Synthesis of Mesoporous TiO2-B Nanobelts with Highly Crystalized Walls toward Efficient H2 Evolution
Abstract
:1. Introduction
2. Materials and Methods
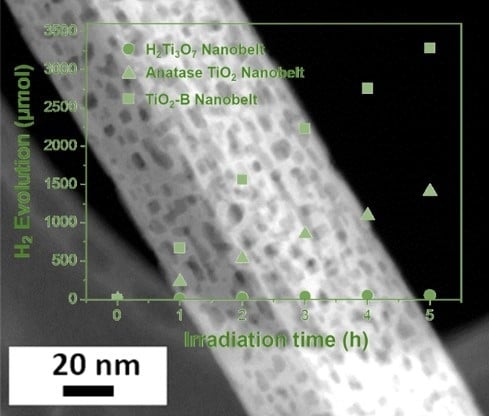
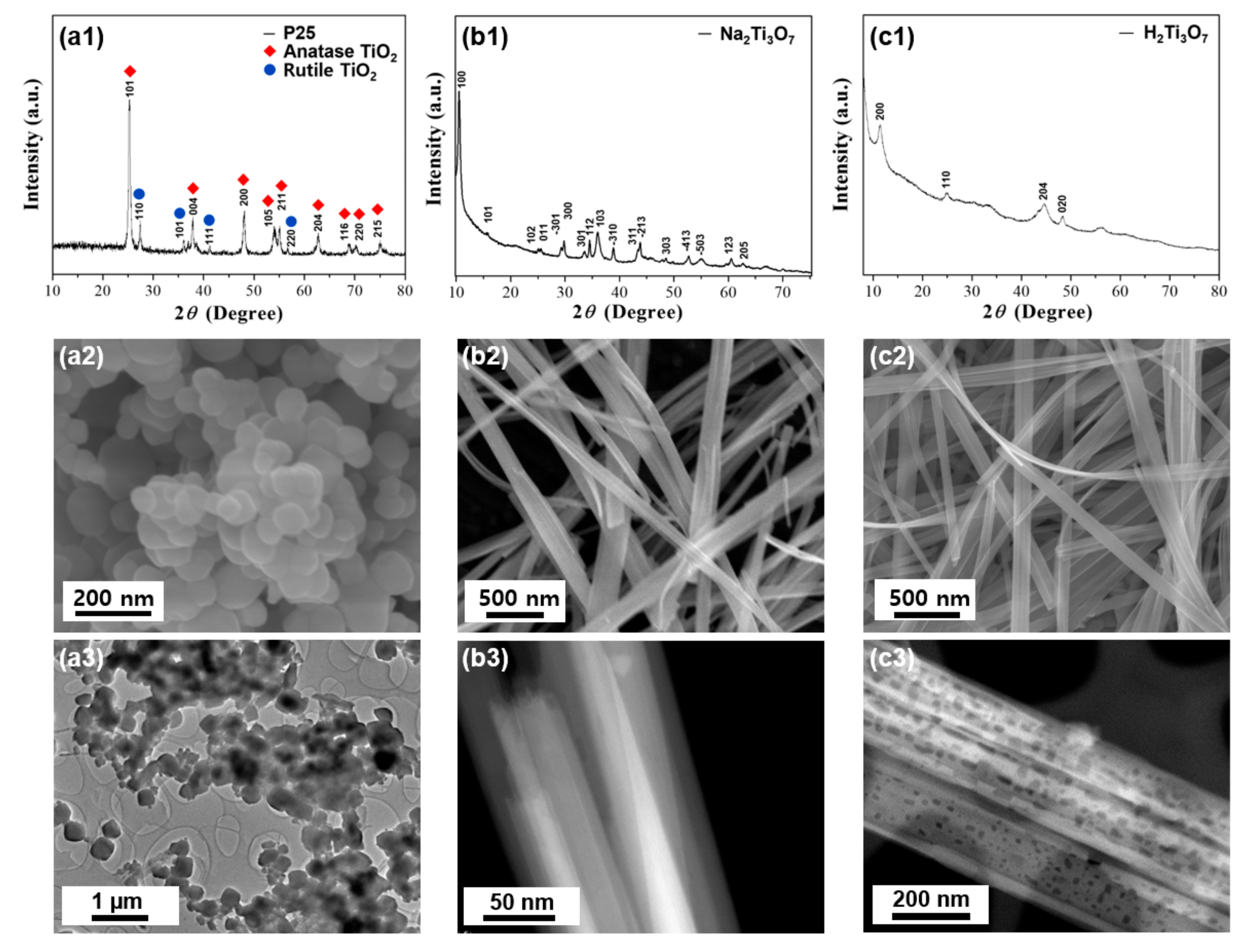
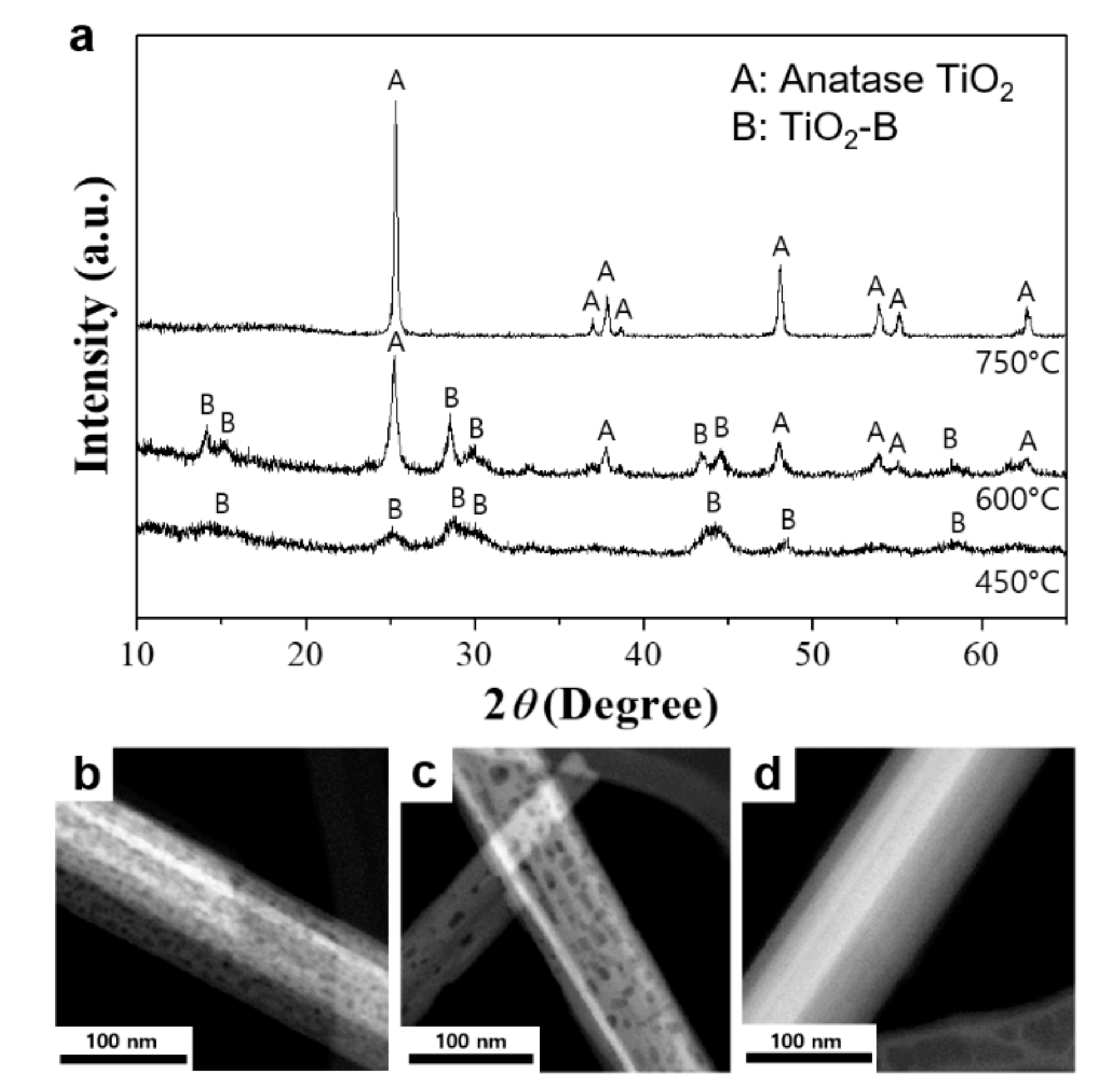
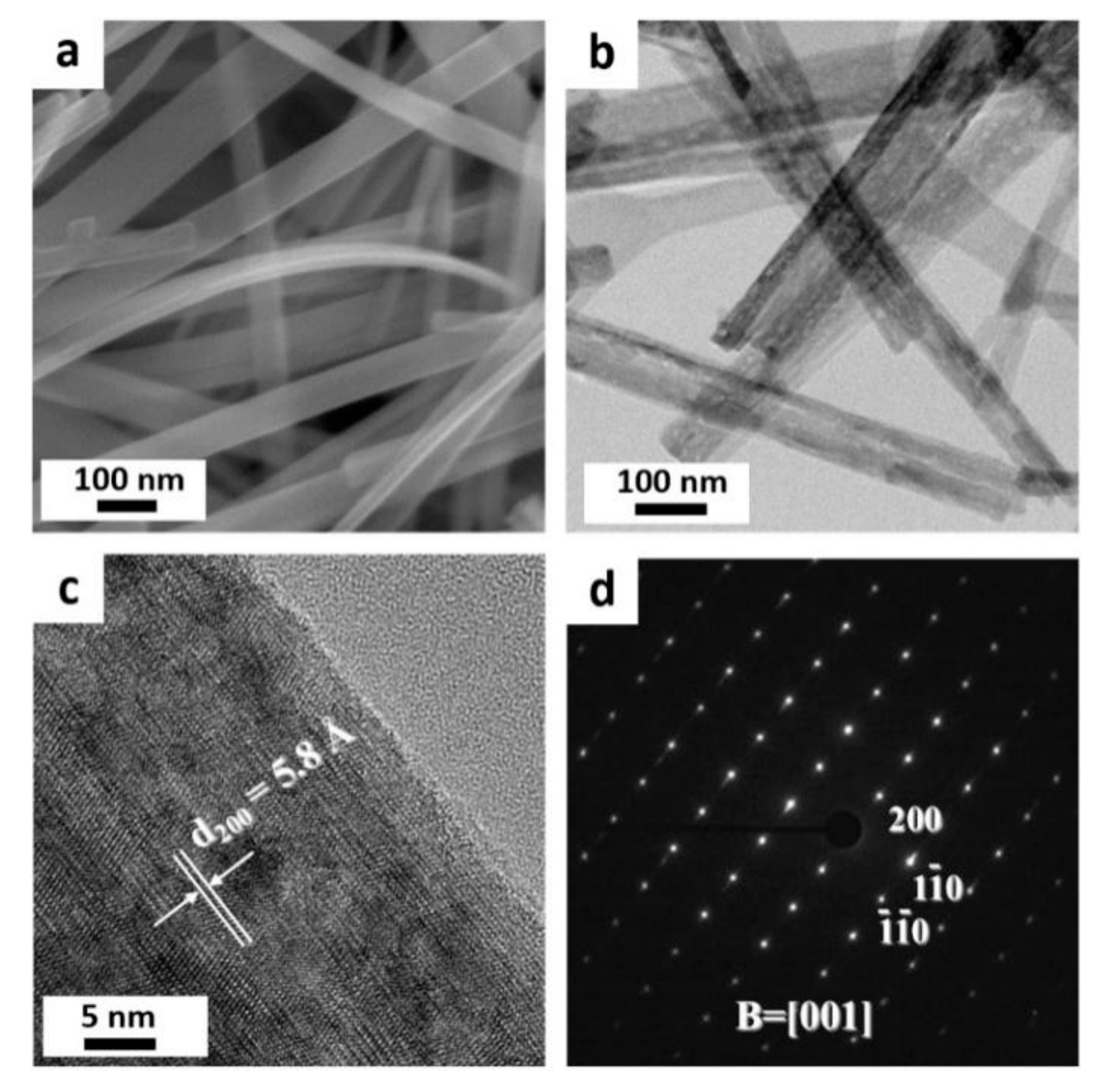
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Zhang, Y.; Malyi, O.I.; Bucher, N.; Xia, H.; Xi, S.; Zhu, Z.; Lv, Z.; Li, W.; Wei, J.; et al. Identifying the Origin and Contribution of Surface Storage in TiO2(B) Nanotube Electrode by In Situ Dynamic Valence State Monitoring. Adv. Mater. 2018, 30, 1802200. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Y.; Yu, L.; Li, J.; Lou, X.W. A universal Cooperative Assembly-directed Method for Coating of Mesoporous TiO2 Nanoshells with Enhanced Lithium Storage Properties. Sci. Adv. 2016, 2, e1501554. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm Rutile Titanium Dioxide Nanoparticles for Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-ordered Large-Pore Mesoporous Anatase TiO2 with Remarkably High Thermal Stability and Improved Crystallinity: Preparation, Characterization, and Photocatalytic Performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Li, X.; Chen, N.; Lin, S.; Wang, J.; Zhang, J. NiO-wrapped Mesoporous TiO2 Microspheres Based Selective Ammonia Sensor at Room Temperature. Sens. Actuators B 2015, 209, 729–734. [Google Scholar] [CrossRef]
- Crossland, E.J.W.; Noel, N.; Sivaram, V.; Leijtens, T.; Alexander-Webber, J.A.; Snaith, H.J. Mesoporous TiO2 Single Crystals Delivering Enhanced Mobility and Optoelectronic Device Performance. Nature 2013, 495, 215. [Google Scholar] [CrossRef]
