Efficient Ni/Au Mesh Transparent Electrodes for ITO-Free Planar Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Metal Mesh Preparation
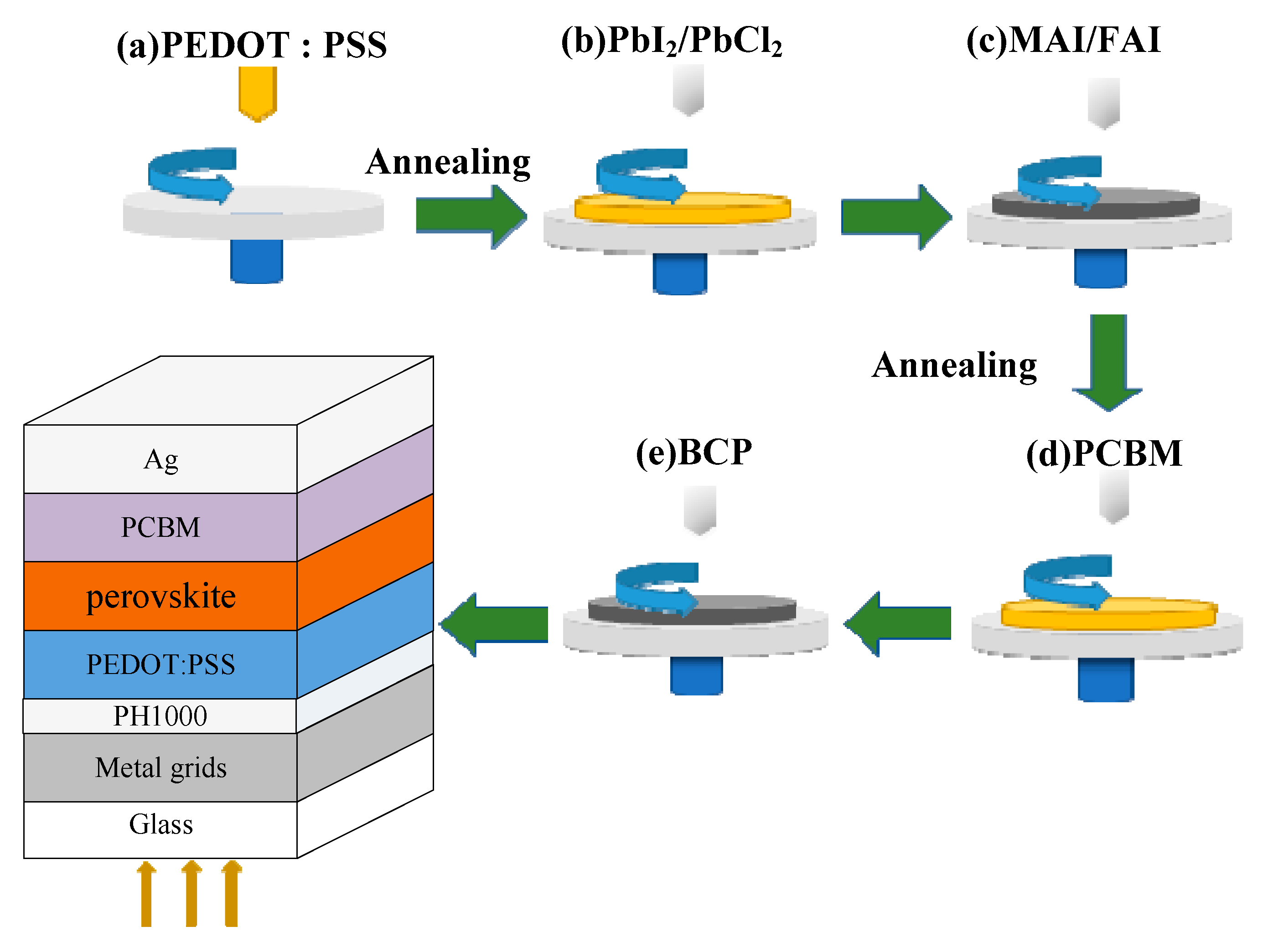
2.3. PSCs Preparation and Characterization
3. Results and Discussion
3.1. Optimization of Ni/Au Mesh Transparent Electrode
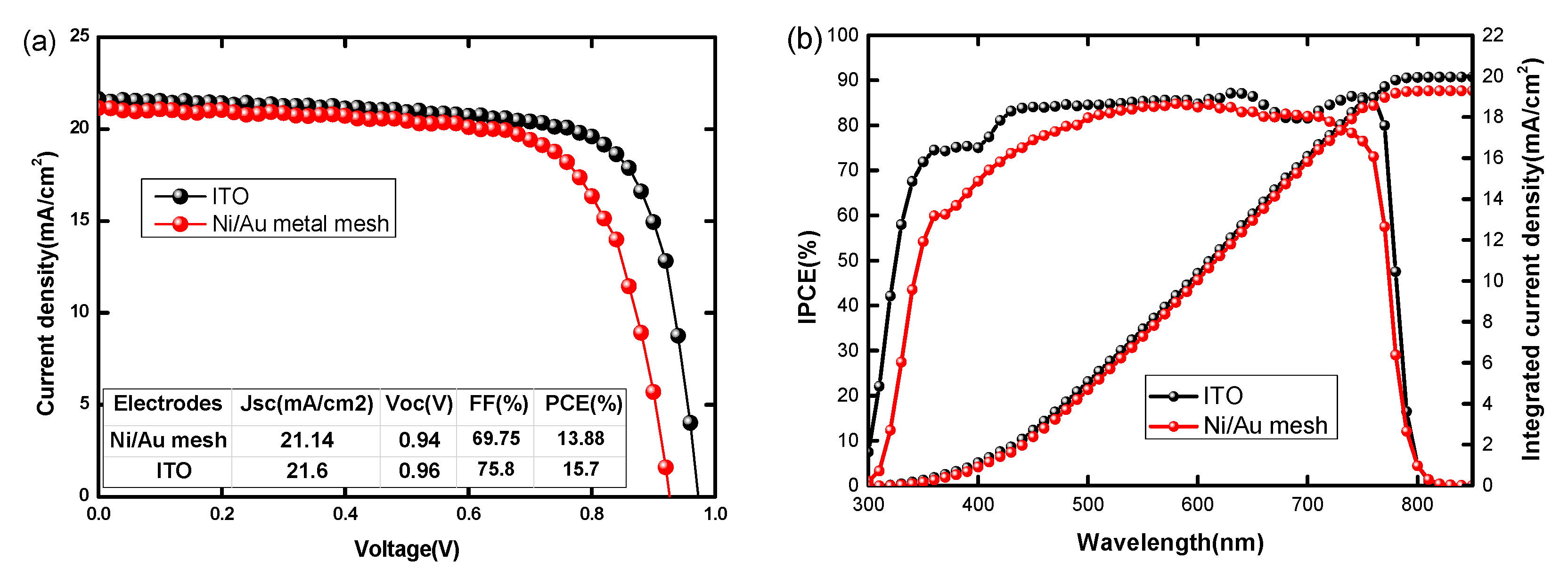
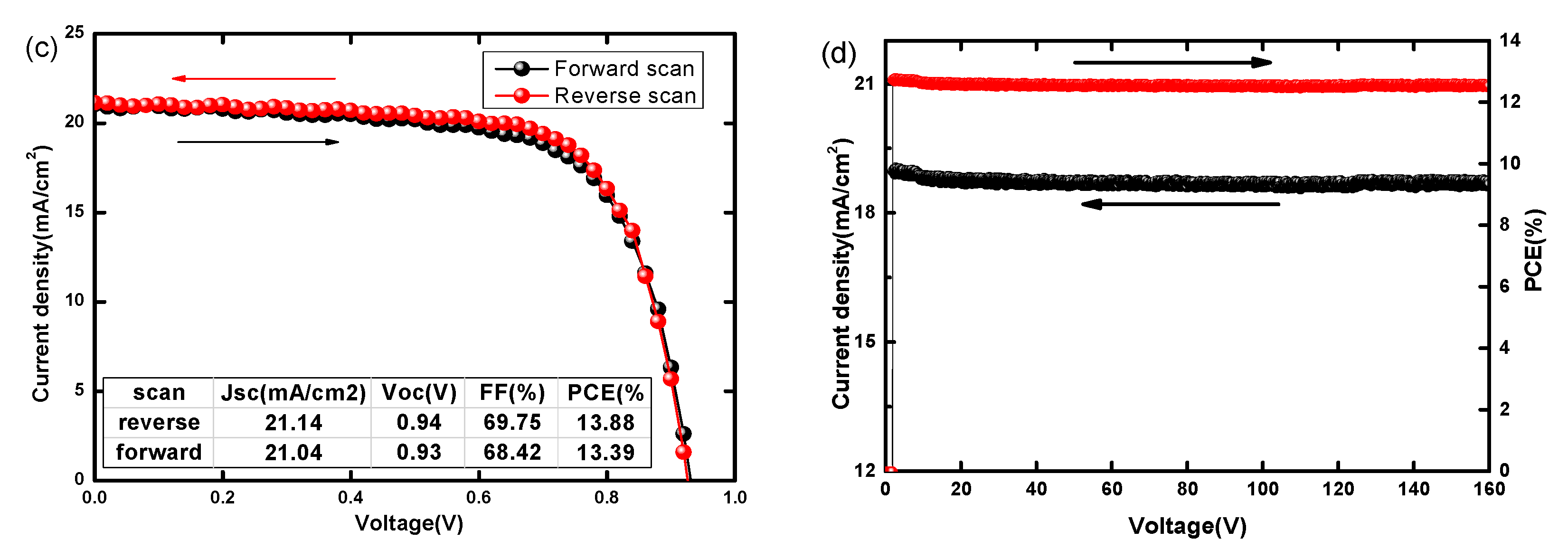
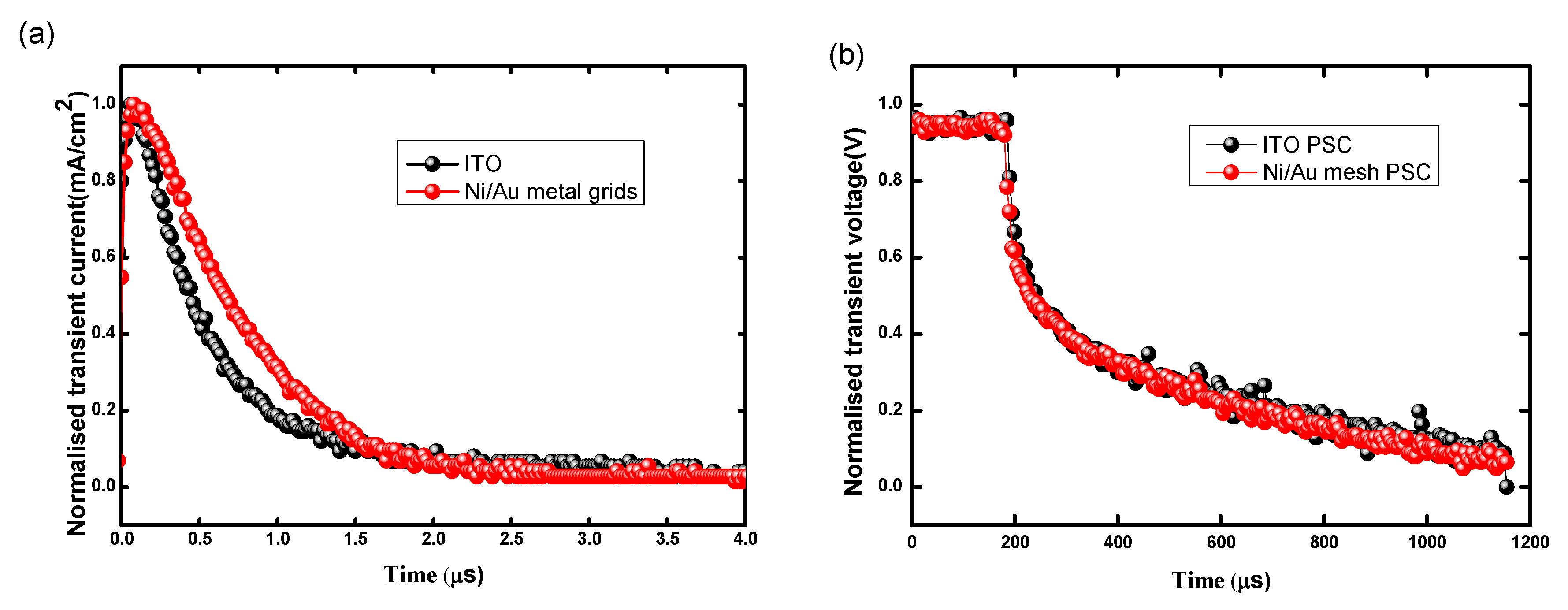
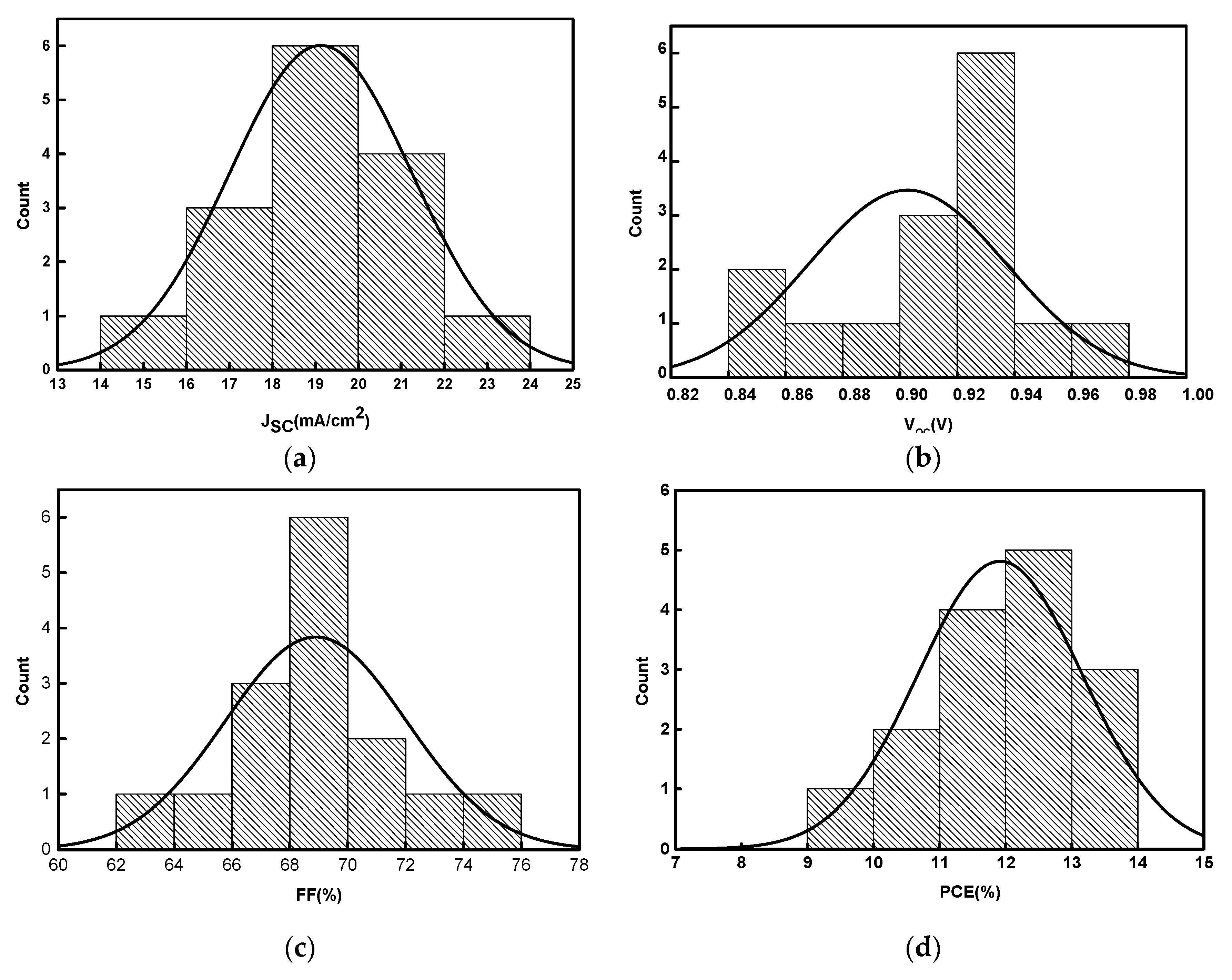
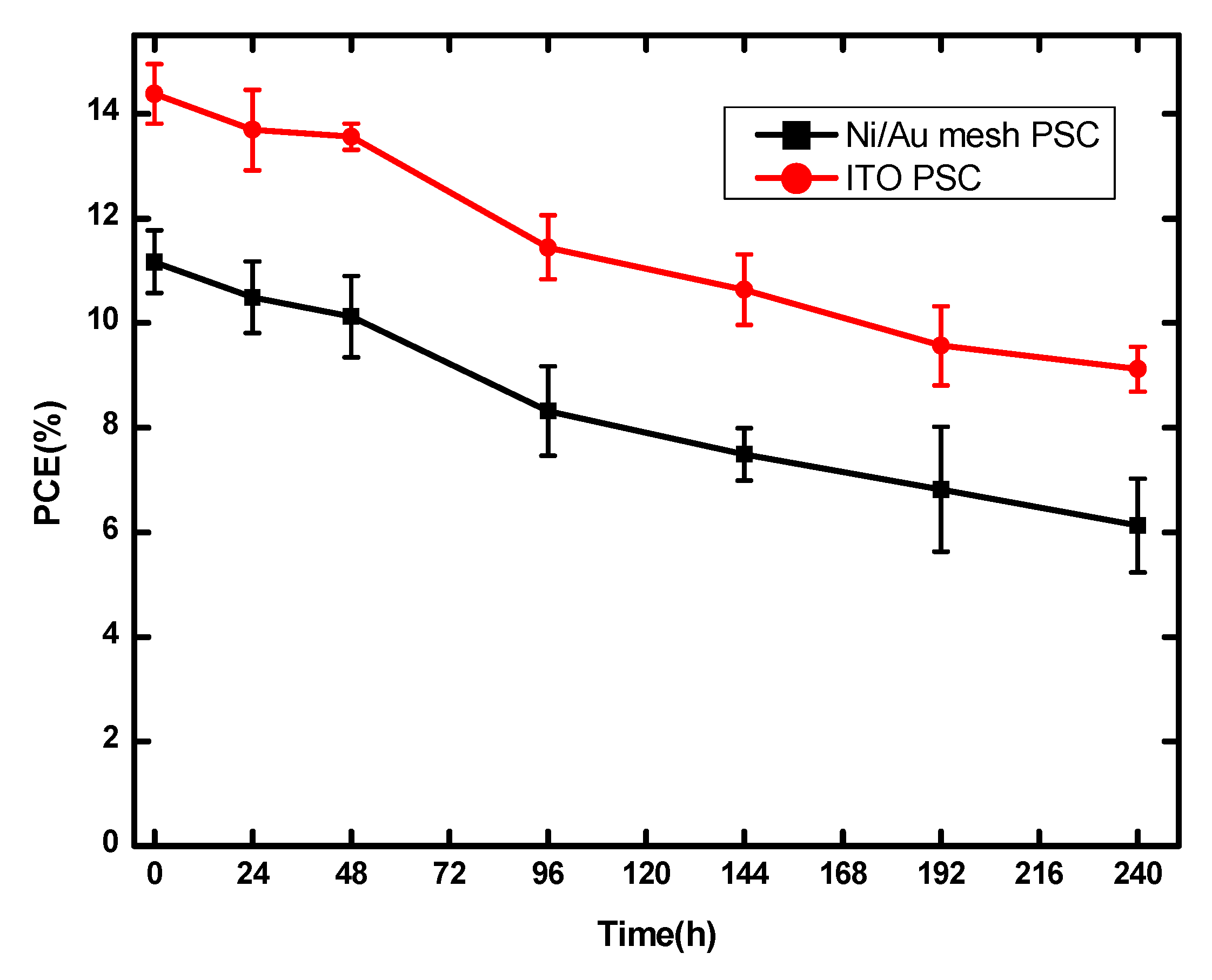
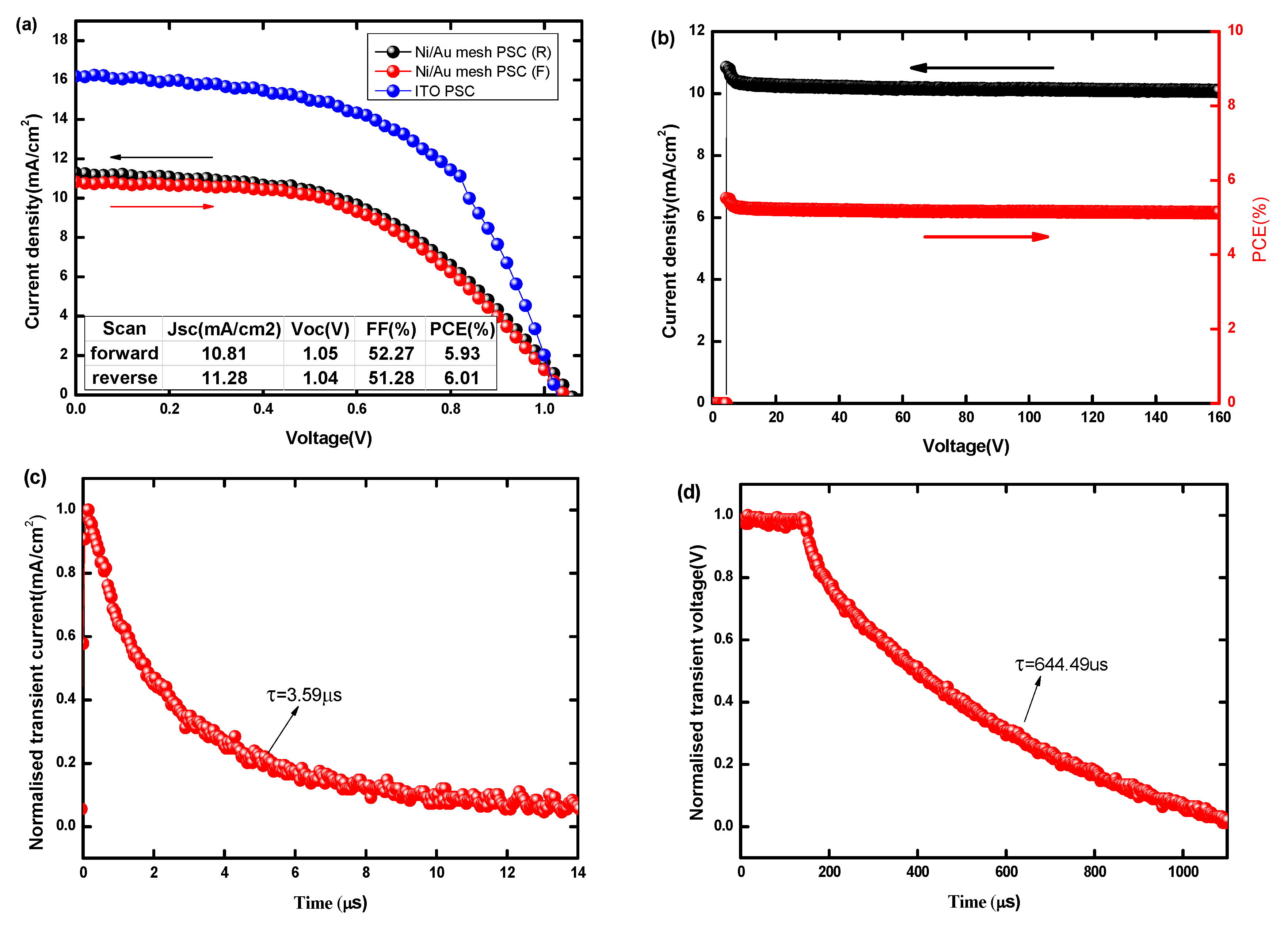
3.2. Performance of PSCs with Optimal Ni/Au Metal Mesh
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, in press. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Warta, W.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.H. Solar cell efficiency tables (version 50). Prog. Photovolt. Res. Appl. 2017, 25, 668–676. [Google Scholar] [CrossRef]
- Liu, Z.; Chang, J.; Lin, Z.; Zhou, L.; Yang, Z.; Chen, D.; Zhang, C.; Liu, S.; Hao, Y. High-performance planar perovskite solar cells using low temperature, solution–combustion-based nickel oxide hole transporting layer with efficiency exceeding 20%. Adv. Energy Mater. 2018, 8, 1703432. [Google Scholar] [CrossRef]
- Pang, S.; Chen, D.; Zhang, C.; Chang, J.; Lin, Z.; Yang, H.; Sun, X.; Mo, J.; Xi, H.; Han, G.; et al. Efficient bifacial semitransparent perovskite solar cells with silver thin film electrode. Sol. Energy Mater. Sol. Cells 2017, 170, 278–286. [Google Scholar] [CrossRef]
