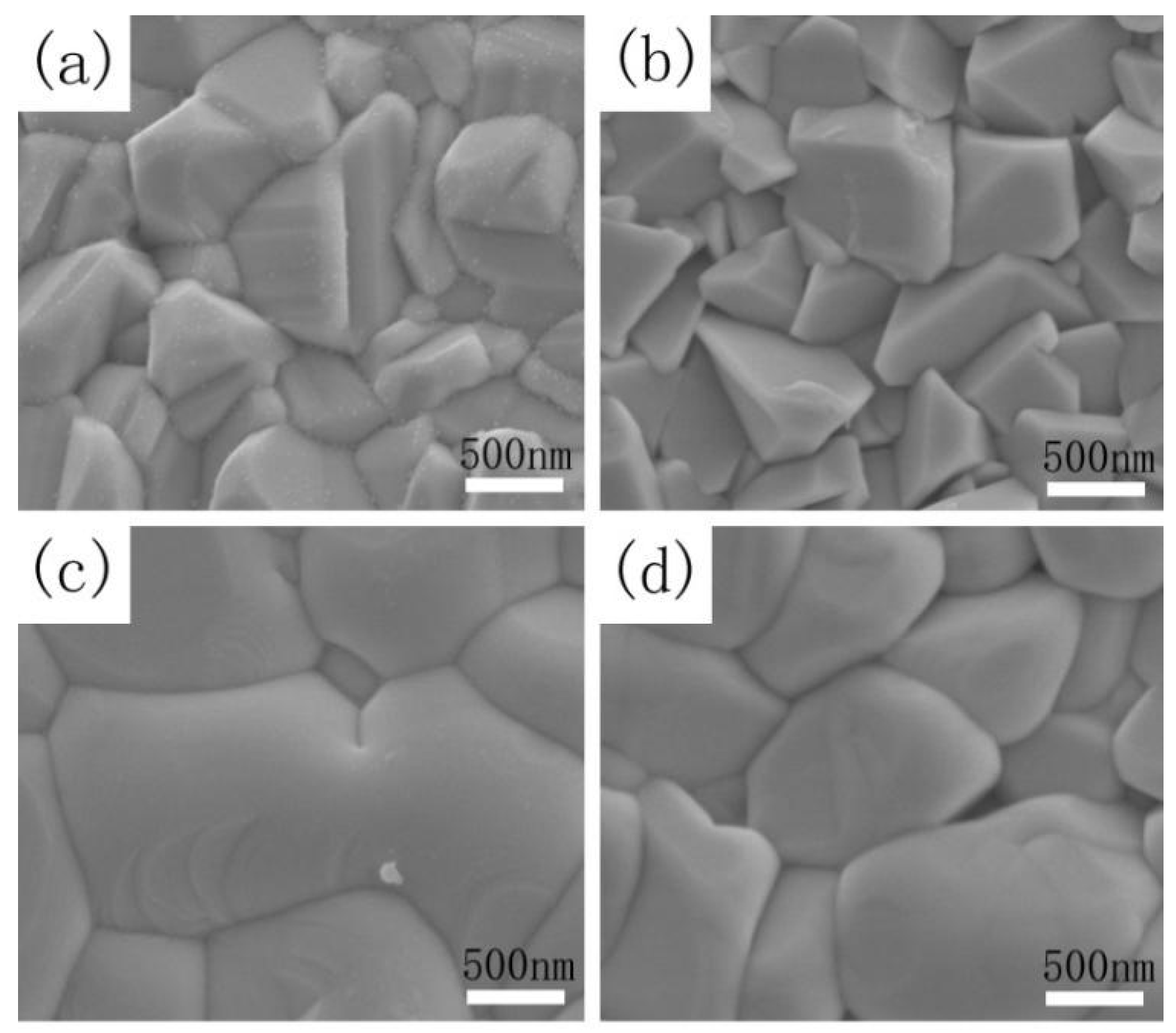
Figure 4a–d shows the scanning electron microscopy (SEM) images of the CZTSSe film annealed at 530 °C for 15 min (sample A) and the CMZTSSe films annealed under atmosphere of Se for 15 min at temperatures of 500, 530, and 560 °C (samples A1, A2, and A3).
Figure 4a shows the surface SEM images of sample A. As we can see from
Figure 4a, irregular and small grain sizes of 0.5–0.8 µm were observed. In addition, it was clearly seen that the surface morphology of Cu
2ZnSn(S,Se)
4 films is very rough.