- Gu, D.; Schüth, F. Synthesis of Non-siliceous Mesoporous Oxides. Chem. Soc. Rev. 2014, 43, 313–344. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A Perspective on Mesoporous TiO2 Materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol-Gel Method. Angew. Chem. Int. Ed. Engl. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Ishihara, S.; Leo, S.Y.; Ariga, K.; Wu, K.C.W.; Yamauchi, Y. Polymeric Micelle Assembly for Preparation of Large-Sized Mesoporous Metal Oxides with Various Compositions. Langmuir 2014, 30, 651. [Google Scholar] [CrossRef]
- Yue, W.; Randorn, C.; Attidekou, P.S.; Su, Z.; Irvine, J.T.S.; Zhou, W. Syntheses, Li Insertion, and Photoactivity of Mesoporous Crystalline TiO2. Adv. Funct. Mater. 2009, 19, 2826–2833. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, B.-W.; Sitti, M. Light-Driven Janus Hollow Mesoporous TiO2-Au Microswimmers. Adv. Funct. Mater. 2018, 28, 1704902. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, K.; Bagabas, A.A.; Zhang, P.; Gao, W.; Wang, J.; Sun, Z.; Fan, J.; Elzatahry, A.A.; Zhao, D. Ordered Macro/Mesoporous TiO2 Hollow Microspheres with Highly Crystalline Thin Shells for High Efficiency Photoconversion. Small 2016, 12, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rodriguez, E.F.; Hollenkamp, A.F.; Bhatt, A.I.; Chen, D.; Caruso, R.A. High Reversible Pseudocapacity in Mesoporous Yolk-Shell Anatase TiO2/TiO2(B) Microspheres Used as Anodes for Li-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1703270. [Google Scholar] [CrossRef]
- Zhao, T.; Luo, W.; Deng, Y.; Luo, Y.; Xu, P.; Liu, Y.; Wang, L.; Ren, Y.; Jiang, W. Monodisperse Mesoporous TiO2 Microspheres for Dye Sensitized Solar Cells. Nano Energy 2016, 26, 16–25. [Google Scholar] [CrossRef]
