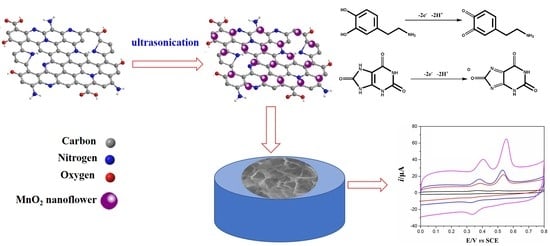
Facile Synthesis of MnO2 Nanoflowers/N-Doped Reduced Graphene Oxide Composite and Its Application for Simultaneous Determination of Dopamine and Uric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials Characterization
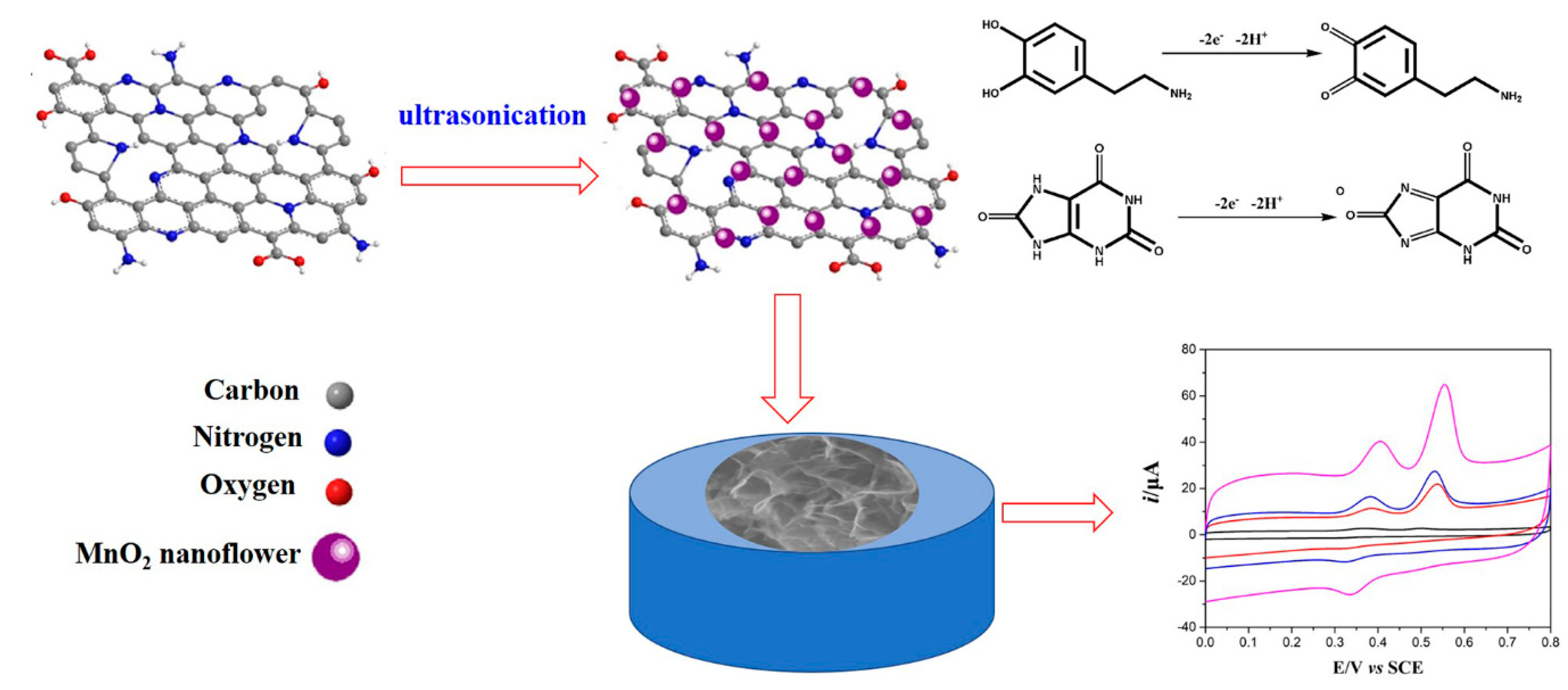
2.3. Synthsis of MnO2NFs/NNrGO Comoposites
2.4. Fabrication of MnO2NFs/NrGO Modified Electrodes
2.5. Procedures for Electrochemical Mesurements
3. Results and Discussion
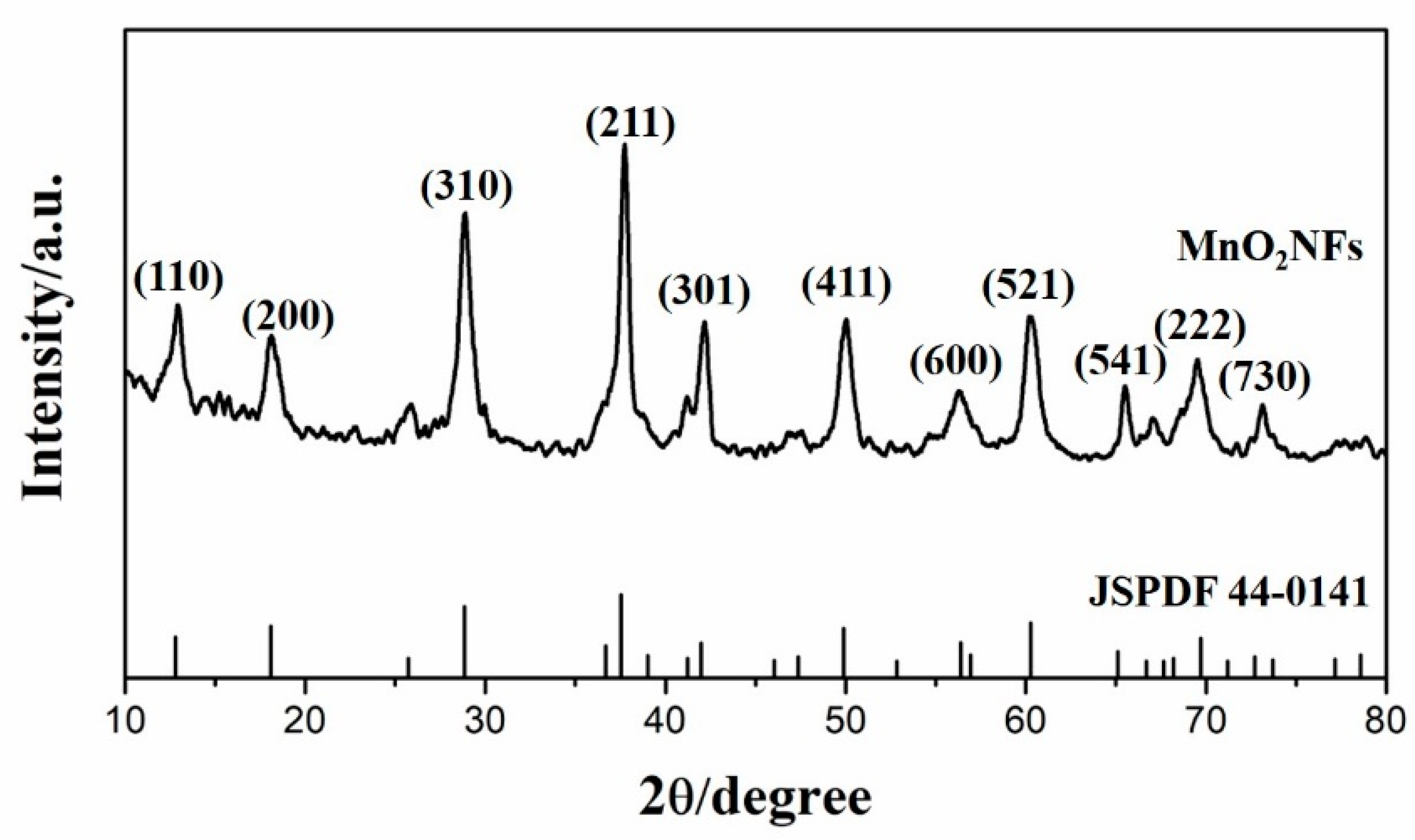
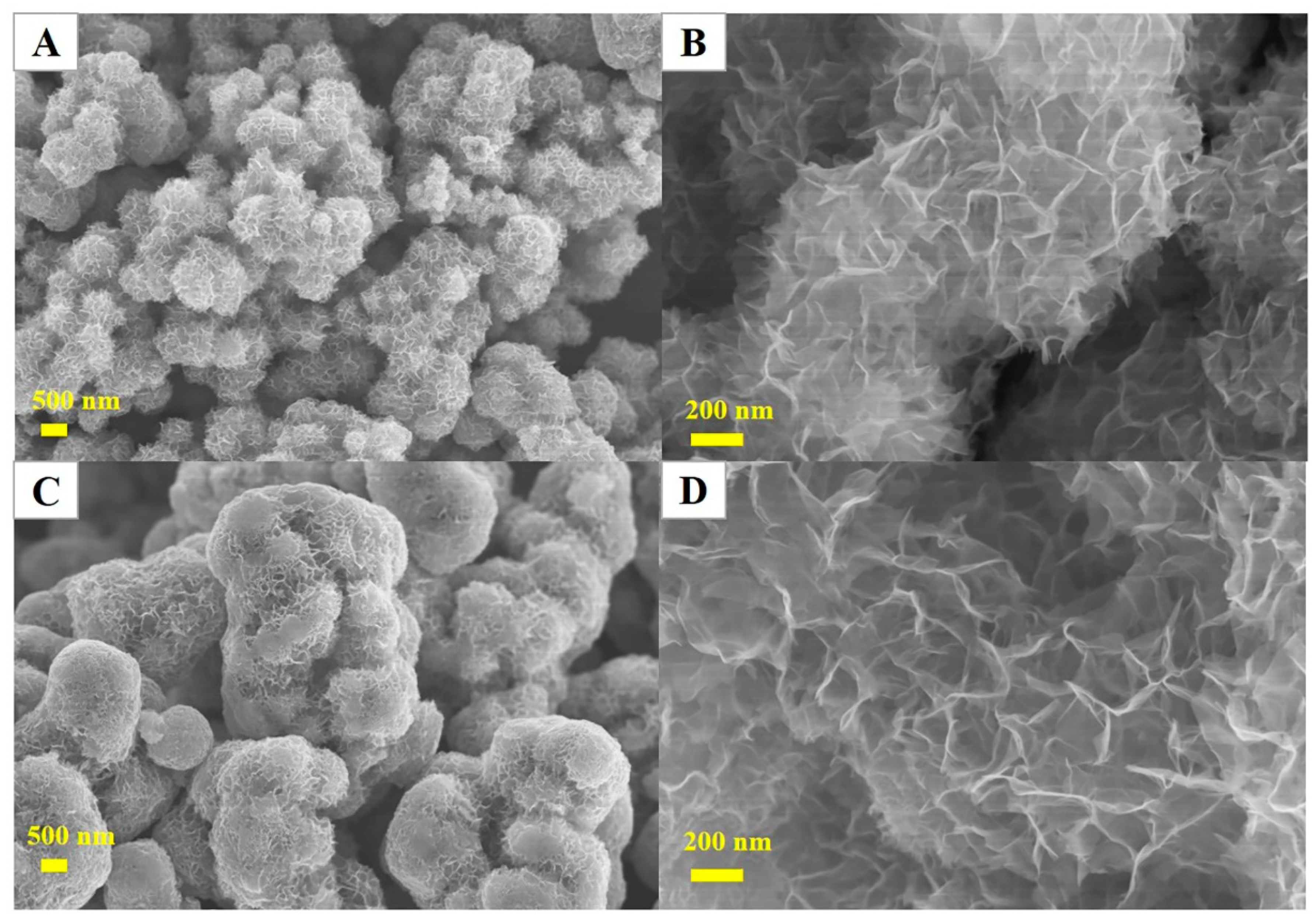
3.1. Physical Chararazation
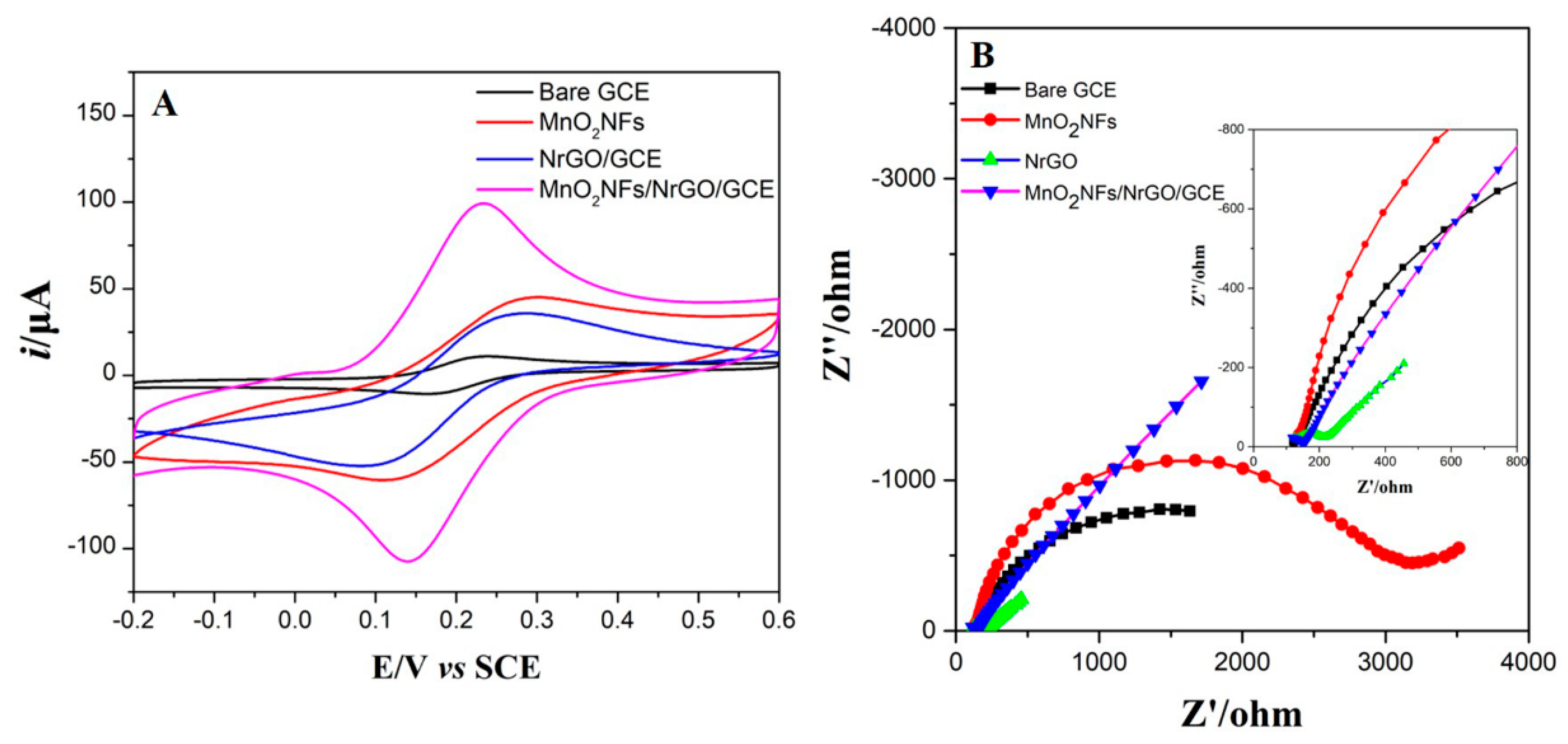
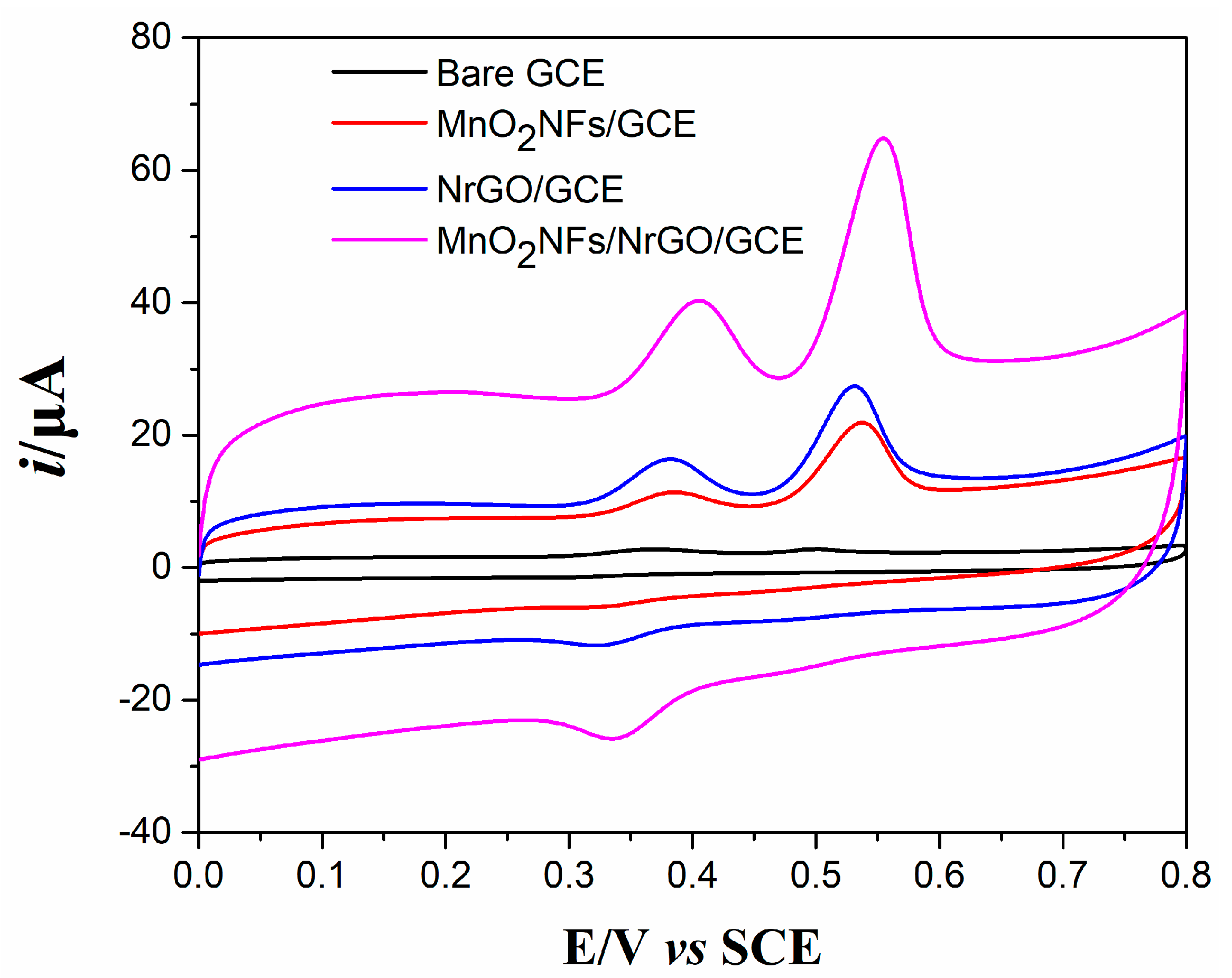
3.2. Evaluation of Electrochemical Performances
3.3. Voltammetric Responses of DA and UA at Various Electrodes
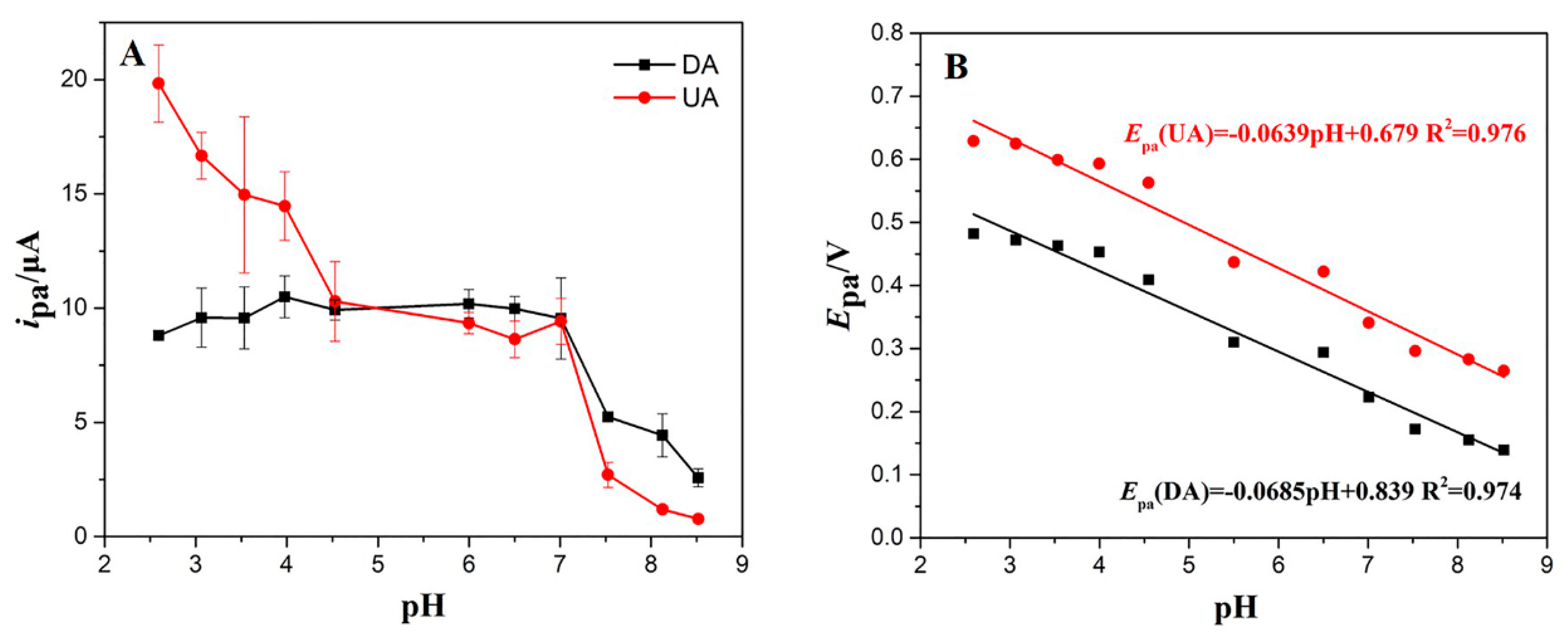
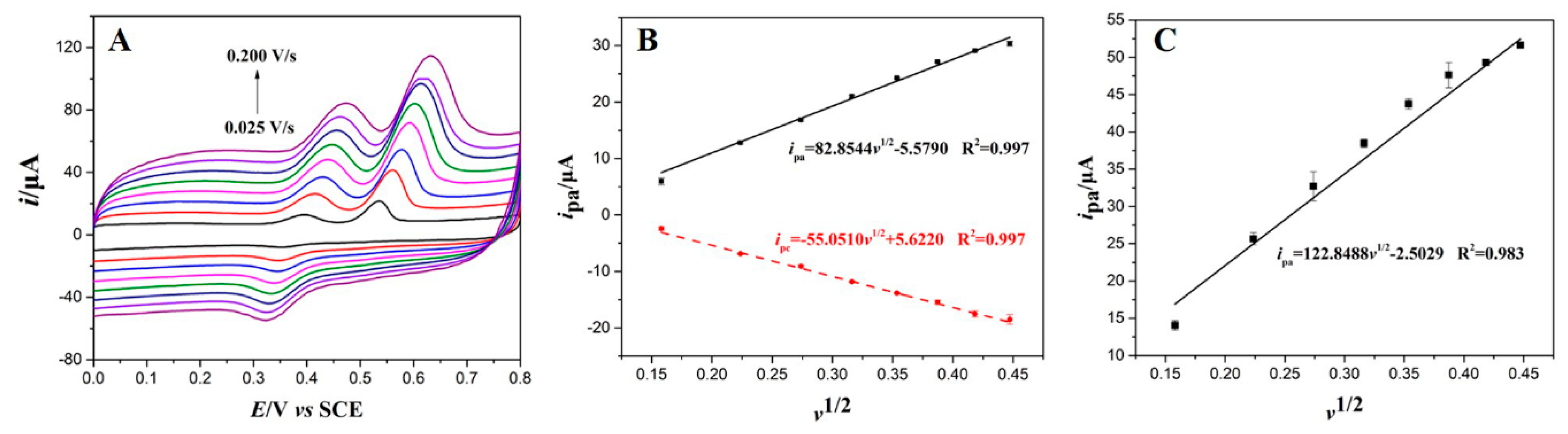
3.4. Optimization of Voltammetrical Parameters
3.4.1. Effect of pH
3.4.2. Effect of Scanning Rate
3.4.3. Influence of Accumulation Parameters
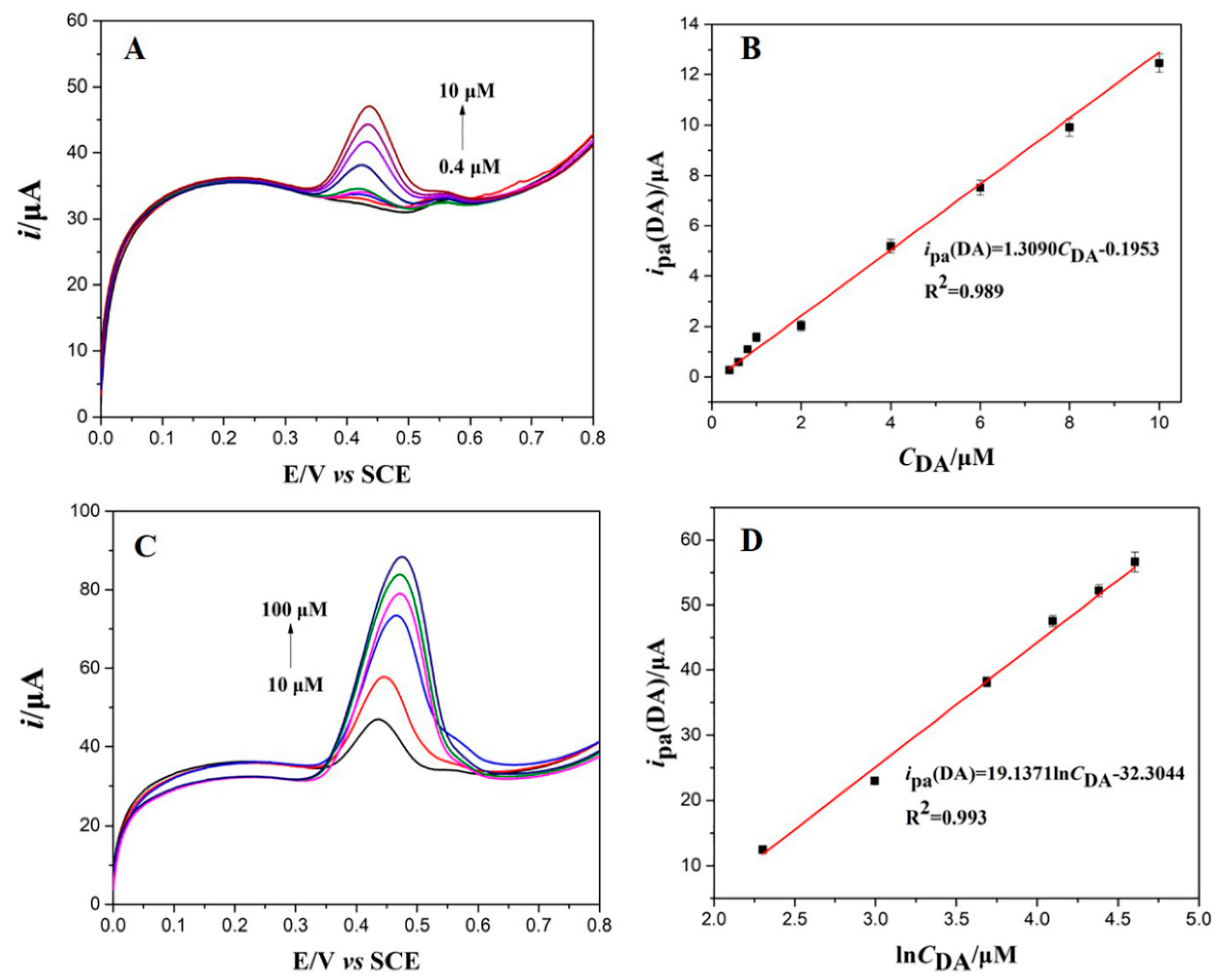
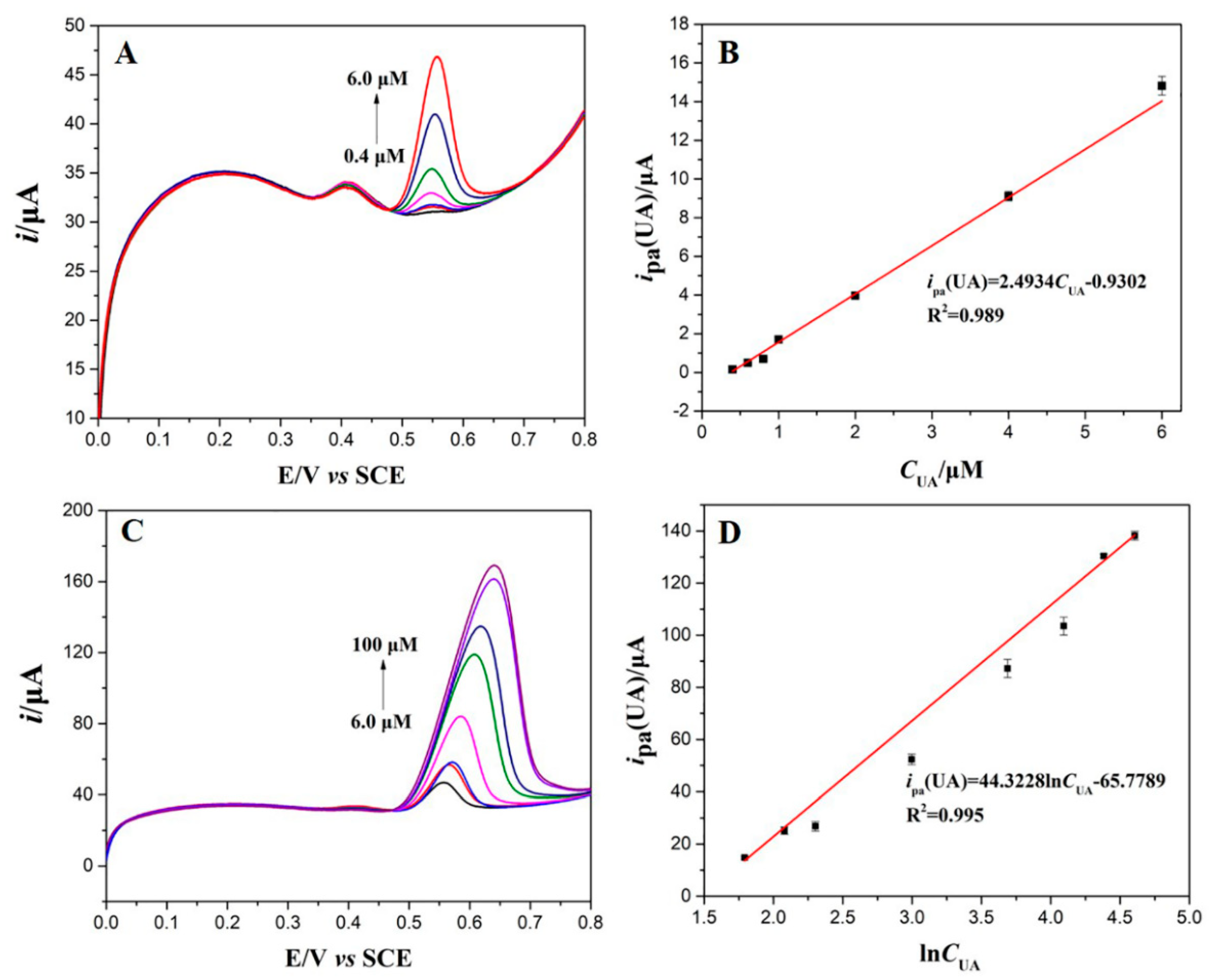
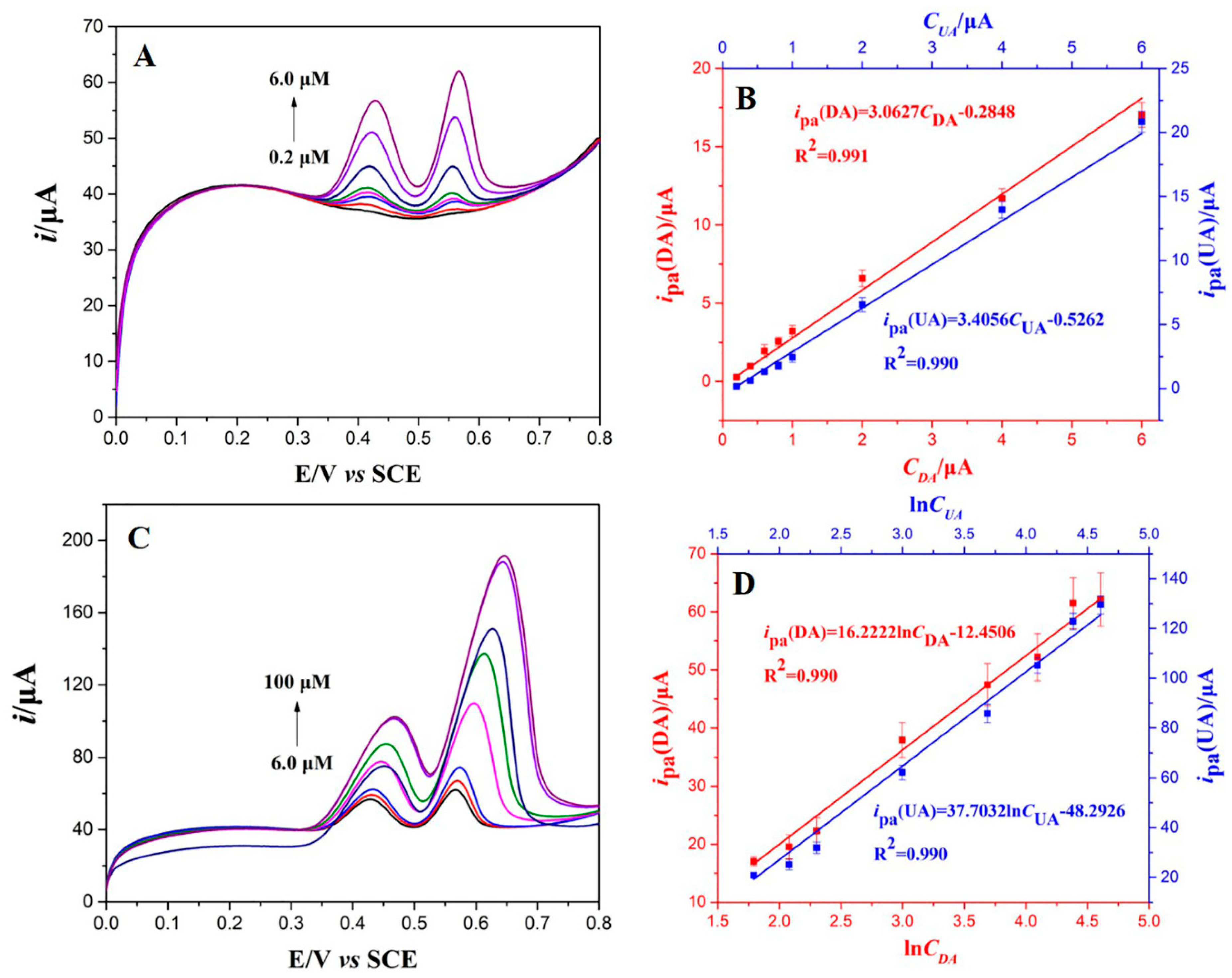
3.5. Individual and Simultaneous Determination of DA and UA
3.6. Selectivity, Repeatability, and Reproducbility Assay
3.7. Determination of DA and UA in Human Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards improvements for penetrating the blood–brain barrier—Recent progress from a material and pharmaceutical perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Dalley, J.W.; Roiser, J.P. Dopamine, serotonin and impulsivity. Neuroscience 2012, 215, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A. Does dopamine play a role in schizophrenia? Psychol. Med. 1977, 7, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liao, C.; Yao, Y.; Liu, Z.; Gong, F.; Yan, F. High-performance dopamine sensors based on whole-graphene solution-gated transistors. Adv. Funct. Mater. 2014, 24, 978–985. [Google Scholar] [CrossRef]
