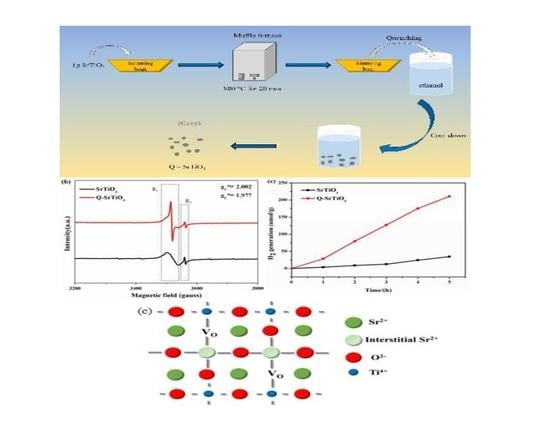
Ethanol-Quenching Introduced Oxygen Vacancies in Strontium Titanate Surface and the Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Q-SrTiO3
2.2. Characterization
2.3. Photocatalytic Test
2.3.1. Photocatalytic Degradation of RhB
2.3.2. Photocatalytic Evolution of hydrogen
3. Results and Discussion
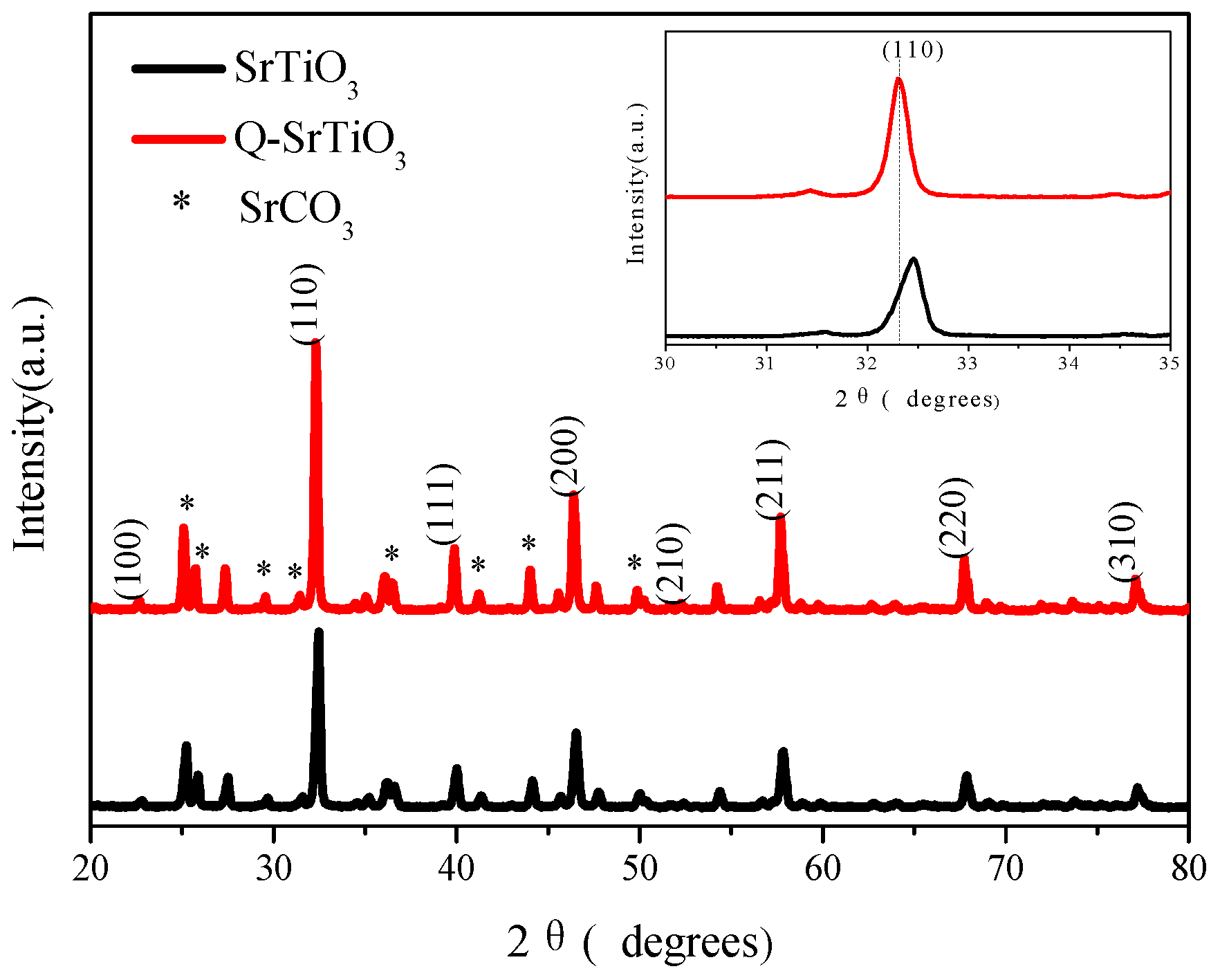
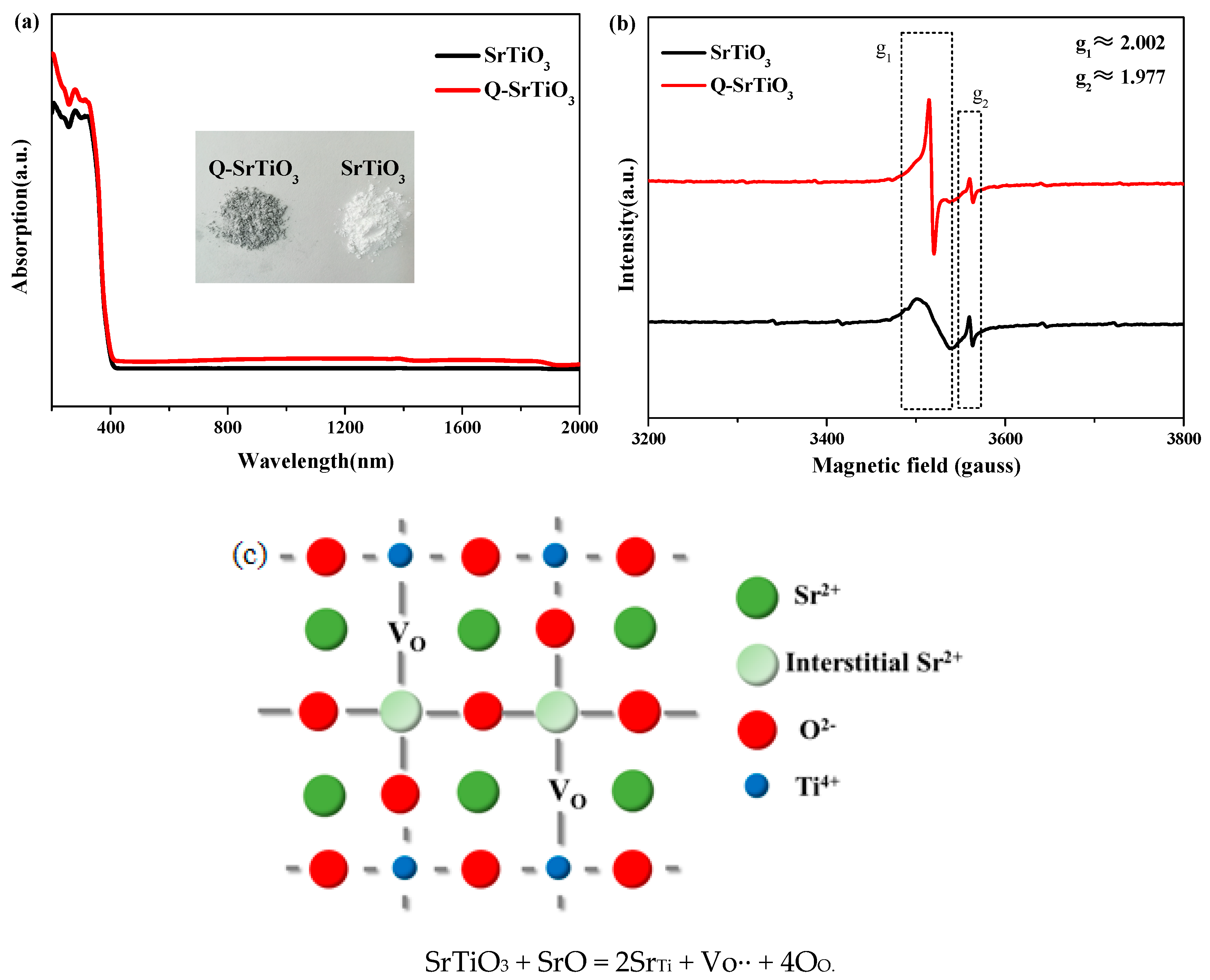
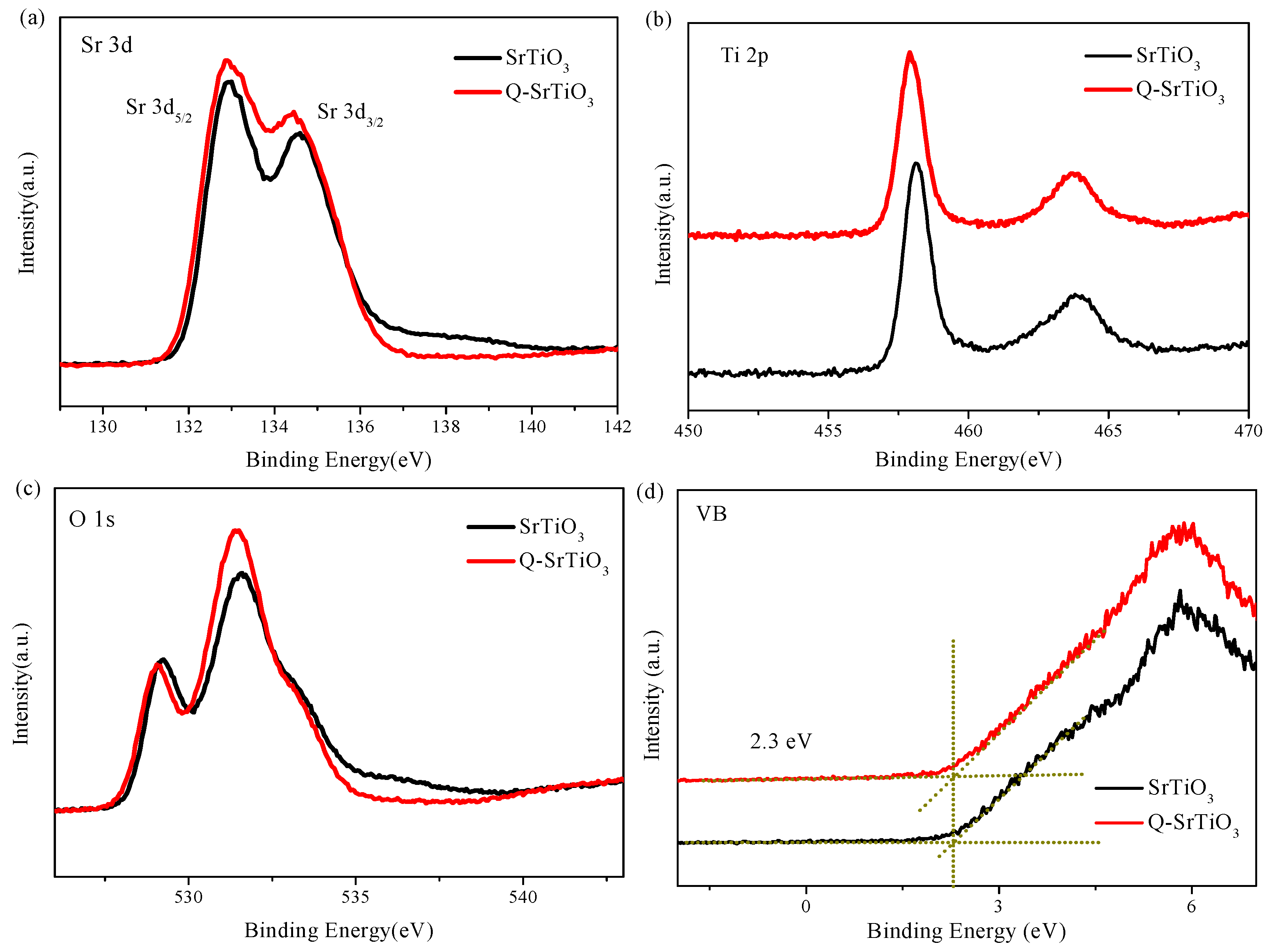
3.1. Characterization of the Photocatalysts
3.2. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.R.; Zhang, J.; Yu, J.G.; Jaroniecc, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor based photocatalytic water splitting. Chem. Soc. Rrv. 2015, 46, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, L.S.; Chen, Z.G.; Hu, J.Q.; Li, S.J.; Wang, Z.H.; Liu, J.S.; Wang, X.C. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rrv. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Pei, Z.X.; Weng, S.X.; Liu, P. Enhanced photocatalytic activity by bulk trapping and spatial separation of charge carriers: A case study of defect and facet mediated TiO2. Appl. Catal. B Environ. 2016, 180, 463–470. [Google Scholar] [CrossRef]
- Wu, Z.; Su, Y.F.; Yu, J.D.; Xiao, W.; Sun, L.; Lin, C.J. Enhanced photoelectrocatalytic hydrogen production activity of SrTiO3-TiO2 heteronanoparticle modified TiO2 nanotube arrays. Int. J. Hydrog. Energy 2015, 40, 9704–9712. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties–A review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Xie, T.H.; Sun, X.Y.; Lin, J. Enhanced photocatalytic degradation of RhB driven by visible light-induced MMCT of Ti(Ⅳ)–O–Fe(Ⅱ) formed in fe–doped SrTiO3. J. Phys. Chem. C 2008, 112, 9753–9759. [Google Scholar] [CrossRef]
