A Facile and Flexible Approach for Large-Scale Fabrication of ZnO Nanowire Film and Its Photocatalytic Applications
Abstract
1. Introduction
2. Materials and Methods
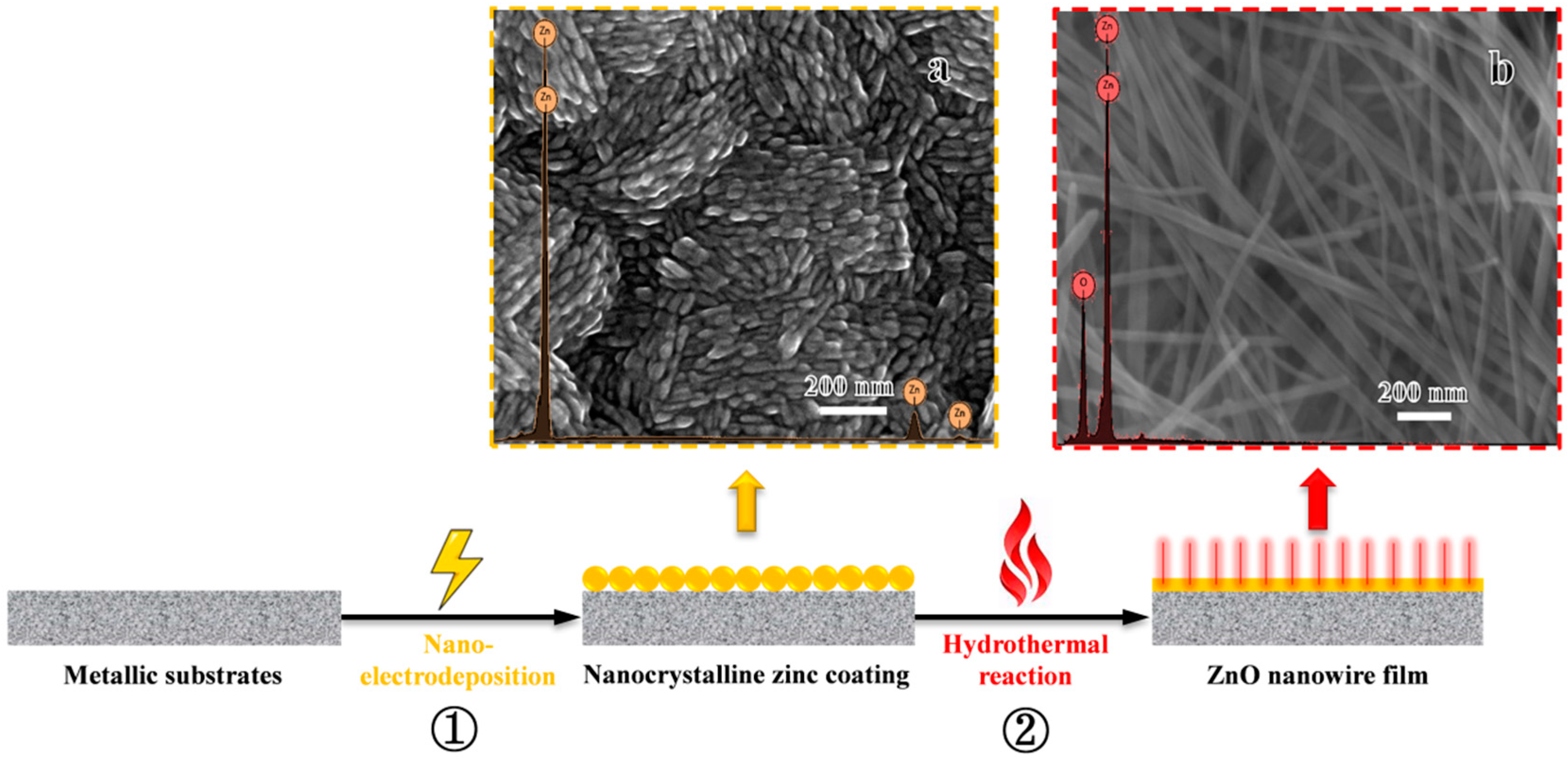
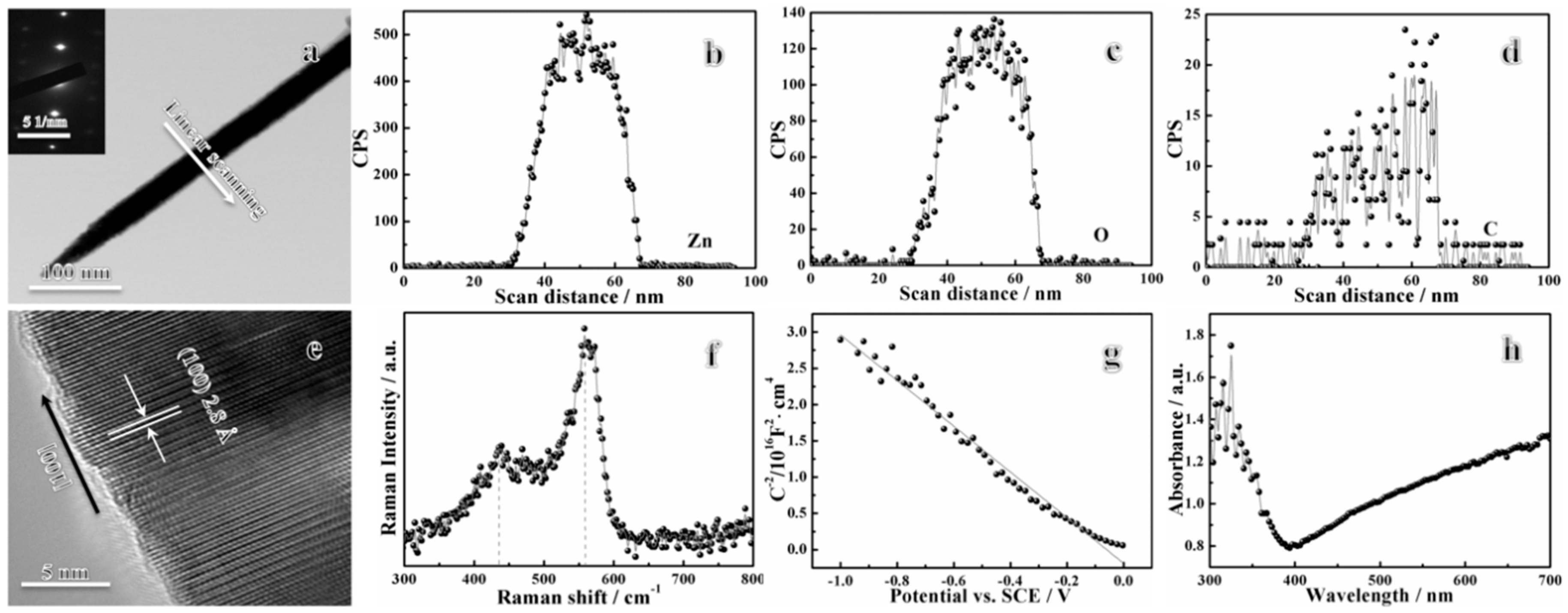
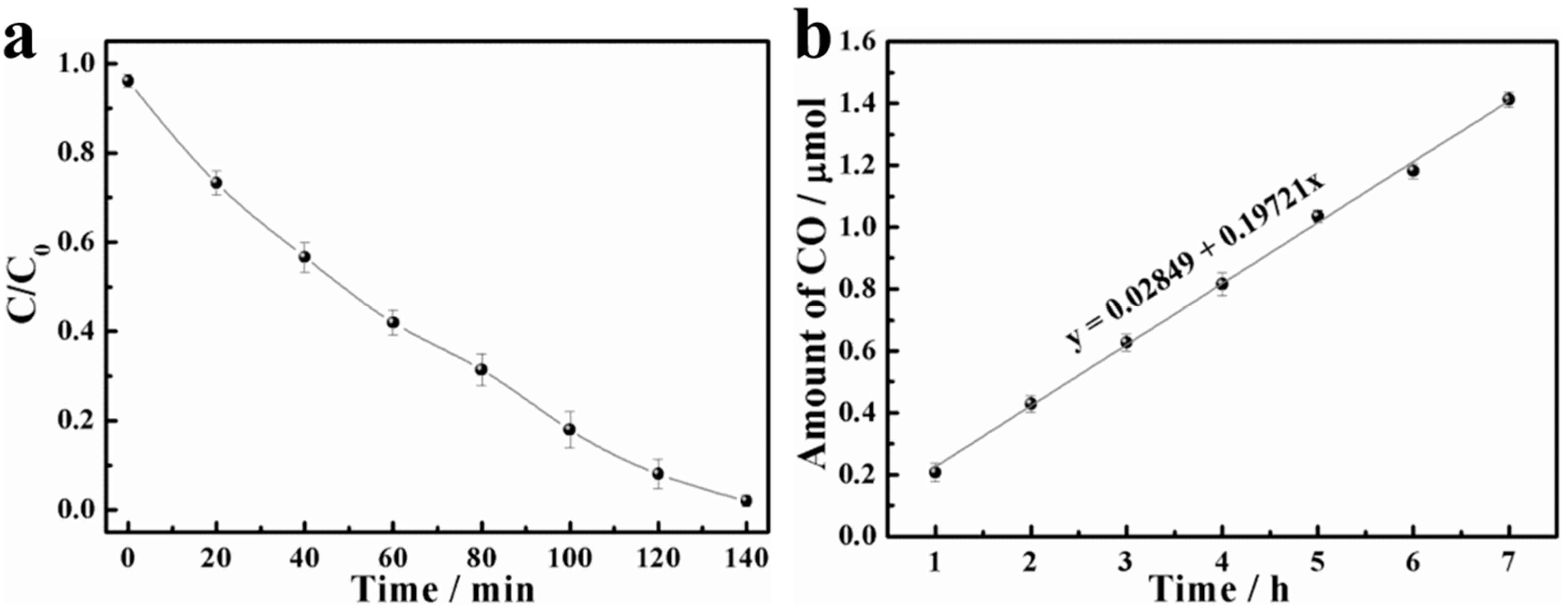
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.L.; Song, J.H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Summers, C.J.; Wang, Z.L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004, 4, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dyesensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.J.; Lin, C.N.; Kuo, C.L.; Huang, M.H. Growth of ultralong ZnO nanowires on silicon substrates by vapor transport and their use as recyclable photocatalysts. Chem. Mater. 2007, 19, 5143–5147. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L. Hydrothermal synthesis and photoluminescence properties of ZnO nanowires. Solid State Commun. 2004, 132, 269–271. [Google Scholar] [CrossRef]
- Chu, S.; Wang, G.; Zhou, W.; Lin, Y.; Chernyak, L.; Zhao, J.; Kong, J.; Li, L.; Ren, J.; Liu, J. Electrically pumped waveguide lasing from ZnO nanowires. Nat. Nanotechnol. 2011, 6, 506. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.R.; Yang, P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.; Zhang, B.R.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407–409. [Google Scholar] [CrossRef]
- Hong, J.; Bae, J.; Wang, Z.L.; Snyder, R.L. Roomtemperature, texture-controlled growth of ZnO thin films and their application for growing aligned ZnO nanowire arrays. Nanotechnology 2009, 20, 085609. [Google Scholar] [CrossRef]
- Chiou, W.T.; Wu, W.Y.; Ting, J.M. Growth of single crystal ZnO nanowires using sputter deposition. Diam. Relat. Mater. 2003, 12, 1841–1844. [Google Scholar] [CrossRef]