- Liu, Y.; Elzatahry, A.A.; Luo, W.; Lan, K.; Zhang, P.; Fan, J.; Wei, Y.; Wang, C.; Deng, Y.; Zheng, G.; et al. Surfactant-templating Strategy for Ultrathin Mesoporous TiO2 Coating on Flexible Graphitized Carbon Supports for High-performance Lithium-ion Battery. Nano Energy 2016, 25, 80–90. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, K.; Li, S.; Liu, Y.; Kong, B.; Wang, G.; Zhang, P.; Wang, R.; He, H.; Ling, Y.; et al. Constructing Three-Dimensional Mesoporous Bouquet-Posy-like TiO2 Superstructures with Radially Oriented Mesochannels and Single-Crystal Walls. J. Am. Chem. Soc. 2017, 139, 517–526. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Zhang, J. Mesoporous TiO2 Nanocrystals Grown in Situ on Graphene Aerogels for High Photocatalysis and Lithium-ion Batteries. J. Am. Chem. Soc. 2014, 136, 5852–5855. [Google Scholar] [CrossRef]
- Riad, K.B.; Wood-Adams, P.M.; Wegner, K. Flame-made TiO2(B). Mater. Res. Bull. 2018, 106, 276–281. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Z.; Pourpoint, F.; Armstrong, A.R.; Grey, C.P.; Bruce, P.G. Nanoparticulate TiO2(B): An Anode for Lithium-ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 2164–2167. [Google Scholar] [CrossRef]
- Wessel, C.; Zhao, L.; Urban, S.; Ostermann, R.; Djerdj, I.; Smarsly, B.M.; Chen, L.; Hu, Y.-S.; Sallard, S. Ionic-Liquid Synthesis Route of TiO2(B) Nanoparticles for Functionalized Materials. Chem.-Eur. J. 2011, 17, 775–779. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; García, R.; Bruce, P.G. Lithium-Ion Intercalation into TiO2-B Nanowires. Adv. Mater. 2005, 17, 862–865. [Google Scholar]
- Lan, T.; Dou, J.; Xie, F.; Xiong, P.; Wei, M. Ultrathin TiO2-B Nanowires with Enhanced Electrochemical Performance for Li-ion Batteries. J. Mater. Chem. A 2015, 3, 10038–10044. [Google Scholar] [CrossRef]
