Characterization of Sn4P3–Carbon Composite Films for Lithium-Ion Battery Anode Fabricated by Aerosol Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication and Characterization of Sn4P3/C Composite Powders
2.2. Fabrication and Characterization of Sn4P3/C Composite Films by AD
3. Results and Discussion
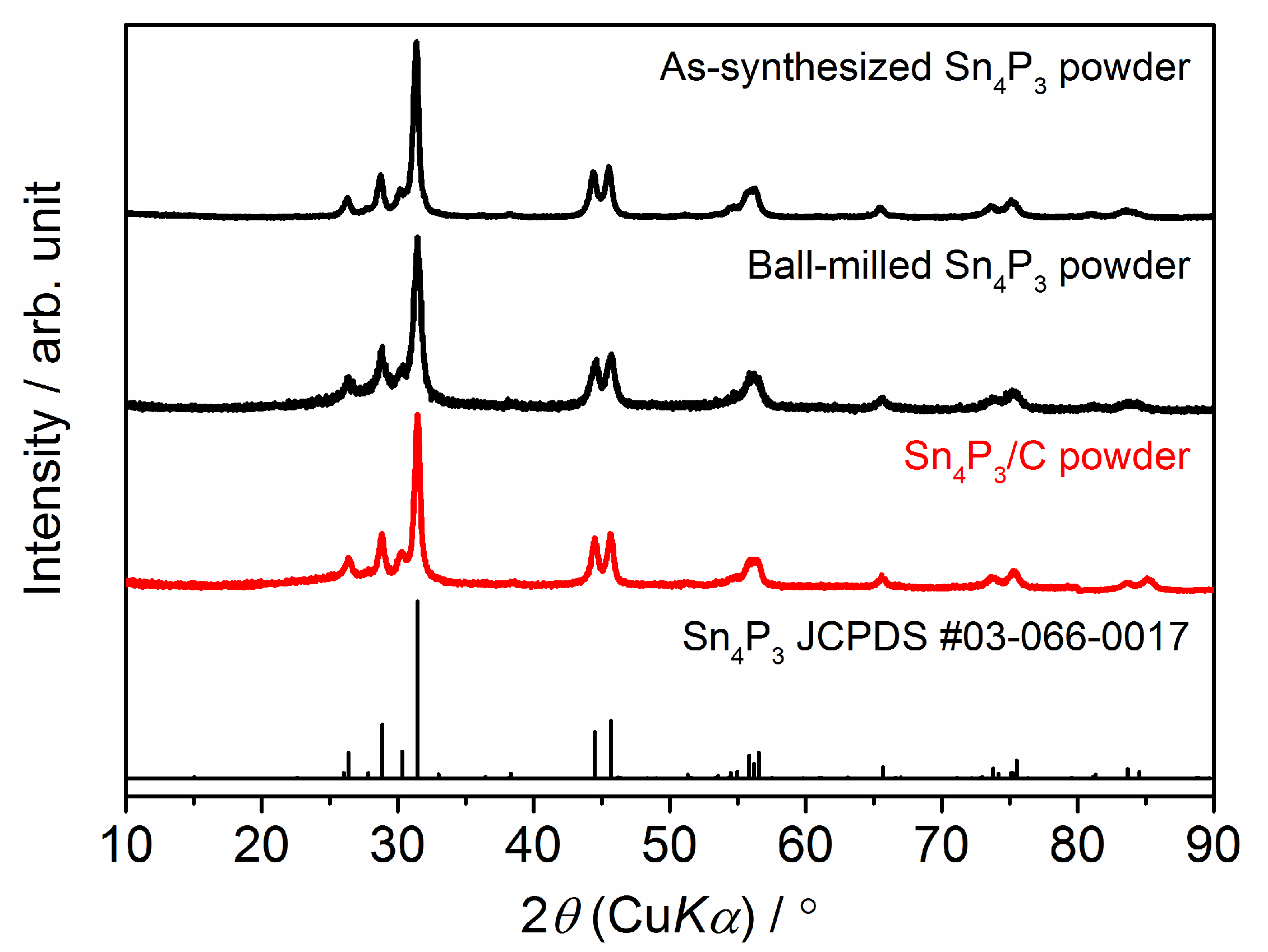
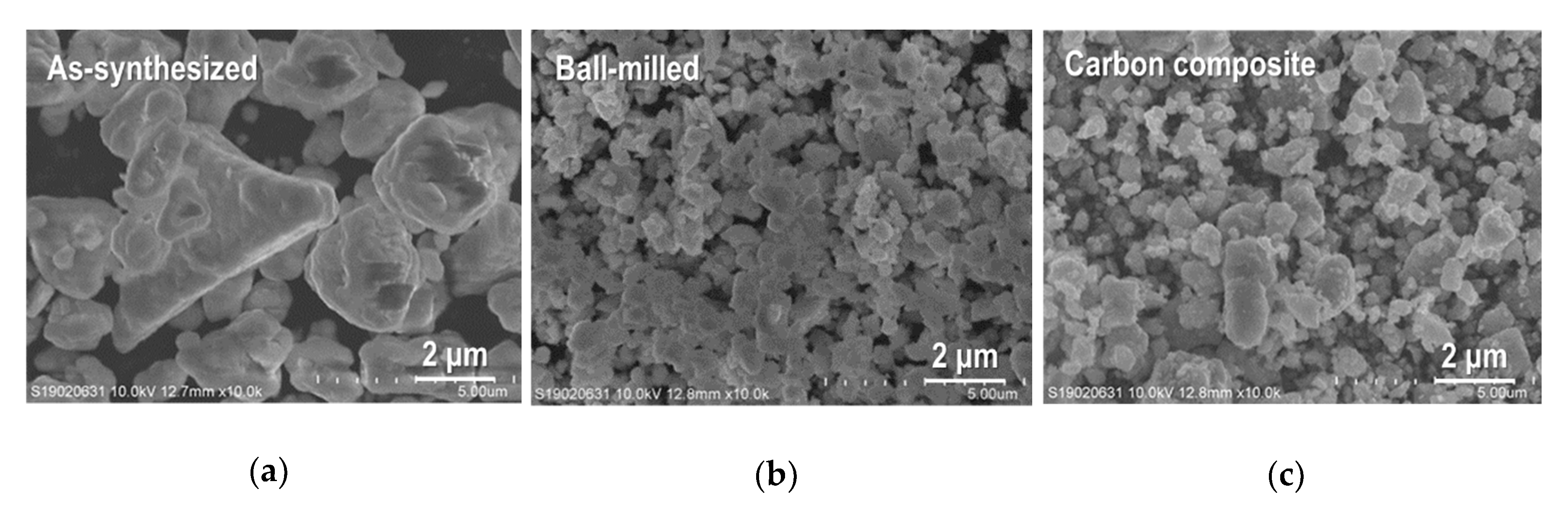
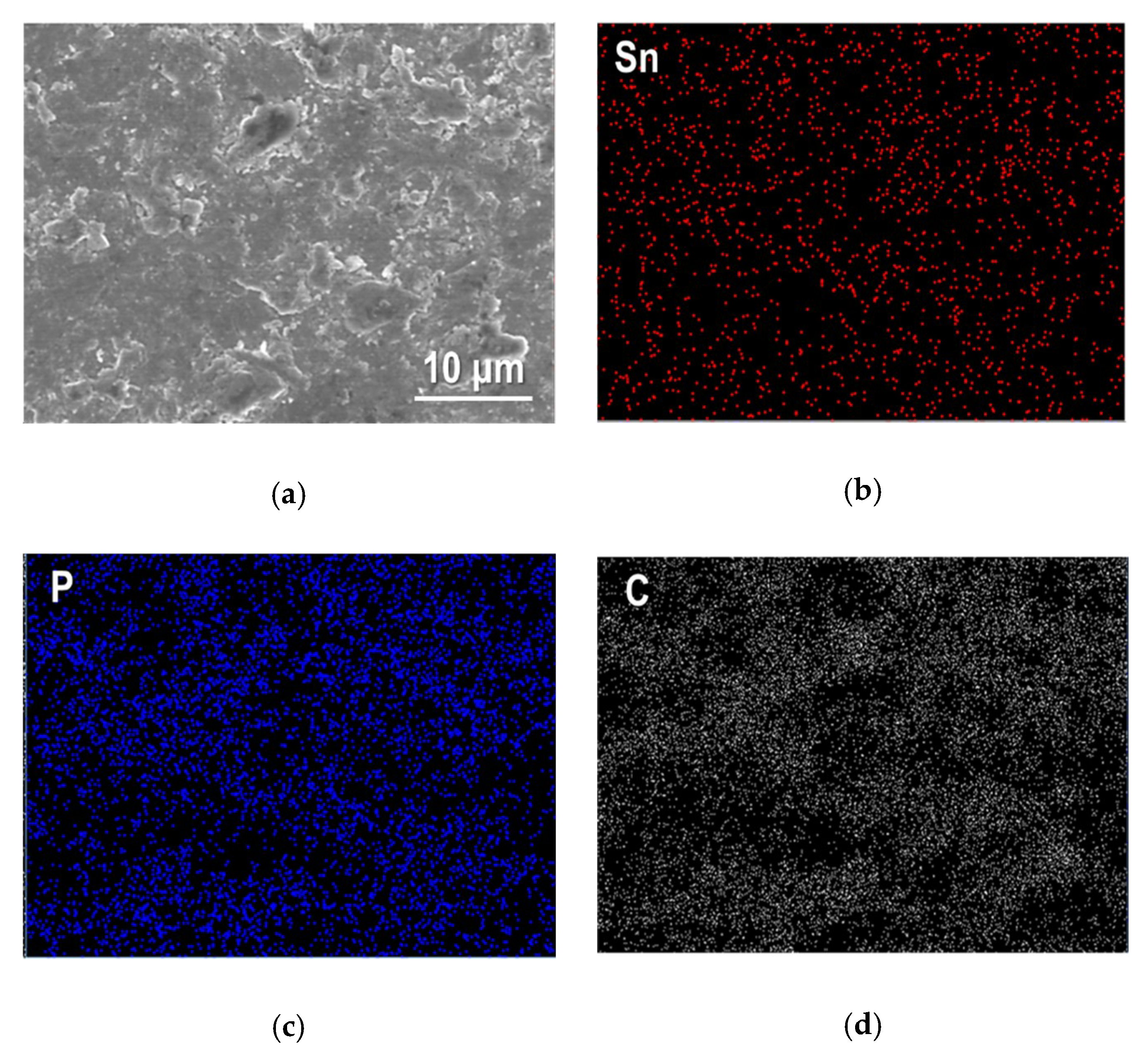
3.1. Crystal Phase and Microstructure of Sn4P3/C Composite Powder and Film
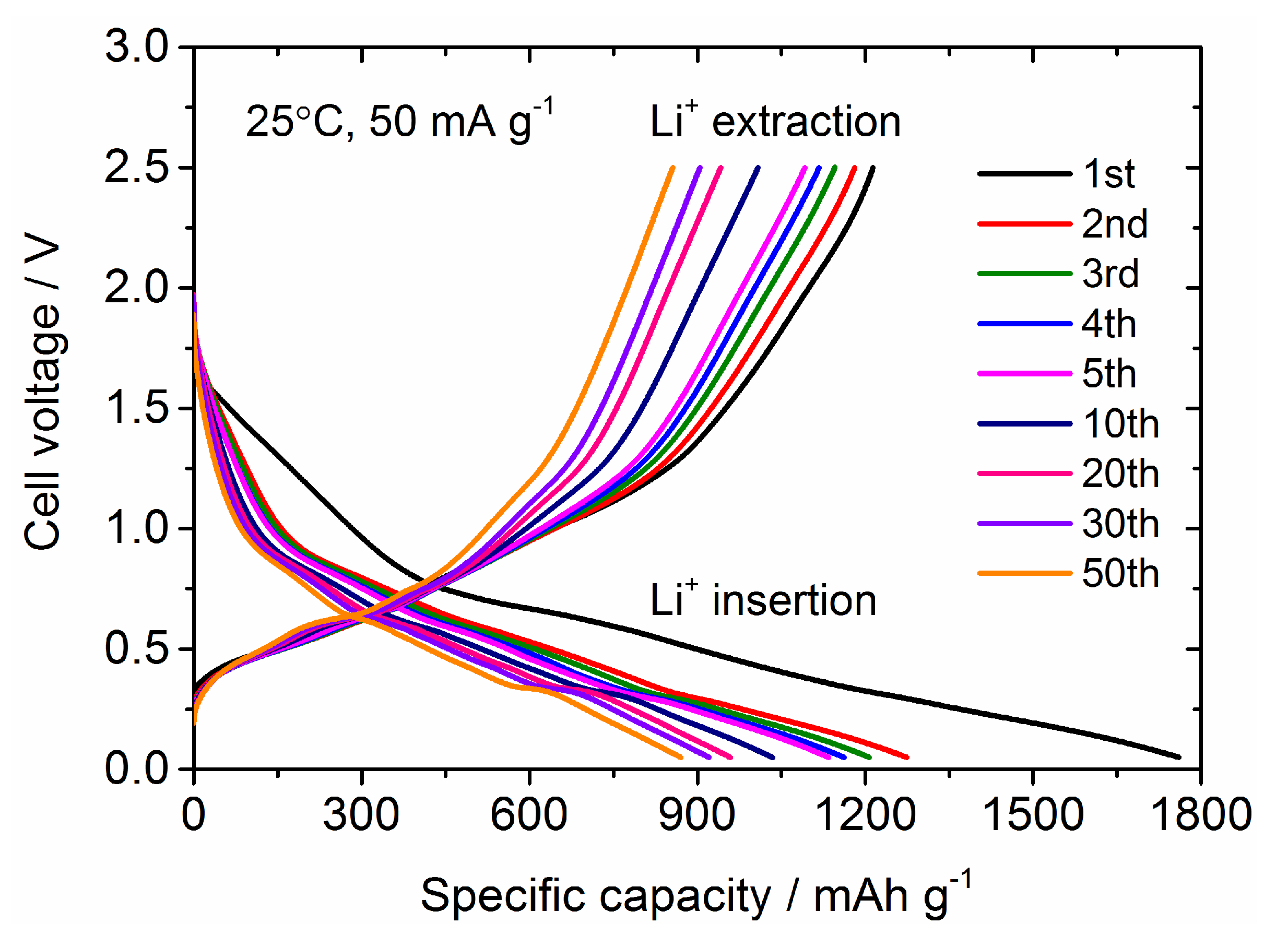
3.2. Electrochemical Performance of Sn4P3 Film Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano- and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50. [Google Scholar] [CrossRef]
- Zhang, W.J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Idota, Y.; Kubota, T.; Matsufuji, A.; Maekawa, Y.; Miyasaka, T. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material. Science 1997, 276, 1395–1397. [Google Scholar] [CrossRef]
- Besenhard, J.; Yang, J.; Winter, M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries? J. Power Sources 1997, 68, 87–90. [Google Scholar] [CrossRef]
- Kepler, K.D.; Vaughey, J.T.; Thackeray, M.M. LixCu6Sn5 (0 < x <13): An intermetallic insertion electrode for rechargeable lithium batteries. Electrochem. Solid-State Lett. 1999, 2, 307–309. [Google Scholar]
- Kim, H.; Choi, J.; Sohn, H.; Kang, T. The insertion mechanism of lithium into Mg2Si anode material for Li-ion batteries. J. Electrochem. Soc. 1999, 146, 4401–4405. [Google Scholar] [CrossRef]