- Wightman, R.M.; May, L.J.; Michael, A.C. Detection of dopamine dynamics in the brain. Anal. Chem. 1988, 60, 769A–779A. [Google Scholar] [CrossRef]
- Jindal, K.; Tomar, M.; Gupta, V. Nitrogen-doped zinc oxide thin films biosensor for determination of uric acid. Analyst 2013, 138, 4353–4362. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Sharaf El Din, U.A.A.; Salem, M.M.; Abdulazim, D.O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 2017, 8, 537–548. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.; Lu, Y.; Yang, H.; Yang, W. Electrogenerated chemiluminescence behavior of peptide nanovesicle and its application in sensing dopamine. Biosens. Bioelectron. 2015, 63, 478–482. [Google Scholar] [CrossRef]
- Xiang, L.-W.; Li, J.; Lin, J.M.; Li, H.F. Determination of gouty arthritis’ biomarkers in human urine using reversed-phase high-performance liquid chromatography. J. Pharm. Anal. 2014, 4, 153–158. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, P.; Yang, L.; Cao, X.; Jin, L. Determination of dopamine in rat striatum by microdialysis and high-performance liquid chromatography with electrochemical detection on a functionalized multi-wall carbon nanotube electrode. Anal. Bioanal. Chem. 2006, 384, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Song, H.; Hao, L.; Liu, X.; Zhang, L.; Lv, Y. Luminescent ZnO quantum dots for sensitive and selective detection of dopamine. Talanta 2013, 107, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.R.; Dadfarnia, S.; Shabani, A.M.H.; Shahbazikhah, P. Chemometric-assisted kinetic–spectrophotometric method for simultaneous determination of ascorbic acid, uric acid, and dopamine. Anal. Biochem. 