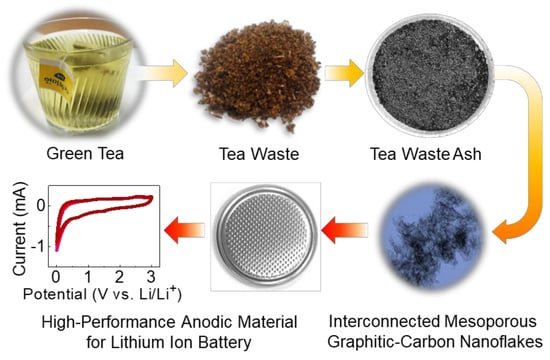
Substantial LIB Anode Performance of Graphitic Carbon Nanoflakes Derived from Biomass Green-Tea Waste
Abstract
1. Introduction
2. Experimental Details
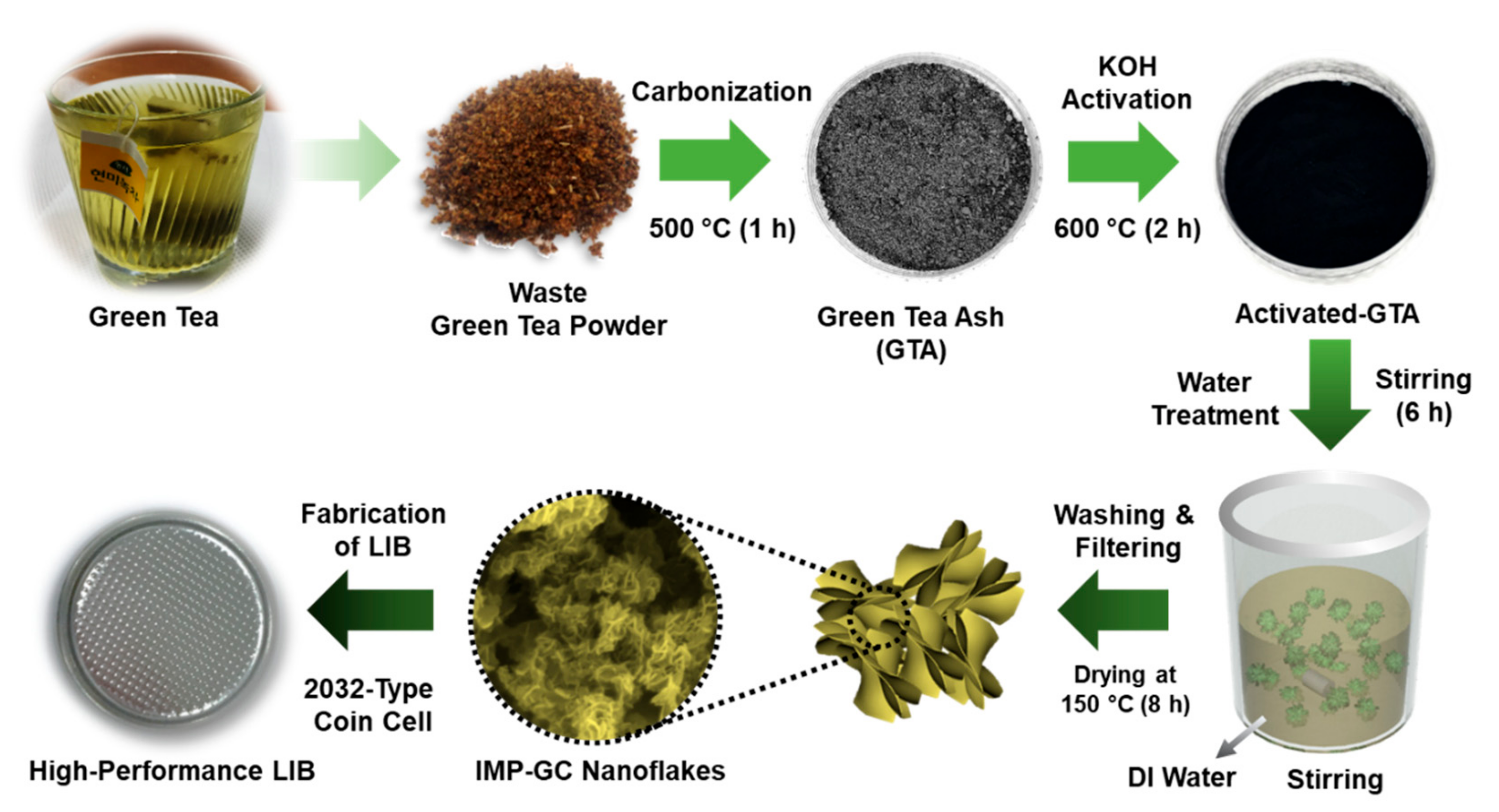
2.1. IMP-GC Nanoflake Synthesis
2.2. Measurements of Material Characteristics
2.3. Electrochemical Performance Measurements
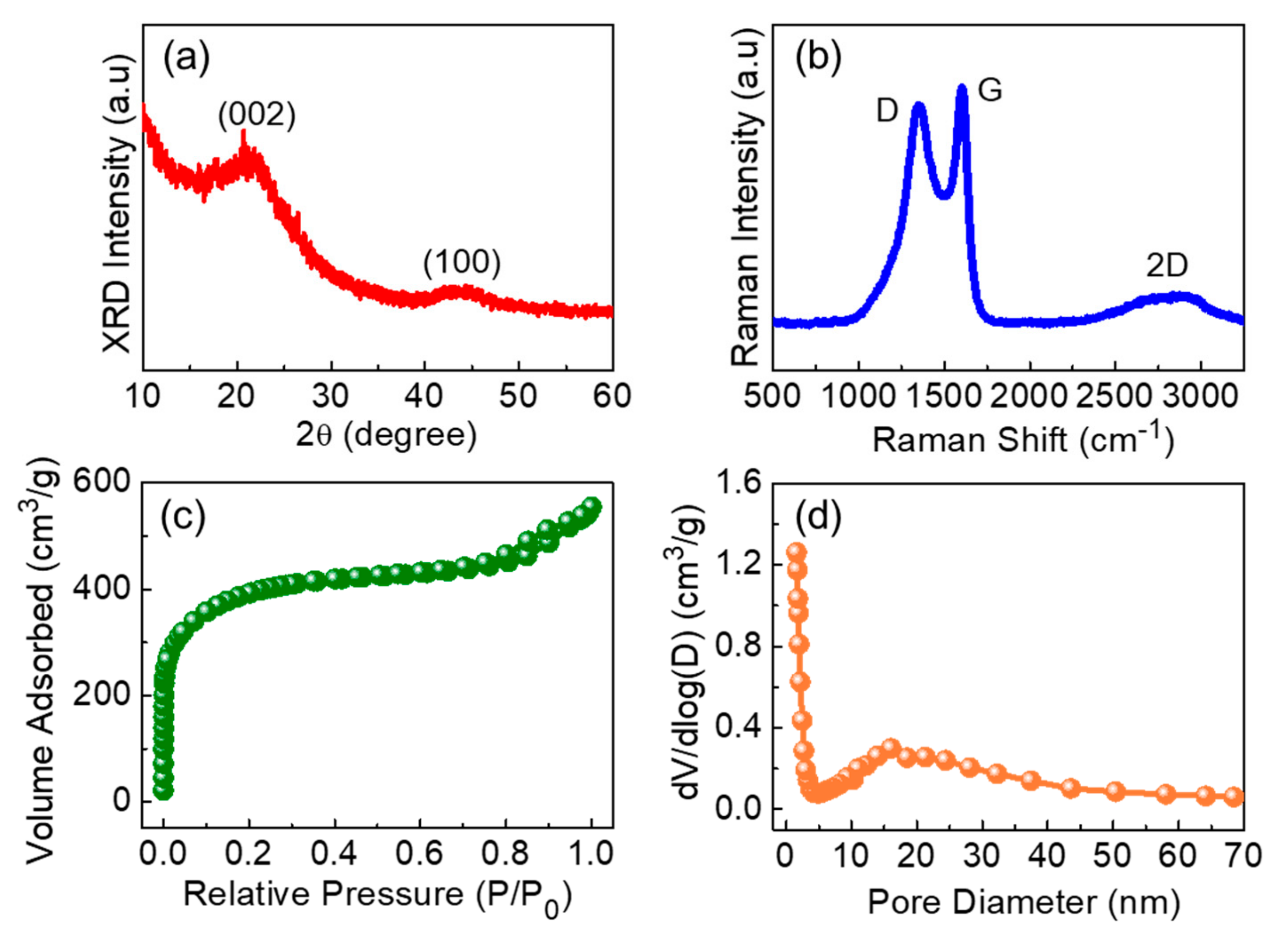
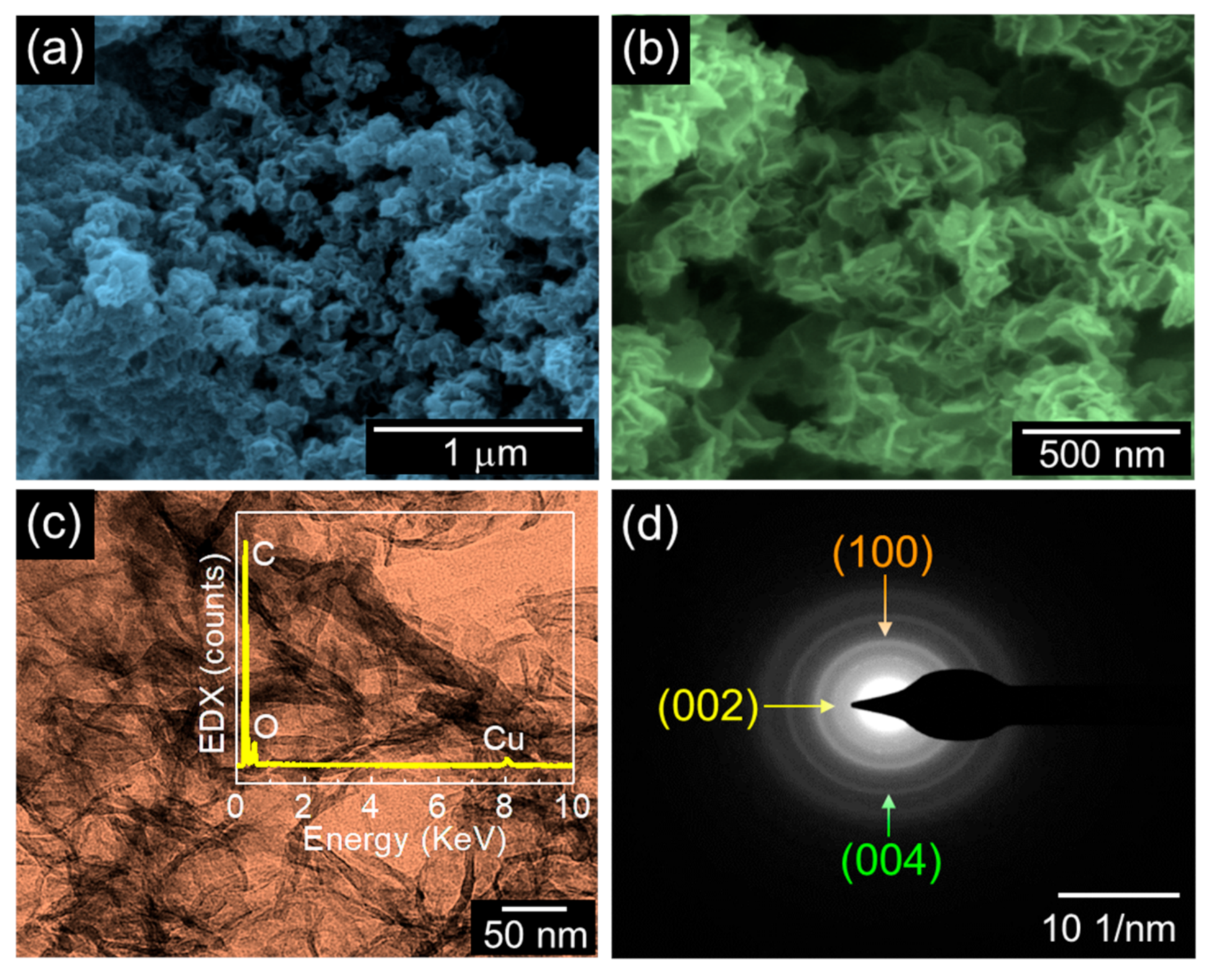
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Poudel, P.; Qiao, Q. Carbon nanostructure counter electrodes for low cost and stable dye-sensitized solar cells. Nano Energy 2014, 4, 157–175. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Decarbonization of fossil fuels as a strategy to control global warming. Renew. Sustain. Energy Rev. 2011, 15, 1828–1834. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-X.; Li, X.-H.; Chen, J.-S. Surface and interface engineering of electrode materials for lithium-ion batteries. Adv. Mater. 2015, 27, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Mariappan, V.K.; Kim, S.J. Copper tungsten sulfide anchored on Ni-foam as a high-performance binder free negative electrode for asymmetric supercapacitor. Chem. Eng. J. 2019, 359, 409–418. [Google Scholar] [CrossRef]
- Mondal, A.K.; Su, D.; Wang, Y.; Chen, S.; Wang, G. Hydrothermal synthesis of nickel oxide nanosheets for lithium-ion batteries and supercapacitors with excellent performance. Chem. Asian J. 2013, 8, 2828–2832. [Google Scholar] [CrossRef] [PubMed]
