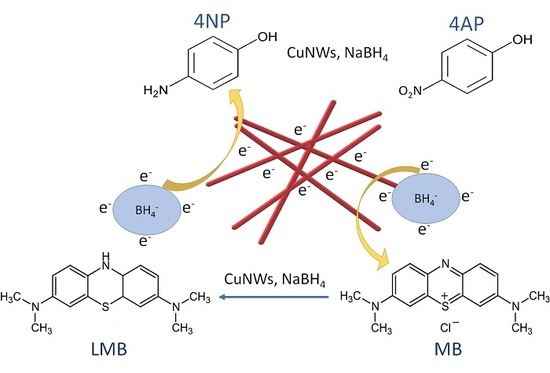
Rapid Catalytic Reduction of 4-Nitrophenol and Clock Reaction of Methylene Blue using Copper Nanowires
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CuNWs
2.3. Preparations of CuNWs Strips
2.4. Catalytic Reduction of 4-NP and Clock Reaction of MB Using CuNWs Strips
2.5. Retreatment of CuNWs Using GAA
2.6. Characterizations
3. Results and Discussion
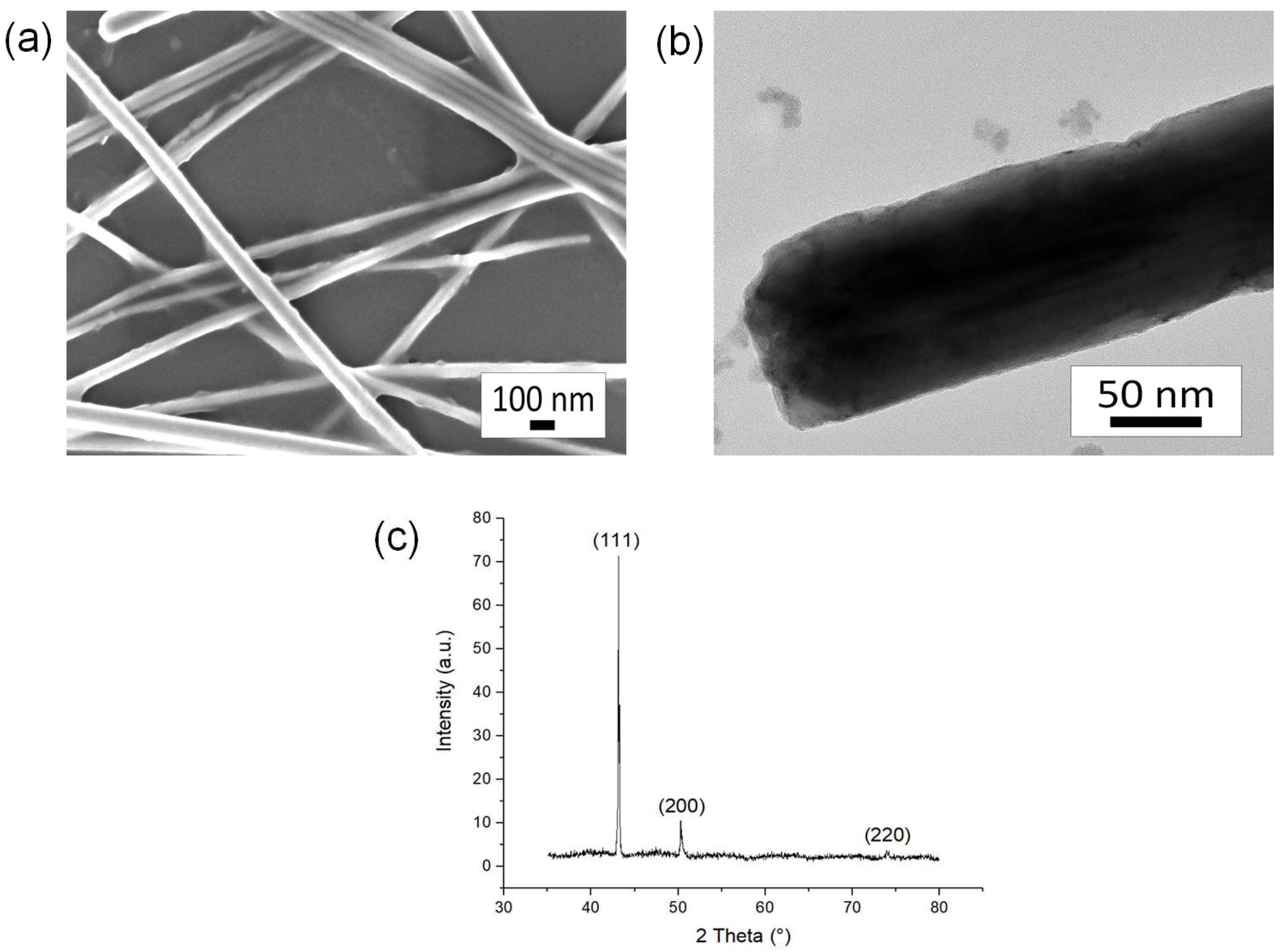
3.1. Characterization of CuNWs
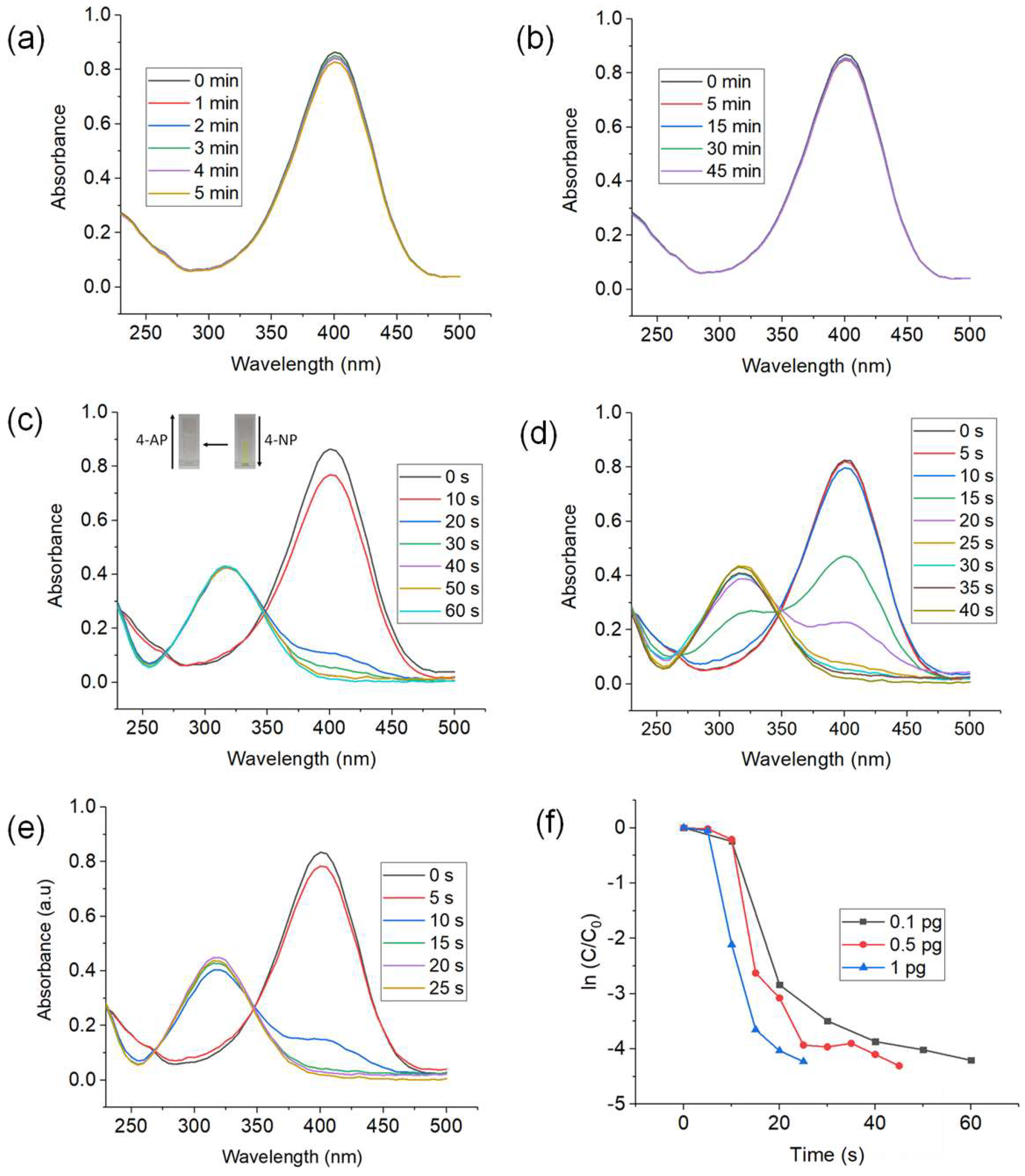
3.2. Catalytic Reduction of 4-NP Using CuNWs
3.2.1. Effect of CuNWs Content
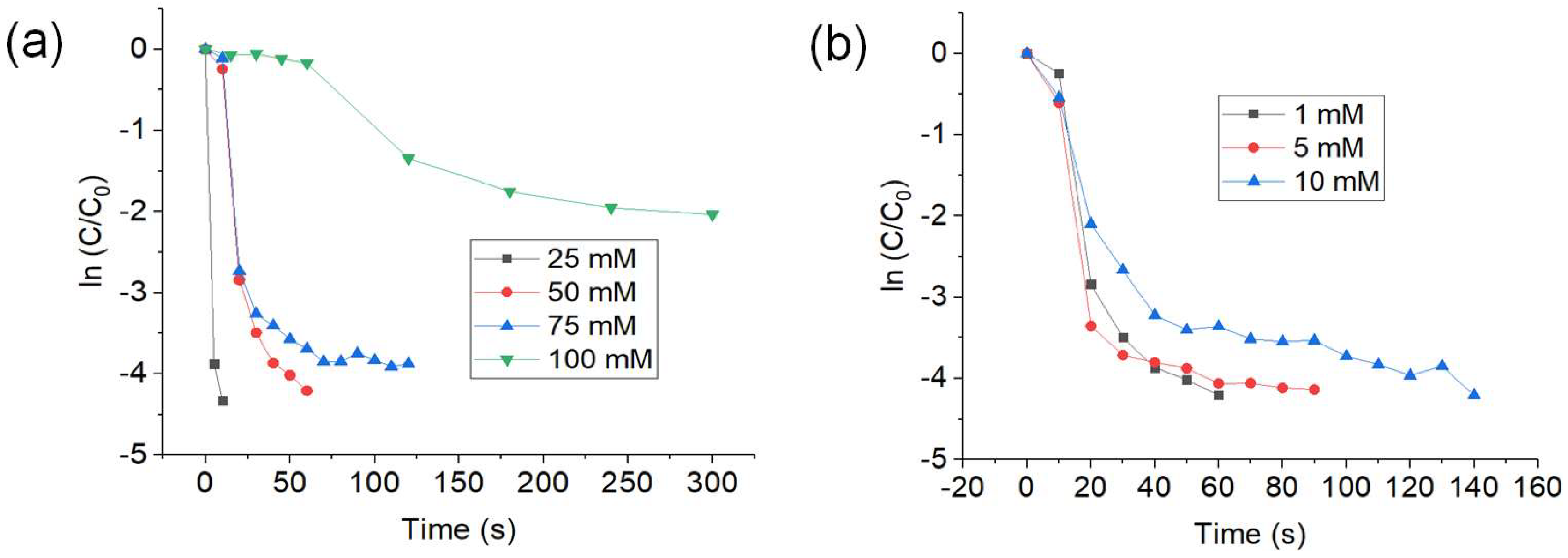
3.2.2. Effect of 4-NP and NaBH4 Concentration
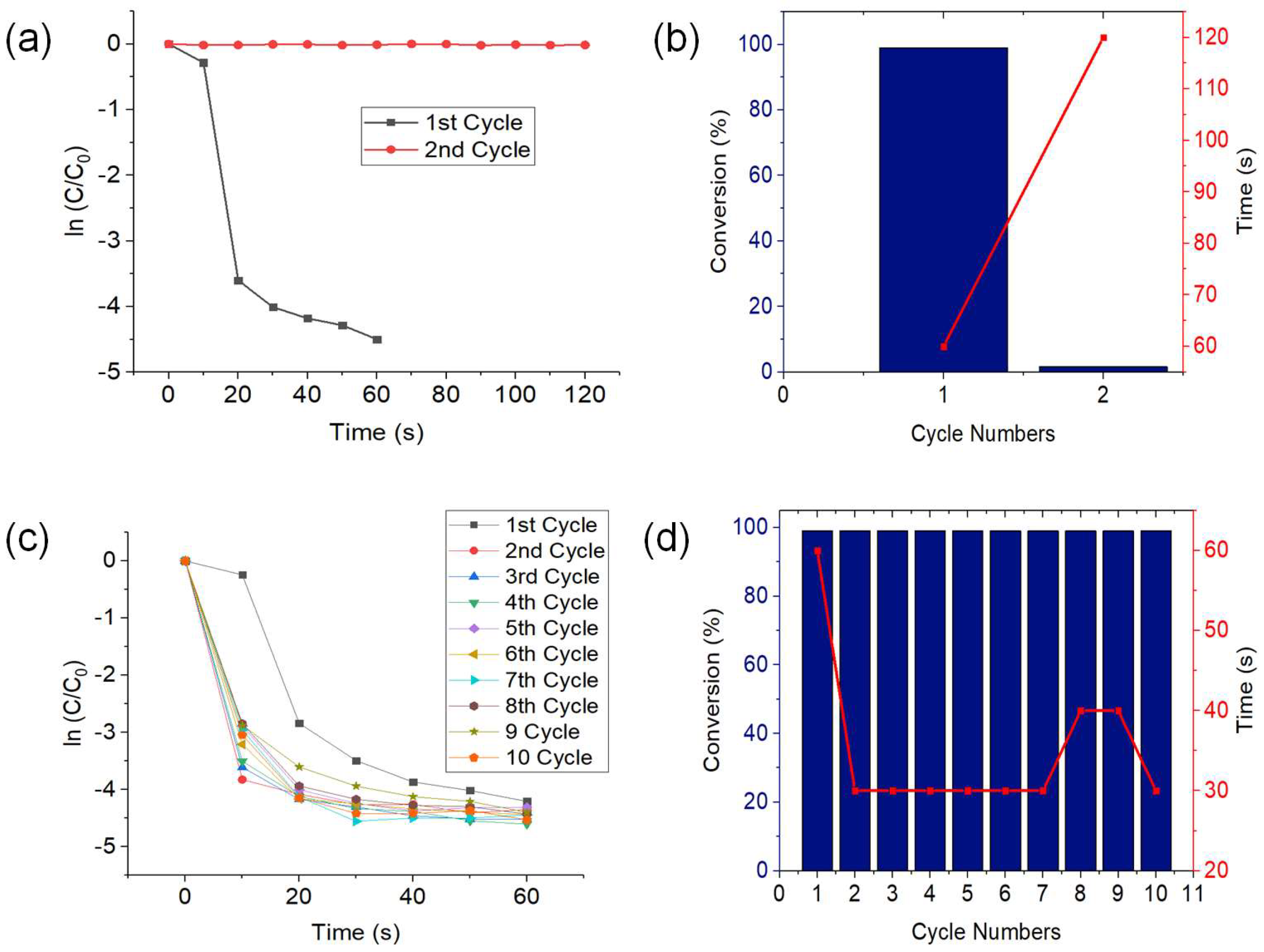
3.2.3. Recyclability Test and Retreatment of CuNWs with GAA
3.3. Clock Reaction of MB
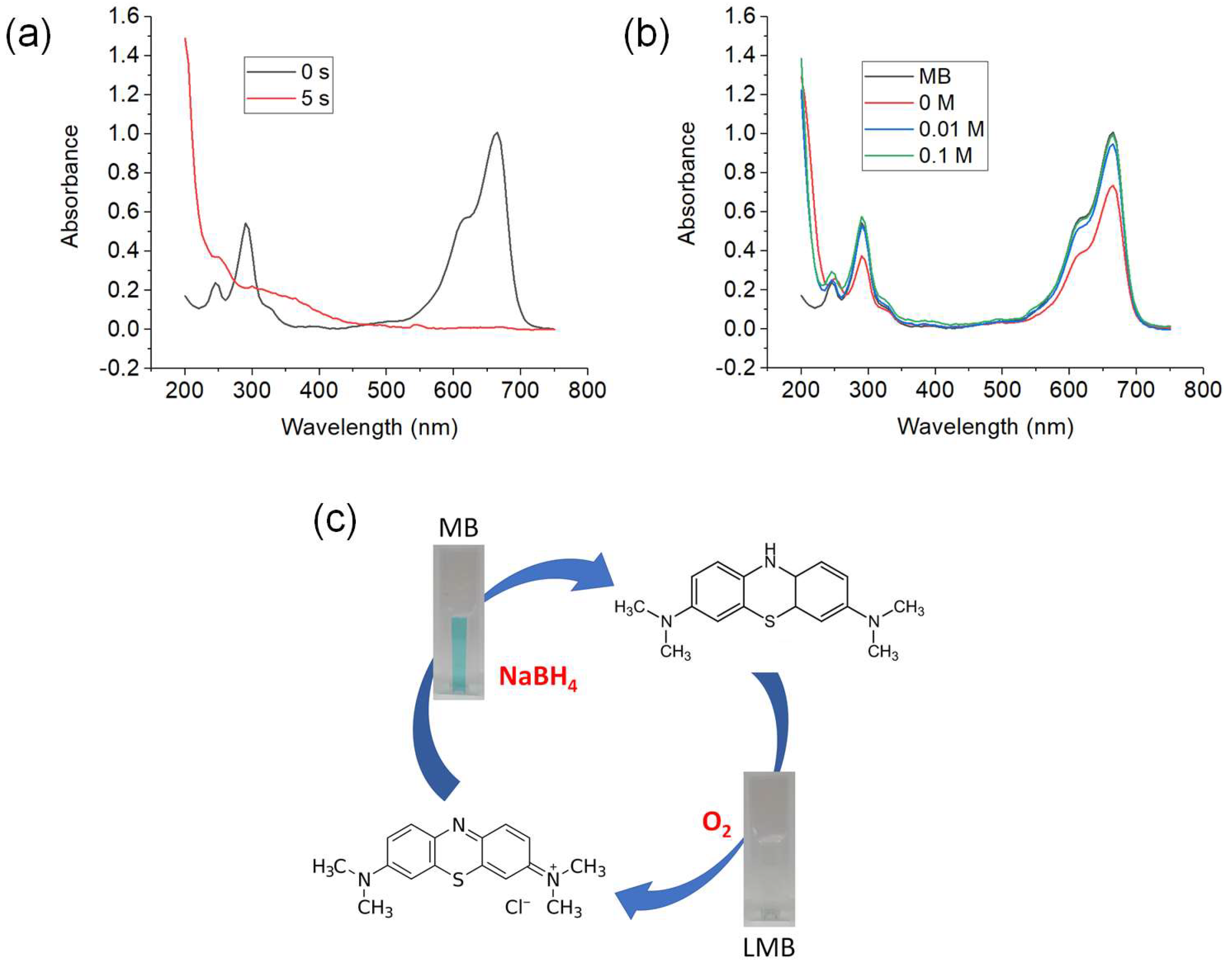
3.3.1. Decolorization of MB using Sodium Borohydride (NaBH4) as Reducing Agent
3.3.2. Comparison of Decolorization of MB using Different Reducing Agents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Chen, L.; Zheng, Y.; Tang, H.; Liu, Z. Determination of 4-nitrophenol in water using free-standing Cu nanowire electrode. Int. J. Electrochem. Sci. 2018, 13, 5698–5708. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Zeng, B.; Liu, L.; Yang, J. Facile synthesis of ultralong and thin copper nanowires and its application to high-performance flexible transparent conductive electrodes. Nano Res. Lett. 2018, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep. 2018, 8, 4589. [Google Scholar] [CrossRef]
- Rahman, M.; Heng, L.Y.; Futra, D.; Chiang, C.P.; Rashid, Z.A.; Ling, T.L. A highly sensitive electrochemical DNA biosensor from acrylic-gold nano-composite for the determination of arowana fish gender. Nanoscale Res. Lett. 2017, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Neoh, H.M.; Jamal, R. Effective immobilization of silver nanoparticles on a regenerated cellulose-chitosan composite membrane and its antibacterial activity. New J. Chem. 2017, 41, 5061–5065. [Google Scholar] [CrossRef]