2011, 410, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kumbhat, S.; Shankaran, D.R.; Kim, S.J.; Gobi, K.V.; Joshi, V.; Miura, N. Surface plasmon resonance biosensor for dopamine using D3 dopamine receptor as a biorecognition molecule. Biosens. Bioelectron. 2007, 23, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, J.; He, G.; Zhong, D.; Chen, J.; Shi, L.; Zhou, X.; Jiang, H. Layer-by-layer assembled multilayer films of reduced graphene oxide/gold nanoparticles for the electrochemical detection of dopamine. J. Electroanal. Chem. 2012, 672, 40–44. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B 2018, 172, 565–572. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of tartrazine based on TiO2-electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef]
- Chen, L.X.; Zheng, J.N.; Wang, A.J.; Wu, L.-J.; Chen, J.R.; Feng, J.J. Facile synthesis of porous bimetallic alloyed PdAg nanoflowers supported on reduced graphene oxide for simultaneous detection of ascorbic acid, dopamine, and uric acid. Analyst 2015, 140, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xi, J.; Wu, Y.; Liu, H.; Fu, C.; Liu, H.; Xiao, F. High loading MnO2 nanowires on graphene paper: Facile electrochemical synthesis and use as flexible electrode for tracking hydrogen peroxide secretion in live cells. Anal. Chim. Acta 2015, 853, 200–206. [Google Scholar] [CrossRef]
- Yang, P.; Hou, Z.; Hang, Y.; Liu, Y.N. Hierarchical architecture of nanographene-coated rice-like manganese dioxide nanorods/graphene for enhanced electrocatalytic activity toward hydrogen peroxide reduction. Mater. Sci. Semicond. Process. 2015, 40, 176–182. [Google Scholar]
- Zeng, F.; Yang, P.; Yong, Y.; Li, Q.; Li, G.; Hou, Z.; Gang, G. Facile construction of Mn3O4-MnO2 hetero-nanorods/graphene nanocomposite for highly sensitive electrochemical detection of hydrogen peroxide. Electrochim. Acta 2016, 196, 587–596. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, J. Synthesis of single-crystal α-MnO2 nanotubes-loaded Ag@C core-shell matrix and their application for electrochemical sensing of nonenzymatic hydrogen peroxide. Talanta 2016, 159, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, M.R.; Alias, Y.; Basirun, W.J.; Pei, M.W.; Sookhakian, M. Facile preparation of MnO2 nanotubes/reduced graphene oxide nanocomposite for electrochemical sensing of hydrogen peroxide. Sens. Actuators B 2014, 201, 526–534. [Google Scholar] [CrossRef]
- Huang, J.; Qiang, Z.; Wang, L. Ultrasensitive electrochemical determination of Ponceau 4R with a novel ε-MnO2 microspheres/chitosan modified glassy carbon electrode. Electrochim. Acta 2016, 206, 176–183. [Google Scholar] [CrossRef]
- Wang, H.E.; Qian, D. Synthesis and electrochemical properties of α-MnO2 microspheres. Mater. Chem. Phys. 2008, 109, 399–403. [Google Scholar] [CrossRef]
- Xue, S.; Jing, P.; Xu, W. Hemin on graphene nanosheets functionalized with flower-like MnO2 and hollow AuPd for the electrochemical sensing lead ion based on the specific DNAzyme. Biosens. Bioelectron. 2016, 86, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.W.; Wei, Z.; Lin, M.; Ma, J.J.; Dong, E.Z.; Zhi, W.T.; Chen, J. Enhanced simultaneous detection of ractopamine and salbutamol—Via electrochemical-facial deposition of MnO2 nanoflowers onto 3D RGO/Ni foam templates. Biosens. Bioelectron. 2016, 78, 259–266. [Google Scholar]
- Gan, T.; Shi, Z.; Deng, Y.; Sun, J.; Wang, H. Morphology–dependent electrochemical sensing properties of manganese dioxide–graphene oxide hybrid for guaiacol and vanillin. Electrochim. Acta 2014, 147, 157–166. [Google Scholar] [CrossRef]
- Ray, C.; Dutta, S.; Roy, A.; Sahoo, R.; Pal, T. Redox mediated synthesis of hierarchical Bi2O3/MnO2 nanoflowers: A Non-Enzymatic Hydrogen Peroxide Electrochemical Sensor. Dalton Trans. 2016, 45, 4780–4790. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, J.; Duan, B.; Zhu, M.; Yang, P.; Du, Y. A three dimensional Pt nanodendrite/graphene/MnO2 nanoflower modified electrode for the sensitive and selective detection of dopamine. J. Mater. Chem. B 2015, 3, 7440–7448. [Google Scholar] [CrossRef]