- Boukhalfa, S.; Evanoff, K.; Yushin, G. Atomic layer deposition of vanadium oxide on carbon nanotubes for high-power supercapacitor electrodes. Energy Environ. Sci. 2012, 5, 6872–6879. [Google Scholar] [CrossRef]
- Yang, C.; Wei, H.; Guan, L.; Guo, J.; Wang, Y.; Yan, X.; Zhang, X.; Wei, S.; Guo, Z. Polymer nanocomposites for energy storage, energy saving, and anticorrosion. J. Mater. Chem. A 2015, 3, 14929–14941. [Google Scholar] [CrossRef]
- Ru, H.; Xiang, K.; Zhou, W.; Zhu, Y.; Zhao, X.S.; Chen, H. Bean-dreg-derived carbon materials used as superior anode material for lithium-ion batteries. Electrochim. Acta 2016, 222, 551–560. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Peng, Y.; Wang, X.; Wang, N.; Wang, J.; Zhao, J. Nitrogen-doped biomass-based hierarchical porous carbon with large mesoporous volume for application in energy storage. Chem. Eng. J. 2018, 348, 850–859. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Lee, J.H.; Je, S.H.; Buyukcakir, O.; Kwon, T.-W.; Polychronopoulou, K.; Choi, J.W.; Coskun, A. Chemical blowing approach for ultramicroporous carbon nitride frameworks and their applications in gas and energy storage. Adv. Funct. Mater. 2017, 27, 1604658. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Talapaneni, S.N.; Coskun, A. Chemically activated covalent triazine frameworks with enhanced textural properties for high capacity gas storage. ACS Appl. Mater. Interfaces 2017, 9, 30679–30685. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Ouyang, H.; Gong, Q.; Li, C.; Huang, J.; Xu, Z. Porphyra derived hierarchical porous carbon with high graphitization for ultra-stable lithium-ion batteries. Mater. Lett. 2019, 235, 111–115. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Graphitic carbon nanostructures from cellulose. Chem. Phy. Lett. 2010, 490, 63–68. [Google Scholar] [CrossRef]
