Acetic-Acid Plasma-Polymerization on Polymeric Substrates for Biomedical Application
Abstract
1. Introduction
2. Materials and Methods
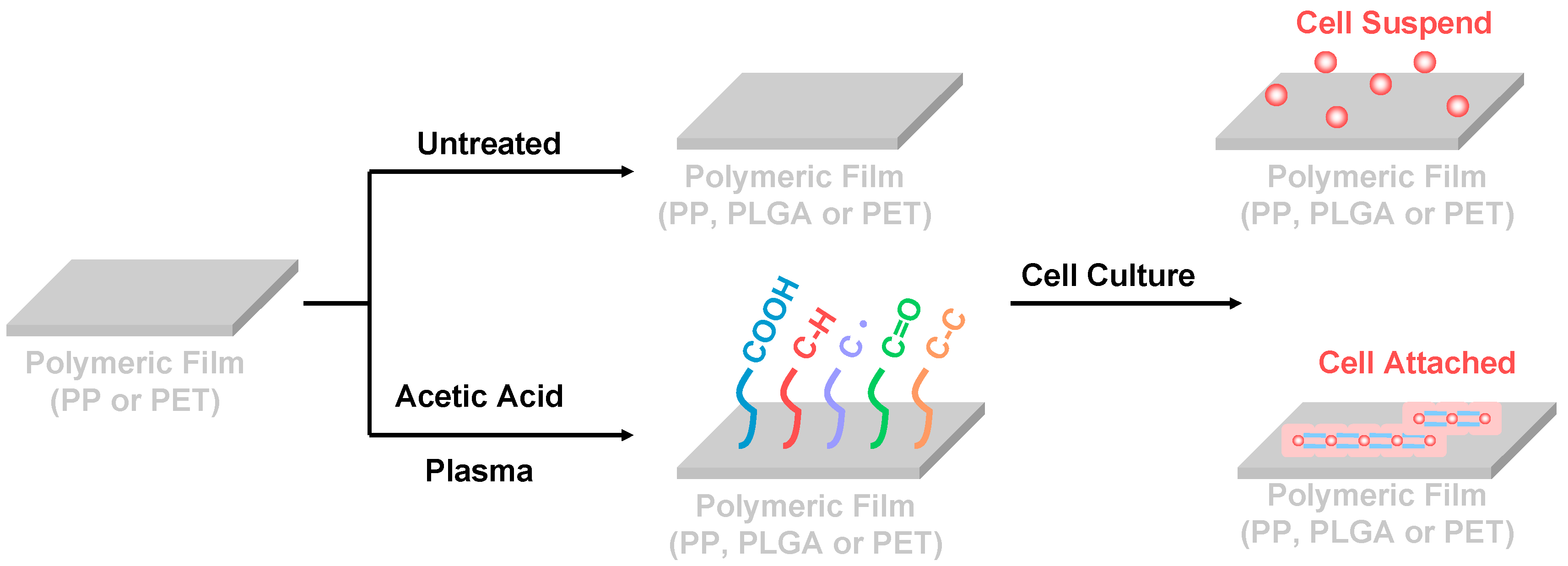
2.1. Pretreatment of Materials
2.2. Cold Plasma Treatment
2.3. Characteristics Analysis
2.3.1. Wettability (Surface Hydrophobicity/Hydrophilicity) Test
2.3.2. Chemical Composition
2.3.3. Cell Culture
2.4. Cell Morphology Analysis
3. Results and Discussion
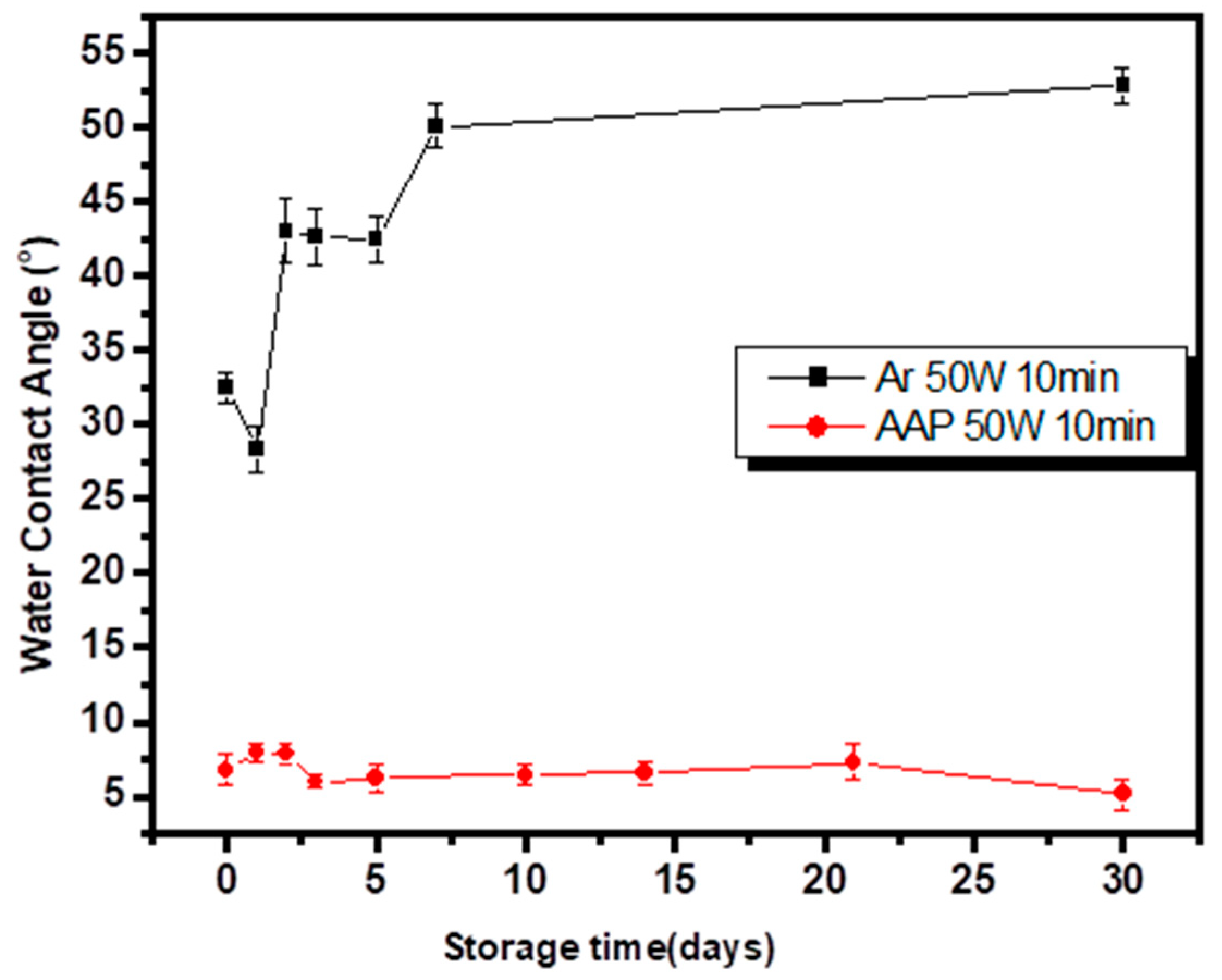
3.1. Wettability of Surface-Modified PET and PP Nonwoven
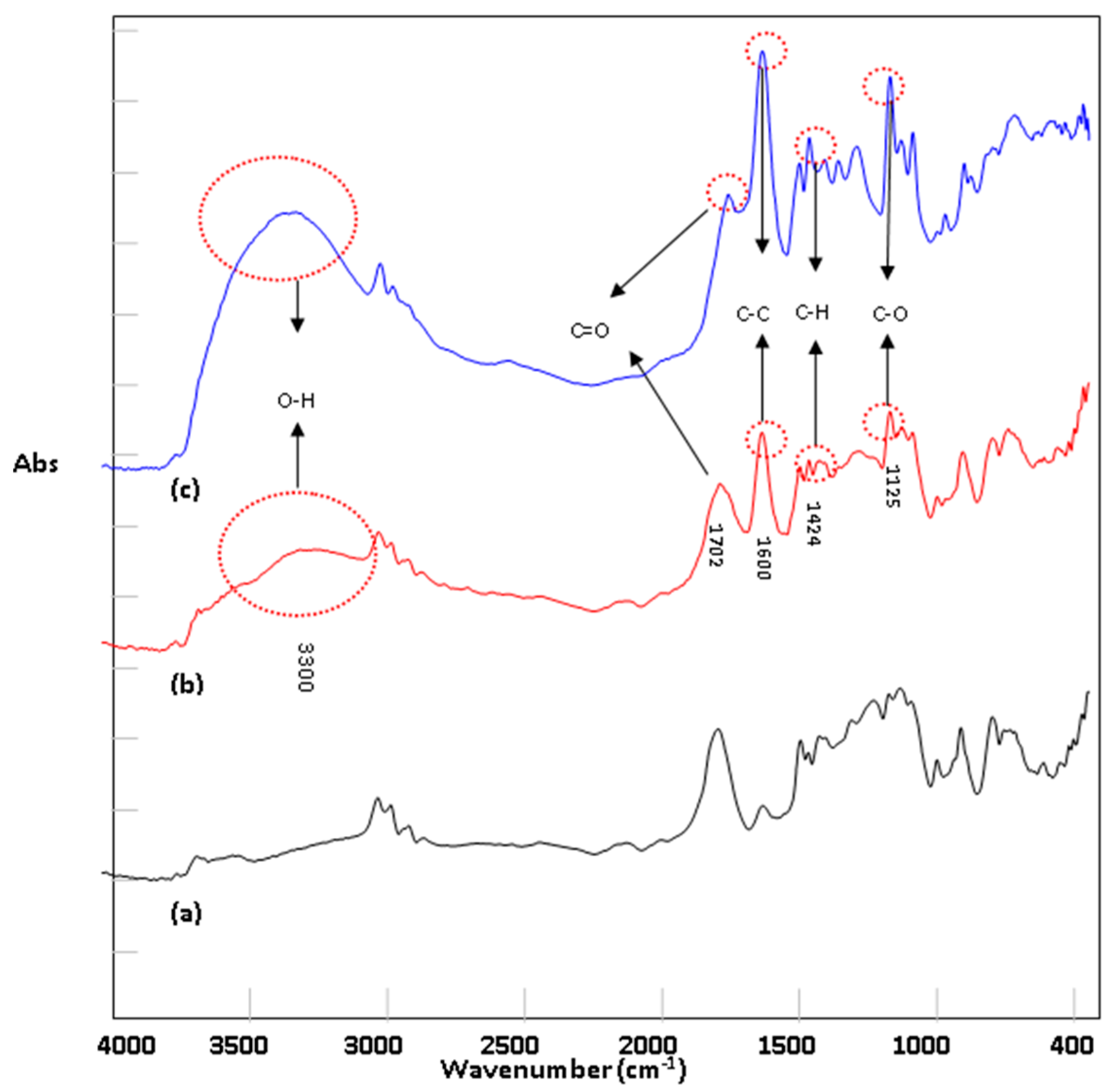
3.2. FTIR Characterization of Acetic Acid Membrane Structure
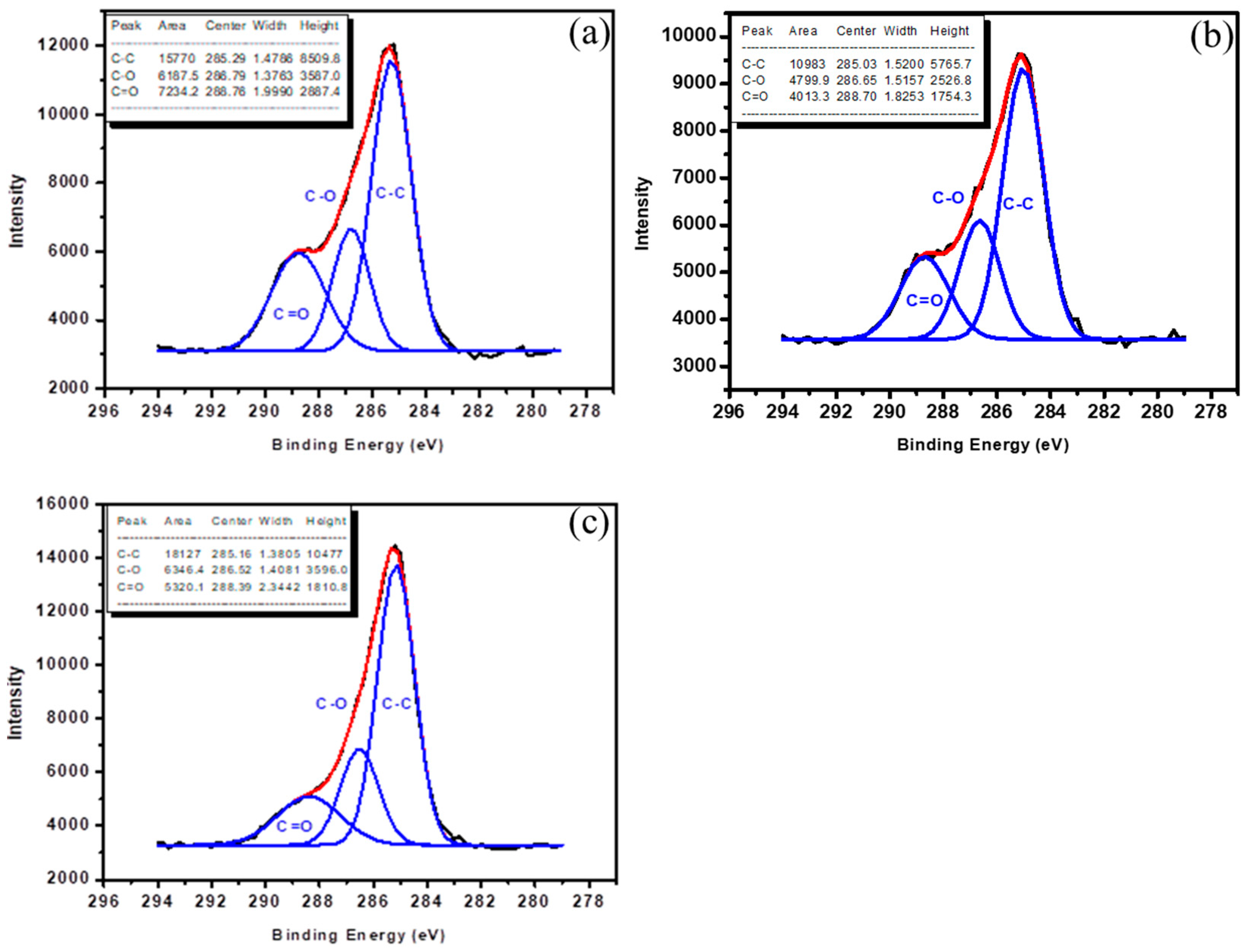
3.3. Chemical Analysis of Acetic Acid Film
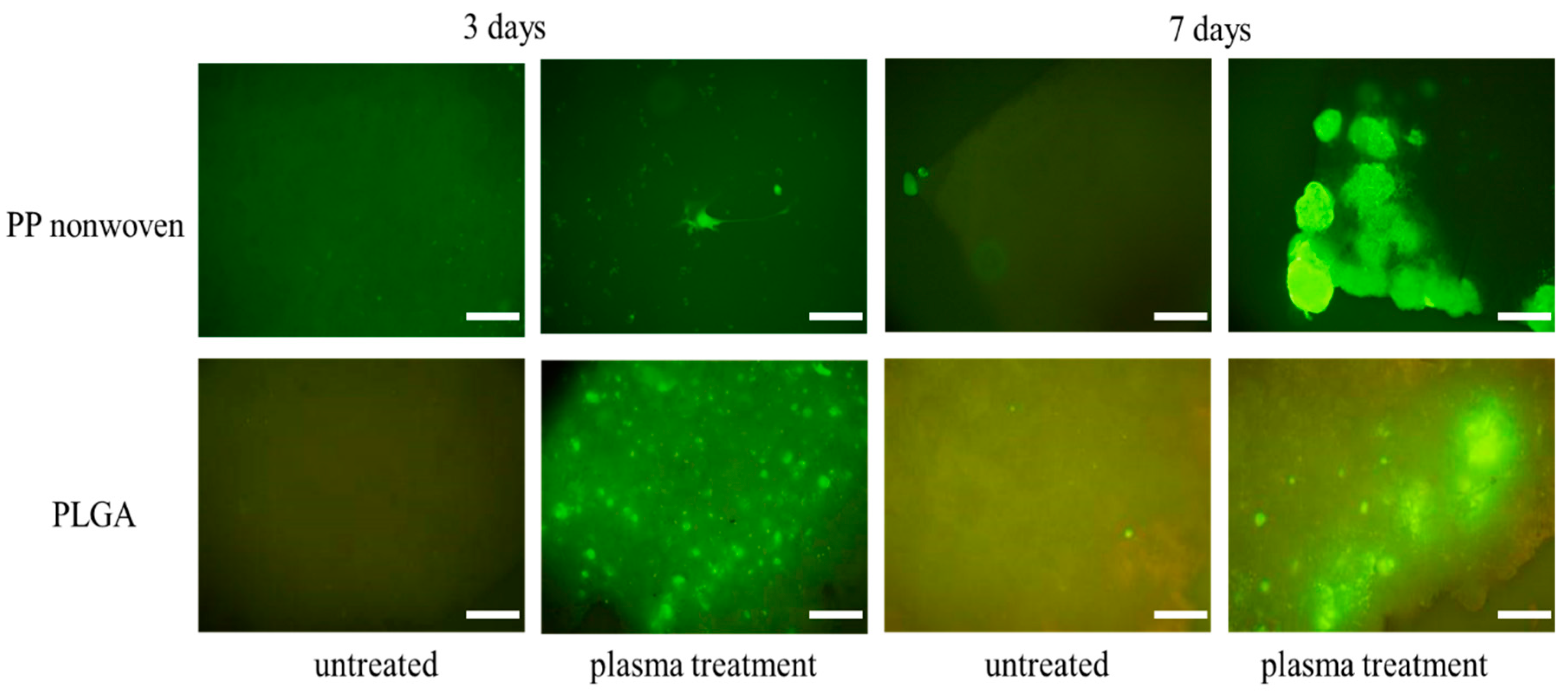
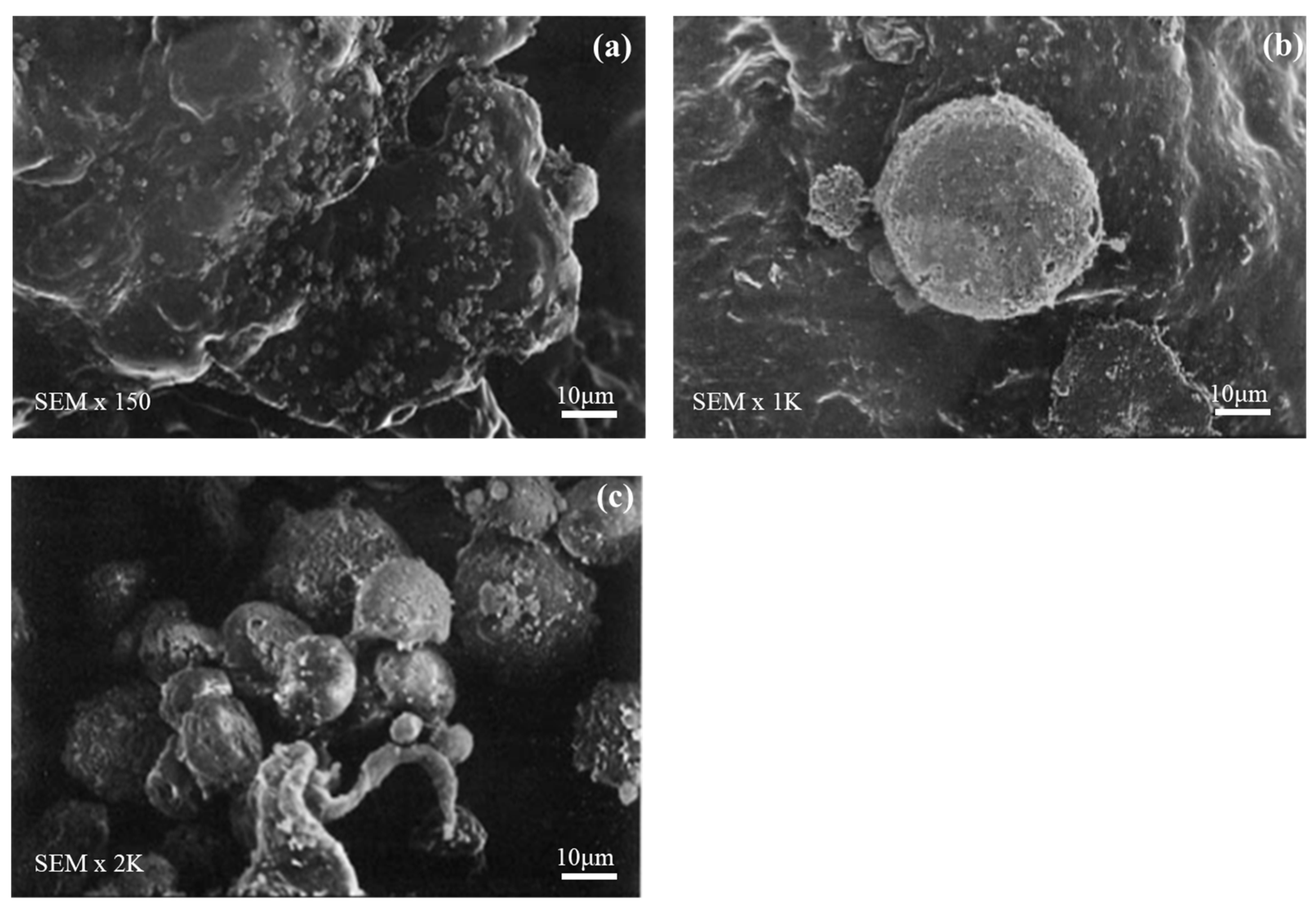
3.4. Cell Morphology Observations
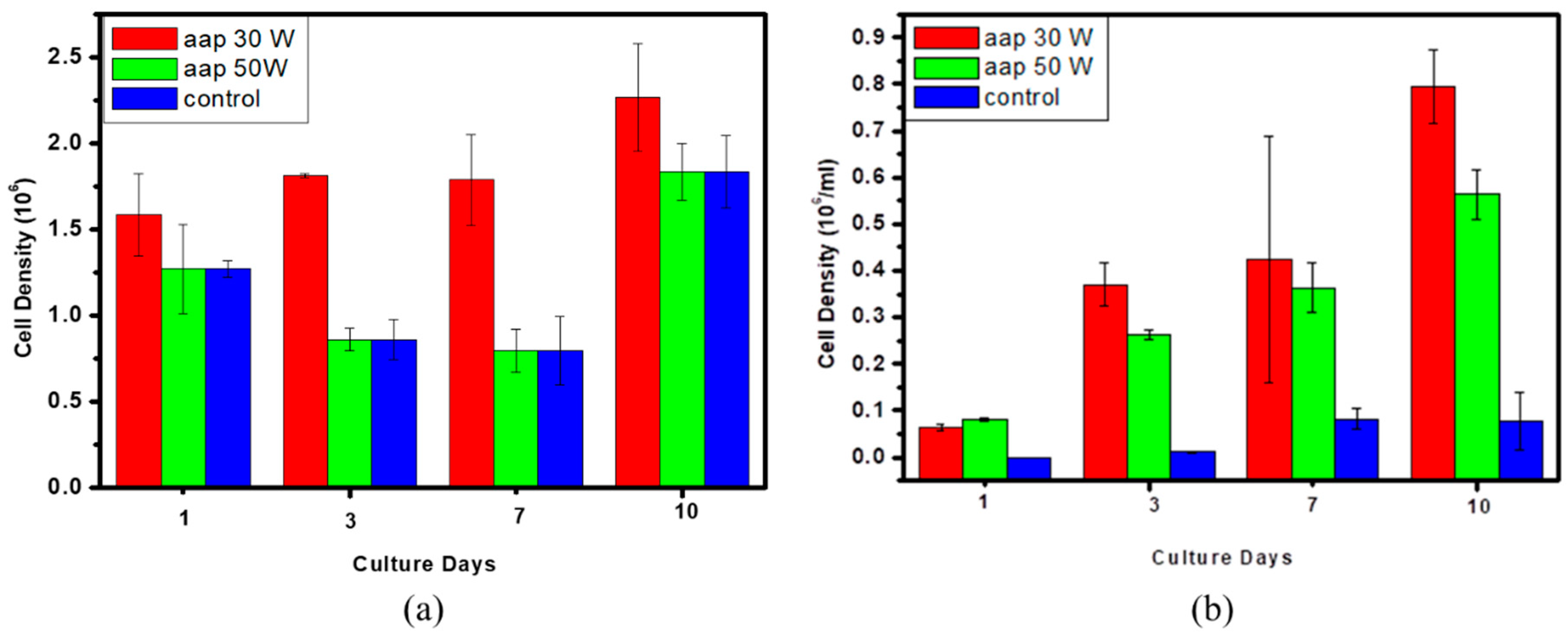
3.5. In Vitro Cytocompatibility Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, J.; Shive, M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Holderegger, C.; Schmidlin, P.; Weber, F.; Mohn, D. Preclinical in vivo Performance of Novel Biodegradable, Electrospun Poly(lactic acid) and Poly(lactic-co-glycolic acid) Nanocomposites: A Review. Materials 2015, 8, 4912–4931. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.; Claes, L. In vitro biocompatibility of bioresorbable polymers: Poly(L, DL-lactide) and poly(L-lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: A factorial design study. Int. J. Pharm. 2002, 238, 77–92. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, Z.; Yu, T.; Chen, X.; Jing, X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Inagaki, N. Surface modification of poly(vinylidene fluoride) film by remote Ar, H2, and O2 plasmas. Polymer 2003, 44, 1569–1575. [Google Scholar] [CrossRef]
- Mattioli, S.; Kenny, J.; Armentano, I. Plasma surface modification of porous PLLA films: Analysis of surface properties and in vitro hydrolytic degradation. J. Appl. Polym. Sci. 2012, 125, E239–E247. [Google Scholar] [CrossRef]
- Shi, T.; Shao, M.; Zhang, H.; Yang, Q.; Shen, X. Surface modification of porous poly(tetrafluoroethylene) film via cold plasma treatment. Appl. Surf. Sci. 2011, 258, 1474–1479. [Google Scholar] [CrossRef]
- Safinia, L.; Datan, N.; Höhse, M.; Mantalaris, A.; Bismarck, A. Towards a methodology for the effective surface modification of porous polymer scaffolds. Biomaterials 2005, 26, 7537–7547. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of Biomacromolecules onto Aminolyzed Poly(L-lactic acid) toward Acceleration of Endothelium Regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Legendre, J.; Créac’hcadec, R.; Gilbert, F.; Jacquet, D. A new method to measure the adhesion capability of the metallic surface under shear loading using a modified Arcan test. J. Adhes. 2017, 94, 1017–1035. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Liu, X.; Yeung, K.; Wu, S. Construction of poly (vinyl alcohol)/poly (lactide-glycolide acid)/vancomycin nanoparticles on titanium for enhancing the surface self-antibacterial activity and cytocompatibility. Coll. Surfaces B Biointerfaces 2017, 151, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Uchida, E.; Uyama, Y.; Ikada, Y. Surface graft polymerization of ionic monomers onto poly(ethylene terephthalate) by UV-irradiation without degassing. J. Appl. Polym. Sci. 1993, 47, 417–424. [Google Scholar] [CrossRef]