- Yao, B.D.; Chan, Y.F.; Wang, N. Formation of ZnO nanostructures by a simple way of thermal evaporation. Appl. Phys. Lett. 2002, 81, 757–759. [Google Scholar] [CrossRef]
- Wu, Z.W.; Tyan, S.L.; Lee, C.R.; Mo, T.S. Bidirectional growth of ZnO nanowires with high optical properties directly on Zn foil. Thin Solid Films 2017, 621, 102–107. [Google Scholar] [CrossRef]
- Li, Y.; Meng, G.W.; Zhang, L.; Phillipp, F. Ordered semiconductor ZnO nanowire arrays and their photoluminescence properties. Appl. Phys. Lett. 2000, 76, 2011–2013. [Google Scholar] [CrossRef]
- Pauporte, T.; Bataille, G.; Joulaud, L.; Vermersch, F.J. Well-aligned ZnO nanowire arrays prepared by seed-layer-free electrodeposition and their cassie-wenzel transition after hydrophobization. J. Phys. Chem. C 2009, 114, 194–202. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of poly (lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C 2017, 79, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Balancing bacteria–osteoblast competition through selective physical puncture and biofunctionalization of ZnO/polydopamine/arginine-glycine-aspartic acid-cysteine nanorods. ACS Nano 2017, 11, 11250–11263. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mat. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Goldberger, J.E.; Kim, F.; Johnson, J.C.; Zhang, Y.; Saykally, R.J.; Yang, P. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 2003, 115, 3139–3142. [Google Scholar] [CrossRef]
- Li, Q.; An, M.; Li, D. In situ growth of ZnO nanowire film on nanocrystalline zinc electrodeposit via a low-temperature hydrothermal reaction. Results Phys. 2019, 12, 1446–1449. [Google Scholar] [CrossRef]