- Yang, J.; Zuo, S. Facile synthesis of graphitic mesoporous carbon materials from sucrose. Diam. Relat. Mater. 2019, 95, 1–4. [Google Scholar] [CrossRef]
- Wang, H.; Yi, H.; Zhu, C.; Wang, X.; Jin Fan, H. Functionalized highly porous graphitic carbon fibers for high-rate supercapacitive electrodes. Nano Energy 2015, 13, 658–669. [Google Scholar] [CrossRef]
- Selvamani, V.; Ravikumar, R.; Suryanarayanan, V.; Velayutham, D.; Gopukumar, S. Garlic peel derived high capacity hierarchical N-doped porous carbon anode for sodium/lithium ion cell. Electrochim. Acta 2016, 190, 337–345. [Google Scholar] [CrossRef]
- Lim, D.G.; Kim, K.; Razdan, M.; Diaz, R.; Osswald, S.; Pol, V.G. Lithium storage in structurally tunable carbon anode derived from sustainable source. Carbon 2017, 121, 134–142. [Google Scholar] [CrossRef]
- Wang, W.X.; Wan, Y.; Wu, S.F.; Li, M.C.; Cao, L.J.; Lv, F.C.; Yang, M.Y.; Sun, Z.F.; Sun, R.; Lu, Z.G. Graphitized porous carbon prepared from pyrolysis of Sterculia scaphigera and its application in lithium ion batteries. RSC Adv. 2015, 5, 46558–46563. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Bai, T.; Long, B.; Liao, Q.; Ren, Y.; Yang, J. Interconnected highly graphitic carbon nanosheets derived from wheat stalk as high performance anode materials for lithium ion batteries. Green Chem. 2016, 18, 2078–2088. [Google Scholar] [CrossRef]
