Reduced Graphene Oxide-Wrapped Super Dense Fe3O4 Nanoparticles with Enhanced Electromagnetic Wave Absorption Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
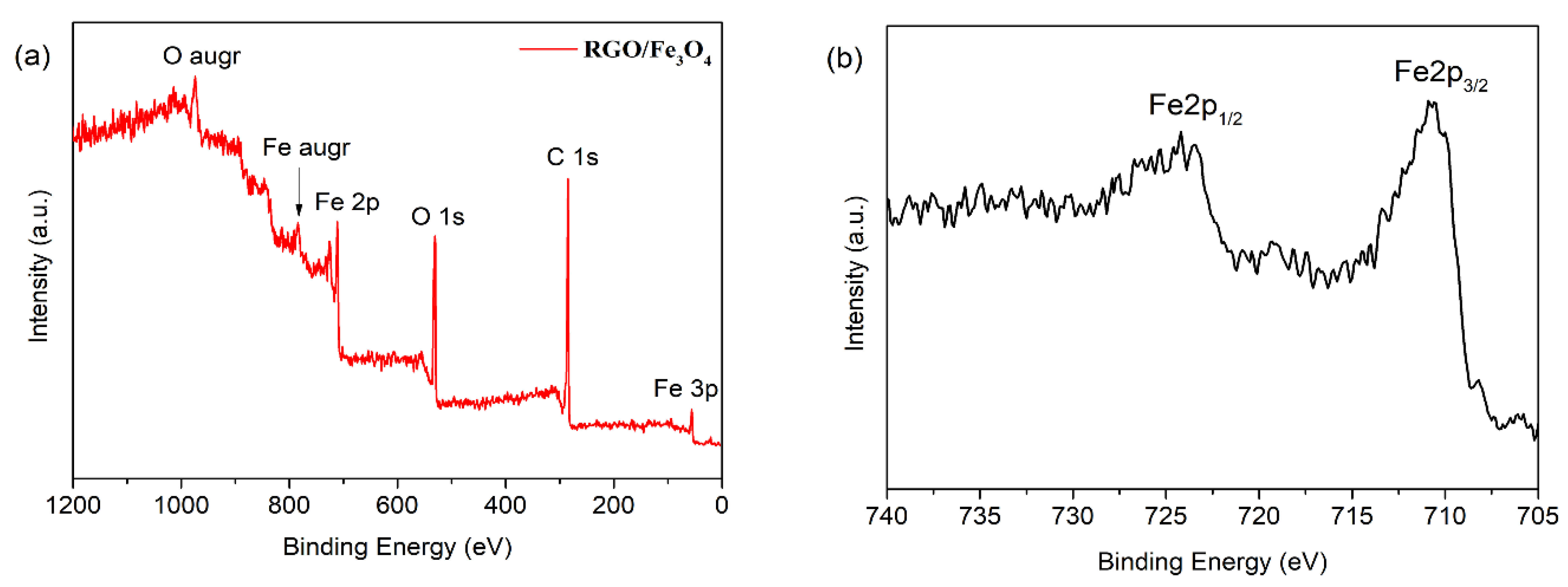
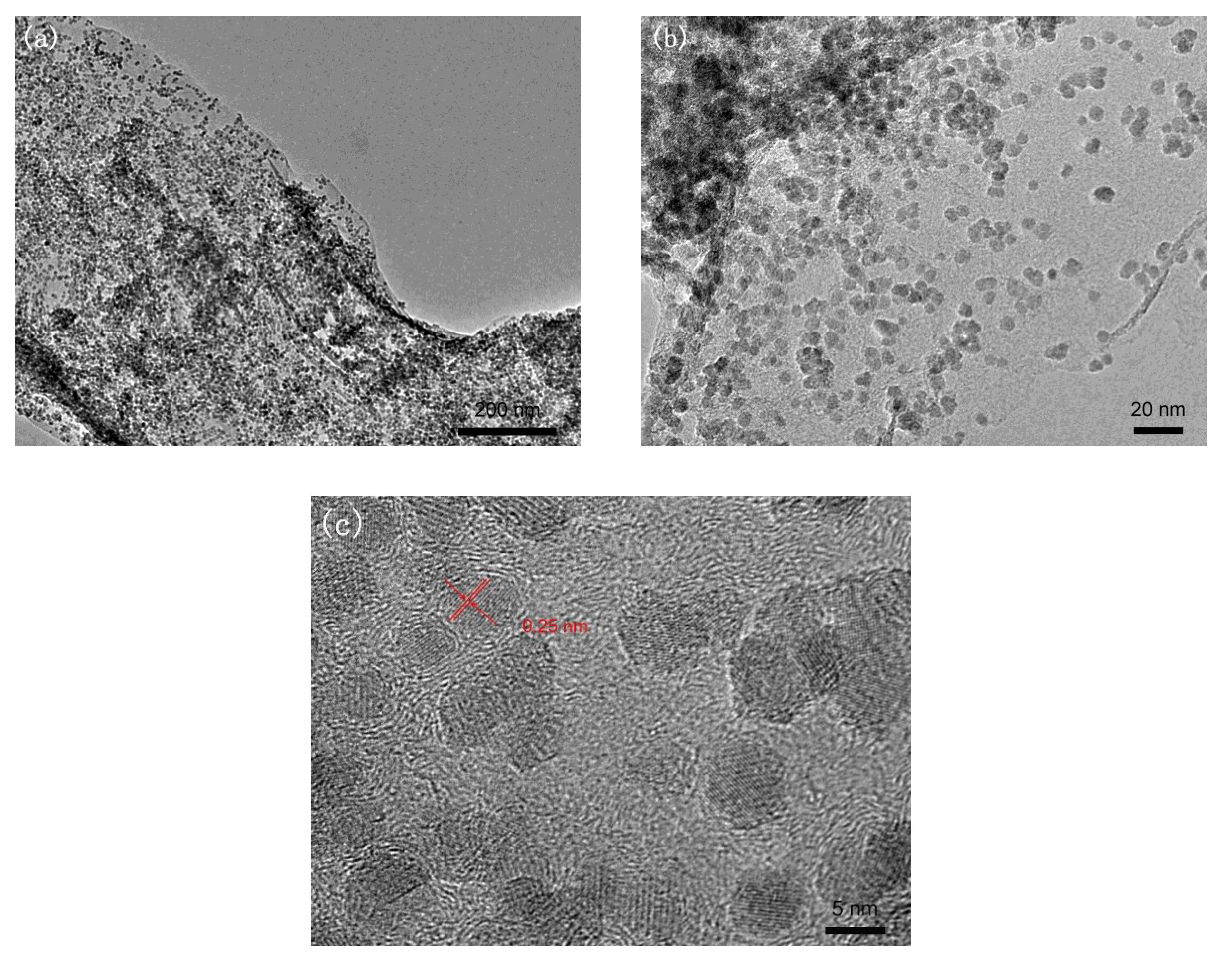
3.1. Chemical Composition and Morphology
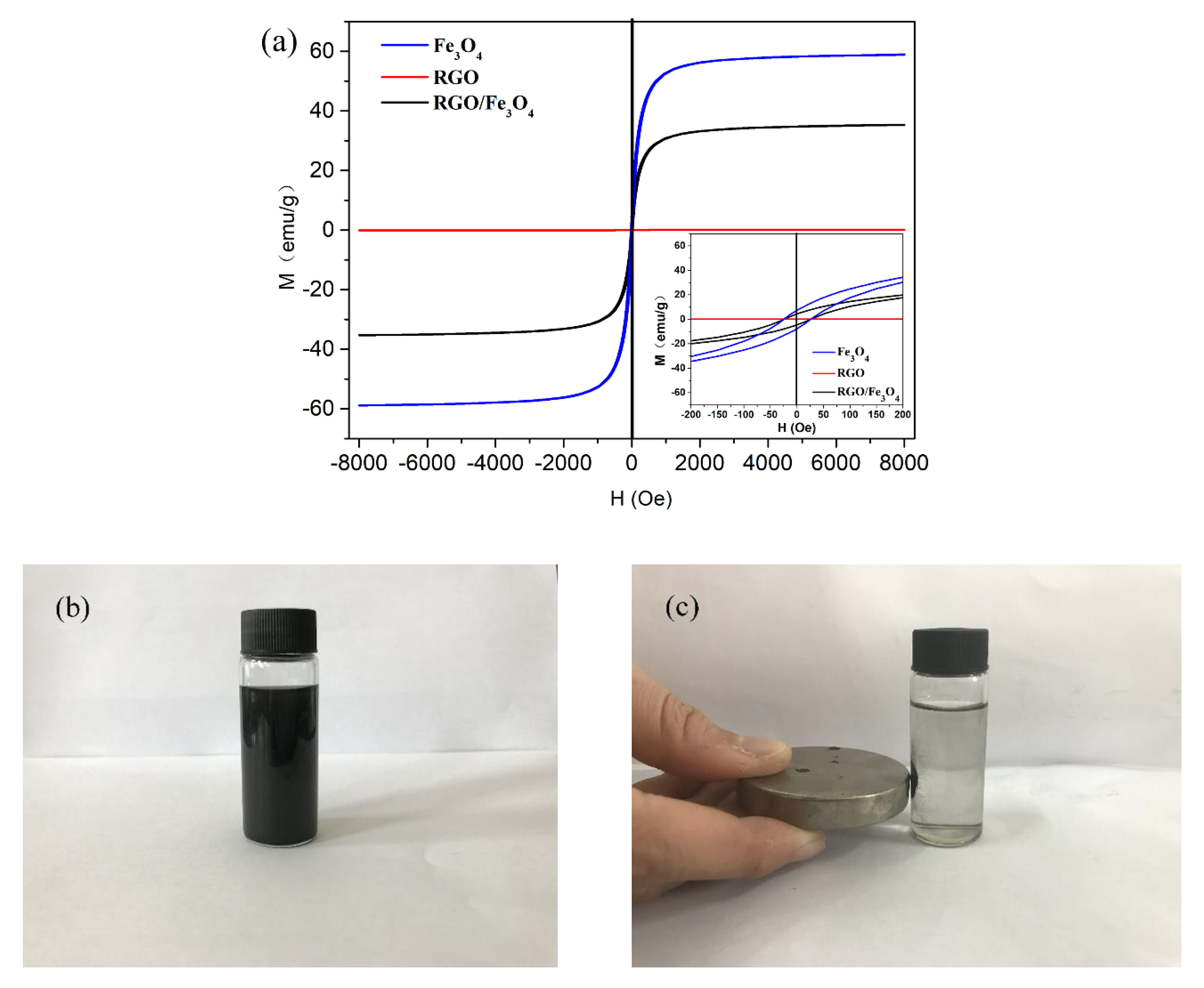
3.2. Magnetic Properties
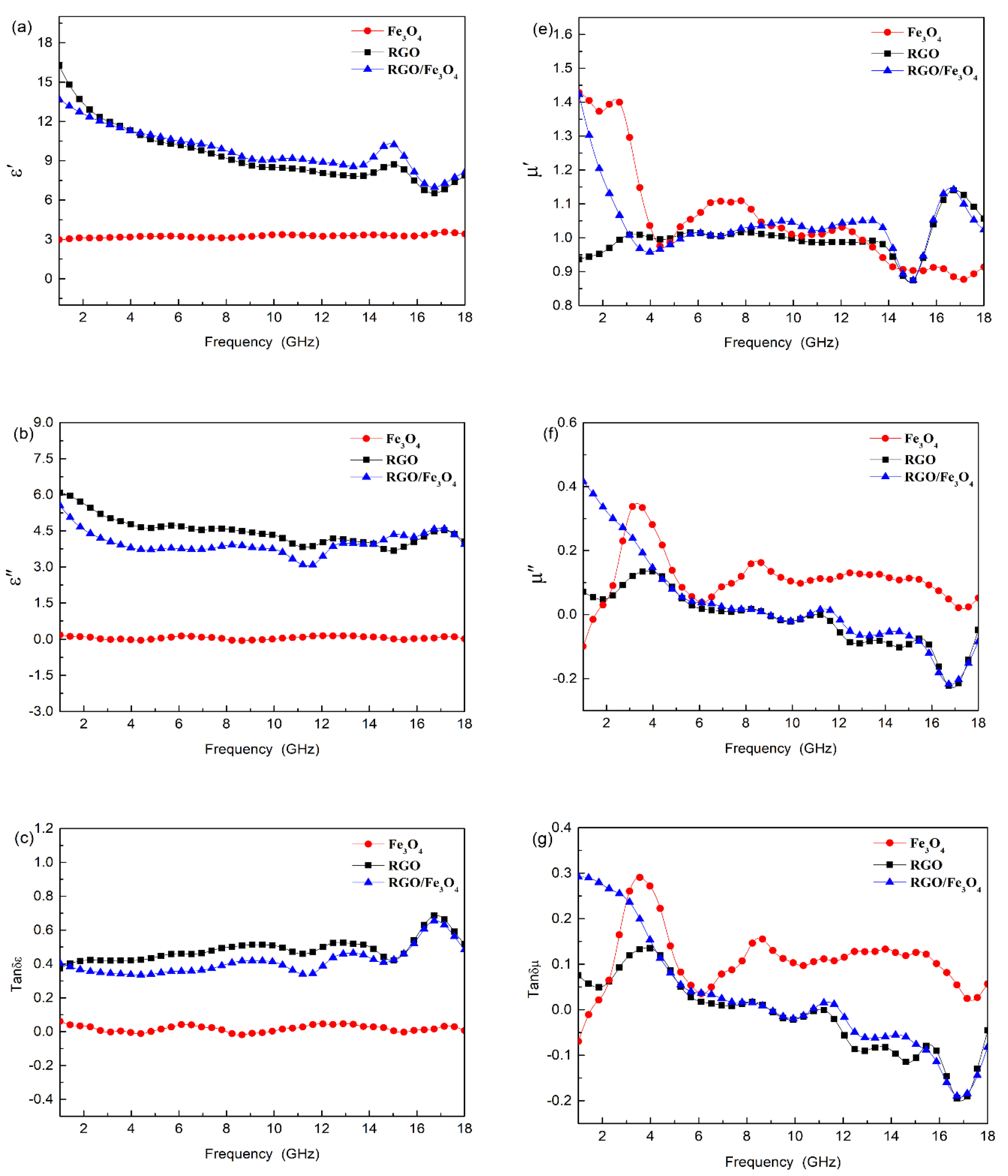
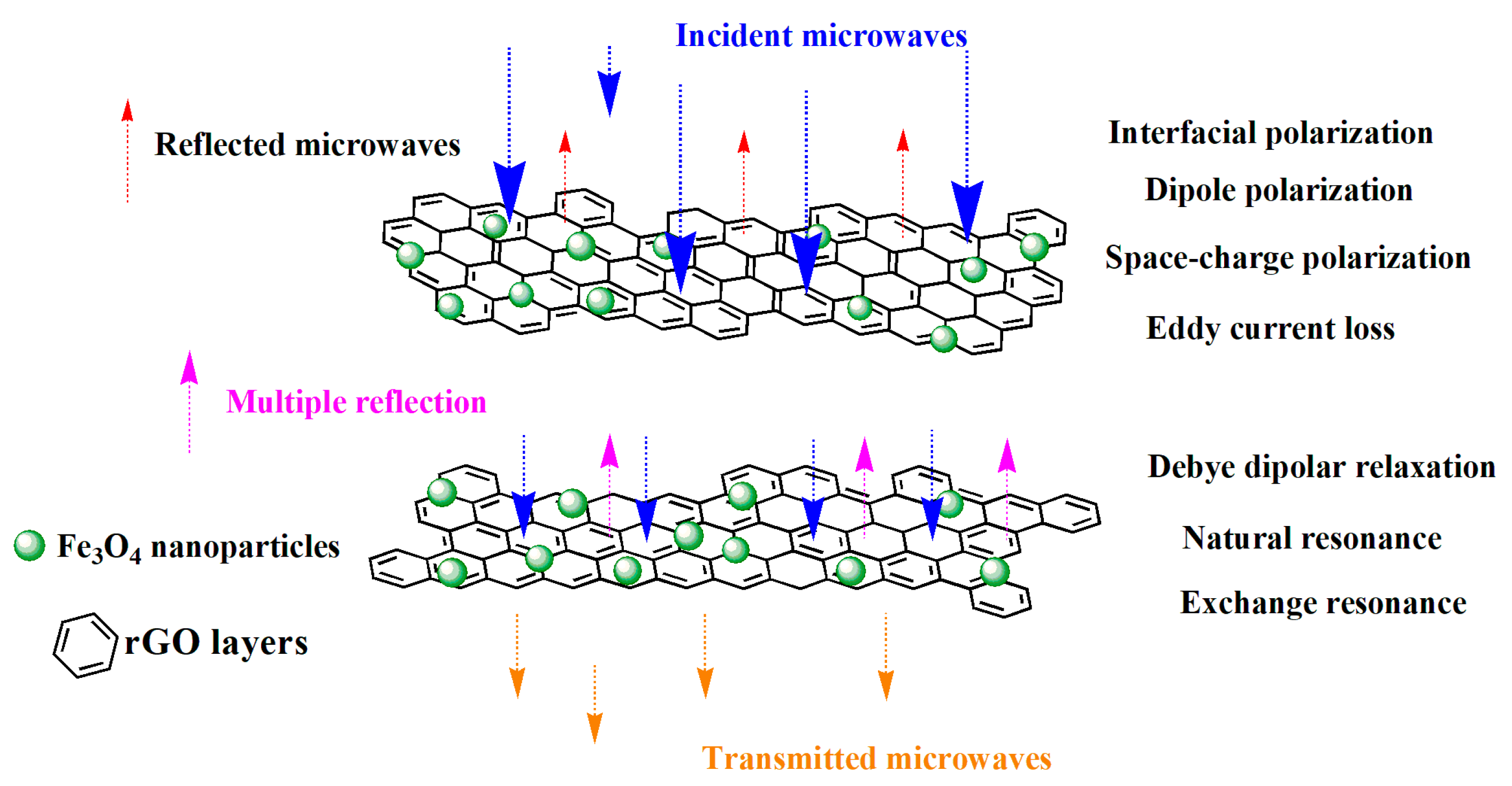
3.3. Electromagnetic Characteristics
3.4. Microwave Absorption Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jian, X.; Wu, B.; Wei, Y.; Dou, S.; Wang, X.; He, W.; Mahmood, N. Facile Synthesis of Fe3O4/GCs Composites and their Enhanced Microwave Absorption Properties. ACS Appl. Mater. Interfaces 2016, 8, 6101–6109. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhu, G.; Ren, P.; Wang, K.; Cui, X.; Yan, X. Cyanate ester resin filled with graphene nanosheets and CoFe2O4-reduced graphene oxide nanohybrids as a microwave absorber. Appl. Surf. Sci. 2015, 351, 40–47. [Google Scholar] [CrossRef]
- Xu, H.; Yin, X.; Zhu, M.; Han, M.; Hou, Z.; Li, X.; Zhang, L.; Cheng, L. Carbon Hollow Microspheres with a Designable Mesoporous Shell for High-Performance Electromagnetic Wave Absorption. ACS Appl. Mater. Interfaces 2017, 9, 