- Kim, Y.U.; Lee, C.K.; Kang, T.; Sohn, H.J. Reaction mechanism of tin phosphide anode by mechanochemical method for lithium secondary batteries. J. Electrochem. Soc. 2004, 151, A933. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, C.; Sohn, H.; Kang, T. Enhancement of capacity and cycle-life of Sn4+δP3 (0 ≤ δ ≤ 1) anode for lithium secondary batteries anode for lithium secondary batteries. J. Power Sources 2005, 141, 163–166. [Google Scholar] [CrossRef]
- León, B.; Corredor, J.I.; Pérez-Vicente, C.; Tirado, J.L.; Tirado, J.L. On the mechanism of the electrochemical reaction of tin phosphide with lithium. J. Electrochem. Soc. 2006, 153, A1829. [Google Scholar] [CrossRef]
- Wu, J.J.; Fu, Z.W. Pulsed-laser-deposited Sn4P3 electrodes for lithium-ion batteries. J. Electrochem. Soc. 2009, 156, A22. [Google Scholar] [CrossRef]
- Nazri, G. Preparation, structure and ionic conductivity of lithium phosphide. Solid State Ion. 1989, 34, 97–102. [Google Scholar] [CrossRef]
- Ueda, A.; Nagao, M.; Inoue, A.; Hayashi, A.; Seino, Y.; Ota, T.; Tatsumisago, M. Electrochemical performance of all-solid-state lithium batteries with Sn4P3 negative electrode. J. Power Sources 2013, 244, 597–600. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Xu, L.; Ma, L. Synthesis of hollow spherical tin phosphides (Sn4P3) and their high adsorptive and electrochemical performance. J. Cryst. Growth 2016, 438, 31–37. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Xu, L.; Ma, L.; Chen, X. Solvothermal preparation of tin phosphide as a long-life anode for advanced lithium and sodium ion batteries. J. Power Sources 2016, 304, 346–353. [Google Scholar] [CrossRef]