- Li, G.; Dawa, C.; Bu, Q.; Zhen, F.; Lu, X.; Ke, Z.; Hong, H.; Yao, C.; Liu, P.; Tong, Y. Electrochemical synthesis of orientation-ordered ZnO nanorod bundles. Electrochem. Commun. 2007, 9, 863–868. [Google Scholar] [CrossRef]
- Lupan, O.; Guerin, V.M.; Tiginyanu, I.M.; Ursaki, V.V.; Chow, L.; Heinrich, H.; Pauporte, T. Well-aligned arrays of vertically oriented ZnO nanowires electrodeposited on ITO-coated glass and their integration in dye sensitized solar cells. J. Photochem. Photobiol. A 2010, 211, 65–73. [Google Scholar] [CrossRef]
- Youssef, K.M.; Koch, C.C.; Fedkiw, P.S. Improved corrosion behavior of nanocrystalline zinc produced by pulse-current electrodeposition. Corros. Sci. 2004, 46, 51–64. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, G.; Li, S.; Xi, Z.; Guo, D. Fabrication of arrays of zinc oxide nanorods and nanotubes in aqueous solution under an external voltage. J. Cryst. Growth 2007, 299, 184–188. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Xie, Y. Selected-control synthesis of ZnO nanowires and nanorods via a PEG-assisted route. Inorg. Chem. 2003, 42, 8105–8109. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Cai, W.; Cao, B.; Hu, J.; Li, Y.; Liu, P. Surface optical phonon Raman scattering in Zn/ZnO core-shell structured nanoparticles. Appl. Phys. Lett. 2006, 88, 181905. [Google Scholar] [CrossRef]
- Morrison, S.R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes; Plenum Press: New York, NY, USA, 1980; pp. 127–128. [Google Scholar]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Nezar, S.; Cherifi, Y.; Barras, A.; Addad, A.; Dogheche, E.; Saoula, N.; Laoufi, N.A.; Roussel, P.; Szunerits, S.; Boukherroub, R. Efficient reduction of Cr (VI) under visible light irradiation using CuS nanostructures. Arab. J. Chem. 2019, 12, 215–224. [Google Scholar] [CrossRef]