6332–6341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Wu, L.N.; Zhou, J.G.; Shen, B.Z.; Jiang, Z.H. Enhanced microwave absorption of Fe3O4 nanocrystals after heterogeneously growing with ZnO nanoshell. Rsc Adv. 2013, 3, 3309–3315. [Google Scholar] [CrossRef]
- Li, C.-J.; Wang, B.; Wang, J.-N. Magnetic and Microwave Absorbing Properties of Electrospun Ba(1−x)LaxFe12O19 Nanofibers. J. Magn. Magn. Mater. 2012, 324, 1305–1311. [Google Scholar] [CrossRef]
- Liu, Q.H.; Cao, Q.; Bi, H.; Liang, C.Y.; Yuan, K.P.; She, W.; Yang, Y.J.; Che, R.C. CoNi@SiO2@TiO2 and CoNi@Air@TiO2 microspheres with strong wide-band micro-wave absorption. Adv. Mater. 2016, 28, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, H.L.; Chen, P.; Wang, Q.; Lu, C.; Jia, C.X. Synthesis and electromagnetic absorption properties of Fe3O4@C nanofibers/bismaleimide nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 2769–2774. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, M.; Chen, P.; Wang, Q.; Lu, C.; Gao, Y.; Wang, R.; Chen, H. Enhanced microwave absorption properties of electrospun PEK-C nanofibers loaded with Fe3O4/CNTs hybrid nanoparticles. Eng. Sci. 2017, 57, 1186–1192. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.-S.; Lu, M.; Cao, W.; Shi, H.; Liu, J.; Wang, X.; Jin, H.; Fang, X.; Wang, W.; et al. Reduced Graphene Oxides: Light-Weight and High-Efficiency Electromagnetic Interference Shielding at Elevated Temperatures. Adv. Mater. 2014, 26, 3484–3489. [Google Scholar] [CrossRef]