- Fan, H.; Yan, L.; Dan, W.; Ma, H.; Mao, K.; Fan, D.; Du, B. Electrochemical bisphenol A sensor based on N-doped graphene sheets. Anal. Chim. Acta 2012, 711, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, Characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An Overview of Advanced Heteroatom Doped Carbons with Boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, X.; Zheng, J. A facile one-step synthesis of Fe2O3/nitrogen-doped reduced graphene oxide nanocomposite for enhanced electrochemical determination of dopamine. J. Alloys Compd. 2017, 709, 581–587. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Yang, L.; Li, J. Selective electrochemical detection of dopamine using nitrogen-doped graphene/manganese monoxide composites. RSC Adv. 2015, 5, 85065–85072. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Bard, A.; Faulkner, L. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2001; pp. 669–676. [Google Scholar]
- Li, G.; Wang, S.; Duan, Y.Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B 2018, 277, 250–260. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Duan, Y.Y. Towards gel-free electrodes: A systematic study of electrode-skin impedance. Sens. Actuators B 2017, 241, 1244–1255. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Wang, S.; Duan, Y.Y. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp. Sens. Actuators B 2016, 237, 167–178. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Naderi, H.R.; Norouzi, P.; Ganjali, M.R. Electrochemical Study of a Novel High Performance Supercapacitor Based on MnO2/Nitrogen-Doped Graphene Nanocomposite. Appl. Surf. Sci. 2016, 366, 552–560. [Google Scholar] [CrossRef]