- Beuvier, T.; Richard-Plouet, M.; Granvalet, M.M.-L.; Brousse, T.; Crosnier, O.; Brohan, L. TiO2(B) Nanoribbons As Negative Electrode Material for Lithium Ion Batteries with High Rate Performance. Inorg. Chem. 2010, 49, 8457–8464. [Google Scholar] [CrossRef]
- Naoi, K.; Kurita, T.; Abe, M.; Furuhashi, T.; Abe, Y.; Okazaki, K.; Miyamoto, J.; Iwama, E.; Aoyagi, S.; Naoi, W.; et al. Ultrafast Nanocrystalline-TiO2(B)/Carbon Nanotube Hyperdispersion Prepared via Combined Ultracentrifugation and Hydrothermal Treatments for Hybrid Supercapacitors. Adv. Mater. 2016, 28, 6751–6757. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, V.; Shubha, N.; Cheah, Y.L.; Prasanth, R.; Chuiling, W.; Prabhakar, R.R.; Madhavi, S. Extraordinary Long-term Cycleability of TiO2-B Nanorods as Anodes in Full-cell Assembly with Electrospun PVdF-HFP Membranes. J. Mater. Chem. A 2013, 1, 308–316. [Google Scholar] [CrossRef]
- Huang, H.; Fang, J.; Xia, Y.; Tao, X.; Gan, Y.; Du, J.; Zhua, W.; Zhang, W. Construction of Sheet-belt Hybrid Nanostructures from One-dimensional Mesoporous TiO2(B) Nanobelts and Graphene Sheets for Advanced Lithium-ion Batteries. J. Mater. Chem. A 2013, 1, 2495–2500. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, J.; Yang, X.; Lu, F.; He, S.; Yang, J.; Fan, H.J.; Wu, M. Ultrathin Anatase TiO2 Nanosheets Embedded with TiO2-B Nanodomains for Lithium-ion Storage: Capacity Enhancement by Phase Boundaries. Adv. Energy Mater. 2015, 5, 1401756. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Liu, X.; Li, W.; Li, M. Orderly Integration of Porous TiO2(B) Nanosheets into Bunchy Hierarchical Structure for High-rate and Ultralong-lifespan Lithium-ion Batteries. Nano Energy 2017, 31, 1–8. [Google Scholar] [CrossRef]
