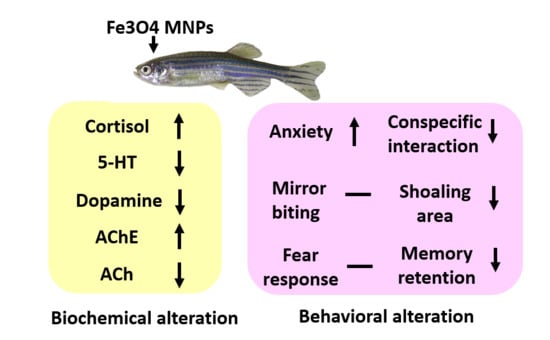
Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
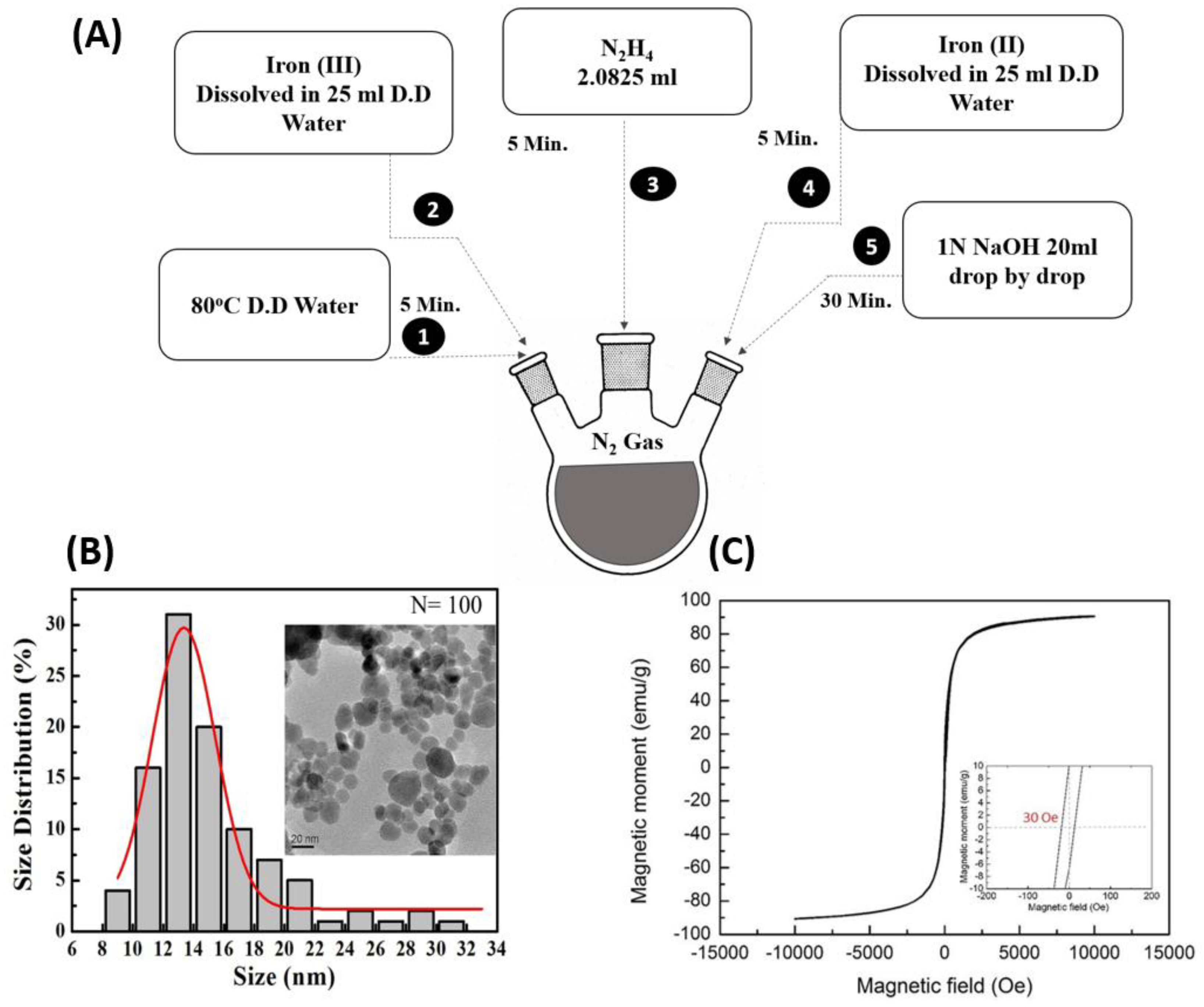
2.2. Synthetization and Characterization of Magnetic Nanoparticles
2.3. Zebrafish Ethics and Husbandry
2.4. Embryo Acute Toxicity Test
2.5. Zebrafish Exposure to Magnetic Nanoparticles
2.6. Novel Tank Test
2.7. Aggressiveness Test
2.8. Predator Avoidance Test
2.9. Shoaling Test
2.10. Social Interaction Test
2.11. Circadian Rhythm Locomotion Activity Test
2.12. Short Term Memory Test
2.13. Tissue Preparation and Total Protein Determination
2.14. Quantification of Oxidative Stress Markers, Stress Hormone, and Ferric (Metal) Content in Brain Tissues
2.15. Determination of Neurotransmitters in Brain Tissues
2.16. Statistical Analysis
3. Results
3.1. Characterization of Magnetic Nanoparticles (MNPs)
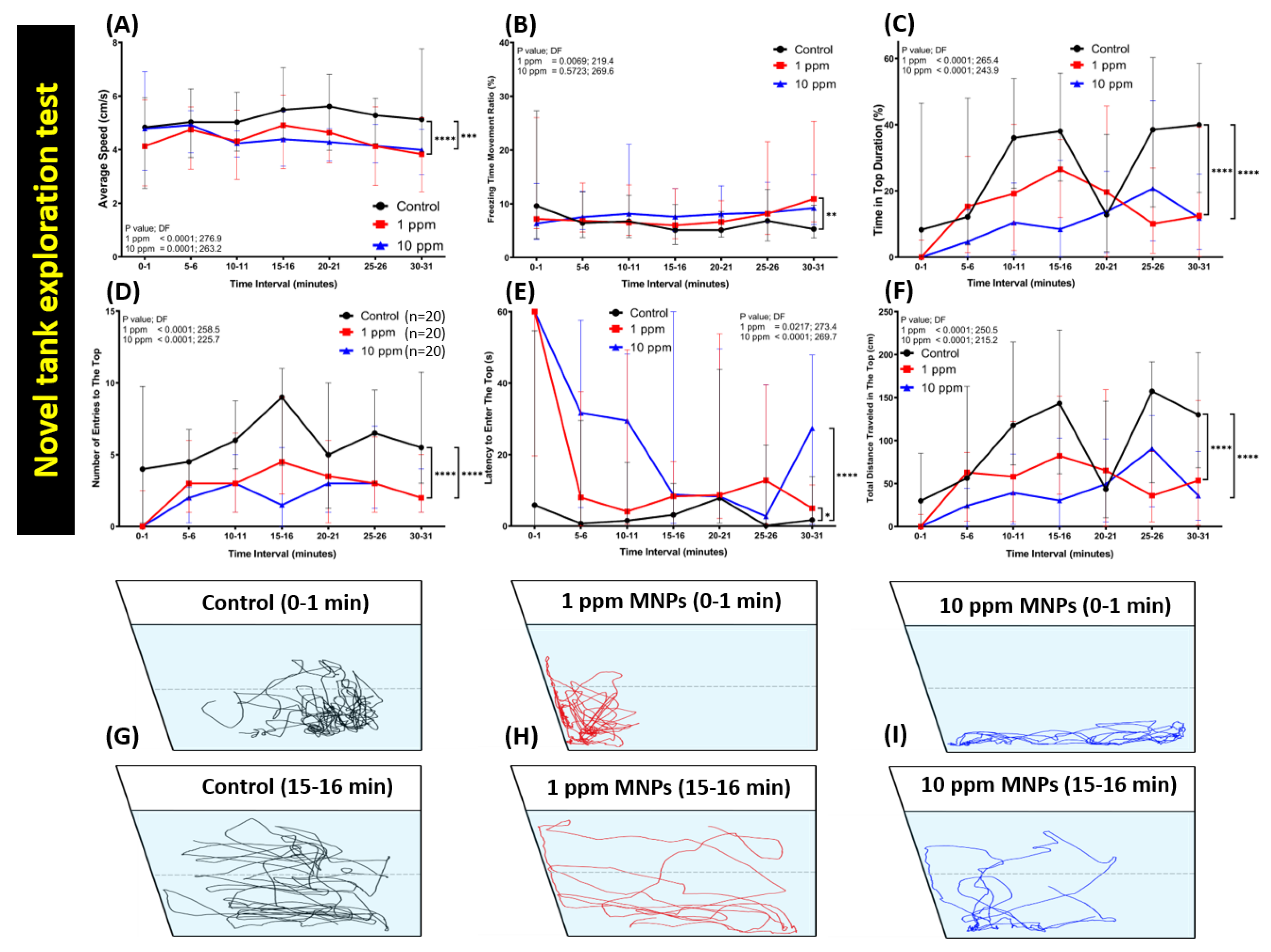
3.2. MNPs Exposure Significantly Reduced the Novel Tank Exploration Behavior of Zebrafish
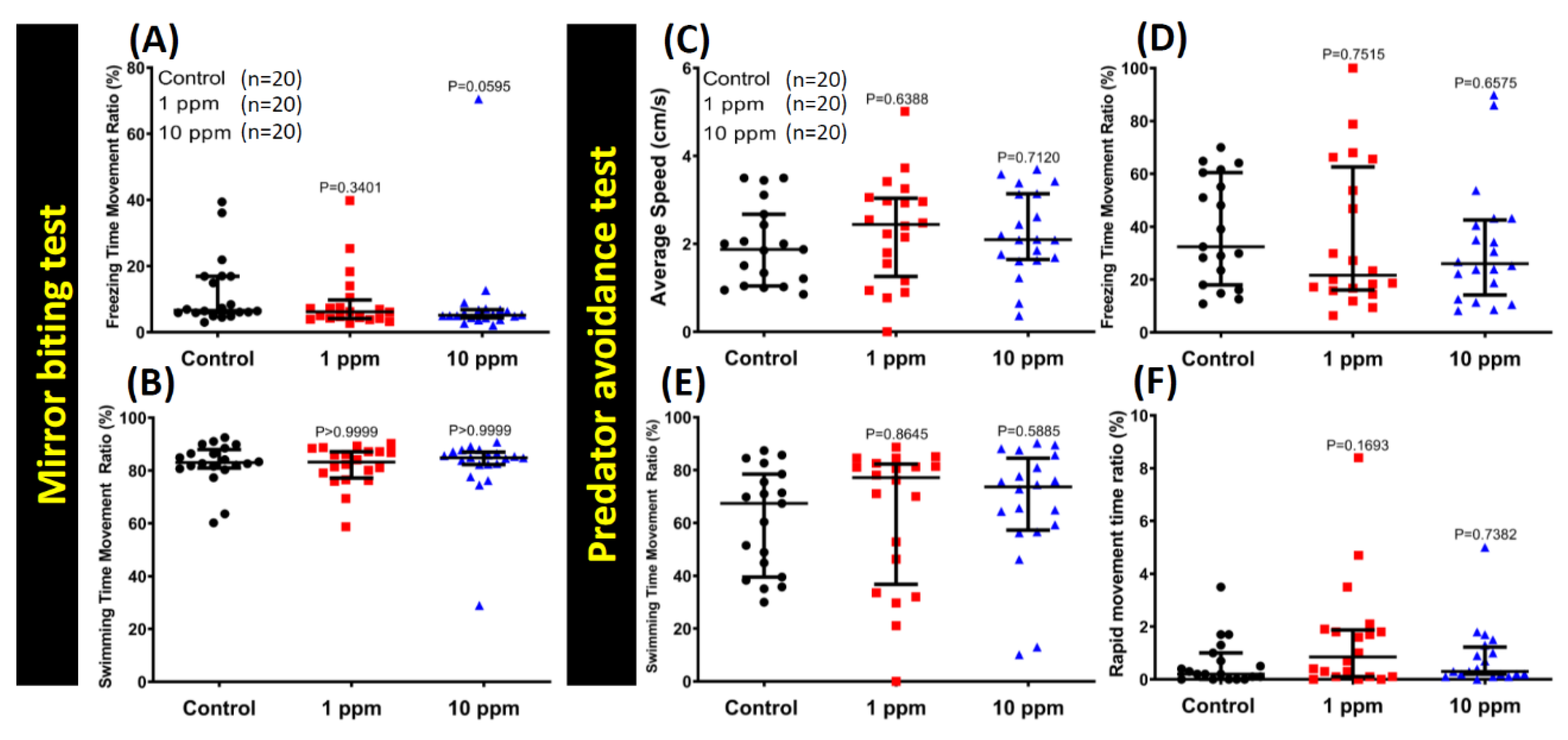
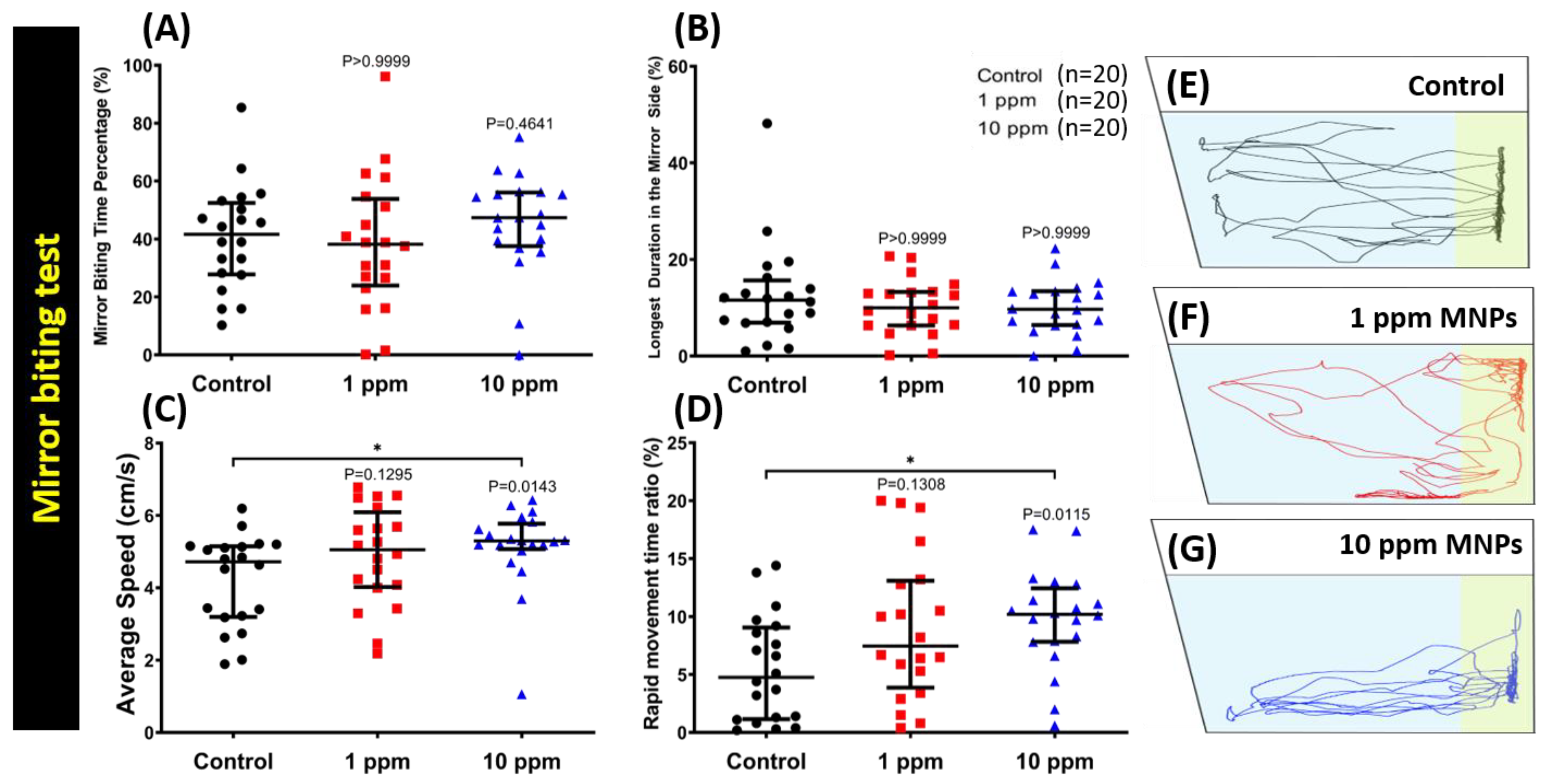
3.3. MNPs Exposure Did Not Alter Aggressiveness in Zebrafish
3.4. MNPs Exposure Did Not Change the Predator Avoidance Behavior
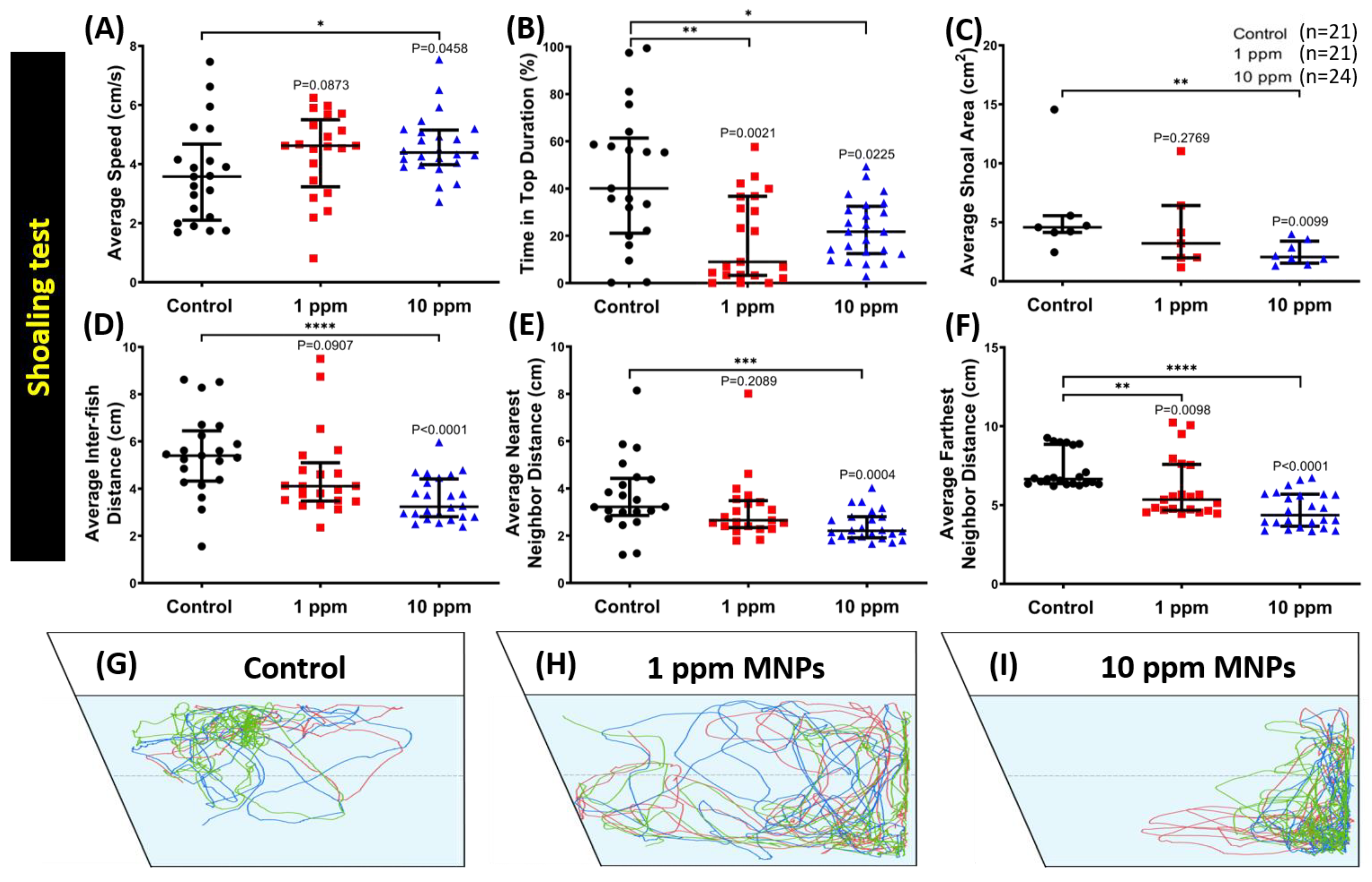
3.5. MNPs Exposure Tightened Shoaling Behavior in the High Concentration MNPs Group
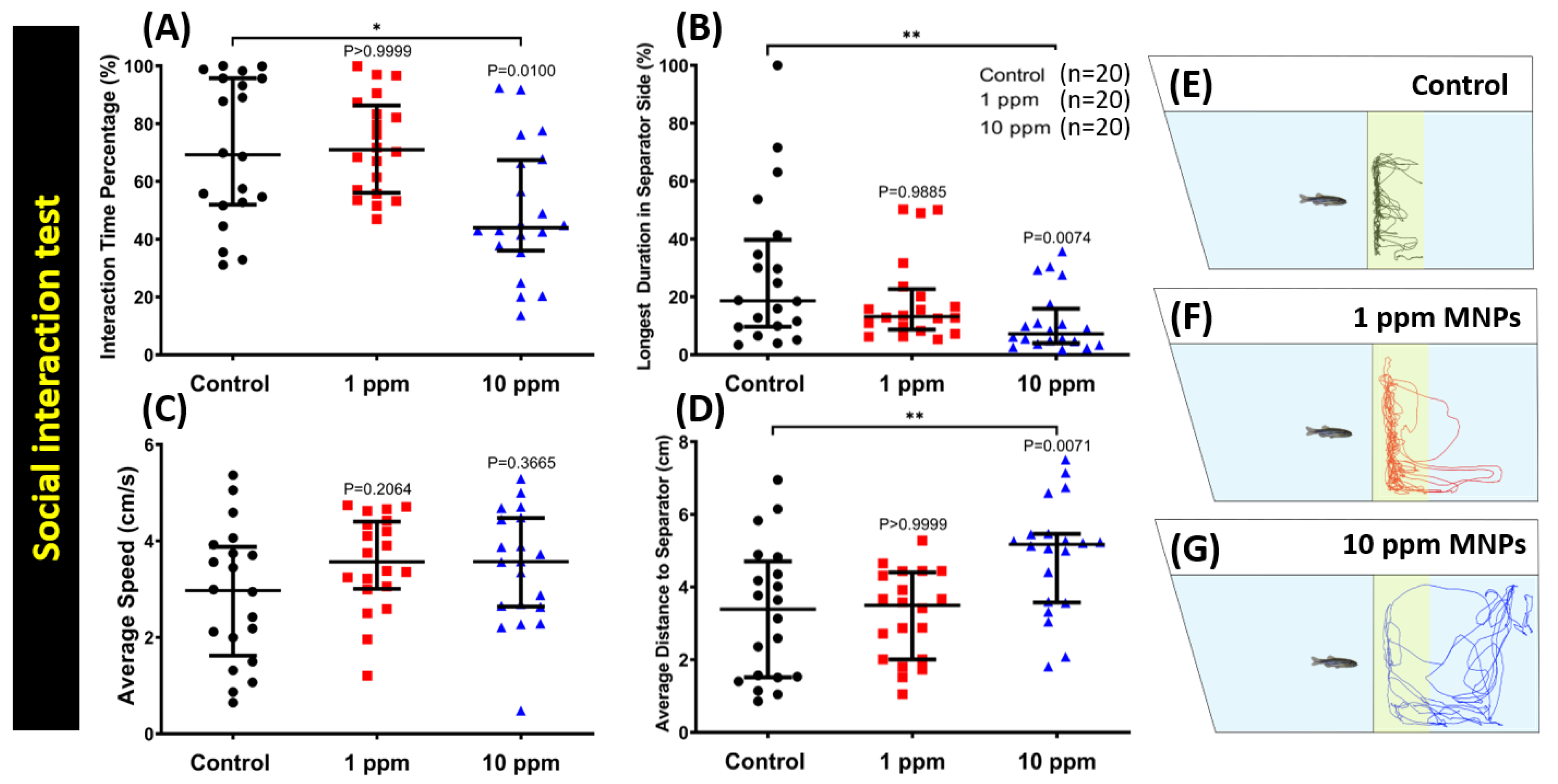
3.6. MNPs Exposure Reduced Conspecific Social Interaction Interest in Zebrafish
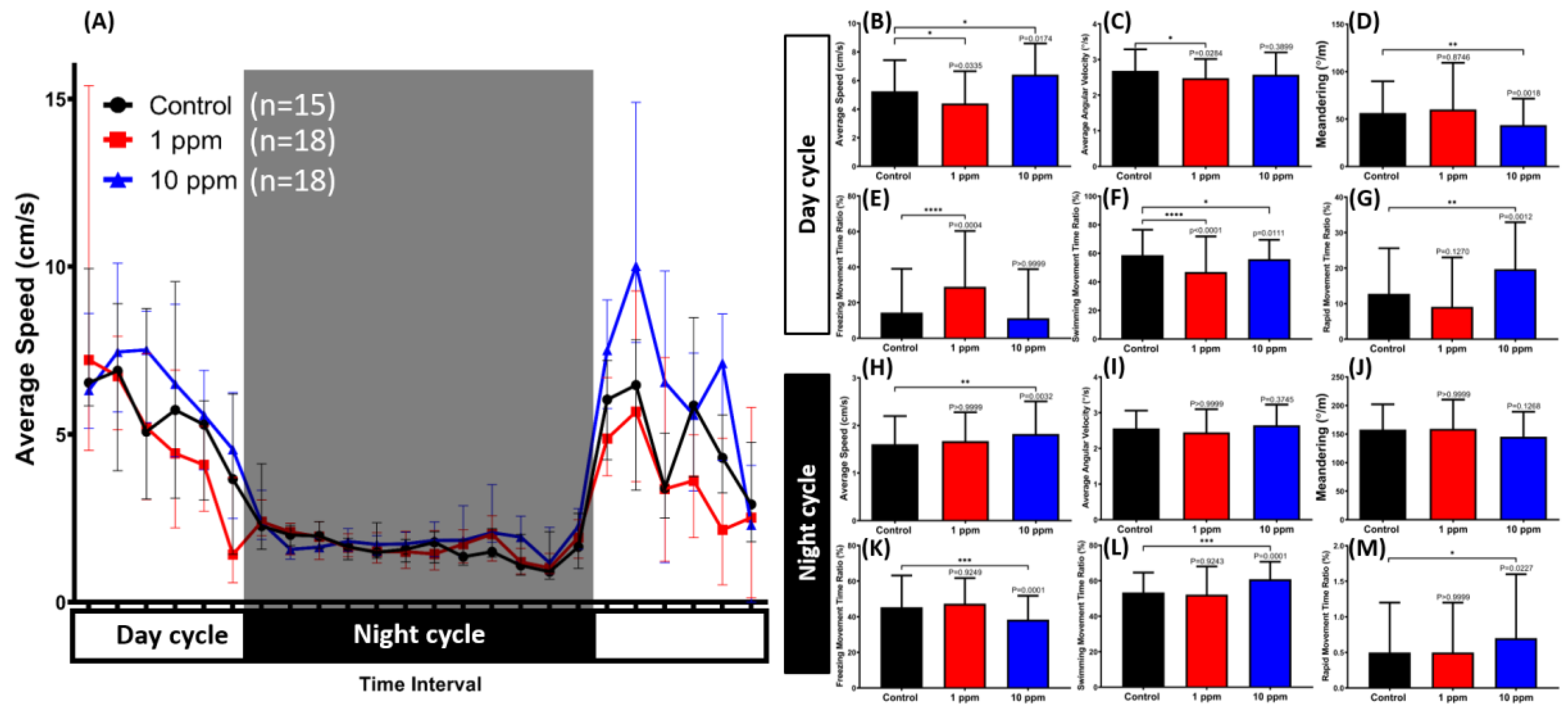
3.7. MNPs Induced Dysregulation of Zebrafish Circadian Rhythm Locomotion Activity
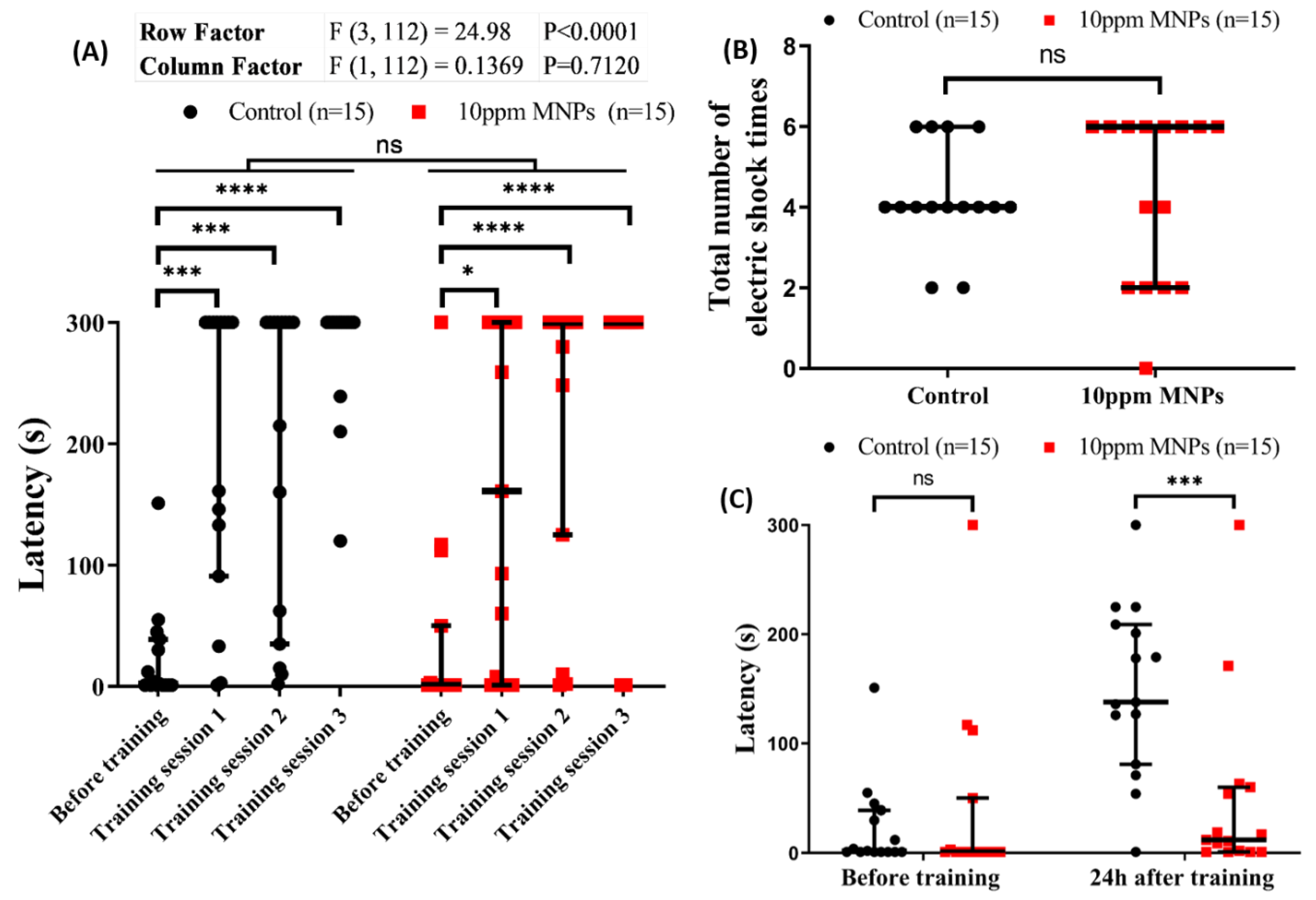
3.8. MNPs Reduced Short-Term Memory Retention in Zebrafish
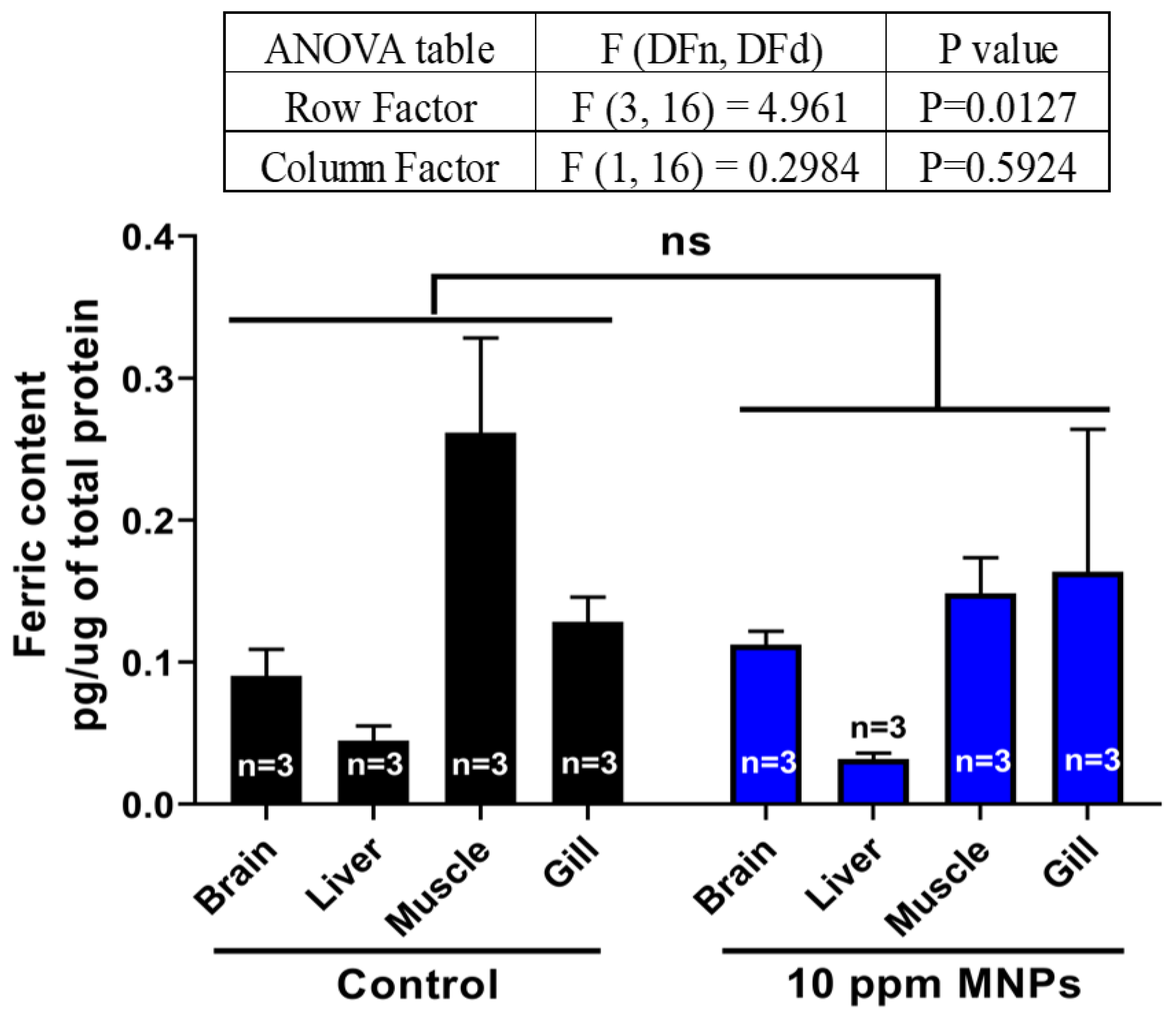
3.9. MNPs Exposure on Ferric (Metal) Content, Reactive Oxygen Species (ROS), and Stress Hormones in the Brain
3.10. MNPs Exposure on the Expression of Neurotransmitters in the Brain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.M.C.; Guo, M.Y.; Leung, Y.H.; Chan, C.M.; Wong, S.W.; Yung, M.M.; Ma, A.P.; Djurišić, A.B.; Leung, F.C.; Leung, K.M. Metal oxide nanoparticles with low toxicity. J. Photochem. Photobiol. B Biol. 2015, 151, 17–24. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.M.T.; de Oliveira, E.M.N.; Pereira, T.C.B.; Papaléo, R.M.; Bogo, M.R. Implications of exposure to dextran-coated and uncoated iron oxide nanoparticles to developmental toxicity in zebrafish. J. Nanopart. Res. 2017, 19, 389. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting mri and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.N.