- Park, G.; Lee, C.; Lee, J.; Choi, J.H.; Lee, Y.S.; Lee, S.M. Effect of Fe substitution on electrochemical properties of Sn3.95Fe0.05P3 alloy anode for lithium ion batteries. J. Alloy. Compd. 2014, 588, 534–539. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Xu, L.; Ma, L.; Hou, X. High lithium storage performance of Mn-doped Sn4P3 nanoparticles. Electrochim. Acta 2016, 210, 888–896. [Google Scholar] [CrossRef]
- Lin, J.; Kopold, P.; Wu, C.; van Aken, P.A.; Maiyer, J.; Yu, Y. Uniform yolk-shell Sn4P3@C nanospheres as high-capacity and cycle-stable anode materials for sodium-ion batteries. Energy Environ. Sci. 2015, 8, 3531–3538. [Google Scholar] [CrossRef]
- Fan, X.; Mao, J.; Zhu, Y.; Luo, C.; Suo, L.; Gao, T.; Han, F.; Liou, S.; Wang, C. Superior stable self-healing SnP3 anode for sodium-ion batteries. Nano. Lett. 2017, 17, 1870–1876. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z.; Tomofeeva, E.V.; Segre, C.U. In situ EXAFS−derived mechanism of highly reversible tin phosphide/graphite composite anode for Li-ion batteries. Adv. Mater. 2018, 8, 1702134. [Google Scholar] [CrossRef]
- Wang, X.; Kim, H.; Xiao, Y.; Sun, Y. Sn4P3–C nanospheres as high capacitive and ultra-stable anodes for sodium ion and lithium ion batteries. J. Mater. Chem. A 2018, 6, 17437–17443. [Google Scholar] [CrossRef]
- Xia, Y.; Han, S.; Zhu, Y.; Liang, Y.; Gu, M. Stable cycling of mesoporous Sn4P3/SnO2@C nanosphere anode with high initial coulombic efficiency for Li-ion batteries. Energy Storage Mater. 2019, 18, 125–132. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Jiang, J.; Liu, S.; Li, W.; Huang, S.; Chen, Z.; Zhao, B. In-situ solvothermal phosphorization from nano-sized tetragonal-Sn to rhombohedral-Sn4P3 embedded in hollow graphene sphere with high capacity and stability. Electrochim. Acta 2019, 312, 263–271. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Feng, J.; Chai, Y.; Luo, X.; Yuan, R.; Yang, X. Controllable synthesis of 3D nitrogen-doped carbon networks supported SnxPy nanoparticles as high performance anode for lithium ion batteries. Appl. Surf. Sci. 2019, 484, 899–905. [Google Scholar] [CrossRef]
- Gómez-Cámer, J.; Arnaiz, M.; Rojo, T.; Ajuria, J. Novel lithium-ion capacitor based on tin phosphide and olive pit derived activated carbon. J. Power Sources 2019, 434, 226695. [Google Scholar] [CrossRef]
- Akedo, J. Aerosol deposition of ceramic thick films at room temperature: densification mechanism of ceramic layers. J. Am. Ceram. Soc. 2006, 89, 1834–1839. [Google Scholar] [CrossRef]
- Akedo, J. Room temperature impact consolidation (RTIC) of fine ceramic powder by aerosol deposition method and applications to microdevices. J. Therm. Spray Technol. 2008, 17, 181–198. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Schubert, M.; Thomas, S.; Fuierer, P.; Moos, R. An overview of the aerosol deposition method: Process fundamentals and new trends in materials applications. J. Ceram. Sci. Technol. 2015, 6, 147–182. [Google Scholar] [CrossRef]
- Usui, H.; Yamamoto, Y.; Yoshiyama, K.; Itoh, T.; Sakaguchi, H. Application of electrolyte using novel ionic liquid to Si thick film anode of Li-ion battery. J. Power Sources 2011, 196, 3911–3915. [Google Scholar] [CrossRef]