- Ouyang, S.; Tong, H.; Umezawa, N.; Cao, J.; Li, P.; Bi, Y.; Zhang, Y.; Ye, J. Surface–alkalinization–induced enhancement of photocatalytic H2 evolution over SrTiO3-Based photocatalysts. J. Am. Chem. Soc. 2012, 134, 1974–1977. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Nisar, J.; Pathak, B.; Ahuja, R. Hybrid density functional study on SrTiO3 for visible light photocatalysis. Int. J. Hydrog. Energy 2012, 37, 11611–11617. [Google Scholar] [CrossRef]
- Yan, J.H.; Zhu, Y.R.; Tang, Y.G.; Zheng, S.Q. Nitrogen-doped SrTiO3/TiO2 composite photocatalysts for hydrogen production under visible light irradiation. J. Alloy. Compd. 2009, 472, 429–433. [Google Scholar] [CrossRef]
- Luo, H.M.; Takata, T.; Lee, Y.; Zhao, J.F.; Domen, K.; Yan, Y.S. Photocatalytic Activity Enhanced for Titanium Dioxide by Co-doping with Bromine and Chlorine. Chem. Mater. 2004, 16, 846–849. [Google Scholar] [CrossRef]
- Enterkin, J.A.; Setthapun, W.; Elam, J.W.; Christensen, S.T.; Rabuffetti, F.A.; Marks, L.D.; Stair, P.C.; Poeppelmeier, K.R.; Marshall, C.L. Propane oxidation over Pt/SrTiO3 nanocuboids. ACS Catal. 2011, 1, 629–635. [Google Scholar] [CrossRef]
- Pan, X.W.; Wang, L.Q.; Ling, F.; Li, Y.H.; Han, D.X.; Pang, Q. A novel biomass assisted synthesis of Au-SrTiO3 as a catalyst for hydrogen generation from formaldehyde aqueous solution at low temperature. Int. J. Hydrog. Energy 2015, 40, 1752–1759. [Google Scholar] [CrossRef]