- Singh, A.P.; Mishra, M.; Hashim, D.P.; Narayanan, T.; Hahm, M.G.; Kumar, P.; Dwivedi, J.; Kedawat, G.; Gupta, A.; Singh, B.P.; et al. Probing the engineered sandwich network of vertically aligned carbon nanotube–reduced graphene oxide composites for high performance electromagnetic interference shielding applications. Carbon 2015, 85, 79–88. [Google Scholar] [CrossRef]
- Wen, B.; Wang, X.X.; Cao, W.Q.; Shi, H.L.; Lu, M.M.; Wang, G.; Jin, H.B.; Wang, W.Z.; Yuan, J.; Cao, M.-S. Reduced graphene oxides: The thinnest and most lightweight materials with highly efficient microwave attenuation performances of the carbon world. Nanoscale 2014, 6, 5754–5761. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, J.; Du, Y.; Bai, J.; Fan, H.M.; Zhang, H.-L.; Peng, Y.; Li, F. One-pot synthesis of CoFe2O4/graphene oxide hybrids and their conversion into FeCo/graphene hybrids for lightweight and highly efficient microwave absorber. J. Mater. Chem. A 2015, 3, 5535–5546. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.-S.; Yin, P.-G. Designed fabrication of reduced graphene oxides/Ni hybrids for effective electromagnetic absorption and shielding. Carbon 2018, 139, 759–767. [Google Scholar] [CrossRef]