Figure 4b shows the SEM of sample A1, and it is clearly seen that the film consists of nanograins, with the grain size being even smaller than that of sample A, and the surface morphology is also rough. As shown in
Figure 4c, the crystal quality was improved for sample A2, and the grain size of the film reached 1.0–2.5 µm while the surface morphology became smooth and compact. When the selenization temperature was increased to 560 °C, the grain size of sample A3 slightly reduced to 1.0–1.5 µm and displayed a rough morphology, as displayed in
Figure 4d. The results indicate that the proper selenization temperature is 530 °C, and that this is beneficial to promote the grain growth. Excessively high or low temperatures will cause the deterioration of film quality. When the selenization temperature is 530 °C, not only does the grain size of CMZTSSe film reach its maximum, but the surface morphology of the film also becomes smooth and compact.
As we all know, the photoelectric properties of CZTSSe-based films need to rely heavily on the stoichiometric ratios of Cu, Zn, Sn, S, and Se in CZTSSe-based films [
34].
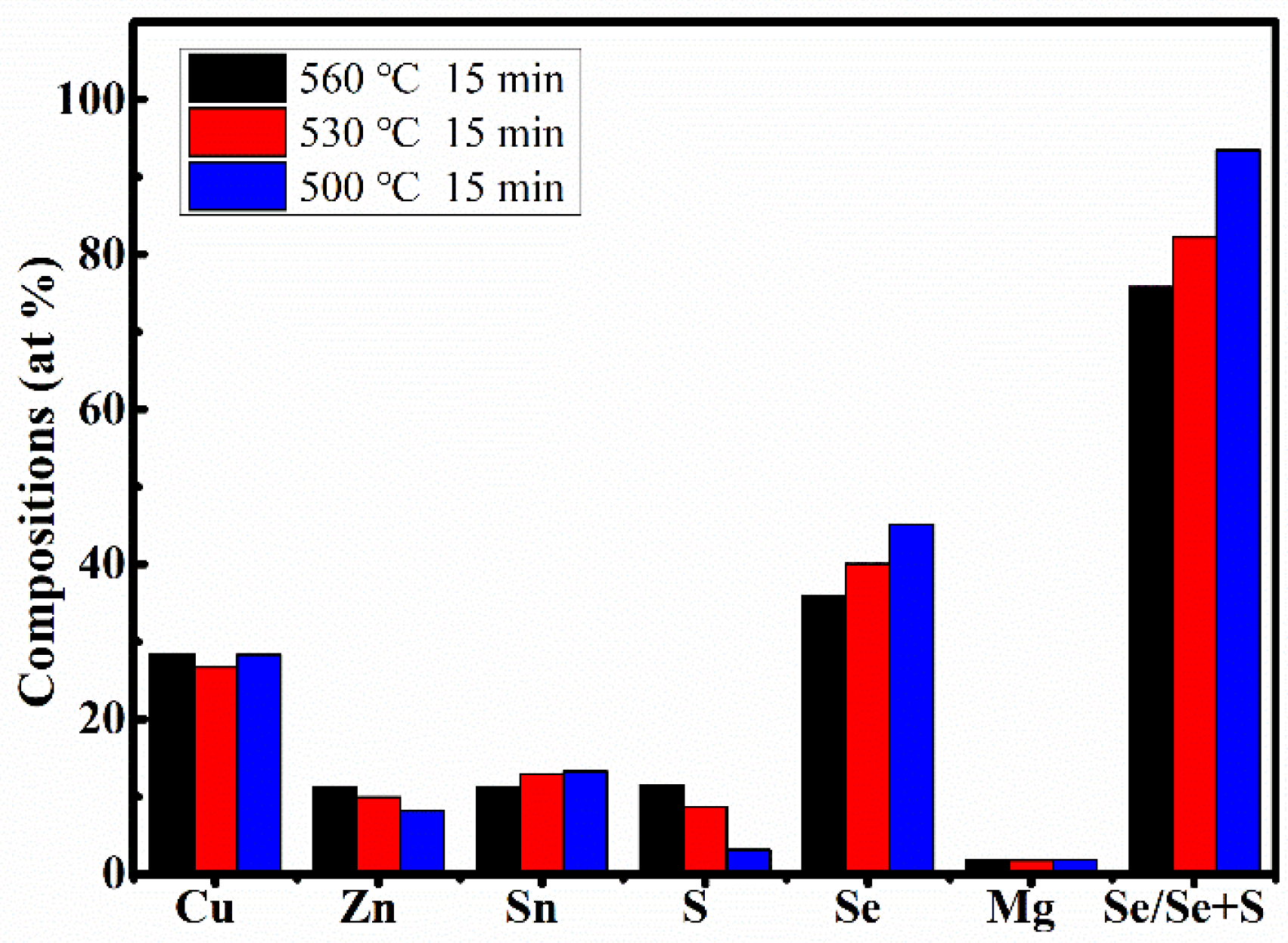
Table 2 displays the EDS results of CMZTSSe films annealed at different temperatures from 500 to 560 °C (samples A1, A2, and A3). According to the EDS results, we confirmed the existence of Cu, Zn, Mg, Sn, S, and Se elements in samples A1, A2, and A3. It was found that the atomic percentages of Se increased from 35.87% to 45.20% and S evidently decreased from 11.46% to 3.16% with increasing selenization temperature from 500 to 560 °C, indicating that Se will partially replace S in the CMZTSSe compound. The compositions of the other elements (Cu, Sn, and Mg) in all three samples were found to be nearly the same, while the atomic percentages of Zn decreased from 11.30% to 8.16%, indicating that Zn loss happened when the selenization temperature changed from 500 to 560 °C. In the precursors, the ratios of Cu/(Zn + Mg + Sn) and Mg/(Mg + Zn) were about 0.82 and 0.2, respectively. The ratios of Mg/(Mg + Zn) in all the films were close to 0.2, but the ratios of Cu/(Zn + Mg + Sn) in all films became significantly larger. This may be due to the decrease of Zn content as the annealing temperature increases. As mentioned before, Se/(S + Se) > 50% is highly suitable for the fabrication of high-efficiency solar cells [