- Usui, H.; Shibata, M.; Nakai, K.; Sakaguchi, H. Anode properties of thick-film electrodes prepared by gas deposition of Ni-coated Si particles. J. Power Sources 2011, 196, 2143–2148. [Google Scholar] [CrossRef]
- Usui, H.; Sakata, T.; Shimizu, M.; Sakaguchi, H. Electrochemical Na-insertion/Extraction Properties of Sn–P Anodes. Electrochemistry 2015, 83, 810–812. [Google Scholar] [CrossRef]
- Takai, S.; Sakaguchi, H.; Tanaka, K.; Nagao, Y.; Esaka, T. Cathode Performance of LiMn2O4 thick films prepared by gas-deposition for lithium rechargeable battery. Electrochemistry 2008, 76, 293–296. [Google Scholar] [CrossRef]
- Kim, I.; Park, J.; Nam, T.H.; Kim, K.W.; Ahn, J.H.; Park, D.S.; Ahn, C.; Wang, G.; Ahn, H.J. Electrochemical properties of an as-deposited LiFePO4 thin film electrode prepared by aerosol deposition. J. Power Sources 2013, 244, 646–651. [Google Scholar] [CrossRef]
- Inada, R.; Shibukawa, K.; Masada, C.; Nakanishi, Y.; Sakurai, Y. Characterization of as-deposited Li4Ti5O12 thin film electrode prepared by aerosol deposition method. J. Power Sources 2014, 253, 181–186. [Google Scholar] [CrossRef]
- Iwasaki, S.; Hamanaka, T.; Yamakawa, T.; West, W.C.; Yamamoto, K.; Motoyama, M.; Hirayama, T.; Iriyama, Y. Preparation of thick-film LiNi1/3Co1/3Mn1/3O2 electrodes by aerosol deposition and its application to all-solid-state batteries. J. Power Sources 2014, 272, 1086–1090. [Google Scholar] [CrossRef]
- Kato, T.; Iwasaki, S.; Ishii, Y.; Motoyama, M.; West, W.C.; Yamamoto, Y.; Iriyama, Y.; Kato, T. Preparation of thick-film electrode-solid electrolyte composites on Li7La3Zr2O12 and their electrochemical properties. J. Power Sources 2016, 303, 65–72. [Google Scholar] [CrossRef]