- Ye, W.; Fu, J.; Wang, Q.; Wang, C.; Xue, D. Electromagnetic wave absorption properties of NiCoP alloy nanoparticles decorated on reduced graphene oxide nanosheets. J. Magn. Magn. Mater. 2015, 395, 147–151. [Google Scholar] [CrossRef]
- Zhang, S.L.; Jiao, Q.Z.; Hua, J.; Li, J.J.; Zhao, Y.; Li, H.S.; Wu, Q. Vapor diffusion synthesis of rugby-shaped CoFe2O4/graphene composites as absorbing materials. J. Alloys Compd. 2015, 630, 195–201. [Google Scholar] [CrossRef]
- Chu, H.-R.; Zeng, Q.; Chen, P.; Yu, Q.; Xu, D.-W.; Xiong, X.-H.; Wang, Q. Synthesis and electromagnetic wave absorption properties of matrimony vine-like iron oxide/reduced graphene oxide prepared by a facile method. J. Alloy Compd. 2017, 719, 296–307. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilčáková, J.; Machovský, M.; Škoda, D.; Urbánek, P.; Masař, M.; Gořalik, M.; Urbánek, M.; Kalina, L.; et al. Polypropylene Nanocomposite Filled with Spinel Ferrite NiFe2O4 Nanoparticles and In-Situ Thermally-Reduced Graphene Oxide for Electromagnetic Interference Shielding Application. Nanomaterials 2019, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A.; Corinaldesi, V.; Donnini, J.; Di Perna, C.; Micheli, D.; Vricella, A.; Pastore, R.; Bastianelli, L.; Moglie, F.; Primiani, V.M. Effect