- Han, S.-W.; Jung, D.-W.; Jeong, J.-H.; Oh, E.-S. Effect of pyrolysis temperature on carbon obtained from green tea biomass for superior lithium ion battery anodes. Chem. Eng. J. 2014, 254, 597–604. [Google Scholar] [CrossRef]
- Choi, C.; Seo, S.-D.; Kim, B.-K.; Kim, D.-W. Enhanced lithium storage in hierarchically porous carbon derived from waste tea leaves. Sci. Rep. 2016, 6, 39099. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Im, Y.B.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Peng, C.; Yan, X.-B.; Wang, R.-T.; Lang, J.-W.; Ou, Y.-J.; Xue, Q.-J. Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electrochim. Acta 2013, 87, 401–408. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Chen, M.; Yan, D.; Zhang, X.; Yu, Z.; Zhu, G.; Zhao, Y.; Lu, S.; Chen, G.; Xu, H.; Yu, A. Activated carbons by a hydrothermal-assisted activated method for Li-ion batteries. Mater. Lett. 2017, 196, 276–279. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Kuang, S.; Xing, W.; Zhuo, S. Hierarchical porous carbon derived from rice husk as a low-cost counter electrode of dye-sensitized solar cells. Renew. Energy 2014, 63, 708–714. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Y.; Tian, C.; Yang, Y.; Wang, L.; Yin, J.; Ma, J.; Wang, R.; Fu, H. Isolated boron and nitrogen sites on porous graphitic carbon synthesized from nitrogen-containing chitosan for supercapacitors. Chem. Sus. Chem. 2014, 7, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.R.; Aravinda, L.S.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Activated carbon derived from non-metallic printed circuit board waste for supercapacitor application. Electrochim. Acta 2016, 211, 488–498. [Google Scholar] [CrossRef]