- Zare, H.R.; Rajabzadeh, N.; Nasirizadeh, N.; Ardakani, M.M. Voltammetric studies of an oracet blue modified glassy carbon electrode and its application for the simultaneous determination of dopamine, ascorbic acid and uric acid. J. Electroanal. Chem. 2006, 589, 60–69. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Zheng, X.-Q.; Xu, J.-Y.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous Determination of Ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, B.; Zhong, J.; Yan, B.; Zhang, K.; Zhai, C.; Shiraishi, Y.; Du, Y.; Yang, P. Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2017, 497, 172–180. [Google Scholar] [CrossRef]
- Xu, T.-Q.; Zhang, Q.L.; Zheng, J.-N.; Lv, Z.Y.; Wei, J.; Wang, A.J.; Feng, J.J. Simultaneous determination of dopamine and uric acid in the presence of ascorbic acid using Pt nanoparticles supported on reduced graphene oxide. Electrochim. Acta 2014, 115, 109–115. [Google Scholar] [CrossRef]
- Kogularasu, S.; Akilarasan, M.; Chen, S.-M.; Chen, T.W.; Lou, B.S. Urea-based morphological engineering of ZnO; for the biosensing enhancement towards dopamine and uric acid in food and biological samples. Mater. Chem. Phys. 2019, 227, 5–11. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Q.; Wu, C.; Zhang, Y.; Zeng, L. PtNi bimetallic nanoparticles loaded MoS2 nanosheets: Preparation and electrochemical sensing application for the detection of dopamine and uric acid. Anal. Chim. Acta 2019, 1055, 17–25. [Google Scholar] [CrossRef]
- Liu, Y.; She, P.; Gong, J.; Wu, W.; Xu, S.; Li, J.; Zhao, K.; Deng, A. A novel sensor based on electrodeposited Au–Pt bimetallic nano-clusters decorated on graphene oxide (GO)–electrochemically reduced GO for sensitive detection of dopamine and uric acid. Sens. Actuators B 2015, 221, 1542–1553. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; Abdelwahab, A.A.; Abdelmottaleb, M.; Khairy, M.; El-Safty, S.A. Nanohexagonal Fe2O3 electrode for one-step selective monitoring of dopamine and uric acid in biological samples. Electrocatalysis 2018, 9, 514–525. [Google Scholar] [CrossRef]