35]. The percentage of Se/(S + Se) is 75.79% for A1, and the percentages of Se/(S + Se) increase to 82.22% and 93.47% for A2 and A3, respectively, indicating that A1, A2, and A3 samples are suitable for the fabrication of efficient solar cell devices. Sample A2 has an appropriate Se/(S + Se) ratio and the grain size became larger compared to samples A1 and A3. Therefore, sample A2 is more suitable to fabricate the high-efficiency solar cells.
Figure 5 summarizes the elements composition analysis of CMZTSSe films according to
Table 2. As shown from
Figure 5, when increasing the selenization temperature from 500 to 560 °C, the proportion of Se increases gradually while the content of S decreases, while the atomic percentages of Cu, Zn, Sn, and Mg remain relatively constant compared with those of S and Se.
In order to research the influence of selenization temperature on the optical bandgaps of CMZTSSe films (samples A1, A2, and A3), we studied the optical absorption measurements of the CMZTSSe films annealed at different selenization temperatures by an UV-vis-NIR spectrophotometer.
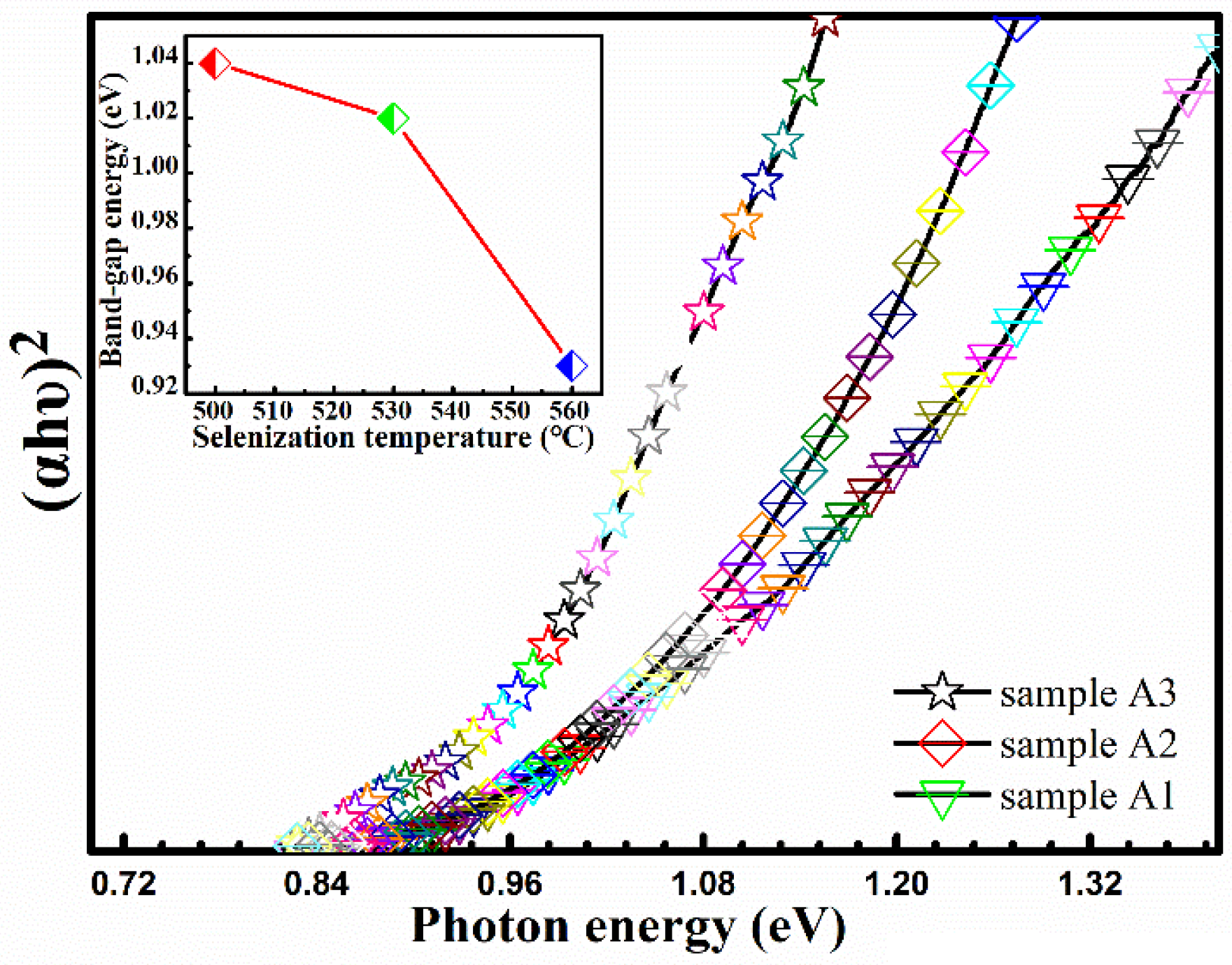
Figure 6 displays the (
αhυ)
2–
hυ plots of CMZTSSe films. We use the solids band theory to express the relation between the absorption coefficient (
α) and the photon energy (
hυ) as follows [
36]:
where
h,
B,
υ, and
Eg are Plank’s constant, a constant, photon frequency, and optical bandgap, respectively. The values of n can employ 3, 2, 3/2, and 1/2, when transitions are indirect unallowed, indirect allowed, direct unallowed, and direct allowed, respectively [
37]. The values of n can employ 1/2 for direct bandgaps of semiconductor CZTSSe [
36]. By using the Equation (1) and the data in
Figure 6, the bandgaps of CMZTSSe are evaluated to be 1.04, 1.02, and 0.93 eV for samples A1, A2, and A3, respectively, as shown in the illustration of
Figure 6. It was found that the bandgap values of samples A1, A2, and A3 gradually decrease with increasing selenization temperature, which can be attributed to the changes of crystal lattice and disparities in electronegativities owing to alloying and modified atomic structures through Se taking the site of S.