- Lan, K.; Liu, Y.; Zhang, W.; Liu, Y.; Elzatahry, A.; Wang, R.; Xia, Y.; Al-Dhayan, D.; Zheng, N.; Zhao, D. Uniform Ordered Two-Dimensional Mesoporous TiO2 Nanosheets from Hydrothermal-Induced Solvent-Confined Monomicelle Assembly. J. Am. Chem. Soc. 2018, 140, 4135–4143. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bi, Z.; Sun, X.-G.; Unocic, R.R.; Paranthaman, M.P.; Dai, S.; Brown, G.M. Mesoporous TiO2-B Microspheres with Superior Rate Performance for Lithium Ion Batteries. Adv. Mater. 2011, 23, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Kuo, Y.; van der Ven, A.; Bartlett, B.M. Mesoporous TiO2-B Microflowers Composed of (110) Facet-exposed Nanosheets for Fast Reversible Lithium-ion Storage. J. Mater. Chem. A 2013, 1, 12028–12032. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Foster, C.W.; Banks, C.E.; Qiu, X.; Ji, X. Oxygen Vaccancies Evoked Blue TiO2(B) Nanobelts with Efficiency Enhancement in Sodium Storage Behaviors. Adv. Funct. Mater. 2017, 27, 1700856. [Google Scholar] [CrossRef]
- Amano, F.; Prieto-Mahaney, O.-O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral Single-Crystalline Particles of Anatase Titanium(IV) Oxide with High Photocatalytic Activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Knyazeva, E.E. Micro-mesoporous Materials Obtained by Zeolite Recrystallization: Synthesis, Characterization and Catalytic Applications. Chem. Soc. Rev. 2013, 42, 3671–3688. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, Y.; Sun, C.; Li, F.; Lu, G.Q.; Cheng, H.-M. Synergistic Effects of B/N Doping on the Visible-Light Photocatalytic Activity of Mesoporous TiO2. Angew. Chem. Int. Ed. 2008, 47, 4516–4520. [Google Scholar] [CrossRef]