- Li, Y.; Meng, L.; Yang, Y.; Xu, G.; Hong, Z.; Chen, Q.; You, J.; Li, G.; Yang, Y.; Li, Y. High-efficiency robust perovskite solar cells on ultrathin flexible substrates. Nat. Commun. 2016, 7, 10214. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Yang, Z.; Berthold, E.E.S.; Chen, W. Recent advances of flexible perovskite solar cells. J. Energy Chem. 2018, 27, 673–689. [Google Scholar] [CrossRef]
- Fu, F.; Feurer, T.; Jäger, T.; Avancini, E.; Bissig, B.; Yoon, S.; Buecheler, S.; Tiwari, A.N. Low-temperature-processed efficient semi-transparent planar perovskite solar cells for bifacial and tandem applications. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 3–8. [Google Scholar] [CrossRef]
- Cardinaletti, I.; Vangerven, T.; Nagels, S.; Cornelissen, R.; Schreurs, D.; Hruby, J.; Vodnik, J.; Devisscher, D.; Kesters, J.; D’Haen, J.; et al. Organic and perovskite solar cells for space applications. Sol. Energy Mater. Sol. Cells 2018, 182, 121–127. [Google Scholar] [CrossRef]
- Heo, J.H.; Lee, D.S.; Shin, D.H.; Im, S.H. Recent advancements in and perspectives on flexible hybrid perovskite solar cells. J. Mater. Chem. A 2019, 7, 888–900. [Google Scholar] [CrossRef]
- Loh, K.P.; Tong, S.W.; Wu, J. Graphene and graphene-like molecules: Prospects in solar cells. J. Am. Chem. Soc. 2016, 138, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Jiang, Y.; Tang, C.W.; Kwok, H.S. Grid optimization of large-area OLED lighting panel electrodes. J. Disp. Technol. 2016, 12, 605–609. [Google Scholar] [CrossRef]