- Yin, A.; Wei, X.; Cao, Y.; Li, H. High-quality molybdenum disulfide nanosheets with 3D structure for electrochemical sensing. Appl. Surf. Sci. 2016, 385, 63–71. [Google Scholar] [CrossRef]
- Ghanbari, K.; Moloudi, M. Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Ma, Z. A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim. Acta 2016, 183, 257–263. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Zhang, X.; Liu, D.; Lv, S.; Zhu, D.; Wu, T.; You, T. Simultaneous determination of ascorbic acid, dopamine and uric acid at a nitrogen-doped carbon nanofiber modified electrode. RSC Adv. 2015, 5, 11925–11932. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Odhikari, N.; Shah, S.S.; Hasan, M.M.; Islam, T.; Pal, P.R.; Ahmed Qasem, M.A.; Aziz, M.A. Porous tal palm carbon nanosheets: Preparation, characterization and application for the simultaneous determination of dopamine and uric acid. Nanoscale Adv. 2019, 1, 613–626. [Google Scholar] [CrossRef]

| Electrodes | Methods | Detection Range (μM) | LOD (μM) | Ref. | ||
|---|---|---|---|---|---|---|
| DA | UA | DA | UA | |||
| Au/Cu2O/rGO/GCE | a DPV | 10–90 | 100–900 | 3.9 | 6.5 | [7] |
| NrGO/GCE | DPV | 0.5–170 | 0.1–20 | 0.25 | 0.045 | [46] |
| Pd/RGO/GCE | DPV | 0.45–71 | 6–469.5 | 0.18 | 1.6 | [47] |
| Pt/RGO/GCE | DPV | 10–170 | 10–130 | 0.25 | 0.45 | [48] |
| ZnO/SPCE | DPV | 0.1–374 | 0.1–169 | 0.004 | 0.00849 | [49] |
| PtNi@MoS2/GCE | DPV | 0.5–150 | 0.5–600 | 0.1 | 0.1 | [50] |
| Au–Pt/GO–ERGO | DPV | 0.0682–49,800 | 0.125–82,800 | 0.0207 | 0.0407 | [51] |
| HFP/GCE | DPV | 1–200 | 20–400 | 0.016 | 0.218 | [52] |
| MoS2/GCE | DPV | 1–900 | 1–60 | 0.15 | 0.06 | [53] |
