Aggregation, Sedimentation, and Dissolution of Copper Oxide Nanoparticles: Influence of Low-Molecular-Weight Organic Acids from Root Exudates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of CuO NPs
2.2. Plant Culture
2.3. Collection of Root Exudates
2.4. Identifications of LMWOAs in Root Exudates
2.5. Aggregation Analysis of CuO NPs
2.6. Sedimentation Measurements of CuO NPs
2.7. Dissolution Detection of CuO NPs
2.8. Statistical Analysis
3. Results
3.1. LMWOAs in Root Exudates
3.2. Aggregation of CuO NPs in LMWOAs from Root Exudates
3.3. Sedimentation of CuO NPs in LMWOAs from Root Exudates
3.4. Dynamic Dissolution of CuO NPs in LMWOAs from Root Exudates
3.5. Effect of an LMWOA Mixture on the Fate of CuO NPs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiesner, M.R.; Lowry, G.V.; Alvarez, P.; Dionysiou, D.; Biswas, P. Assessing the risks of manufactured nanomaterials. Environ. Sci. Technol. 2006, 40, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agricul. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 2013, 47, 11592–11598. [Google Scholar] [CrossRef]
- Hawthorne, J.; Roche, R.D.; Xing, B.S.; Newman, L.A.; Ma, X.M.; Majumdar, S.; Gardea-Torresdey, J.; White, J.C. Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ. Sci. Technol. 2014, 48, 13102–13109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Karn, B. Nanoscale environmental science and technology: Challenges and opportunities. Environ. Sci. Technol. 2005, 39, 94a–95a. [Google Scholar] [CrossRef]
- Larue, C.; Castillo–Michel, H.; Sobanska, S.; Cecillon, L.; Bureau, S.; Barthes, V.; Ouerdane, L.; Carriere, M.; Sarret, G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 2014, 264, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; McGrath, S.P. Biofortification and phytoremediation. Curr. Opin. Plant Biol. 2009, 12, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Whipps, J.M. Substrate flow in the rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Lu, L.L.; Tian, S.K.; Yang, X.E.; Peng, H.Y.; Li, T.Q. Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii: The impact of citric acid and tartaric acid. J. Zhejiang Univ-Sci. B 2013, 14, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ott, D.W.; Cutright, T.J. Accumulation and histological location of heavy metals in Phragmites australis grown in acid mine drainage contaminated soil with or without citric acid. Environ. Exp. Bot. 2014, 105, 46–54. [Google Scholar] [CrossRef]
- Najeeb, U.; Xu, L.; Ali, S.; Jilani, G.; Gong, H.J.; Shen, W.Q.; Zhou, W.J. Citric acid enhances the phytoextraction of manganese and plant growth by alleviating the ultrastructural damages in Juncus effusus L. J. Hazard. Mater. 2009, 170, 1156–1163. [Google Scholar] [CrossRef]
- Sinhal, V.K.; Srivastava, A.; Singh, V.P. EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta). J. Environ. Biol. 2010, 31, 255–259. [Google Scholar]
- Zhan, F.D.; Qin, L.; Guo, X.H.; Tan, J.B.; Liu, N.N.; Zu, Y.Q.; Li, Y. Cadmium and lead accumulation and low-molecular-weight organic acids secreted by roots in an intercropping of a cadmium accumulator Sonchus asper L. with Vicia faba L. RSC Adv. 2016, 6, 33240–33248. [Google Scholar] [CrossRef]