- Abdul Halim, N.H.; Lee, Y.H.; Marugan, R.S.P.M.; Hashim, U. Mediatorless impedance studies with titanium dioxide conjugated gold nanoparticles for hydrogen peroxide detection. Biosensors 2017, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Chook, S.W.; Chia, C.H.; Kaco, H.; Zakaria, S.; Huang, N.M.; Neoh, H.M. Highly porous chitosan beads embedded with silver-graphene oxide nanocomposites for antibacterial application. Sains Malaysiana 2016, 45, 1663–1667. [Google Scholar]
- Mock, J.; Bobinger, M.; Bogner, C.; Lugli, P.; Becherer, M. Aqueous synthesis, degradation, and encapsulation of copper nanowires for transparent electrodes. Nanomaterials 2018, 8, 767. [Google Scholar] [CrossRef]
- Das, J.; Velusamy, P. Catalytic reduction of methylene blue using biogenic gold nanoparticles from Sesbania grandiflora L. J. Taiwan Inst. Chem. Eng. 2014, 45, 2280–2285. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Imran, M.; Khan, Z.U.H.; Tahir, K.; Khan, A.U.; Raza, M.; Khan, Q.; Yuan, Q. Size dependent catalytic activities of green synthesized gold nanoparticles and electro-catalytic oxidation of catechol on gold nanoparticles modified electrode. RSC Adv. 2015, 5, 99364–99377. [Google Scholar] [CrossRef]
- Mayousse, C.; Celle, C.; Carella, A.; Simonato, J.-P. Synthesis and purification of long copper nanowires. Application to high performance flexible transparent electrodes with and without PEDOT: PSS. Nano Res. 2014, 7, 315–324. [Google Scholar] [CrossRef]
- Du, C.; He, S.; Gao, X.; Chen, W. Hierarchical Cu@MnO2 core–shell nanowires: A nonprecious-metal catalyst with an excellent catalytic activity toward the reduction of 4-nitrophenol. ChemCatChem 2016, 8, 2885–2889. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Al-Sharif, M.S. Visible light assisted reduction of 4-nitrophenol to 4-aminophenol on Ag/TiO2 photocatalysts synthesized by hybrid templates. Appl. Catal. B Environ. 2013, 142, 432–441. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Catalytic reduction of p-nitrophenol by using platinum nanoparticles stabilised by guar gum. Carbohydr. Polym. 2014, 113, 525–531. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Soares, O.S.G.P.; Pinho, M.T.; Pereira, M.F.R.; Madeira, L.M. p-Nitrophenol degradation by heterogeneous Fenton’s oxidation over activated carbon-based catalysts. Appl. Catal. B Environ. 2017, 219, 109–122. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Z.; Fu, L.; Zhang, Y.; Yang, H.; Ouyang, J.; Chen, D. Silver nanoparticles assembled on modified sepiolite nanofibers for enhanced catalytic reduction of 4-nitrophenol. Appl. Clay Sci. 2018, 166, 166–173. [Google Scholar] [CrossRef]
- Deka, P.; Deka, R.C.; Bharali, P. In situ generated copper nanoparticle catalyzed reduction of 4-nitrophenol. New J. Chem. 2014, 38, 1789–1793. [Google Scholar] [CrossRef]
- Tan, S.T.; Umar, A.A.; Salleh, M.M. (001)-Faceted hexagonal ZnO nanoplate thin film synthesis and the heterogeneous catalytic reduction of 4-nitrophenol characterization. J. Alloys Compd. 2015, 650, 299–304. [Google Scholar] [CrossRef]
- Li, B.; Hao, Y.; Zhang, B.; Shao, X.; Hu, L. A multifunctional noble-metal-free catalyst of CuO/TiO2 hybrid nanofibers. Appl. Catal. A Gen. 2016, 531, 1–12. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.C.; Wang, X.; Wang, Z.; Liu, G.; Zhou, W.; Wen, L.; Li, Q.; Wang, X.; Chen, X.; et al. Facile synthesis of pentacle gold-copper alloy nanocrystals and their plasmonic and catalytic properties. Nat. Commun. 2014, 5, 4327. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, F.; Xu, L.; Yin, Z.; Song, X. Roughness-controlled copper nanowires and Cu nanowires-Ag heterostructures: Synthesis and their enhanced catalysis. J. Mater. Chem. A 2014, 2, 18583–18592. [Google Scholar] [CrossRef]
- Zhang, P.; Sui, Y.; Xiao, G.; Wang, Y.; Wang, C.; Liu, B.; Zou, G.; Zou, B. Facile fabrication of faceted copper nanocrystals with high catalytic activity for p-nitrophenol reduction. J. Mater. Chem. A 2013, 1, 1632–1638. [Google Scholar] [CrossRef]
- Lente, G.; Bazsa, G.; Fábián, I. What is and what isn’t a clock reaction? New J. Chem. 2007, 31, 1707. [Google Scholar] [CrossRef]