- Dou, B.; Whitaker, J.B.; Bruening, K.; Moore, D.T.; Wheeler, L.M.; Ryter, J.; Breslin, N.J.; Berry, J.J.; Garner, S.M.; Barnes, F.S.; et al. Roll-to-roll printing of perovskite solar cells. ACS Energy Lett. 2018, 3, 2558–2565. [Google Scholar] [CrossRef]
- Han, T.H.; Lee, Y.; Choi, M.R.; Woo, S.H.; Bae, S.H.; Hong, B.H.; Ahn, J.H.; Lee, T.W. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar] [CrossRef]
- Kang, J.; Jang, Y.; Kim, Y.; Cho, S.H.; Suhr, J.; Hong, B.H.; Choi, J.B.; Byun, D. An Ag-grid/graphene hybrid structure for large-scale, transparent, flexible heaters. Nanoscale 2015, 7, 6567–6573. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Venugopal, A.; Pirkle, A.; McDonnell, S.; Hinojos, D.; Magnuson, C.W.; Ruoff, R.S.; Colombo, L.; Wallace, R.M.; Vogel, E.M. Reducing extrinsic performance-limiting factors in graphene grown by chemical vapor deposition. ACS Nano 2012, 6, 3224–3229. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Rathmell, A.R.; Chen, Z.; Stewart, I.E.; Wiley, B.J. Metal nanowire networks: The next generation of transparent conductors. Adv. Mater. 2014, 26, 6670–6687. [Google Scholar] [CrossRef]
- Wu, J.; Que, X.; Hu, Q.; Luo, D.; Liu, T.; Liu, F.; Russell, T.P.; Zhu, R.; Gong, Q. Multi-length scaled silver nanowire grid for application in efficient organic solar cells. Adv. Funct. Mater. 2016, 26, 4822–4828. [Google Scholar] [CrossRef]