- Liao, S.; Chen, K.; Chen, W.; Chou, C.; Wai, K. Surface Graft Polymerization of Acrylamide onto Plasma Activated Nylon Microfiber Artificial Leather for Improving Dyeing Properties. Int. J. Chem. Eng. Appl. 2013, 78–81. [Google Scholar] [CrossRef][Green Version]
- Chen, K.; Chang, S.; Feng, C.; Lin, W.; Liao, S. Plasma Deposition and UV Light Induced Surface Grafting Polymerization of NIPAAm on Stainless Steel for Enhancing Corrosion Resistance and Its Drug Delivery Property. Polymers 2018, 10, 1009. [Google Scholar] [CrossRef]
- Chen, K.; Ku, Y.; Lin, H.; Yan, T.; Sheu, D.; Chen, T. Surface grafting polymerization of N-vinyl-2-pyrrolidone onto a poly(ethylene terephthalate) nonwoven by plasma pretreatment and its antibacterial activities. J. Appl. Polym. Sci. 2006, 100, 803–809. [Google Scholar] [CrossRef]
- Guex, A.; Frobert, A.; Valentin, J.; Fortunato, G.; Hegemann, D.; Cook, S.; Carrel, T.P.; Tevaearai, H.T.; Giraud, M.N. Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization. Acta Biomater. 2014, 10, 2996–3006. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Lucarini, G.; Virgili, L.; Biagini, G.; Detomaso, L.; Favia, P.; D’Agostino, R.; Gristina, R.; Gigante, A.; Bevilacqua, C. Mesenchymal Stem Cells on Plasma-Deposited Acrylic Acid Coatings: An In Vitro Investigation to Improve Biomaterial Performance in Bone Reconstruction. J. Bioact. Compat. Polym. 