- Khan, A.A.; Kumar, M.; Khan, K.; Molla, A.; Hussain, S. Photoinduced oxygen prompted iron-iron oxide catalyzed clock reaction: Mimic of the blue bottle experiment. New J. Chem. 2017, 41, 6420–6426. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Faria, R.B. The chlorate-iodine clock reaction. J. Am. Chem. Soc. 2005, 127, 18022–18023. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, U.; Negron, A.; Jensen, K.F. A clock reaction based on molybdenum blue. J. Phys. Chem. A 2013, 117, 4343–4351. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Dutta, S.; Sarkar, S.; Sahoo, R.; Roy, A.; Pal, T. A facile synthesis of 1D nano structured selenium and Au decorated nano selenium: Catalysts for the clock reaction. RSC Adv. 2013, 3, 24313–24320. [Google Scholar] [CrossRef]
- Galagan, Y.; Su, W.F. Reversible photoreduction of methylene blue in acrylate media containing benzyl dimethyl ketal. J. Photochem. Photobiol. A Chem. 2008, 195, 378–383. [Google Scholar] [CrossRef]
- Dilgin, Y.; Nişli, G. Fluorimetric determination of ascorbic acid in vitamin c tablets using methylene blue. Chem. Pharm. Bull. 2005, 53, 1251–1254. [Google Scholar] [CrossRef]
- Wang, W.; Ye, Y.; Feng, J.; Chi, M.; Guo, J.; Yin, Y. Enhanced photoreversible color switching of redox dyes catalyzed by barium-doped TiO2 nanocrystals. Angew. Chemie Int. Ed. 2015, 54, 1321–1326. [Google Scholar] [CrossRef]
- Lee, S.K.; Mills, A.; Lepre, A. An intelligence ink for oxygen. Chem. Commun. 2004, 1912–1913. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ye, M.; He, L.; Yin, Y. Nanocrystalline TiO2-catalyzed photoreversible color switching. Nano Lett. 2014, 14, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, S.; Ji, Q.; Zhang, J.; Zhang, Z.; Wang, Z. Ultrathin Cu7S4 nanosheets constructed hierarchical hollow cubic cages: One-step synthesis based on Kirkendall effect and catalysis property. J. Mater. Chem. A 2014, 2, 4574–4579. [Google Scholar] [CrossRef]
- Pal, J.; Ganguly, M.; Dutta, S.; Mondal, C.; Negishi, Y.; Pal, T. Hierarchical Au-CuO nanocomposite from redox transformation reaction for surface enhanced Raman scattering and clock reaction. CrystEngComm 2014, 16, 883–893. [Google Scholar] [CrossRef]