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Lévy, M.; Bacri, J.-C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative organization in iron oxide multi-core nanoparticles potentiates their efficiency as heating mediators and mri contrast agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Zheng, M.; Lu, J.; Zhao, D. Effects of starch-coating of magnetite nanoparticles on cellular uptake, toxicity and gene expression profiles in adult zebrafish. Sci. Total Environ. 2018, 622, 930–941. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, S.; Cai, Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (danio rerio) early life stages. PLoS ONE 2012, 7, e46286. [Google Scholar] [CrossRef]
- Rana, S.; Kalaichelvan, P. Ecotoxicity of nanoparticles. Isrn Toxicol. 2013, 2013, 574648. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R., II; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Dutz, S.; Clement, J.H.; Eberbeck, D.; Gelbrich, T.; Hergt, R.; Müller, R.; Wotschadlo, J.; Zeisberger, M. Ferrofluids of magnetic multicore nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2009, 321, 1501–1504. [Google Scholar] [CrossRef]
- Aurich, K.; Schwalbe, M.; Clement, J.H.; Weitschies, W.; Buske, N. Polyaspartate coated magnetite nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2007, 311, 1–5. [Google Scholar] [CrossRef]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.-J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Handy, R.D.; Shaw, B.J. Ecotoxicity of nanomaterials to fish: Challenges for ecotoxicity testing. Integr. Environ. Assess. Manag. 2007, 3, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, C.; Barandika, G.; Igartua, A.; Areitioaurtena, O.; Mendoza, G. Key challenges for nanotechnology: Standardization of ecotoxicity testing. J. Environ. Sci. Health Part C 2017, 35, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Moore, M. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef]
- Bundschuh, M.; Seitz, F.; Rosenfeldt, R.R.; Schulz, R. Effects of nanoparticles in fresh waters: Risks, mechanisms and interactions. Freshw. Biol. 2016, 61, 2185–2196. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Gajewicz, A.; Rasulev, B.; Dinadayalane, T.C.; Urbaszek, P.; Puzyn, T.; Leszczynska, D.; Leszczynski, J. Advancing risk assessment of engineered nanomaterials: Application of computational approaches. Adv. Drug Deliv. Rev. 2012, 64, 1663–1693. [Google Scholar] [CrossRef] [PubMed]