The electrical properties of the absorbing layer are also important factors affecting the efficiency of solar cells.
Table 3 displays the electrical characteristics of CMZTSSe annealed at different temperatures (samples A1, A2, and A3) by the Vander Paw method at room temperature. It was found that the CMZTSSe films annealed at different temperatures behave with p-type semiconductor characteristics. When the selenization temperature is increased from 500 to 530 °C, the resistivity first decreases from 6.18 × 10
0 to 2.85 × 10
−1 Ω·cm, then increases to 1.10 × 10
2 Ω·cm at the selenization temperature of 560 °C. Obviously, when the selenization temperature is 530 °C, the resistivity is optimal. Simultaneously, it is clear that the corresponding carrier concentration shows a best value of 6.47 × 10
18 cm
−3 at the selenization temperature of 530 °C. In addition, the mobility reduced from 1.09 × 10
0 cm
2V
−1s
−1 (500 °C) to 3.31 × 10
−1 cm
2V
−1s
−1 (530 °C), but increased to 1.04 × 10
−1 cm
2V
−1s
−1 at the selenization temperature of 560 °C. We analyzed the reasons for the change of CMZTSSe electrical properties, combined with the characterization of SEM. It was concluded that the defects at the surfaces of the absorbing layers are passivated, and owing to the crystal quality of CMZTSSe films improving with the selenization temperature increasing from 500 to 530 °C, the resistivity and carrier concentration achieve the best values with sample A2. It is obvious that the deterioration of resistivity and carrier concentration is owing to the deterioration of crystal quality when the selenization temperature further increases from 530 to 560 °C.
3.2. Effect of Selenization Time on Properties of CMZTSSe Films
After a series of analyses and characterizations, it was proved that the best selenization temperature is 530 °C. As we all know, selenization time is also one of the major parameters influencing the performance of the absorber layer. In our study, after optimizing the annealing temperature, the influence of annealing time on the properties of CMZTSSe films has been studied. The CMZTSSe films were annealed for 10, 15, and 20 min at 530 °C under atmosphere of Se, afterward referred as B1, B2, and B3, respectively.