- Ahmadi, R.; Jafarzadeh, M.; Khodaei, M.M.; Adnan, R. Encapsulation of Ag nanoparticles in magnetically modified silica nanostructures for reduction of 4-nitrophenol. Monatshefte fur Chemie 2017, 148, 1423–1431. [Google Scholar] [CrossRef]
- Qian, F.; Lan, P.C.; Olson, T.; Zhu, C.; Duoss, E.B.; Spadaccini, C.M.; Han, T.Y.-J. Multiphase separation of copper nanowires. Chem. Commun. 2016, 52, 11627–11630. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Ruan, H.; Wang, R.; Luo, Y.; Liu, H.; Han, T.; Yang, L. Study on synthesis and growth mechanism of copper nanowires via a facile oleylamine-mediated process. J. Mater. Sci. Mater. Electron. 2016, 27, 9405–9409. [Google Scholar] [CrossRef]
- Tan, M.; Balela, M.D. One-pot synthesis of high aspect ratio copper nanowires in aqueous solution. Adv. Mater. Res. 2015, 1119, 34–37. [Google Scholar] [CrossRef]
- Wang, R.; Ruan, H. Synthesis of copper nanowires and its application to flexible transparent electrode. J. Alloys Compd. 2016, 656, 936–943. [Google Scholar] [CrossRef]
- Chavez, K.L.; Hess, D.W. A novel method of etching copper oxide using acetic acid. J. Electrochem. Soc. 2001, 148, G640–G643. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Kou, Q.; Liu, Y.; Han, D.; Wang, D.; Sun, Y.; Zhang, Y.; Wang, Y.; Lu, Z.; et al. Enhanced catalytic reduction of 4-nitrophenol driven by Fe3O4-Au magnetic nanocomposite interface engineering: From facile preparation to recyclable application. Nanomaterials 2018, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Pal, A.; Kundu, S.; Basu, S.; Pal, T. Photochemical green synthesis of calcium-alginate-stabilized Ag and Au nanoparticles and their catalytic application to 4-nitrophenol reduction. Langmuir 2009, 26, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lan, J.Y.; Liu, J.; Yu, J.; Luo, Z.; Wang, W.; Sun, L. Synthesis of gold nanoparticles on rice husk silica for catalysis applications. Ind. Eng. Chem. Res. 2015, 54, 5656–5663. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, L.; Xiao, C.; Li, L.; Liao, F. Single metal of silver nanoparticles in the microemulsion for recyclable catalysis of 4-nitrophenol reduction. J. Adv. Nanomater. 2017, 2, 31–40. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Yin, Z.; Song, X. Synthesis of copper submicro/nanoplates with high stability and their recyclable superior catalytic activity towards 4-nitrophenol reduction. J. Mater. Chem. A 2013, 1, 12361–12370. [Google Scholar] [CrossRef]