2005, 20, 343–360. [Google Scholar] [CrossRef]
- Siow, K.; Britcher, L.; Kumar, S.; Griesser, H. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Kim, S.; Pham, V.; Kim, C. Cell adhesion to cathodic arc plasma deposited CrAlSiN thin films. Appl. Surf. Sci. 2012, 258, 7202–7206. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, L.; Chai, H.; Chen, H. Surface modification of poly(tetrafluoroethylene) films via plasma treatment and graft copolymerization of acrylic acid. Desalination 2006, 192, 271–279. [Google Scholar] [CrossRef]
- Dolci, L.; Quiroga, S.; Gherardi, M.; Laurita, R.; Liguori, A.; Sanibondi, P.; Fiorani, A.; Calzà, L.; Colombo, V.; Focarete, M.L. Carboxyl Surface Functionalization of Poly(L-lactic acid) Electrospun Nanofibers through Atmospheric Non-Thermal Plasma Affects Fibroblast Morphology. Plasma Process. Polym. 2013, 11, 203–213. [Google Scholar] [CrossRef]
- Chen, W.; Matthews, A.; Jones, F.; Chen, K. Immobilization of carboxylic acid groups on polymeric substrates by plasma-enhanced chemical vapor or atmospheric pressure plasma deposition of acetic acid. Thin Solid Films 2018, 666, 54–60. [Google Scholar] [CrossRef]
- Chen, W.; Matthews, A.; Jones, F.; Chen, K. Deposition of a stable and high concentration of carboxylic acid functional groups onto a silicon surface via a tailored remote atmospheric pressure plasma process. Surf. Coat. 2018, 336, 67–71. [Google Scholar] [CrossRef]
- Dhayal, M.; Cho, S. Leukemia cells interaction with plasma-polymerized acrylic acid coatings. Vacuum 2006, 80, 636–642. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Ikada, Y. Cell adhesion to plasma-treated polymer surfaces. Polymer 1993, 34, 2208–2212. [Google Scholar] [CrossRef]
- Zare, Y.; Shabani, I. Polymer/metal nanocomposites for biomedical applications. Mater. Sci. Eng. C 2016, 60, 195–203. [Google Scholar] [CrossRef]
- Moroishi, H.; Sonotaki, S.; Murakami, Y. PLA- and PLA/PLGA-Emulsion Composite Biomaterial Sheets for the Controllable Sustained Release of Hydrophilic Compounds. Materials 2018, 11, 2588. [Google Scholar] [CrossRef]