- Wehmas, L.C.; Anders, C.; Chess, J.; Punnoose, A.; Pereira, C.B.; Greenwood, J.A.; Tanguay, R.L. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol. Rep. 2015, 2, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Kovrižnych, J.A.; Sotníková, R.; Zeljenková, D.; Rollerová, E.; Szabová, E.; Wimmerová, S. Acute toxicity of 31 different nanoparticles to zebrafish (danio rerio) tested in adulthood and in early life stages—Comparative study. Interdiscip. Toxicol. 2013, 6, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A.; Alshamsan, A. Cobalt iron oxide nanoparticles induce cytotoxicity and regulate the apoptotic genes through ros in human liver cells (hepg2). J. Colloids Surf. B Biointerfaces 2016, 148, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S.; Biplab, K.; Paudel, S.N.; Karna, D.; Shrestha, B.G. Synthesis, characterization, and study of in vitro cytotoxicity of zno-fe3o4 magnetic composite nanoparticles in human breast cancer cell line (mda-mb-231) and mouse fibroblast (nih 3t3). Nanoscale Res. Lett. 2016, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Gutiérrez, L.; Cáceres-Vélez, P.; Santos, D.; Chaves, S.; Fascineli, M.; Garcia, M.; Azevedo, R.; Morales, M. Biotransformation of magnetic nanoparticles as a function of coating in a rat model. Nanoscale 2015, 7, 16321–16329. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; Westerhoff, P.; Posner, J.D. Biological accumulation of engineered nanomaterials: A review of current knowledge. Environ. Sci. Process. 2013, 15, 103–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, L.; Zhou, Y.; Chen, J. Accumulation and elimination of iron oxide nanomaterials in zebrafish (danio rerio) upon chronic aqueous exposure. J. Environ. Sci. 2015, 30, 223–230. [Google Scholar] [CrossRef]
- Vargas, R.A.; Sarmiento, K.; Vásquez, I.C. Zebrafish (danio rerio): A potential model for toxinological studies. Zebrafish 2015, 12, 320–326. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Bohnsack, J.P.; Assemi, S.; Miller, J.D.; Furgeson, D.Y. The primacy of physicochemical characterization of nanomaterials for reliable toxicity assessment: A review of the zebrafish nanotoxicology model. In Nanotoxicity; Springer: Berlin, Germany, 2012; pp. 261–316. [Google Scholar]
- Blaney, L. Magnetite (fe3o4): Properties, synthesis, and applications. Lehigh Rev. 2007, 15, 33–81. [Google Scholar]
- Sneddon, L.U.; Halsey, L.G.; Bury, N.R. Considering aspects of the 3rs principles within experimental animal biology. J. Exp. Biol. 2017, 220, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A versatile setup for measuring multiple behavior endpoints in zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef]