- Nagalakshmi, M.; Kalaiselvi, N. Mesoporous dominant cashewnut sheath derived bio-carbon anode for LIBs and SIBs. Electrochim. Acta 2019, 304, 175–183. [Google Scholar] [CrossRef]
- Bazan, A.; Nowicki, P.; Półrolniczak, P.; Pietrzak, R. Thermal analysis of activated carbon obtained from residue after supercritical extraction of hops. J. Therm. Anal. Calorim. 2016, 125, 1199–1204. [Google Scholar] [CrossRef]
- ALOthman, Z. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874. [Google Scholar] [CrossRef]
- Sankar, S.; Lee, H.; Jung, H.; Kim, A.; Ahmed, A.T.A.; Inamdar, A.I.; Kim, H.; Lee, S.; Im, H.; Young Kim, D. Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. New J. Chem. 2017, 41, 13792–13797. [Google Scholar] [CrossRef]
- Cui, J.; Xi, Y.; Chen, S.; Li, D.; She, X.; Sun, J.; Han, W.; Yang, D.; Guo, S. Prolifera-green-tide as sustainable source for carbonaceous aerogels with hierarchical pore to achieve multiple energy storage. Adv. Funct. Mater. 2016, 26, 8487–8495. [Google Scholar] [CrossRef]
- Cui, J.; Cheng, F.; Lin, J.; Yang, J.; Jiang, K.; Wen, Z.; Sun, J. High surface area C/SiO2 composites from rice husks as a high-performance anode for lithium ion batteries. Powder Technol. 2017, 311, 1–8. [Google Scholar] [CrossRef]
- Tang, J.; Etacheri, V.; Pol, V.G. Wild Fungus Derived carbon fibers and hybrids as anodes for lithium-ion batteries. ACS Sustain. Chem. Eng. 2016, 4, 2624–2631. [Google Scholar] [CrossRef]