of graphene oxide and metallic fibers on the electromagnetic shielding effect of engineered cementitious composites. J. Eng. 2018, 18, 33–39. [Google Scholar] [CrossRef]
- Qing, Y.C.; Min, D.D.; Zhou, Y.Y.; Luo, F.; Zhou, W.C. Graphene nanosheet-and flake carbonyl iron particle-filled epoxy-silicone composites asthin-thickness and wide-bandwidth microwave absorber. Carbon 2015, 86, 98–107. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Vricella, A.; Marchetti, M. Matter’s Electromagnetic Signature Reproduction by Graded-Dielectric Multilayer Assembly. IEEE Trans. Microw. Theory Tech. 2017, 65, 2801–2809. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Delfini, A.; Giusti, A.; Vricella, A.; Tolochko, O.; Vasilyeva, E.; Santoni, F.; Marchetti, M. Electromagnetic characterization of advanced nanostructured materials and multilayer design optimization for metrological and low radar observability applications. Acta Astronaut. 2017, 134, 33–40. [Google Scholar] [CrossRef]
- Xu, D.W.; Yang, S.; Chen, P.; Yu, Q.; Xiong, X.H.; Wang, J. Synthesis of magnetic graphene aerogels for microwave absorption by a in-situ pyrolysis. Carbon 2019, 146, 301–312. [Google Scholar] [CrossRef]