- Song, J.; Kulinich, S.A.; Li, J.; Liu, Y.; Zeng, H. A general one-pot strategy for the synthesis of high-performance transparent-conducting-oxide nanocrystal inks for all-solution-processed devices. Angew. Chem. Int. Ed. 2015, 127, 472–476. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, G.; Wu, J.; Lin, J.Y. Efficient bifacial perovskite solar cell based on a highly transparent poly(3,4-Ethylenedioxythiophene) as the P-type hole-transporting material. J. Power Sour. 2016, 306, 171–177. [Google Scholar] [CrossRef]
- Sun, K.; Li, P.; Xia, Y.; Chang, J.; Ouyang, J. Transparent conductive oxide-free perovskite solar cells with PEDOT:PSS as transparent electrode. ACS Appl. Mater. Interfaces 2015, 7, 15314–15320. [Google Scholar] [CrossRef] [PubMed]
- Abachi, T.; Cattin, L.; Louarn, G.; Lare, Y.; Bou, A.; Makha, M.; Torchio, P.; Fleury, M.; Morsli, M.; Addou, M.; et al. Highly flexible, conductive and transparent MoO3/Ag/MoO3 multilayer electrode for organic photovoltaic cells. Thin Solid Films 2013, 545, 438–444. [Google Scholar] [CrossRef]
- Pang, S.; Li, X.; Dong, H.; Chen, D.; Zhu, W.; Chang, J.; Lin, Z.; Xi, H.; Zhang, J.; Zhang, C.; et al. Efficient bifacial semitransparent perovskite solar cells using Ag/V 2 O 5 as transparent anodes. ACS Appl. Mater. Interfaces 2018, 10, 12731–12739. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Chang, Y.