- Chen, S.; Yeoh, W.; Liu, Q.; Wang, G. Chemical-free synthesis of graphene–carbon nanotube hybrid materials for reversible lithium storage in lithium-ion batteries. Carbon 2012, 50, 4557–4565. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.-M.; Wang, Z.-H.; Shao, Q.-G.; Li, X.; Yuan, L.-X.; Hu, X.-L.; Zhang, W.-X.; Huang, Y.-H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.; Liu, H.; Fan, H.; Wang, G. Naturally nitrogen doped porous carbon derived from waste shrimp shells for high-performance lithium ion batteries and supercapacitors. Microporous Mesoporous Mater. 2017, 246, 72–80. [Google Scholar] [CrossRef]
- Tao, L.; Zheng, Y.; Zhang, Y.; Ma, H.; Di, M.; Zheng, Z. Liquefied walnut shell-derived carbon nanofibrous mats as highly efficient anode materials for lithium ion batteries. RSC Adv. 2017, 7, 27113–27120. [Google Scholar] [CrossRef]
- Lv, W.; Wen, F.; Xiang, J.; Zhao, J.; Li, L.; Wang, L.; Liu, Z.; Tian, Y. Peanut shell derived hard carbon as ultralong cycling anodes for lithium and sodium batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Fey, G.T.-K.; Chen, K.-L.; Chang, Y.-C. Effects of surface modification on the electrochemical performance of pyrolyzed sugar carbons as anode materials for lithium-ion batteries. Mater. Chem. Phy. 2002, 76, 1–6. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Hernán, L.; Morales, J.; Olivares-Marín, M.; Gómez-Serrano, V. Improving the performance of biomass-derived carbons in li-ion batteries by controlling the lithium insertion process. J. Electrochem. Soc. 2010, 157, A791–A797. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, W.; Mu, C.; Zhao, J.; Wang, B. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Muñoz-Batista, M.J.