- Gao, S.; Jia, X.; Yang, J.; Wei, X. Hierarchically micro/nanostructured porous metallic copper: Convenient growth and superhydrophilic and catalytic performance. J. Mater. Chem. 2012, 22, 21733–21739. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Hydroxyapatite-supported cobalt(0) nanoclusters as efficient and cost-effective catalyst for hydrogen generation from the hydrolysis of both sodium borohydride and ammonia-borane. Catal. Today 2012, 183, 17–25. [Google Scholar] [CrossRef]
- Seoudi, R.; Al-Marhaby, F.A. Synthesis, characterization and photocatalytic application of different sizes of gold nanoparticles on 4-nitrophenol. World J. Nano Sci. Eng. 2016, 6, 120. [Google Scholar] [CrossRef]
- Deka, P.; Sarmah, P.; Deka, R.C.; Bharali, P. Hetero-nanostructured Ni/α-Mn2O3 as highly active catalyst for aqueous phase reduction reactions. ChemistrySelect 2016, 1, 4726–4735. [Google Scholar] [CrossRef]
- Guerrero-Araque, D.; Acevedo-Peña, P.; Ramírez-Ortega, D.; Gómez, R. Improving photocatalytic reduction of 4-nitrophenol over ZrO2-TiO2 by synergistic interaction between methanol and sulfite ions. New J. Chem. 2017, 41, 12655–12663. [Google Scholar] [CrossRef]
- Khalavka, Y.; Becker, J.; Sönnichsen, C. Synthesis of rod-shaped gold nanorattles with improved plasmon sensitivity and catalytic activity. J. Am. Chem. Soc. 2009, 131, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Antonels, N.C.; Meijboom, R. Preparation of well-defined dendrimer encapsulated ruthenium nanoparticles and their evaluation in the reduction of 4-nitrophenol according to the Langmuir-Hinshelwood approach. Langmuir 2013, 29, 13433–13442. [Google Scholar] [CrossRef] [PubMed]