-C.; Huang, W.-K.; Liao, W.-C.; Wang, H.; Yeh, C.; Tsai, B.-C.; Huang, Y.-C.; Tsao, C.-S. Achieving high efficiency and improved stability in large-area ito-free perovskite solar cells with thiol-functionalized self-assembled monolayers. J. Mater. Chem. A 2016, 4, 7903–7913. [Google Scholar] [CrossRef]
- Mao, L.; Chen, Q.; Li, Y.; Li, Y.; Cai, J.; Su, W.; Bai, S.; Jin, Y.; Ma, C.Q.; Cui, Z.; et al. Flexible silver grid/PEDOT: PSS hybrid electrodes for large area inverted polymer solar cells. Nano Energy 2014, 10, 259–267. [Google Scholar] [CrossRef]
- Sam, F.L.M.; Mills, C.A.; Rozanski, L.J.; Silva, S.R.P. Thin film hexagonal gold grids as transparent conducting electrodes in organic light emitting diodes. Laser Photonics Rev. 2014, 8, 172–179. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.; Kang, I.; Jung, M.S.; Kim, S.J.; Kim, J.K.; Cho, S.M.; Kim, J.H.; Park, J.H. Hybrid silver mesh electrode for ITO-free flexible polymer solar cells with good mechanical stability. ChemSusChem 2016, 9, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.D.; Kwon, J.; Lee, J.; Lee, H.; Jeong, S.; Kim, D.; Cho, H.; Yeo, J.; Ko, S.H. Maskless fabrication of highly robust, flexible transparent Cu conductor by random crack network assisted Cu nanoparticle patterning and laser sintering. Adv. Electron. Mater. 2016, 7, 5024–5031. [Google Scholar] [CrossRef]