- Wu, L.H.; Luo, Y.M.; Christie, P.; Wong, M.H. Effects of EDTA and low molecular weight organic acids on soil solution properties of a heavy metal polluted soil. Chemosphere 2003, 50, 819–822. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Schwab, F.; Colman, B.P.; Webb, S.M.; Newville, M.; Lanzirotte, A.; Winkler, C.; Wiesner, M.R.; Lowry, G.V. Speciation matters: Bioavailability of silver and silver sulfide nanoparticles to Alfalfa (Medicago sativa). Environ. Sci. Technol. 2015, 49, 8451–8460. [Google Scholar] [CrossRef]
- Zhang, W.; Dan, Y.; Shi, H.; Ma, X. Elucidating the mechanisms for plant uptake and in-planta speciation of cerium in radish (Raphanus sativus L.) treated with cerium oxide nanoparticles. J. Environ. Chem. Eng. 2017, 5, 572–577. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Keller, A.A. Interactions, transformations, and bioavailability of nano-copper exposed to root exudates. Environ. Sci. Technol. 2017, 51, 9774–9783. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ. Toxicol. Chem. 2018, 37, 11–20. [Google Scholar] [CrossRef]
- Peng, C.; Chen, S.; Shen, C.S.; He, M.; Zhang, Y.Q.; Ye, J.; Liu, J.S.; Shi, J.Y. Iron plaque: A barrier layer to the uptake and translocation of copper oxide nanoparticles by rice plants. Environ. Sci. Technol. 2018, 52, 12244–12254. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Manila, Philippines, 1976. [Google Scholar]
- Zhang, N.; Wang, D.D.; Liu, Y.P.; Li, S.Q.; Shen, Q.R.; Zhang, R.F. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, H.; Fang, H.X.; Xu, C.; Huang, H.M.; Wang, Y.; Sun, L.J.; Yuan, X.F.; Chen, Y.X.; Shi, J.Y. Natural organic matter-induced alleviation of the phytotoxicity to rice (Oryza sativa L.) caused by copper oxide nanoparticles. Environ. Toxicol. Chem. 2015, 34, 1996–2003. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, W.T.; Dai, Y.H.; Liu, L.J.; Wang, Z.Y.; Yu, X.Y.; Zhang, J.Z.; Wang, X.K.; Xing, B.S. Uptake, distribution, and transformation of CuO NPs in a floating plant Eichhornia crassipes and related stomatal responses. Environ. Sci. Technol. 2017, 51, 7686–7695. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Copper oxide nanoparticles and arsenic interact to alter seedling growth of rice (Oryza sativa japonica). Chemosphere 2018, 206, 330–337. [Google Scholar] [CrossRef]
- Peng, C.; Shen, C.S.; Zheng, S.Y.; Yang, W.L.; Hu, H.; Liu, J.S.; Shi, J.Y. Transformation of CuO nanoparticles in the aquatic environment: Influence of pH, electrolytes and natural organic matter. Nanomaterials 2017, 7, 326. [Google Scholar] [CrossRef]
- Dahle, J.T.; Livi, K.; Arai, Y. Effects of pH and phosphate on CeO2 nanoparticle dissolution. Chemosphere 2015, 119, 1365–1371. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Luo, Y.; Yu, Y.; Lin, Q.; Wong, M. Physiological mechanism of plant roots exposed to cadmium. Chemosphere 2003, 50, 789–793. [Google Scholar] [CrossRef]
- Liu, J.G.; Qian, M.; Cai, G.L.; Zhu, Q.S.; Wong, M.H. Variations between rice cultivars in root secretion of organic acids and the relationship with plant cadmium uptake. Environ. Geochem. Health 2007, 29, 189–195. [Google Scholar] [CrossRef]