Figure 7a–c shows the XRD patterns of CMZTSSe films annealed for different times from 10 to 20 min (samples B1, B2, and B3). For the samples B1, B2, and B3, five diffraction peaks located at 28.53°, 47.33°, 56.17°, 69.27°, and 76.44° were observed, conforming to the (112), (220), (312), (008), and (332) planes of kesterite CZTS respectively [
25,
26]. The characteristic diffraction peaks of other impurity phases were not observed in
Figure 7. We can explore the influence of annealing time on the structural performance of CMZTSSe films by observing the peak intensity and peak shift. It is observed from
Figure 7a,b that the intensity of the (112) peak is increased, which indicates that the crystal quality is enhanced by increasing the annealing time from 10 to 15 min. Furthermore, the characteristic peak intensity is almost unchanged with the increase of annealing time from 15 to 20 min, as displayed in
Figure 7c. In addition, the position of the (112) peaks are shifted to lower 2θ angle with selenization time increasing, as shown in the illustration of
Figure 7. Since the concentration of Se in the CMZTSSe matrix increases with the increase of selenization time, there is enlargement of unit cell size, causing the change of the lattice distance in the films. According to the analysis results of XRD, it was found that the crystal structure of CMZTSSe was not changed with the increase of annealing time, and we speculate that the crystal growth of CMZTSSe was completed when the annealing time reached 15 min.
The full width at half-maximum (FWHM), grain size, a-axis lattice constant (a), and c-axis lattice constant (c) of the films annealed for different times from 10 to 20 min (samples B1, B2, and B3) are displayed in
Table 4. It was found that by increasing the annealing time from 10 to 20 min, the grain size first increases and then decreases, while the FWHM value first decreases and then increases. When the annealing time is 15 min, the grain size has a maximum value, while the FWHM has a minimum value. The a-axis lattice constant gradually increases from 5.665 to 5.684 Å as the annealing time increases from 10 to 20 min. Meanwhile, the c-axis lattice constant also increases from 11.334 to 11.453 Å. It was concluded that the optimal annealing time is 15 min.