- Zeng, Q.; Xiong, X.-H.; Chen, P.; Yu, Q.; Wang, Q.; Wang, R.-C.; Chu, H.-R. Air@rGO€Fe3O4 microspheres with spongy shells: Self-assembly and microwave absorption performance. J. Mater. Chem. C 2016, 4, 10518–10528. [Google Scholar] [CrossRef]
- He, H.; Gao, C. Supraparamagnetic, Conductive, and Processable Multifunctional Graphene Nanosheets Coated with High-Density Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- He, J.-Z.; Wang, X.-X.; Zhang, Y.-L.; Cao, M.-S. Small magnetic nanoparticles decorating reduced graphene oxides to tune the electromagnetic attenuation capacity. J. Mater. Chem. C 2016, 4, 7130–7140. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, E.; Cao, T.; Wang, H.; Shi, C.; Li, J.; He, C.; He, F.; Ma, L.; Zhao, N. Sandwiched graphene inserted with graphene-encapsulated yolk–shell γ-Fe2O3 nanoparticles for efficient lithium ion storage. J. Mater. Chem. A 2017, 5, 7035–7042. [Google Scholar] [CrossRef]
- Kou, L.; He, H.; Gao, C. Click chemistry approach to functionalize two-dimensional macromolecules of graphene oxide nanosheets. Nano-Micro Lett. 2010, 2, 177–183. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, D.; Yang, Y.; Kang, F.; Gu, J. Fe3O4/carbon composite nanofiber absorber with enhanced microwave absorption performance. Mater. Sci. Eng. B 2013, 178, 1–9. [Google Scholar] [CrossRef]