- Moretz, J.A.; Martins, E.P.; Robison, B.D. The effects of early and adult social environment on zebrafish (danio rerio) behavior. Environ. Biol. Fishes 2007, 80, 91–101. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.; Liang, S.-T.; Hao, E.; Lai, Y.-H.; Hsiao, C.-D. Zinc chloride exposure inhibits brain acetylcholine levels, produces neurotoxic signatures, and diminishes memory and motor activities in adult zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.; Lai, Y.-H.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Evaluation of the effects of carbon 60 nanoparticle exposure to adult zebrafish: A behavioral and biochemical approach to elucidate the mechanism of toxicity. Int. J. Mol. Sci. 2018, 19, 3853. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Joo, S.W.; Anzaby, M.; Hanifehpour, Y.; Nasrabadi, H.T.; Davaran, S. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on a549 lung cancer cell line. J. Nanobiotechnol. 2012, 10, 46. [Google Scholar] [CrossRef]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef]
- Pham, M.; Raymond, J.; Hester, J.; Kyzar, E.; Gaikwad, S.; Bruce, I.; Fryar, C.; Chanin, S.; Enriquez, J.; Bagawandoss, S. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In Zebrafish Protocols for Neurobehavioral Research; Springer: Berlin, Germany, 2012; pp. 231–246. [Google Scholar]
- Ahmed, O.; Seguin, D.; Gerlai, R. An automated predator avoidance task in zebrafish. Behav. Brain Res. 2011, 216, 166–171. [Google Scholar] [CrossRef]
- Abaid, N.; Spinello, C.; Laut, J.; Porfiri, M. Zebrafish (danio rerio) responds to images animated by mathematical models of animal grouping. Behav. Brain Res. 2012, 232, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Collins, C.; Kyzar, E.J.; Pham, M.; Roth, A.; Gaikwad, S.; Cachat, J.; Stewart, A.M.; Landsman, S.; Grieco, F. Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 2012, 210, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.; Kasumyan, A. Patterns and mechanisms of schooling behavior in fish: A review. J. Ichthyol. 2000, 40, S163. [Google Scholar]
- Shaw, E. The development of schooling behavior in fishes. Physiol. Zool. 1960, 33, 79–86. [Google Scholar] [CrossRef]
- Miller, N.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Engeszer, R.E.; Da Barbiano, L.A.; Ryan, M.J.; Parichy, D.M. Timing and plasticity of shoaling behaviour in the zebrafish, danio rerio. Anim. Behav. 2007, 74, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Kelln, K.L. Toxicant exposure and bioaccumulation: A common and potentially reversible cause of cognitive dysfunction and dementia. Behav. Neurol. 2015, 2015, 620143. [Google Scholar] [CrossRef]