- Alegria, E.; Ribeiro, A.; Mendes, M.; Ferraria, A.; do Rego, A.; Pombeiro, A. Effect of phenolic compounds on the synthesis of gold nanoparticles and its catalytic activity in the reduction of nitro compounds. Nanomaterials 2018, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Ranchani, A.A.J.; Parthasarathy, V.; Devi, A.A.; Meenarathi, B.; Anbarasan, R. Synthesis, characterization and catalytic activity of nanosized Ni complexed aminoclay. Appl. Nanosci. 2017, 7, 577–588. [Google Scholar] [CrossRef]
- Akhondi, M.; Jamalizadeh, E. Fabrication of silver-modified halloysite nanotubes and their catalytic performance in rhodamine 6G and methyl orange reduction. Acta Chim. Slov. 2019, 66, 136–144. [Google Scholar] [CrossRef]
- Priya, D.B.; Asharani, I.V. Catalytic reduction in 4-nitrophenol using Actinodaphne madraspatana Bedd leaves-mediated palladium nanoparticles. IET Nanobiotechnol. 2017, 12, 116–126. [Google Scholar] [CrossRef]
- Kalekar, A.M.; Sharma, K.K.K.; Luwang, M.N.; Sharma, G.K. Catalytic activity of bare and porous palladium nanostructures in the reduction of 4-nitrophenol. RSC Adv. 2016, 6, 11911–11920. [Google Scholar] [CrossRef]
- Vilar-Vidal, N.; Rivas, J.; Lopez-Quintela, M. Size dependent catalytic activity of reusable subnanometer copper(0) clusters. ACS Catal. 2012, 2, 1693–1697. [Google Scholar] [CrossRef]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Pal, A.; Mondal, C.; Pal, T. Methylene blue-Cu2O reaction made easy in acidic medium. J. Phys. Chem. C 2012, 116, 25741–25747. [Google Scholar] [CrossRef]
- Du, S.; Liao, Z.; Qin, Z.; Zuo, F.; Li, X. Polydopamine microparticles as redox mediators for catalytic reduction of methylene blue and rhodamine B. Catal. Commun. 2015, 72, 86–90. [Google Scholar] [CrossRef]
- Saad, A.; Snoussi, Y.; Abderrabba, M.; Chehimi, M.M. Ligand-modified mesoporous silica SBA-15/silver hybrids for the catalyzed reduction of methylene blue. RSC Adv. 2016, 6, 57672–57682. [Google Scholar] [CrossRef]
- Das, B.; Sharma, M.; Sarmah, J.C.; Bania, K.K. Rapid reduction of dye pollutants and hexavalent chromium by silver-sulphur oxido-vanadium cluster. J. Environ. Chem. Eng. 2017, 5, 4212–4219. [Google Scholar] [CrossRef]