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Non-porous carbonaceous materials derived from coffee waste grounds as highly sustainable anodes for lithium-ion batteries. J. Clean. Prod. 2019, 207, 411–417. [Google Scholar] [CrossRef]
- Wu , X.-L.; Chen , L.-L.; Xin, S.; Yin, Y.-X.; Guo, Y.-G.; Kong, Q.-S.; Xia, Y.-Z. Preparation and li storage properties of hierarchical porous carbon fibers derived from alginic acid. ChemSusChem 2010, 3, 703–707. [Google Scholar] [CrossRef]
- Caballero, A.; Hernán, L.; Morales, J. Limitations of Disordered Carbons Obtained from Biomass as Anodes for Real Lithium-Ion Batteries. ChemSusChem 2011, 4, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kong, R.; Wang, X.; Xu, Y.; Wang, F.; Ren, W.; Wang, Y.; Su, F.; Jiang, J.-X. Porous carbons derived from hypercrosslinked porous polymers for gas adsorption and energy storage. Carbon 2017, 114, 608–618. [Google Scholar] [CrossRef]

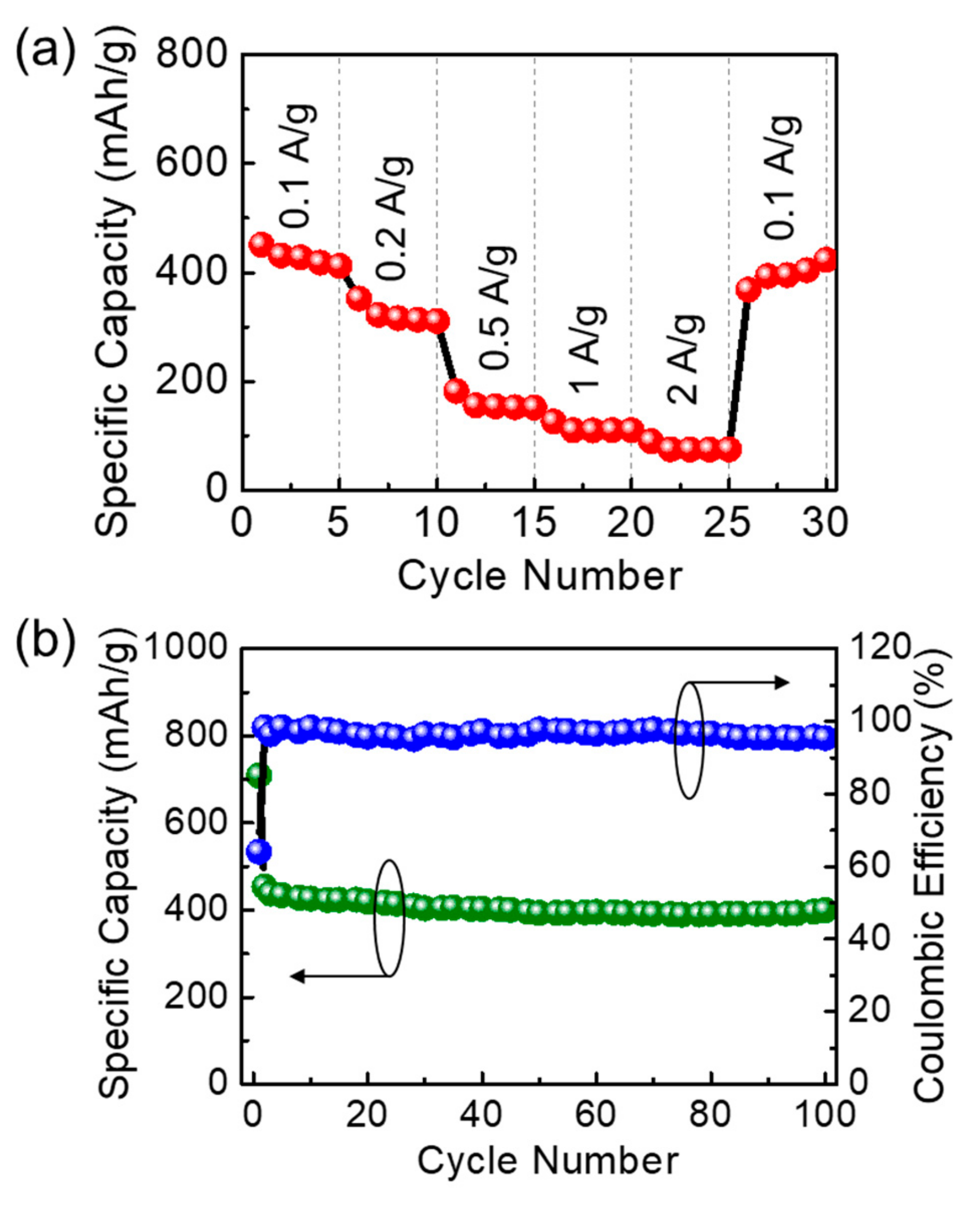
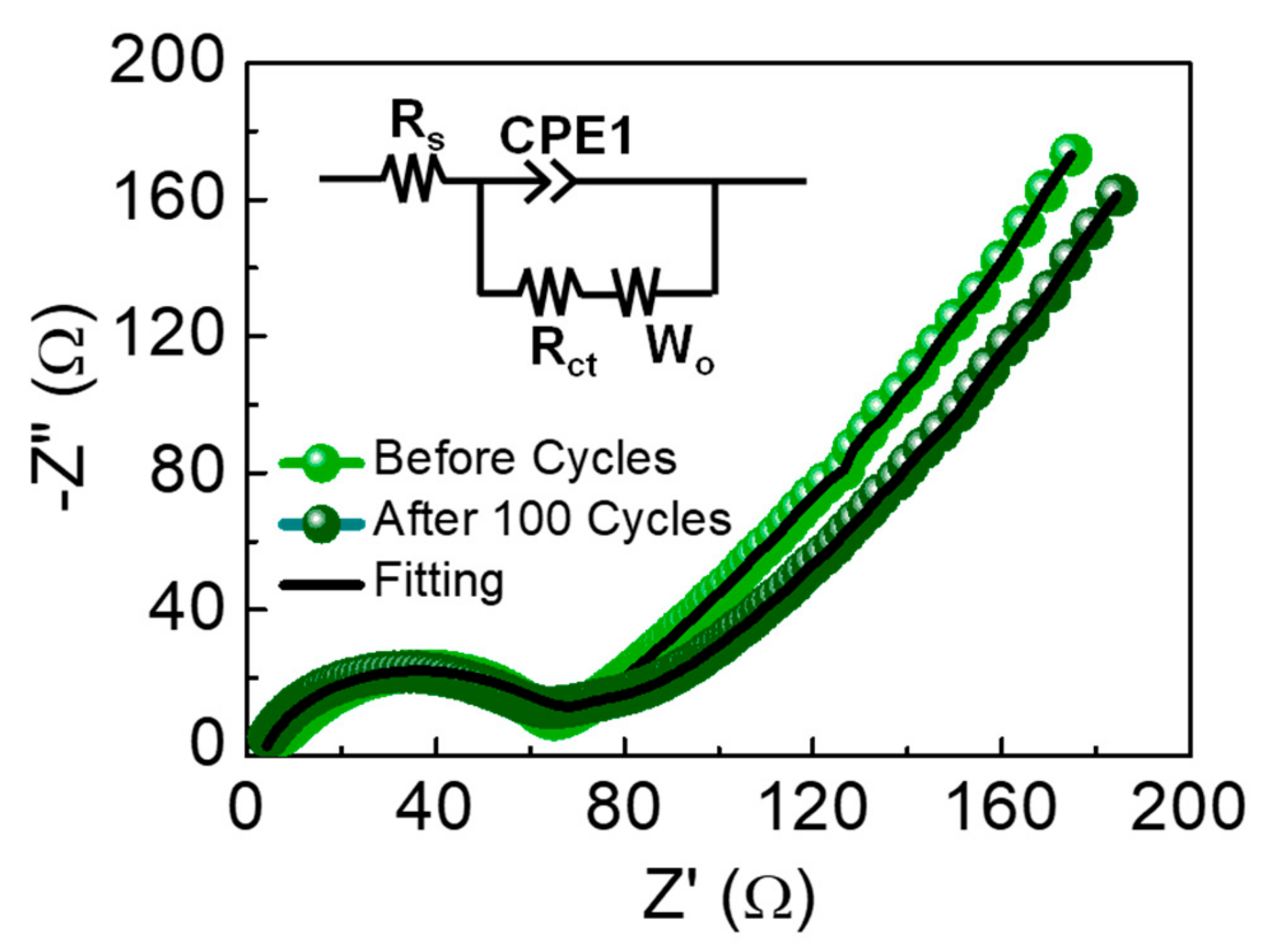
| Biomass Resource | Measurement Condition | Initial Capacity (mAh/g) | Capacity Retention (mAh/g) | Reference |
|---|---|---|---|---|
| Waste green tea | 0.1 A/g | 706 | 400 at 0.1 A/g after 100 cycles | This work |
| Garlic peel | 0.1 A/g | 551 | 540 at 0.1 A/g after 100 cycles | 20 |
| Wheat flour | 1 C | 728 | 217 at 1 C after 100 cycles | 21 |
| Sterculia scaphigera | 0.1 C | 1539 | 423 at 0.1 C after 100 cycles | 22 |
| Wheat stalk | 0.1 C | 502 | ~140 at 10 C after 3000 cycles | 23 |
| Green tea leave | 0.1 C | 530 | ~450 at 0.1 C after 50 cycles | 24 |