- Meng, Q.; Zhou, Y.; Chen, G.; Hu, Y.; Lv, C.; Qiang, L.; Xing, W. Integrating both homojunction and heterojunction in QDs self-decorated Bi2MoO6/BCN composites to achieve an efficient photocatalyst for Cr (VI) reduction. Chem. Eng. J. 2018, 334, 334–343. [Google Scholar] [CrossRef]
- Jing, F.; Liang, R.; Xiong, J.; Chen, R.; Zhang, S.; Li, Y.; Wu, L. MIL-68 (Fe) as an efficient visible-light-driven photocatalyst for the treatment of a simulated waste-water contain Cr (VI) and Malachite Green. Appl. Catal. B Environ. 2017, 206, 9–15. [Google Scholar] [CrossRef]
- Fellahi, O.; Barras, A.; Pan, G.H.; Coffinier, Y.; Hadjersi, T.; Maamache, M.; Szunerits, S.; Boukherroub, R. Reduction of Cr(VI) to Cr(III) using silicon nanowire arrays under visible light irradiation. J. Hazard. Mater. 2016, 304, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Naimi-Joubani, M.; Shirzad-Siboni, M.; Yang, J.K.; Gholami, M.; Farzadkia, M. Photocatalytic reduction of hexavalent chromium with illuminated ZnO/TiO2 composite. J. Ind. Eng. Chem. 2015, 22, 317–323. [Google Scholar] [CrossRef]
- Yu, J.; Zhuang, S.; Xu, X.; Zhu, W.; Feng, B.; Hu, J. Photogenerated electron reservoir in hetero-p–n CuO-ZnO nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr(VI). J. Mater. Chem. A 2015, 3, 1199–1207. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jing, Q.; Tang, Q.; Mu, Y.; Du, A.K. Facile synthesis of g-C3N4 nanosheets/ZnO nanocomposites with enhanced photocatalytic activity in reduction of aqueous chromium(VI) under visible light. Nanomaterials 2016, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Li, J.; Xu, H.Y. One-step in situ solvothermal synthesis of SnS2/TiO2 nanocomposites with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI). Appl. Catal. B Environ. 