- Iwashina, K.; Kudo, A. Rh-doped SrTiO3 photocatalyst electrode showing cathodic photocurrent for water splitting under visible-light irradiation. J. Am. Chem. Soc. 2011, 133, 13272–13275. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.A.; Engelhard, M.H.; Shutthanandan, V.; Zhu, Z.; Droubay, T.C.; Qiao, L.; Sushko, P.V.; Feng, T.; Lee, H.D.; Gustafsson, T.; et al. Instability, intermixing and electronic structure at the epitaxial LaAlO3/SrTiO3(001) heterojunction. Surf. Sci. Rep. 2010, 65, 317–352. [Google Scholar] [CrossRef]
- Tan, H.Q.; Zhao, Z.; Zhu, W.B.; Coker, E.N.; Li, B.S.; Zheng, M.; Yu, W.X.; Fan, H.Y.; Sun, Z.C. Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef]
- Wang, G.M.; Ling, Y.C.; Li, Y. Oxygen-deficient metal oxide nanostructures for photoelectrochemical water oxidation and other applications. Nanoscale 2012, 4, 6682–6691. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T., Jr. TiO2-based photocatalysis: surface defects, oxygen and charge transfer. Top. Catal. 2005, 35, 3–4. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Han, M.Y.; Konkin, A.; Koppe, T.; Wang, D.; Andreu, T.; Chen, G.; Vetter, U.; Morante, J.R.; Schaaf, P. Slightly hydrogenated TiO2 with enhanced photocatalytic performance. J. Mater. Chem. A 2014, 2, 12708–12716. [Google Scholar] [CrossRef]
- Wang, G.M.; Wang, H.Y.; Ling, Y.C.; Tang, Y.C.; Yang, X.Y.; Fitzmorris, R.C.; Wang, C.C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Ling, Y.C.; Wang, H.Y.; Yang, X.Y.; Wang, C.C.; Zhang, J.Z.; Li, Y. Hydrogen-treated WO3 nanoflakes show enhanced photostability. Energy Environ. Sci. 2012, 5, 6180–6187. [Google Scholar] [CrossRef]