As we all know, the diffraction peaks of Cu
3SnS
4 with a tetragonal structure and ZnS with a cubic structure are close to the diffraction peaks of CZTSSe with a kesterite structure [
38,
39]. Thus, it is very difficult to detect possible secondary phases by XRD only. In order to further detect the phase compositions of CMZTSSe films, Raman scattering spectra were measured for the films annealed for different times from 10 to 20 min (samples B1, B2, and B3), as shown in
Figure 8. The Raman spectrum of sample B1 was fitted using the Gaussian fitting method, and three Raman peaks located at ~193 (A1 mode), 173 (A2 mode), and 234 cm
−1 (E mode) were observed, which conform to the Raman characteristic peaks of CZTSSe [
29]. The characteristic peaks of some possible impurity phases were not detected. It is suggested that the CMZTSSe films are composed of a single phase of kesterite CZTSSe. In addition, these characteristic peaks slightly shift to lower values as the selenization time increases, owing to the incorporation of Se in the CMZTSSe compound. It should be noted that the Raman spectra are consistent with the XRD patterns, and some possible secondary phases (Cu
2SnSe
3, SnSe
2, SnSe, ZnSe, and MgSe) were observed. Furthermore, the phase change of the CMZTSSe films did not occur with increasing selenization time from 10 to 20 min. It was concluded that the pure-phase CMZTSSe films were successfully synthesized.
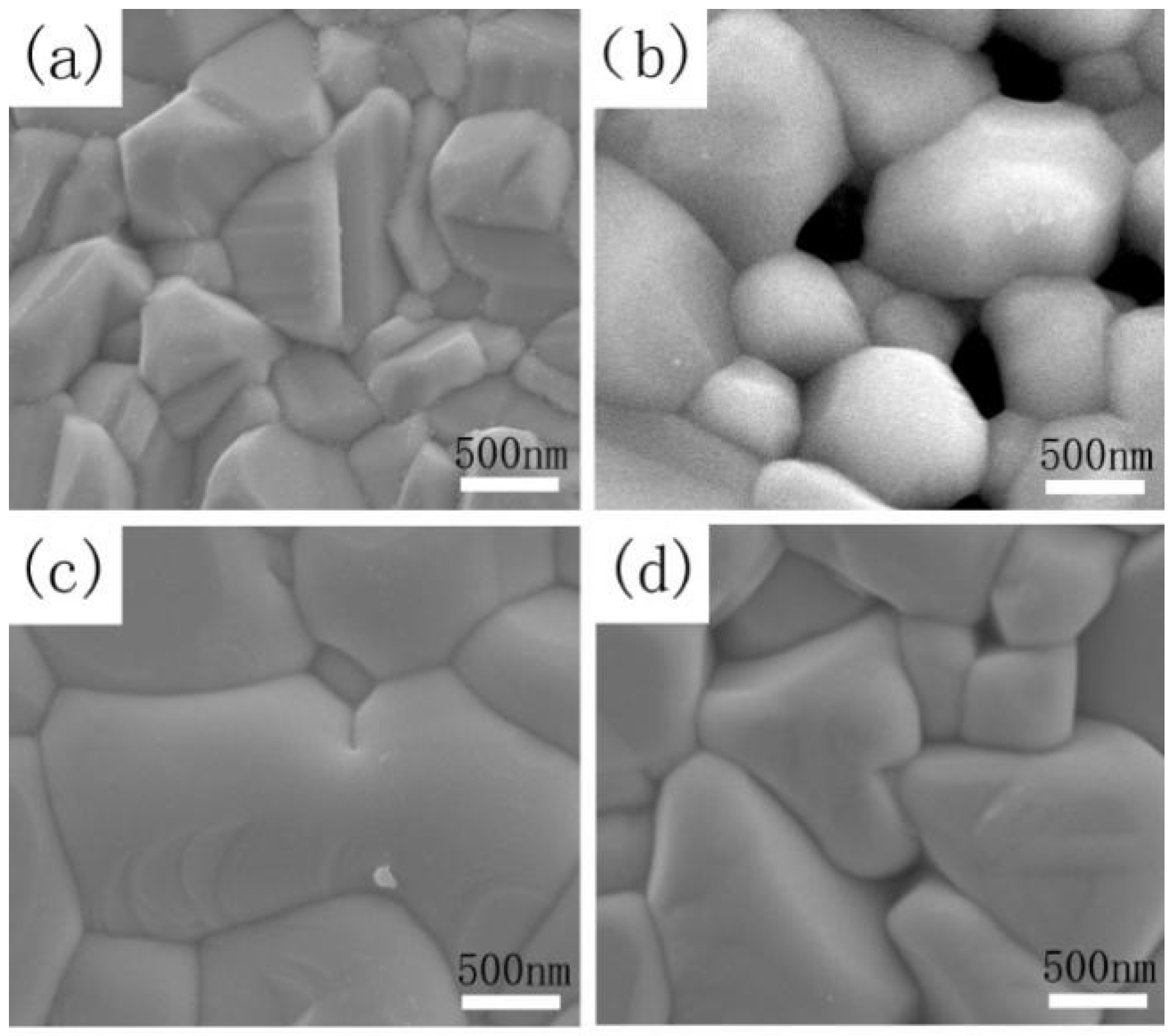
Figure 9a–d depicts the SEM surface images of CZTSSe annealed at 530 °C for 15 min (sample A) and CMZTSSe films annealed at 530 °C for different times from 10 to 20 min (samples B1, B2, and B3), respectively. As shown in
Figure 9a, the surface of the sample A displays pin-hole free morphology and small grain size between 500–800 nm, with a rough surface.
Figure 9b shows the surface morphology of sample B1, and it was clearly observed that the CMZTSSe film still consists of nanograins, where the grain size is between 500 and 1000 nm with a large number of holes on the surface. As the annealing time increased to 15 min, obvious morphological change is observed (