- Ahn, C.W.; Choi, J.J.; Ryu, J.; Hahn, B.D.; Kim, J.W.; Yoon, W.H.; Choi, J.H.; Park, D.S. Microstructure and electrochemical properties of iron oxide film fabricated by aerosol deposition method for lithium ion battery. J. Power Sources 2015, 275, 336–340. [Google Scholar] [CrossRef]
- Inada, R.; Yasuda, S.; Tojo, M.; Tsuritani, K.; Tojo, T.; Sakurai, Y. Development of lithium-stuffed garnet-type oxide solid electrolytes with high ionic conductivity for application to all-solid-state batteries. Front. Energy Res. 2016, 4, 28. [Google Scholar] [CrossRef]
- Iriyama, Y.; Wadaguchi, M.; Yoshida, K.; Yamamoto, Y.; Motoyama, M.; Yamamoto, T. 5V-class bulk-type all-solid-state rechargeable lithium batteries with electrode-solid electrolyte composite electrodes prepared by aerosol deposition. J. Power Sources 2018, 385, 55–61. [Google Scholar] [CrossRef]
- Inada, R.; Okuno, K.; Kito, S.; Tojo, T.; Sakurai, Y. Properties of Lithium Trivanadate Film Electrodes Formed on Garnet-Type Oxide Solid Electrolyte by Aerosol Deposition. Materials 2018, 11, 1570. [Google Scholar] [CrossRef]
- Popovici, D.; Nagai, H.; Fujishima, S.; Akedo, J. Preparation of Lithium Aluminum Titanium Phosphate Electrolytes Thick Films by Aerosol Deposition Method. J. Am. Ceram. Soc. 2011, 94, 3847–3850. [Google Scholar] [CrossRef]
- Inada, R.; Ishida, K.I.; Tojo, M.; Okada, T.; Tojo, T.; Sakurai, Y. Properties of aerosol deposited NASICON-type Li1.5All0.5Ge1.5(PO4)3 solid electrolyte thin films. Ceram. Int. 2015, 41, 11136–11142. [Google Scholar] [CrossRef]