- Lu, X.H.; Wang, G.M.; Xie, S.L.; Shi, J.Y.; Li, W.; Tong, Y.X.; Li, Y. Efficient photocatalytic hydrogen evolution hydrogenated ZnO nanorod arrays. Chem. Commun. 2012, 48, 7717–7719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.L.; Zhao, W.; Zhu, G.L.; Lin, T.Q.; Xu, F.F.; Huang, F.Q. Black strontium titanate nanocrystals of enhanced solar absorption for photocatalysis. CrystEngComm 2015, 17, 7528–7534. [Google Scholar] [CrossRef]
- Supphasrirongjaroen, P.; Kongsuebchart, W.; Panpranot, J.; Mekasuwandumrong, O.; Satayaprasert, C.; Praserthdam, P. Dependence of quenching process on the photocatalytic activity of solvothermal-derived TiO2 with various crystallite sizes. Ind. Eng. Chem. Res. 2008, 47, 693–697. [Google Scholar] [CrossRef]
- Dong, W.J.; Li, X.Y.; Yu, J.; Guo, W.C.; Li, B.J.; Tan, L.; Li, C.R.; Shi, J.J.; Wang, G. Porous SrTiO3 spheres with enhanced photocatalytic performance. Mater. Lett. 2012, 67, 131–134. [Google Scholar] [CrossRef]
- Feng, L.L.; Zou, X.X.; Zhao, J.; Zhou, L.J.; Wang, D.J.; Zhang, X.; Li, G.D. Nanoporous Sr-rich strontium titanate: a stable and superior photocatalyst for H2 evolution. Chem. Commun. 2013, 49, 9788–9790. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Domen, K. Defect engineering of photocatalysts by doping of aliovalent metal cations for efficient water splitting. J. Phys. Chem. C 2009, 113, 19386–19388. [Google Scholar] [CrossRef]
- Hofman, W.; Hoffmann, R.W. Dopant influence on dielectric losses, leakage behaviour, and resistance degradation of SrTiO3 thin films. Thin Solid Film 1997, 305, 66–73. [Google Scholar] [CrossRef]
- Liu, B.S.; Cheng, K.; Nie, S.C.; Zhao, X.J.; Yu, H.G.; Yu, J.G.; Fujishima, A.; Nakata, K. Ice–water quenching induced Ti3+ Self-doped TiO2 with surface lattice distortion and the increased photocatalytic activity. J. Phys. Chem. C 2017, 121, 19836–19848. [Google Scholar] [CrossRef]