- Kim, W.K.; Lee, S.; Hee Lee, D.; Hee Park, I.; Seong Bae, J.; Woo Lee, T.; Kim, J.Y.; Hun Park, J.; Chan Cho, Y.; Ryong Cho, C.; et al. Cu mesh for flexible transparent conductive electrodes. Sci. Rep. 2015, 5, 10715–10722. [Google Scholar] [CrossRef]
- Guo, C.F.; Sun, T.; Liu, Q.; Suo, Z.; Ren, Z. Highly stretchable and transparent nanomesh electrodes made by grain boundary lithography. Nat. Commun. 2014, 5, 3121. [Google Scholar] [CrossRef]
- Galagan, Y.; Rubingh, J.E.J.; Andriessen, R.; Fan, C.C.; Blom, P.W.; Veenstra, S.C.; Kroon, J.M. ITO-free flexible organic solar cells with printed current collecting grids. Sol. Energy Mater. Sol. Cells 2011, 17, 349–354. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Photolithography (accessed on 8 June 2019).
- Abdollahi Nejand, B.; Nazari, P.; Gharibzadeh, S.; Ahmadi, V.; Moshaii, A. All-inorganic large-area low-cost and durable flexible perovskite solar cells using copper foil as a substrate. Chem. Commun. 2017, 53, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, X.; Liu, Z.; Lee, E.C. A transparent poly(3,4-ethylenedioxylenethiophene):poly(styrene sulfonate) cathode for low temperature processed, metal-oxide free perovskite solar cells. J. Mater. Chem. A 2017, 5, 6974–6980. [Google Scholar] [CrossRef]
- Troughton, J.; Bryant, D.; Wojciechowski, K.; Carnie, M.J.; Snaith, H.; Worsley, D.A.; Watson, T.M. Highly efficient, flexible, indium-free perovskite solar cells employing metallic substrates. J. Mater. Chem. A 2015, 3, 9141–9145. [Google Scholar] [CrossRef]