| ZnO/PANI/rGO/GCE | DPV | 0.1–90 | 0.5–90 | 0.017 | 0.12 | [54] |
| MoS2/rGO/GCE | DPV | 5–545 | 25–2745 | 0.05 | 0.46 | [55] |
| NCNF/GCE | DPV | 1–10; 10–200 | 5–200 | 0.5 | 1 | [56] |
| PTPCNs/GCE | DPV | 1–100 | 5–200 | 0.078 | 0.17 | [57] |
| MnO2NFs/NrGO/GCE | LSV | 0.2–6.0; 6.0–100 | 0.2–6.0; 6.0–100 | 0.036 | 0.029 | This work |
| a Samples | Detected (μM) | Added (μM) | Found (μM) | RSD (%) | Recovery (%) | |
|---|---|---|---|---|---|---|
| Serum | DA | b ND | 20 | 18.98 | 4.25 | 94.9 |
| 40 | 38.48 | 3.76 | 96.2 | |||
| UA | 21.8 | 20 | 42.92 | 2.98 | 105.6 | |
| 40 | 63.74 | 2.45 | 104.9 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Yang, S.; Cai, Z.; He, Q.; Ye, Y.; Xia, Y.; Li, G.; Liu, J. Facile Synthesis of MnO2 Nanoflowers/N-Doped Reduced Graphene Oxide Composite and Its Application for Simultaneous Determination of Dopamine and Uric Acid. Nanomaterials 2019, 9, 847. https://doi.org/10.3390/nano9060847
Wan X, Yang S, Cai Z, He Q, Ye Y, Xia Y, Li G, Liu J. Facile Synthesis of MnO2 Nanoflowers/N-Doped Reduced Graphene Oxide Composite and Its Application for Simultaneous Determination of Dopamine and Uric Acid. Nanomaterials. 2019; 9(6):847. https://doi.org/10.3390/nano9060847
Chicago/Turabian StyleWan, Xuan, Shihui Yang, Zhaotian Cai, Quanguo He, Yabing Ye, Yonghui Xia, Guangli Li, and Jun Liu. 2019. "Facile Synthesis of MnO2 Nanoflowers/N-Doped Reduced Graphene Oxide Composite and Its Application for Simultaneous Determination of Dopamine and Uric Acid" Nanomaterials 9, no. 6: 847. https://doi.org/10.3390/nano9060847
APA StyleWan, X., Yang, S., Cai, Z., He, Q., Ye, Y., Xia, Y., Li, G., & Liu, J. (2019). Facile Synthesis of MnO2 Nanoflowers/N-Doped Reduced Graphene Oxide Composite and Its Application for Simultaneous Determination of Dopamine and Uric Acid. Nanomaterials, 9(6), 847. https://doi.org/10.3390/nano9060847