- Wagner, C.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1979. [Google Scholar]
- Schaub, R.; Thostrup, P.; Lopez, N.; Lægsgaard, E.; Stensgaard, I.; Nørskov, J.K.; Besenbacher, F. Oxygen vacancies as active sites for water dissociation on rutile TiO2(110). Phys. Rev. Lett. 2006, 87, 266104-1–266104-4. [Google Scholar] [CrossRef] [PubMed]


| Sample | Atomic Concentration (%) | Atomic Ratio | |||
|---|---|---|---|---|---|
| Ti | Sr | O | C | Sr/Ti | |
| SrTiO3 | 3.73 | 6.14 | 31.36 | 58.76 | 1.64 |
| Q-SrTiO3 | 2.83 | 6.10 | 29.75 | 61.32 | 2.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Chen, S.; Wang, Y.; Hu, Z.; Zhao, H.; Xie, W. Ethanol-Quenching Introduced Oxygen Vacancies in Strontium Titanate Surface and the Enhanced Photocatalytic Activity. Nanomaterials 2019, 9, 883. https://doi.org/10.3390/nano9060883
Xiao Y, Chen S, Wang Y, Hu Z, Zhao H, Xie W. Ethanol-Quenching Introduced Oxygen Vacancies in Strontium Titanate Surface and the Enhanced Photocatalytic Activity. Nanomaterials. 2019; 9(6):883. https://doi.org/10.3390/nano9060883
Chicago/Turabian StyleXiao, Yang, Shihao Chen, Yinhai Wang, Zhengfa Hu, Hui Zhao, and Wei Xie. 2019. "Ethanol-Quenching Introduced Oxygen Vacancies in Strontium Titanate Surface and the Enhanced Photocatalytic Activity" Nanomaterials 9, no. 6: 883. https://doi.org/10.3390/nano9060883
APA StyleXiao, Y., Chen, S., Wang, Y., Hu, Z., Zhao, H., & Xie, W. (2019). Ethanol-Quenching Introduced Oxygen Vacancies in Strontium Titanate Surface and the Enhanced Photocatalytic Activity. Nanomaterials, 9(6), 883. https://doi.org/10.3390/nano9060883