- Zhang, H.X.; Chen, D.Z.; Zhang, C.F. ITO-free perovskite solar cells using photolithography processed metal grids as transparent anodes. In Proceedings of the 2016 13th IEEE International Conference on Solid-State and Integrated Circuit Technology (ICSICT 2016), Hangzhou, China, 25–28 October 2016; pp. 1026–1028. [Google Scholar]
- Lai, W.C.; Lin, K.W.; Wang, Y.T.; Chiang, T.Y.; Chen, P.; Guo, T.F. Oxidized Ni/Au transparent electrode in efficient CH3NH3PbI3 perovskite/fullerene planar heterojunction hybrid solar cells. Adv. Mater. 2016, 28, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Hao, Y.; Lin, Z.; Zhu, C. A simple and efficient solar cell parameter extraction method from a single current-voltage curve. J. Appl. Phys. 2011, 110, 064504. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Batmunkh, M.; Lin, C.-T.; Kim, J.; Tune, D.D.; Amboz, F.; Li, X.; Xu, S.; Sol, C.; Papakonstantinou, I.; et al. Origin of performance enhancement in TiO2-carbon nanotube composite perovskite solar cells. Small Methods 2019, 1900164, Early View. [Google Scholar] [CrossRef]
- Chen, D.; Pang, S.; Zhou, L.; Li, X.; Su, A.; Zhu, W.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Efficient TeO2/Ag transparent top electrode for 20%-efficiency bifacial perovskite solar cells with a bifaciality factor exceeding 80%. J. Mater. Chem. A 2019. accepted manuscript. [Google Scholar] [CrossRef]
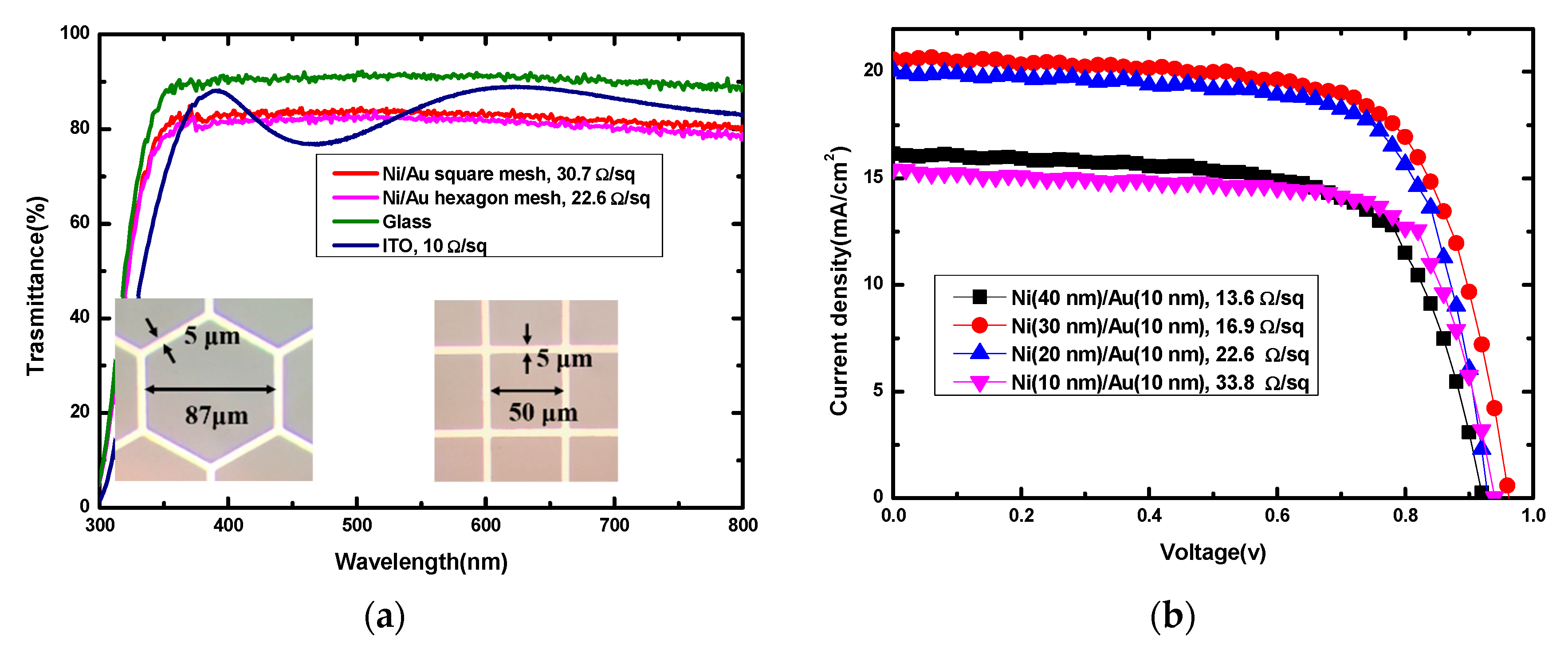