Figure 9c), with the grains size of sample B2 increasing sharply to the micron level (1.0–2.5 μm) and the surface becoming dense and flat. By further extending the annealing time to 20 min, the surface morphology of sample B3 becomes rough but still dense, and the grains size slightly decreases to 0.8–1.3 μm. It was concluded that the optimal selenization time is 15 min, and the CMZTSSe films obtained at this time have the best crystallinity, the largest crystal grain size, and their surfaces are dense and flat.
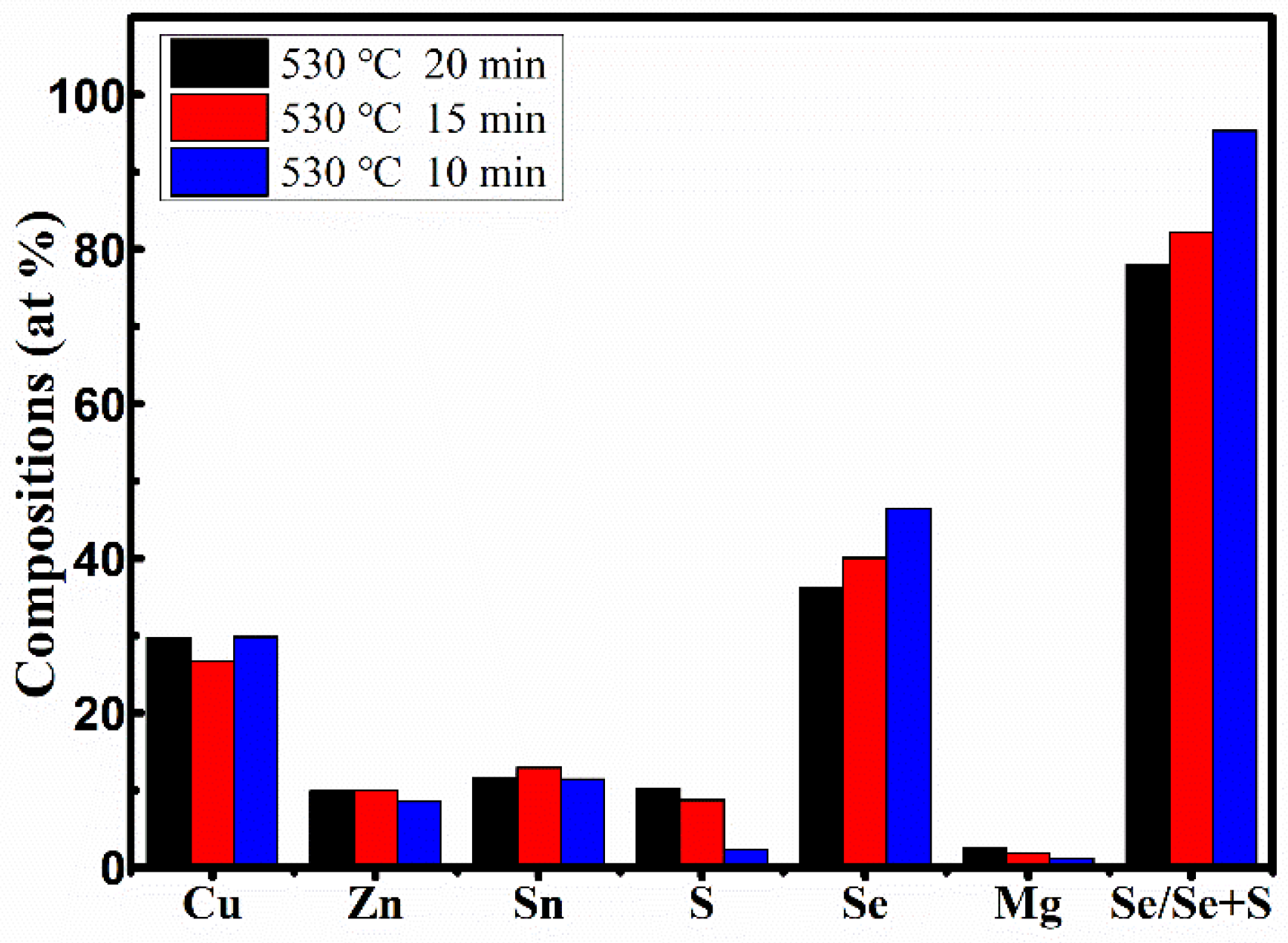
The EDS results of the films annealed for different times from 10 to 20 min (samples B1, B2, and B3) are displayed in
Table 5. It was found that by increasing the annealing time from 10 to 20 min, the atomic percentage of S evidently decreases from 10.23% to 2.26%, the atomic percentage of Se increases from 36.24% to 46.44%, and the ratio of Se/(Se + S) significant increases from 77.99% to 95.36%. The ratios of Cu/(Zn + Mg + Sn) in all films were significantly larger than those in the precursor, and the ratios of Mg/(Mg + Zn) in all films significantly decreased from 0.19 to 0.12 with increasing annealing time from 10 to 20 min. These changes were ascribed the decrease of Mg content with the increase of the annealing time, as shown in
Table 5.
Figure 10 represents the elemental composition analysis of the films annealed for different times from 10 to 20 min. It can be clearly seen that the atomic percentages of Se increase, while the atomic percentages of S decrease with the increase of annealing time. Compared with the increase of Se content and the decrease of S content, the atomic percentages of other elements (Cu, Zn, and Sn) changed only slightly. Furthermore, the changes were irregular and almost negligible, and the changes had less effect on the crystal quality of CMZTSSe films. According to the analysis of elemental composition, the change of selenization time mainly affects the atomic percentages of Se and S elements, and has little effect on other elements.