- Saito, K.; Tominaka, S.; Yoshihara, S.; Ohara, K.; Sugahara, Y.; Ide, Y. Room-Temperature Rutile TiO2 Nanoparticle Formation on Protonated Layered Titanate for High-Performance Heterojunction Creation. ACS Appl. Mater. Interfaces 2017, 9, 24538–24544. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, Y.; Zhu, Y.; Wu, M.; Zhang, H.; Li, X.; Jiang, Z.; Meng, M. In Situ Formation of Disorder-Engineered TiO2(B)-Anatase Heterophase Junction for Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 24987–24992. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Cao, Q.; Zheng, D.; Alshehri, A.A.; Alghamidi, Y.G.; Alzahrani, K.A.; Kim, M.; Hou, J.; Lai, L.; Yamauchi, Y.; et al. Synthesis of Mesoporous TiO2-B Nanobelts with Highly Crystalized Walls toward Efficient H2 Evolution. Nanomaterials 2019, 9, 919. https://doi.org/10.3390/nano9070919
Li P, Cao Q, Zheng D, Alshehri AA, Alghamidi YG, Alzahrani KA, Kim M, Hou J, Lai L, Yamauchi Y, et al. Synthesis of Mesoporous TiO2-B Nanobelts with Highly Crystalized Walls toward Efficient H2 Evolution. Nanomaterials. 2019; 9(7):919. https://doi.org/10.3390/nano9070919
Chicago/Turabian StyleLi, Ping, Qing Cao, Dehua Zheng, Abdulmohsen Ali Alshehri, Yousef Gamaan Alghamidi, Khalid Ahmed Alzahrani, Minjun Kim, Jie Hou, Linfei Lai, Yusuke Yamauchi, and et al. 2019. "Synthesis of Mesoporous TiO2-B Nanobelts with Highly Crystalized Walls toward Efficient H2 Evolution" Nanomaterials 9, no. 7: 919. https://doi.org/10.3390/nano9070919
APA StyleLi, P., Cao, Q., Zheng, D., Alshehri, A. A., Alghamidi, Y. G., Alzahrani, K. A., Kim, M., Hou, J., Lai, L., Yamauchi, Y., Ide, Y., Bando, Y., Kim, J., Malgras, V., & Lin, J. (2019). Synthesis of Mesoporous TiO2-B Nanobelts with Highly Crystalized Walls toward Efficient H2 Evolution. Nanomaterials, 9(7), 919. https://doi.org/10.3390/nano9070919