2012, 123, 18–26. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yao, L.; Zhang, G.; Dionysiou, D.D.; Li, J.; Du, X. One-step hydrothermal synthesis of high-performance visible-light-driven SnS2/SnO2 nanoheterojunction photocatalyst for the reduction of aqueous Cr(VI). Appl. Catal. B Environ. 2014, 144, 730–738. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Kovacevic, M.; Mojet, B.L.; van Ommen, J.G.; Lefferts, L. Effects of morphology of cerium oxide catalysts for reverse water gas shift reaction. Catal. Lett. 2016, 146, 770–777. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, Q.; Chen, Z.; Ma, Q.; An, M. A Facile and Flexible Approach for Large-Scale Fabrication of ZnO Nanowire Film and Its Photocatalytic Applications. Nanomaterials 2019, 9, 846. https://doi.org/10.3390/nano9060846
Li Q, Wang Q, Chen Z, Ma Q, An M. A Facile and Flexible Approach for Large-Scale Fabrication of ZnO Nanowire Film and Its Photocatalytic Applications. Nanomaterials. 2019; 9(6):846. https://doi.org/10.3390/nano9060846
Chicago/Turabian StyleLi, Qingyang, Qiwei Wang, Zaijun Chen, Quanxin Ma, and Maozhong An. 2019. "A Facile and Flexible Approach for Large-Scale Fabrication of ZnO Nanowire Film and Its Photocatalytic Applications" Nanomaterials 9, no. 6: 846. https://doi.org/10.3390/nano9060846
APA StyleLi, Q., Wang, Q., Chen, Z., Ma, Q., & An, M. (2019). A Facile and Flexible Approach for Large-Scale Fabrication of ZnO Nanowire Film and Its Photocatalytic Applications. Nanomaterials, 9(6), 846. https://doi.org/10.3390/nano9060846