- Schmitt, S.; Trebatoski, D.; Krutty, J.; Xie, A.; Rollins, B.; Murphy, W.; Gopalan, P. Peptide Conjugation to a Polymer Coating via Native Chemical Ligation of Azlactones for Cell Culture. Biomacromolecules 2016, 17, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D. Testing polymeric materials for biomedical applications. Polym. Test. 2019, 73, A1. [Google Scholar] [CrossRef]
- Esposito, A.; Kamikawa, C.; Lucchesi, C.; Ferreira, B.; Duek, E. Benefits of oxygen and nitrogen plasma treatment in Vero cell affinity to poly(lactide-co-glycolide acid). Mater. Res. 2013, 16, 695–702. [Google Scholar] [CrossRef]
| Substrates | Control | Acetic Acid Plasma Treated |
|---|---|---|
| PP | 85.5 ± 6.9 | 12.1 ± 1.5 |
| PET | 67.5 ± 4.5 | 9.6 ± 2.3 |
| PLGA | 58.0 ± 4.5 | 28.8 ± 4.1 |
| Dried | Immerse in Water | Absorb Ratio (%) | |
|---|---|---|---|
| Untreated Weight (mg) | 9.2 | 9.2 | 0 |
| After Plasma treated 100 mTorr Weight (mg) | 9.5 | 47.5 | 400 |
| After Plasma treated 150 mTorr Weight (mg) | 10.2 | 50.9 | 46.9 |
| After Plasma treated 200 mTorr Weight (mg) | 9.1 | 46.9 | 420 |
| Atomic Mole Fraction % | |||
|---|---|---|---|
| C | O | O/C | |
| 10 W, 10 min | 54.35 | 45.65 | 0.84 |
| 30 W, 10 min | 68.85 | 31.15 | 0.45 |