- Hu, H. Human Health and Heavy Metals. Life Support: The Environment Human Health; MIT Press: Cambridge, MA, USA, 2002; Volume 65. [Google Scholar]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (pb, cd, as and mehg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Kasprzak, K.S. Possible role of oxidative damage in metal-induced carcinogenesis. Cancer Investig. 1995, 13, 411–430. [Google Scholar] [CrossRef]
- Vallyathan, V.; Shi, X.; Castranova, V. Reactive oxygen species: Their relation to pneumoconiosis and carcinogenesis. Environ. Health Perspect. 1998, 106, 1151–1155. [Google Scholar]
- Liu, T.; Wang, R.; Cao, H.; Lin, A. Polyaspartic acid alleviates heavy metal toxicity in zebrafish (danio rerio). Chem. Ecol. 2017, 33, 684–693. [Google Scholar] [CrossRef]
- Jemec, A.; Drobne, D.; Tišler, T.; Sepčić, K. Biochemical biomarkers in environmental studies—Lessons learnt from enzymes catalase, glutathione s-transferase and cholinesterase in two crustacean species. Environ. Sci. Pollut. Res. 2010, 17, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhu, L.; Dong, M.; Wang, J.; Wang, J.; Xie, H.; Liu, T.; Guo, Y. Oxidative stress and genotoxicity of the ionic liquid 1-octyl-3-methylimidazolium bromide in zebrafish (danio rerio). Arch. Environ. Contam. Toxicol. 2014, 67, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Schetinger, M.R.; Porto, N.M.; Moretto, M.B.; Morsch, V.M.; da Rocha, J.B.T.; Vieira, V.; Moro, F.; Neis, R.T.; Bittencourt, S.; Bonacorso, H.G. New benzodiazepines alter acetylcholinesterase and atpdase activities. Neurochem. Res. 2000, 25, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ji, L.; Zhang, X.; Fan, Y.; Ren, Z. The relationship between behavior responses and brain acetylcholinesterase (ache) activity of zebrafish (danio rerio) in cadmium stress. Int. J. Fish. Sci. Res. 2017, 1, 1002. [Google Scholar]
- Ren, Q.; Zhang, T.; Li, S.; Ren, Z.; Yang, M.; Pan, H.; Xu, S.; Qi, L.; Chon, T.-S. Integrative characterization of toxic response of zebra fish (danio rerio) to deltamethrin based on ache activity and behavior strength. Biomed Res. Int. 2016, 2016, 7309184. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Li, T.; Li, X.; Feng, D.; Kuang, X.; Xu, J.; Zhao, X.; Sun, M.; Chen, D. Sio2 nanoparticles cause depression and anxiety-like behavior in adult zebrafish. RSC Adv. 2017, 7, 2953–2963. [Google Scholar] [CrossRef]