| Waste green tea | 0.1 C | 869 | 479 at 0.2 C after 200 cycles | 25 |
| Walnut shell | 0.1 A/g | 150 | 150 at 0.1 A/g after 100 cycles | 43 |
| Peanut shell | 1 A/g | 761 | 314 at 1 A/g after 400 cycles | 44 |
| Sugar | 0.1 A/g | 477 | - | 45 |
| Cherry stones | 0.1 C | 790 | 210 at 0.1 C after 100 cycles | 46 |
| Orange peel | 1 A/g | 878 | 301 at 1 A/g after 100 cycles | 47 |
| Petroleum coke | 0.1 C | 320 | 293 at 0.1 C after 300 cycles | 48 |
| Coffee waste | 0.1 A/g | 359 | 262 at 0.1 A/g after 100 cycles | 49 |
| Alginic acid | 0.7 C | 420 | 80 at 45 C after 1500 cycles | 50 |
| Olive stones | 0.2 C | 615 | 170 at 0.2 C after 100 cycles | 51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Lee, Y.; Kim, D.Y.; Lee, S. Substantial LIB Anode Performance of Graphitic Carbon Nanoflakes Derived from Biomass Green-Tea Waste. Nanomaterials 2019, 9, 871. https://doi.org/10.3390/nano9060871
Sekar S, Lee Y, Kim DY, Lee S. Substantial LIB Anode Performance of Graphitic Carbon Nanoflakes Derived from Biomass Green-Tea Waste. Nanomaterials. 2019; 9(6):871. https://doi.org/10.3390/nano9060871
Chicago/Turabian StyleSekar, Sankar, Youngmin Lee, Deuk Young Kim, and Sejoon Lee. 2019. "Substantial LIB Anode Performance of Graphitic Carbon Nanoflakes Derived from Biomass Green-Tea Waste" Nanomaterials 9, no. 6: 871. https://doi.org/10.3390/nano9060871
APA StyleSekar, S., Lee, Y., Kim, D. Y., & Lee, S. (2019). Substantial LIB Anode Performance of Graphitic Carbon Nanoflakes Derived from Biomass Green-Tea Waste. Nanomaterials, 9(6), 871. https://doi.org/10.3390/nano9060871