- Peacock, A.J. Oxygen at high altitude. Bmj 1998, 317, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Zhou, X.; Wang, X.; Liang, K.; Yang, Z.K.; Shen, C.C.; Imran, M.; Sahar, S.; Xu, A.W. Hydrogenation/oxidation induced efficient reversible color switching between methylene blue and leuco-methylene blue. RSC Adv. 2017, 7, 30080–30085. [Google Scholar] [CrossRef]
- Yang, D.; Guo, G.; Hu, J.; Wang, C.; Jiang, D. Hydrothermal treatment to prepare hydroxyl group modified multi-walled carbon nanotubes. J. Mater. Chem. 2008, 18, 350–354. [Google Scholar] [CrossRef]
| Catalyst | kapp (s−1) | Activity Factor, K (s−1 mg−1) | Reference |
|---|---|---|---|
| CuNWs (0.1 pg) | 0.076 | 7.6 × 108 | This study |
| Cu@MnO2 | 0.01142 | 0.571 | [12] |
| CuNPs (12.5 mg) | 0.0016 | 0.00013 | [17] |
| CuNWs | 0.0042 | 0.046 | [21] |
| CuNW–Ag | 0.0067 | 0.074 | [21] |
| CuCubes (9.5 nm) | 0.0101 | 0.105 | [22] |
| CuNanoplate | 0.0095 | 0.136 | [46] |
| Porous Cu microsphere | 0.0043 | 0.072 | [47] |
| Reducing Agent | Time of Reaction (s) | Percentage of Decolorization of MB (%) |
|---|---|---|
| Glucose | 1800 | 35 |
| AA | 1800 | 43 |
| NaBH4 | 5 | 99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimi, A.S.; Nohan, M.A.N.M.; Chin, S.X.; Zakaria, S.; Chia, C.H. Rapid Catalytic Reduction of 4-Nitrophenol and Clock Reaction of Methylene Blue using Copper Nanowires. Nanomaterials 2019, 9, 936. https://doi.org/10.3390/nano9070936
Hashimi AS, Nohan MANM, Chin SX, Zakaria S, Chia CH. Rapid Catalytic Reduction of 4-Nitrophenol and Clock Reaction of Methylene Blue using Copper Nanowires. Nanomaterials. 2019; 9(7):936. https://doi.org/10.3390/nano9070936
Chicago/Turabian StyleHashimi, Aina Shasha, Muhammad Amirul Nazhif Mohd Nohan, Siew Xian Chin, Sarani Zakaria, and Chin Hua Chia. 2019. "Rapid Catalytic Reduction of 4-Nitrophenol and Clock Reaction of Methylene Blue using Copper Nanowires" Nanomaterials 9, no. 7: 936. https://doi.org/10.3390/nano9070936
APA StyleHashimi, A. S., Nohan, M. A. N. M., Chin, S. X., Zakaria, S., & Chia, C. H. (2019). Rapid Catalytic Reduction of 4-Nitrophenol and Clock Reaction of Methylene Blue using Copper Nanowires. Nanomaterials, 9(7), 936. https://doi.org/10.3390/nano9070936