- Thörnqvist, P.-O.; McCarrick, S.; Ericsson, M.; Roman, E.; Winberg, S. Bold zebrafish (danio rerio) express higher levels of delta opioid and dopamine d2 receptors in the brain compared to shy fish. Behav. Brain Res. 2019, 359, 927–934. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin, circadian rhythms, and sleep. N. Engl. J. Med. 2000, 343, 1114–1116. [Google Scholar] [CrossRef]
- Cahill, G.M. Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 1996, 708, 177–181. [Google Scholar] [CrossRef]
- Falcon, J.; Migaud, H.; Munoz-Cueto, J.A.; Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, T.-J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.-K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2005, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-T.; Feng, W.-Y.; Wang, B.; Wang, T.-C.; Gu, Y.-Q.; Wang, M.; Wang, Y.; Ouyang, H.; Zhao, Y.-L.; Chai, Z.-F. Comparative study of pulmonary responses to nano-and submicron-sized ferric oxide in rats. Toxicology 2008, 247, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Montes, M.O.; Hanna, S.K.; Lenihan, H.S.; Keller, A.A. Uptake, accumulation, and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. J. Hazard. Mater. 2012, 225, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Lavelle, C.M.; Kane, A.S.; Denslow, N.D.; Barber, D.S. Chronic nanoparticulate silver exposure results in tissue accumulation and transcriptomic changes in zebrafish. Aquat. Toxicol. 2013, 130, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Descamps, L.; Dehouck, M.-P.; Torpier, G.; Cecchelli, R. Receptor-mediated transcytosis of transferrin through blood-brain barrier endothelial cells. In Biology and Physiology of the Blood-Brain Barrier; Springer: Berlin, Germany, 1996; pp. 51–54. [Google Scholar]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Rai, A.; Maurya, S.K.; Khare, P.; Srivastava, A.; Bandyopadhyay, S. Characterization of developmental neurotoxicity of as, cd, and pb mixture: Synergistic action of metal mixture in glial and neuronal functions. Toxicol. Sci. 2010, 118, 586–601. [Google Scholar] [CrossRef]
- Acquas, E.; Wilson, C.; Fibiger, H.C. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: Effects of novelty, habituation, and fear. J. Neurosci. 1996, 16, 3089–3096. [Google Scholar] [CrossRef]
- Miranda, M.a.I.; Ramírez-Lugo, L.; Bermúdez-Rattoni, F. Cortical cholinergic activity is related to the novelty of the stimulus. Brain Res. 2000, 882, 230–235. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Bartolini, L.; Kopf, S.R.; Pepeu, G. Acetylcholine release from the frontal cortex during exploratory activity. Brain Res. 1998, 784, 218–227. [Google Scholar] [CrossRef]