- Choi, J.-J.; Ahn, C.-W.; Ryu, J.; Hahn, B.-D.; Kim, J.-W.; Yoon, W.-H.; Park, D.-S. Li-ion conducting Li0.35La0.55TiO3 electrolyte thick films fabricated by aerosol deposition. J. Korean Phys. Soc. 2016, 68, 12–16. [Google Scholar] [CrossRef]
- Inada, R.; Okada, T.; Bando, A.; Tojo, T.; Sakurai, Y. Properties of garnet-type Li6La3ZrTaO12 solid electrolyte films fabricated by aerosol deposition method. Prog. Nat. Sci. 2017, 27, 350–355. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Moos, R. Thick-films of garnet-type lithium ion conductor prepared by the Aerosol Deposition Method: The role of morphology and annealing treatment on the ionic conductivity. J. Power Sources 2017, 361, 61–69. [Google Scholar] [CrossRef]
- Park, C.M.; Sohn, H.J. Black phosphorus and its composite for lithium rechargeable batteries. Adv. Mater. 2007, 19, 2465–2468. [Google Scholar] [CrossRef]
- Tojo, T.; Yamaguchi, S.; Furukawa, Y.; Aoyanagi, K.; Umezaki, K.; Inada, R.; Sakurai, Y. Electrochemical performance of lithium ion battery anode using phosphorus encapsulated into nanoporous carbon nanotubes. J. Electrochem. Soc. 2018, 165, A1231–A1237. [Google Scholar] [CrossRef]


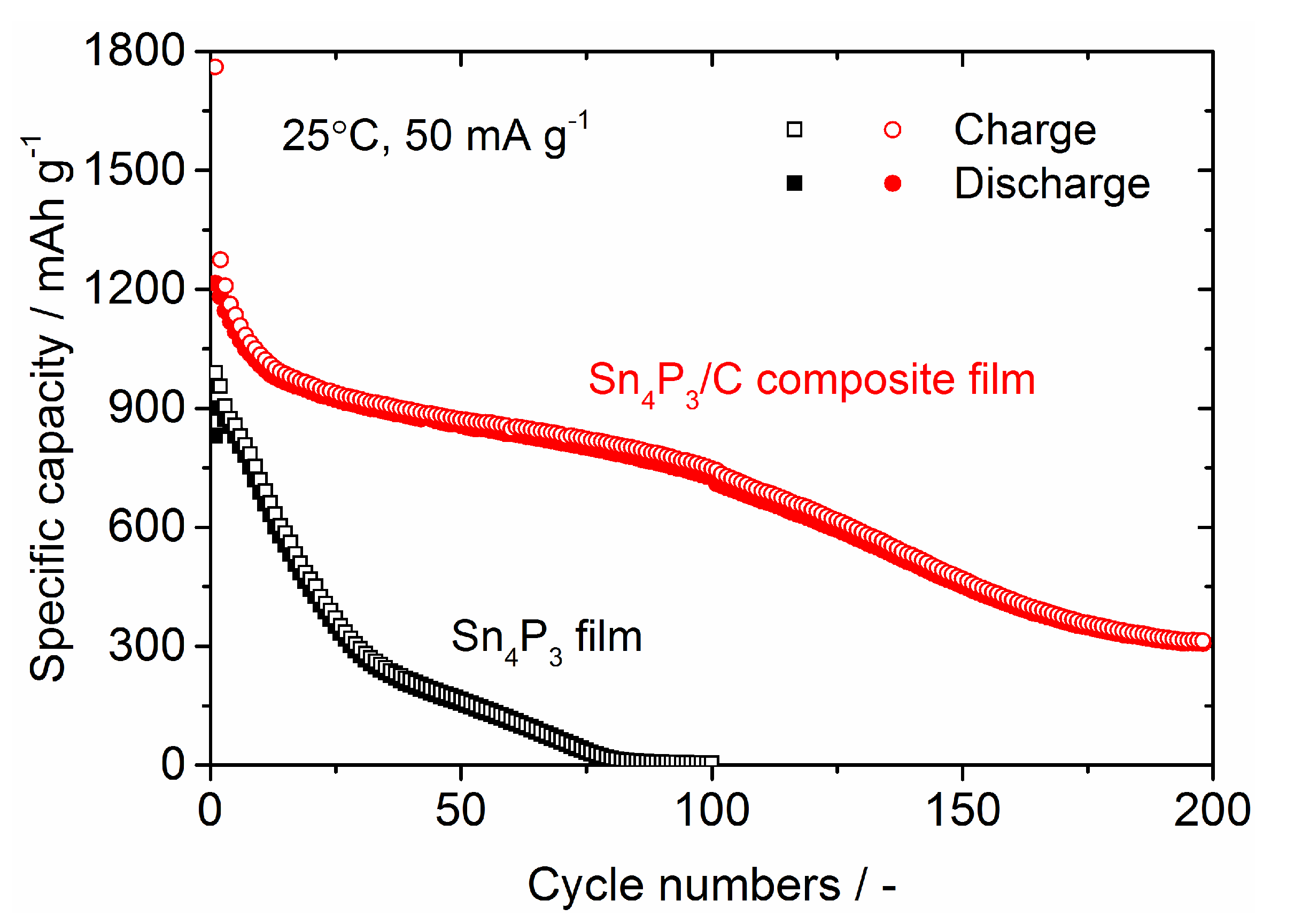
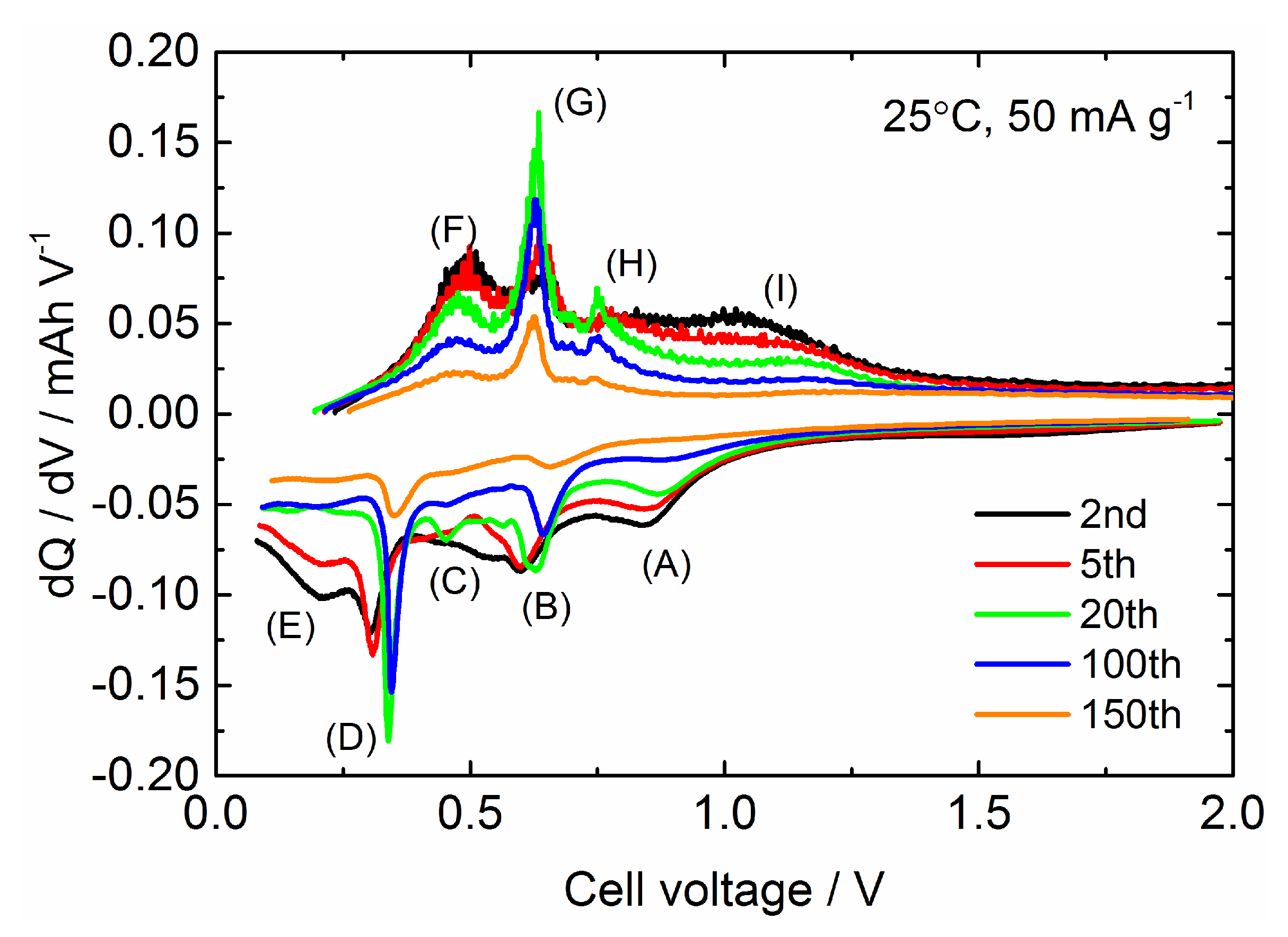
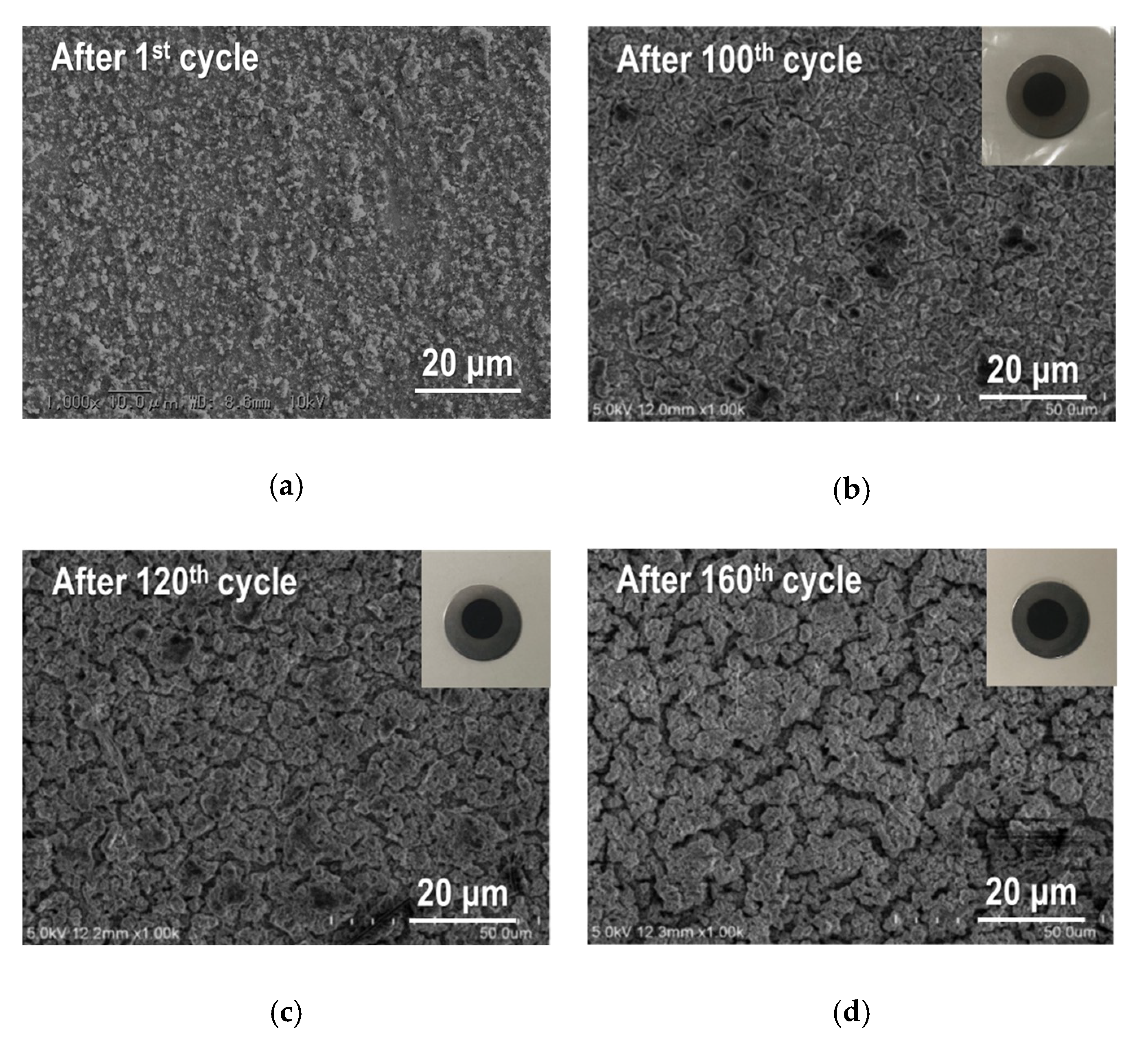
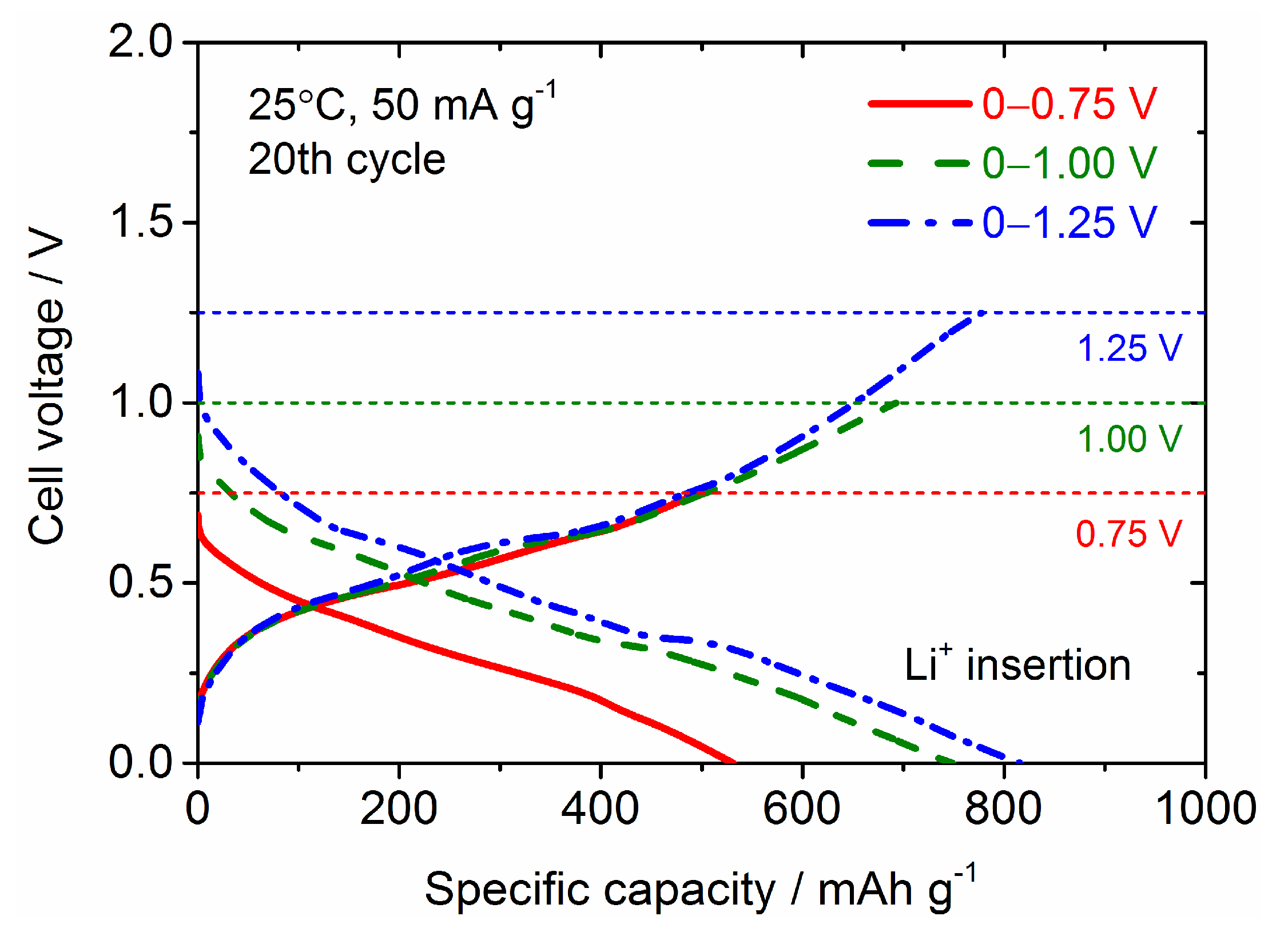
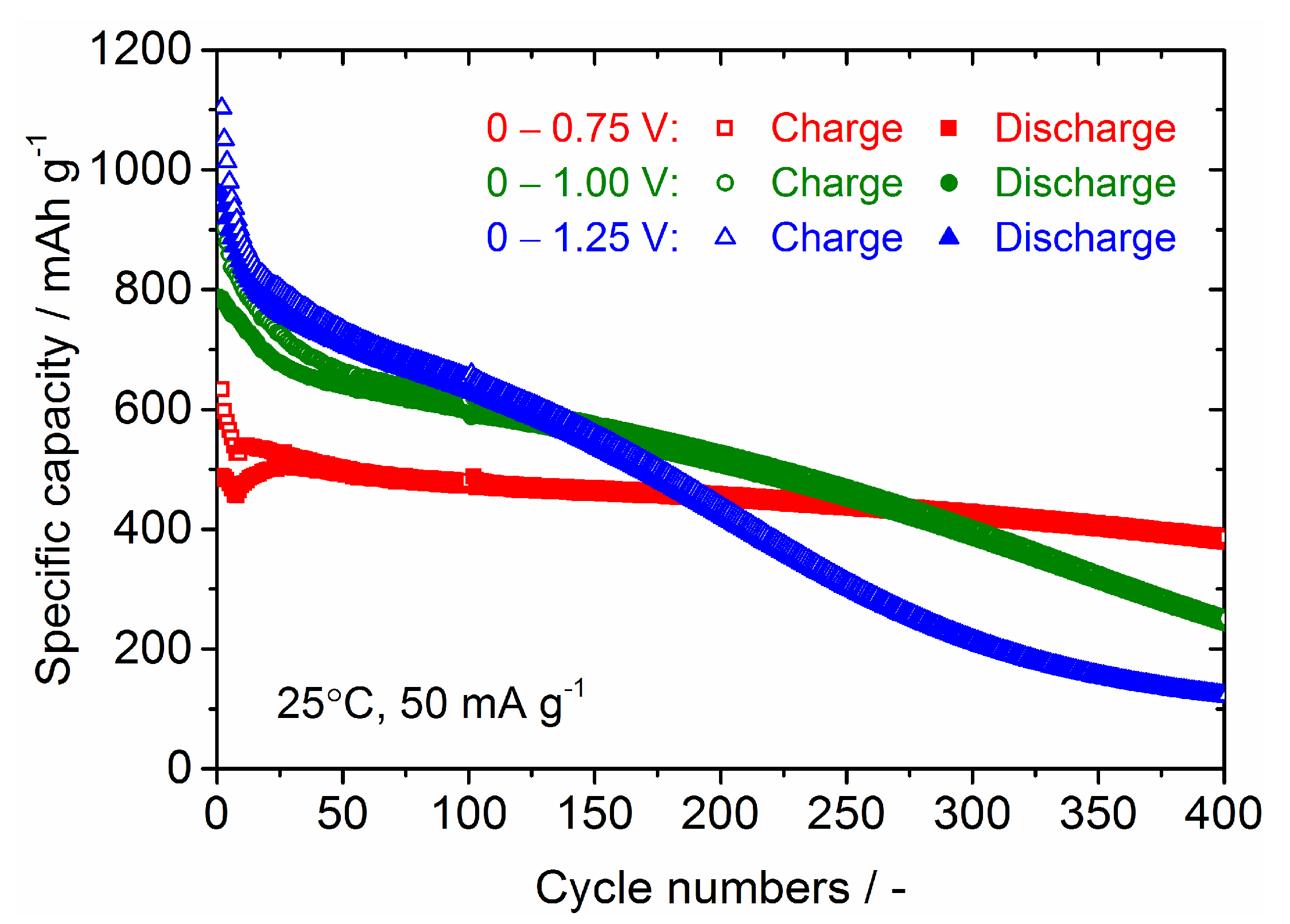
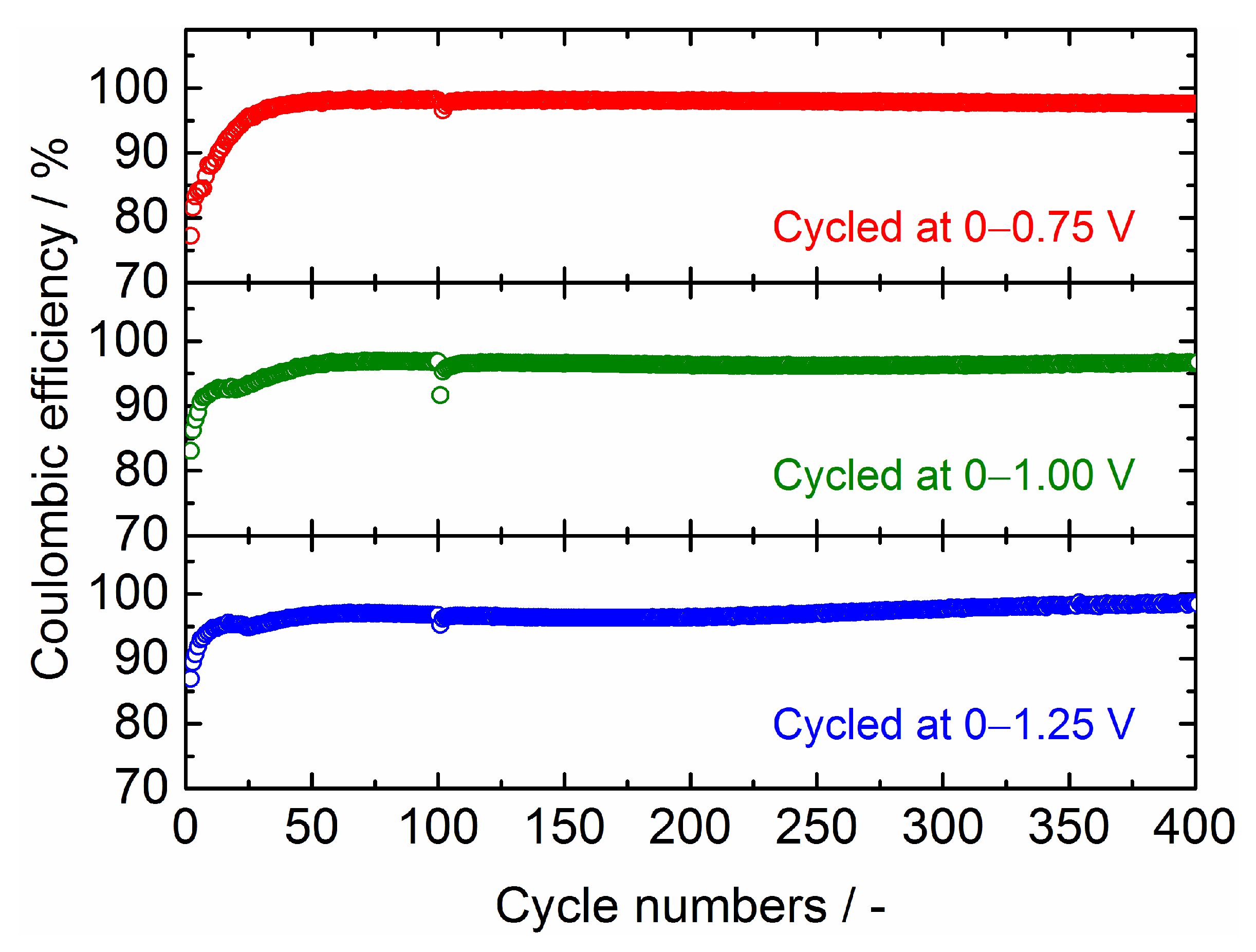
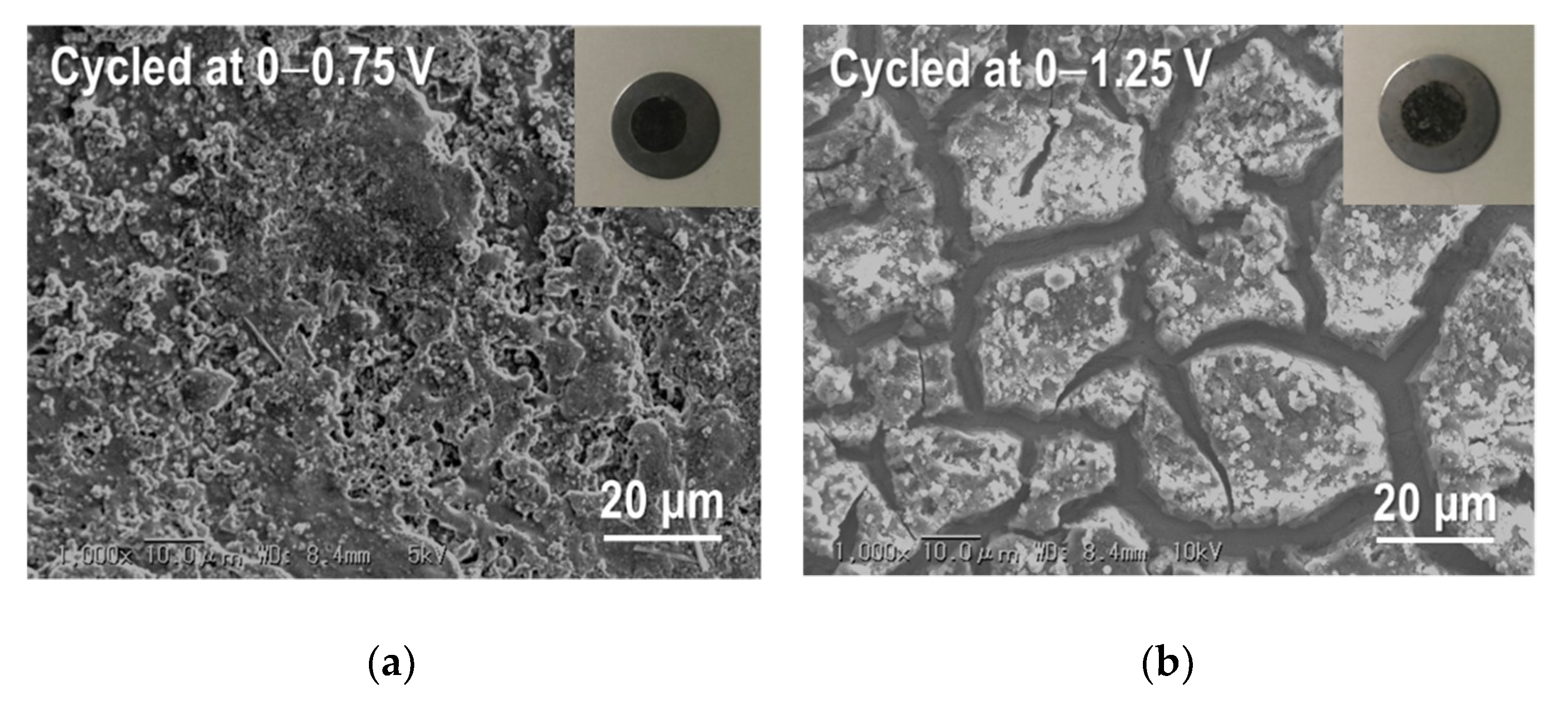
| Samples | Current Density/mA g−1 | Cycle Numbers | Specific Capacity/mAh g−1 | References |
|---|---|---|---|---|
| Sn4P3 | 100 | 50 | 370 | [8] |
| Sn4+xP3 | 100 | 50 | 530 (x = 1) 430 (x = 0.5) | [9] |