- Hou, Z.L.; Zhang, M.; Kong, L.B.; Fang, H.M.; Li, Z.J.; Zhou, H.F.; Jin, H.B.; Cao, M.S. Microwave permittivity and permeability experiments in high-loss dielectrics: Caution with implicit Fabry-Perot resonance for negative imaginary permeability. Appl. Phys. Lett. 2013, 103, 162905. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Li, Y.; Yang, F.; Zhang, L.; Liu, L.; Ren, X.; Yang, H. Sythesis and microwave absorption property of flexible magnetic film based on graphene oxide/carbon nanotubes and Fe3O4 nanoparticles. J. Mater. Chem. A 2014, 2, 14940–14946. [Google Scholar] [CrossRef]
- Yang, H.; Ye, T.; Lin, Y.; Liu, M. Preparation and microwave absorption property of graphene/BaFe12O19/CoFe2O4 nanocomposite. Appl. Surf. Sci. 2015, 357, 1289–1293. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Zhou, J.; Jiang, Z.; Shen, B. Chemoselectivity-induced multiple interfaces in MWCNT/Fe3O4@ZnO heterotrimers for whole X-band microwave absorption. Nanoscale 2014, 6, 12298–12302. [Google Scholar] [CrossRef] [PubMed]


| Absorber | Loading Ratio (wt%) | RLmin (dB) | Effective Bandwidth (GHz) (RL < −10dB) | Thickness (mm) | Refs |
|---|---|---|---|---|---|
| RGO/Ni | 50 | −39.03 | 4.3 | 2.0 | [13] |
| RGO/NiCoP | 50 | −17.84 | 3.5 | 1.5 | [14] |
| Fe3O4/GO/CNT | 30 | −37.3 | 2.2 | 5 | [30] |