| 50 W,10 min | 76.45 | 23.55 | 0.31 |
| Function Groups | 10 W, 10 min | 30 W, 10 min | 50 W, 10 min |
|---|---|---|---|
| C–C | 54.02% | 55.48% | 60.84% |
| C–O | 21.20% | 24.25% | 21.30% |
| C=O | 24.78% | 20.27% | 17.86% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.-C.; Chen, K.-S.; Chien, J.-L.; Chen, S.-C.; Lin, W.-L. Acetic-Acid Plasma-Polymerization on Polymeric Substrates for Biomedical Application. Nanomaterials 2019, 9, 941. https://doi.org/10.3390/nano9070941
Liao S-C, Chen K-S, Chien J-L, Chen S-C, Lin W-L. Acetic-Acid Plasma-Polymerization on Polymeric Substrates for Biomedical Application. Nanomaterials. 2019; 9(7):941. https://doi.org/10.3390/nano9070941
Chicago/Turabian StyleLiao, Shu-Chuan, Ko-Shao Chen, Jui-Lung Chien, Su-Chen Chen, and Win-Li Lin. 2019. "Acetic-Acid Plasma-Polymerization on Polymeric Substrates for Biomedical Application" Nanomaterials 9, no. 7: 941. https://doi.org/10.3390/nano9070941
APA StyleLiao, S.-C., Chen, K.-S., Chien, J.-L., Chen, S.-C., & Lin, W.-L. (2019). Acetic-Acid Plasma-Polymerization on Polymeric Substrates for Biomedical Application. Nanomaterials, 9(7), 941. https://doi.org/10.3390/nano9070941