- McIntyre, C.K.; Pal, S.N.; Marriott, L.K.; Gold, P.E. Competition between memory systems: Acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J. Neurosci. 2002, 22, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Acosta, D.; Danielle, N.M.; Altenhofen, S.; Luzardo, M.D.; Costa, P.G.; Bianchini, A.; Bonan, C.D.; da Silva, R.S.; Dafre, A.L. Copper at low levels impairs memory of adult zebrafish (danio rerio) and affects swimming performance of larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 185, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Strungaru, S.-A.; Robea, M.A.; Plavan, G.; Todirascu-Ciornea, E.; Ciobica, A.; Nicoara, M. Acute exposure to methylmercury chloride induces fast changes in swimming performance, cognitive processes and oxidative stress of zebrafish (danio rerio) as reference model for fish community. J. Trace Elem. Med. Biol. 2018, 47, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 2011, 32, 116–122. [Google Scholar] [CrossRef]
- Winberg, S.; Nilsson, G.E. Induction of social dominance by l-dopa treatment in arctic charr. Neurorep. Int. J. Rapid Commun. Res. Neurosci. 1992, 3, 243–246. [Google Scholar] [CrossRef]
- Cabib, S.; Puglisi-Allegra, S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012, 36, 79–89. [Google Scholar] [CrossRef]
- Kulisevsky, J. Role of dopamine in learning and memory. Drugs Aging 2000, 16, 365–379. [Google Scholar] [CrossRef]
- El-Ghundi, M.; O’Dowd, B.F.; George, S.R. Insights into the role of dopamine receptor systems in learning and memory. Rev. Neurosci. 2007, 18, 37–66. [Google Scholar] [CrossRef]

| Biomarker | WT | MNP (1 ppm) | – | MNP (10 ppm) | – | Unit | Significance | ANOVA F (2,6) Value | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Ferric ion | 0.04 ± 0.01 | 0.09 ± 0.04 | ns | 0.04 ± 0.01 | ns | pg/ug of total protein | NO | 1.04 | 0.4107 |
| Metallothionein | 0.30 ± 0.04 | 0.32 ± 0.06 | ns | 0.27 ± 0.05 | ns | pg/ug of total protein | NO | 0.26 | 0.7737 |
| ROS | 86.19 ± 23.91 | 97.30 ± 16.03 | ns | 69.03 ± 18.82 | ns | nmol/ug of total protein | NO | 0.51 | 0.6220 |
| Catalase | 3.85 ± 0.46 | 5.17 ± 1.13 | ns | 9.35 ± 1.63 | * | U/ug of total protein | YES | 5.91 | 0.0380 |
| TBARS | 46.19 ± 15.77 | 41.76 ± 6.76 | ns | 34.15 ± 7.35 | ns | ng/ug of total protein | NO | 0.31 | 0.7383 |