| G/BaFe12O19/CoFe2O4 | 50 | −32.4 | 3.0 | [31] | |
| Fe3O4/CNT | 50 | −20.1 | 1.4 | 3.5 | [32] |
| RGO/CoFe2O4 | 60 | −39.0 | 4.7 | 2.0 | [15] |
| RGO/matrimony vine-like Fe3O4 | 50 | −42.8 | 4.6 | 1.8 | [22] |
| RGO/Fe3O4 | 50 | −55.71 | 4.76 | 1.7 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Wang, Y.; Chen, P.; Nie, W.; Chen, H.; Zhou, J. Reduced Graphene Oxide-Wrapped Super Dense Fe3O4 Nanoparticles with Enhanced Electromagnetic Wave Absorption Properties. Nanomaterials 2019, 9, 845. https://doi.org/10.3390/nano9060845
Yu Q, Wang Y, Chen P, Nie W, Chen H, Zhou J. Reduced Graphene Oxide-Wrapped Super Dense Fe3O4 Nanoparticles with Enhanced Electromagnetic Wave Absorption Properties. Nanomaterials. 2019; 9(6):845. https://doi.org/10.3390/nano9060845
Chicago/Turabian StyleYu, Qi, Yiyi Wang, Ping Chen, Weicheng Nie, Hanlin Chen, and Jun Zhou. 2019. "Reduced Graphene Oxide-Wrapped Super Dense Fe3O4 Nanoparticles with Enhanced Electromagnetic Wave Absorption Properties" Nanomaterials 9, no. 6: 845. https://doi.org/10.3390/nano9060845
APA StyleYu, Q., Wang, Y., Chen, P., Nie, W., Chen, H., & Zhou, J. (2019). Reduced Graphene Oxide-Wrapped Super Dense Fe3O4 Nanoparticles with Enhanced Electromagnetic Wave Absorption Properties. Nanomaterials, 9(6), 845. https://doi.org/10.3390/nano9060845