| Sn4P3 film by pulsed laser deposition (PLD) | 0.2 mA cm−2 | 10 | 553 | [11] |
| Fe doped Sn4P3 | 100 | 100 | 420 | [14] |
| Mn doped Sn4P3 | 100 | 150 | 488 | [15] |
| Sn4P3 | 100 | 20 | 261 | [16] |
| Sn4P3 | 100 | 300 | 442 | [17] |
| Sn4P3/graphite | 100 | 100 | 651 | [20] |
| Sn4P3/C nanosphere | 200 2000 | 50 500 | 1050 440 | [21] |
| Sn4P3/SnO2–C | 400 | 200 | 733 | [22] |
| Sn4P3/hollow graphene sphere | 100 | 100 | 606 | [23] |
| Sn4P3/N doped C | 100 | 120 | 718 | [24] |
| Sn4P3/C film by AD | 50 | 100 400 | 726 380 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritaka, T.; Yamashita, Y.; Tojo, T.; Inada, R.; Sakurai, Y. Characterization of Sn4P3–Carbon Composite Films for Lithium-Ion Battery Anode Fabricated by Aerosol Deposition. Nanomaterials 2019, 9, 1032. https://doi.org/10.3390/nano9071032
Moritaka T, Yamashita Y, Tojo T, Inada R, Sakurai Y. Characterization of Sn4P3–Carbon Composite Films for Lithium-Ion Battery Anode Fabricated by Aerosol Deposition. Nanomaterials. 2019; 9(7):1032. https://doi.org/10.3390/nano9071032
Chicago/Turabian StyleMoritaka, Toki, Yuh Yamashita, Tomohiro Tojo, Ryoji Inada, and Yoji Sakurai. 2019. "Characterization of Sn4P3–Carbon Composite Films for Lithium-Ion Battery Anode Fabricated by Aerosol Deposition" Nanomaterials 9, no. 7: 1032. https://doi.org/10.3390/nano9071032
APA StyleMoritaka, T., Yamashita, Y., Tojo, T., Inada, R., & Sakurai, Y. (2019). Characterization of Sn4P3–Carbon Composite Films for Lithium-Ion Battery Anode Fabricated by Aerosol Deposition. Nanomaterials, 9(7), 1032. https://doi.org/10.3390/nano9071032