| ssDNA | 2.06 ± 0.35 | 2.11 ± 0.24 | ns | 3.21 ± 0.20 | * | U/ug of total protein | YES | 5.56 | 0.042 |
| ATP | 240.70 ± 62.62 | 383.00 ± 108.50 | ns | 355.50 ± 84.78 | ns | pg/ug of total protein | NO | 0.74 | 0.5132 |
| Creatine Kinase | 10.30 ± 2.37 | 11.59 ± 2.13 | ns | 10.59 ± 3.10 | ns | pg/ug of total protein | NO | 0.06 | 0.9342 |
| Hif-1α | 34.20 ± 7.35 | 37.29 ± 6.06 | ns | 19.60 ± 0.10 | ns | pg/ug of total protein | NO | 2.94 | 0.1284 |
| Cortisol | 18.34 ± 1.30 | 19.42 ± 0.53 | ns | 37.15 ± 4.13 | ** | pg/ug of total protein | YES | 17.42 | 0.0032 |
| Catecholamine | 10.33 ± 2.24 | 17.44 ± 2.73 | ns | 11.73 ± 1.39 | ns | ng/ug of total protein | NO | 2.94 | 0.1283 |
| Acetylcholine esterase | 43.35 ± 5.17 | 22.36 ± 5.84 | * | 18.95 ± 4.41 | * | U/ug of total protein | YES | 6.51 | 0.0313 |
| Acetylcholine | 19.60 ± 3.55 | 22.55 ± 0.70 | ns | 33.07 ± 1.86 | * | U/ug of total protein | YES | 9.06 | 0.0154 |
| Dopamine | 105.90 ± 14.10 | 97.79 ± 3.40 | ns | 55.26 ± 6.35 | * | pg/ug of total protein | YES | 8.84 | 0.0163 |
| GABA | 0.16 ± 0.05 | 0.16 ± 0.04 | ns | 0.08 ± 0.01 | ns | U/ug of total protein | NO | 1.08 | 0.3962 |
| 5-HT | 1.39 ± 0.17 | 1.21 ± 0.03 | ns | 0.64 ± 0.17 | * | ng/ug of total protein | YES | 7.29 | 0.0247 |
| Melatonin | 36.32 ± 10.99 | 33.61 ± 4.73 | ns | 34.20 ± 5.60 | ns | pg/ug of total protein | NO | 0.03 | 0.9660 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, N.; Chen, J.-R.; Sarasamma, S.; Audira, G.; Siregar, P.; Liang, S.-T.; Lai, Y.-H.; Lin, G.-M.; Ger, T.-R.; Hsiao, C.-D. Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing. Nanomaterials 2019, 9, 873. https://doi.org/10.3390/nano9060873
Malhotra N, Chen J-R, Sarasamma S, Audira G, Siregar P, Liang S-T, Lai Y-H, Lin G-M, Ger T-R, Hsiao C-D. Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing. Nanomaterials. 2019; 9(6):873. https://doi.org/10.3390/nano9060873
Chicago/Turabian StyleMalhotra, Nemi, Jung-Ren Chen, Sreeja Sarasamma, Gilbert Audira, Petrus Siregar, Sung-Tzu Liang, Yu-Heng Lai, Geng-Ming Lin, Tzong-Rong Ger, and Chung-Der Hsiao. 2019. "Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing" Nanomaterials 9, no. 6: 873. https://doi.org/10.3390/nano9060873
APA StyleMalhotra, N., Chen, J.-R., Sarasamma, S., Audira, G., Siregar, P., Liang, S.-T., Lai, Y.-H., Lin, G.-M., Ger, T.-R., & Hsiao, C.-D. (2019). Ecotoxicity Assessment of Fe3O4 Magnetic Nanoparticle Exposure in Adult Zebrafish at an Environmental Pertinent Concentration by Behavioral and Biochemical Testing. Nanomaterials, 9(6), 873. https://doi.org/10.3390/nano9060873