| Ni Thickness (nm) | Ni/Au Resistance (Ω/sq) | JSC (mA/cm2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| 40 | 13.6 | 16.16 | 0.90 | 68.76 | 10.13 |
| 30 | 16.9 | 20.67 | 0.96 | 69.09 | 13.72 |
| 20 | 22.6 | 20.04 | 0.92 | 71.22 | 13.14 |
| 10 | 33.8 | 15.43 | 0.94 | 71.68 | 10.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Fan, G.; Zhang, H.; Zhou, L.; Zhu, W.; Xi, H.; Dong, H.; Pang, S.; He, X.; Lin, Z.; et al. Efficient Ni/Au Mesh Transparent Electrodes for ITO-Free Planar Perovskite Solar Cells. Nanomaterials 2019, 9, 932. https://doi.org/10.3390/nano9070932
Chen D, Fan G, Zhang H, Zhou L, Zhu W, Xi H, Dong H, Pang S, He X, Lin Z, et al. Efficient Ni/Au Mesh Transparent Electrodes for ITO-Free Planar Perovskite Solar Cells. Nanomaterials. 2019; 9(7):932. https://doi.org/10.3390/nano9070932
Chicago/Turabian StyleChen, Dazheng, Gang Fan, Hongxiao Zhang, Long Zhou, Weidong Zhu, He Xi, Hang Dong, Shangzheng Pang, Xiaoning He, Zhenhua Lin, and et al. 2019. "Efficient Ni/Au Mesh Transparent Electrodes for ITO-Free Planar Perovskite Solar Cells" Nanomaterials 9, no. 7: 932. https://doi.org/10.3390/nano9070932
APA StyleChen, D., Fan, G., Zhang, H., Zhou, L., Zhu, W., Xi, H., Dong, H., Pang, S., He, X., Lin, Z., Zhang, J., Zhang, C., & Hao, Y. (2019). Efficient Ni/Au Mesh Transparent Electrodes for ITO-Free Planar Perovskite Solar Cells. Nanomaterials, 9(7), 932. https://doi.org/10.3390/nano9070932