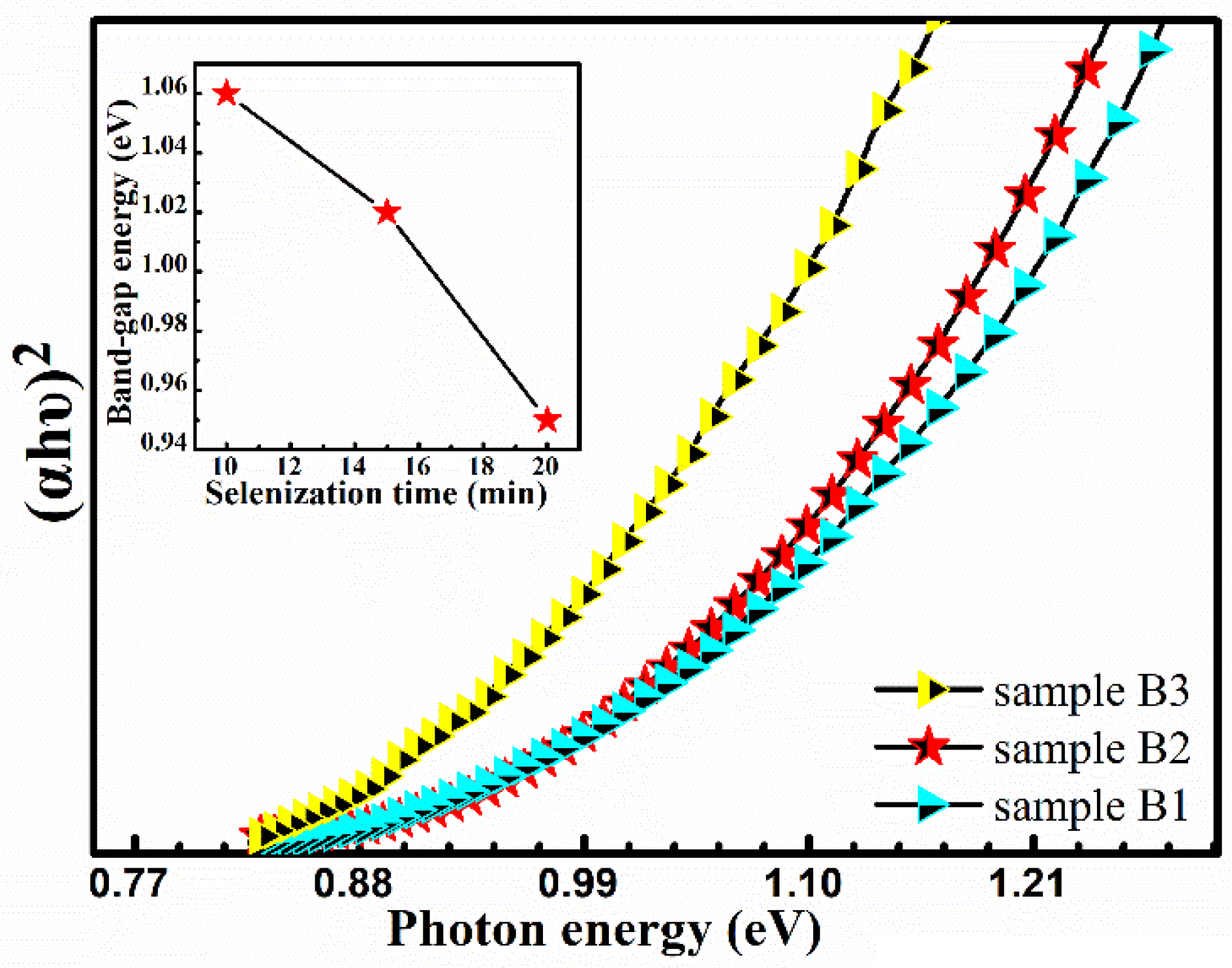
Microstructure, composition, and grain size have great influences on the crystal quality of CMZTSSe, and the bandgaps are also crucial. According to the previous analysis, the change in the ratio of Se/(Se + S) with the increase of selenization time significantly influences the crystal quality, composition, and grain size. The effect of annealing time on the optical bandgaps of the CMZTSSe films has been evaluated by a UV-vis-NIR spectrophotometer. The theoretical basis of bandgap calculation is consistent with Formula 1. As shown in
Figure 11, the bandgaps of the CMZTSSe films show a declining trend (1.06–0.95 eV) with increasing annealing time from 10 to 20 min. The illustration of
Figure 11 displays the dependence of bandgaps on selenization time for CMZTSSe films. We can clearly see that the values of bandgap are 1.06, 1.02, and 0.95 for sample B1, sample B2, and sample B3, respectively. Combined with the analysis results of XRD, Raman, and EDS, the decline in bandgaps with the increase of the selenization time is ascribed to the increase of elemental Se.
As shown in
Table 6, the impacts of selenization time on the conductivity, carrier concentration, and mobility of CMZTSSe (samples B1, B2, and B3) were investigated by Hall measurements at room temperature. It was observed that the p-type conductivity of CMZTSSe was not changed with the increase of annealing time from 10 to 20 min. As shown in
Table 6, when the annealing time increased from 10 to 15 min, the hole concentration of the CMZTSSe films increased obviously from 9.22 × 10
17 to 6.47 × 10
18 cm
−3, the resistivity decreased from 6.18 × 10
0 to 2.85 × 10
−1 Ω·cm, and the mobility decreased from 1.09 × 10
0 to 3.31 × 10
−1 cm
2V
−1s
−1. Combined with the analysis results of SEM, by increasing annealing time from 10 to 15 min, the grain size of CMZTSSe becomes bigger and the surface becomes smooth and hole-free, which leads to the improvement of electrical performance. When the annealing time increases from 15 to 20 min, the crystallinity of CMZTSSe films deteriorates, and hence the hole concentration and resistivity decrease to 5.54 × 10
17 cm
−3 and 1.10 × 10
2 Ω·cm, respectively. It was found that when the selenization temperature and selenization time are 530 °C and 10 min, the best electrical properties of the films are obtained.