- Fu, H.; Yu, H.; Li, T.; Zhang, X. Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol. Environ. Safe 2018, 150, 168–175. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Kreuzwieser, J.; Rennenberg, H. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol. 2001, 3, 139–148. [Google Scholar] [CrossRef]
- Zhao, L.J.; Huang, Y.X.; Hu, J.; Zhou, H.J.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef]
- Louie, S.M.; Tilton, R.D.; Lowry, G.V. Effects of molecular weight distribution and chemical properties of natural organic matter on gold nanoparticle aggregation. Environ. Sci. Technol. 2013, 47, 4245–4254. [Google Scholar] [CrossRef]
- Majedi, S.M.; Kelly, B.C.; Lee, H.K. Role of combinatorial environmental factors in the behavior and fate of ZnO nanoparticles in aqueous systems: A multiparametric analysis. J. Hazard. Mater. 2014, 264, 370–379. [Google Scholar] [CrossRef]
- Pettibone, J.M.; Cwiertny, D.M.; Scherer, M.; Grassian, V.H. Adsorption of organic acids on TiO2 nanoparticles: Effects of pH, nanoparticle size, and nanoparticle aggregation. Langmuir 2008, 24, 6659–6667. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, X.; Cao, Z.; Wang, H.; Chen, Y.; Lian, H.-Z. Aggregation and dissolution of engineering nano Ag and ZnO pretreated with natural organic matters in the simulated lung biological fluids. Chemosphere 2019, 225, 668–677. [Google Scholar] [CrossRef]
- Peng, C.; Xu, C.; Liu, Q.; Sun, L.; Luo, Y.; Shi, J. Fate and transformation of CuO nanoparticles in the soil–rice system during the life cycle of rice plants. Environ. Sci. Technol. 2017, 51, 4907–4917. [Google Scholar] [CrossRef]
- Vindedahl, A.M.; Strehlau, J.H.; Arnold, W.A.; Penn, R.L. Organic matter and iron oxide nanoparticles: Aggregation, interactions, and reactivity. Environ. Sci.-Nano 2016, 3, 494–505. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wang, C.; Hou, J.; Wang, P.F.; Miao, L.Z.; Lv, B.W.; Yang, Y.Y.; You, G.X.; Xu, Y.; Zhang, M.Z.; et al. Aggregation, sedimentation, and dissolution of CuO and ZnO nanoparticles in five waters. Environ. Sci. Pollut. R. 2018, 25, 31240–31249. [Google Scholar] [CrossRef]
- Praetorius, A.; Scheringer, M.; Hungerbuhler, K. Development of environmental fate models for engineered nanoparticles-A case study of TiO2 nanoparticles in the Rhine River. Environ. Sci. Technol. 2012, 46, 6705–6713. [Google Scholar] [CrossRef]
- Babakhani, P.; Doong, R.A.; Bridge, J. Significance of early and late stages of coupled aggregation and sedimentation in the fate of nanoparticles: Measurement and modeling. Environ. Sci. Technol. 2018, 52, 8419–8428. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Pettibone, J.M.; Grassian, V.H. Environmental implications of nanoparticle aging in the processing and fate of copper-based nanomaterials. Environ. Sci. Technol. 2012, 46, 7001–7010. [Google Scholar] [CrossRef]
- Juang, R.S.; Wu, F.C.; Tseng, R.L. Adsorption removal of copper(II) using chitosan from simulated rinse solutions containing chelating agents. Water Res. 1999, 33, 2403–2409. [Google Scholar] [CrossRef]
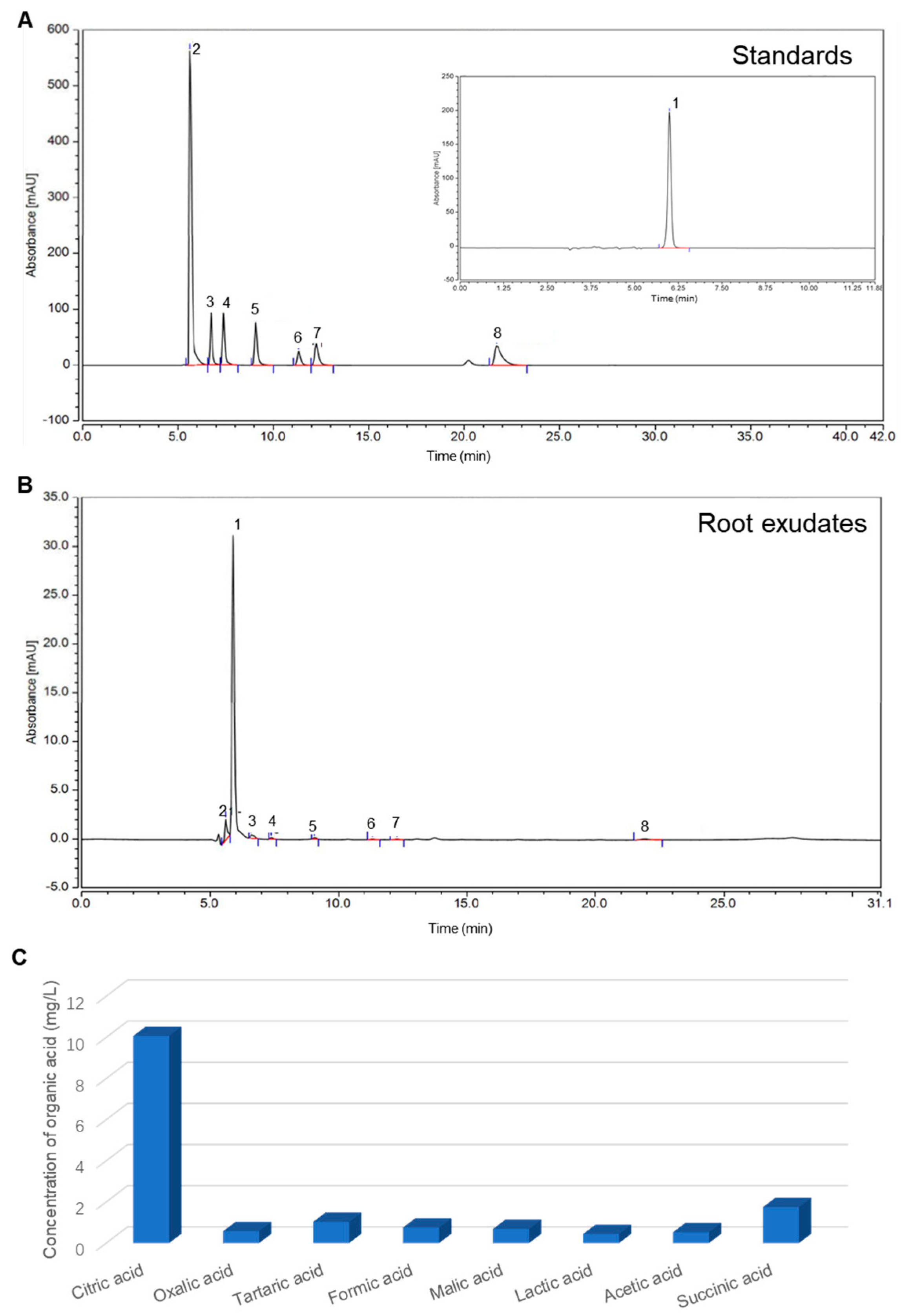
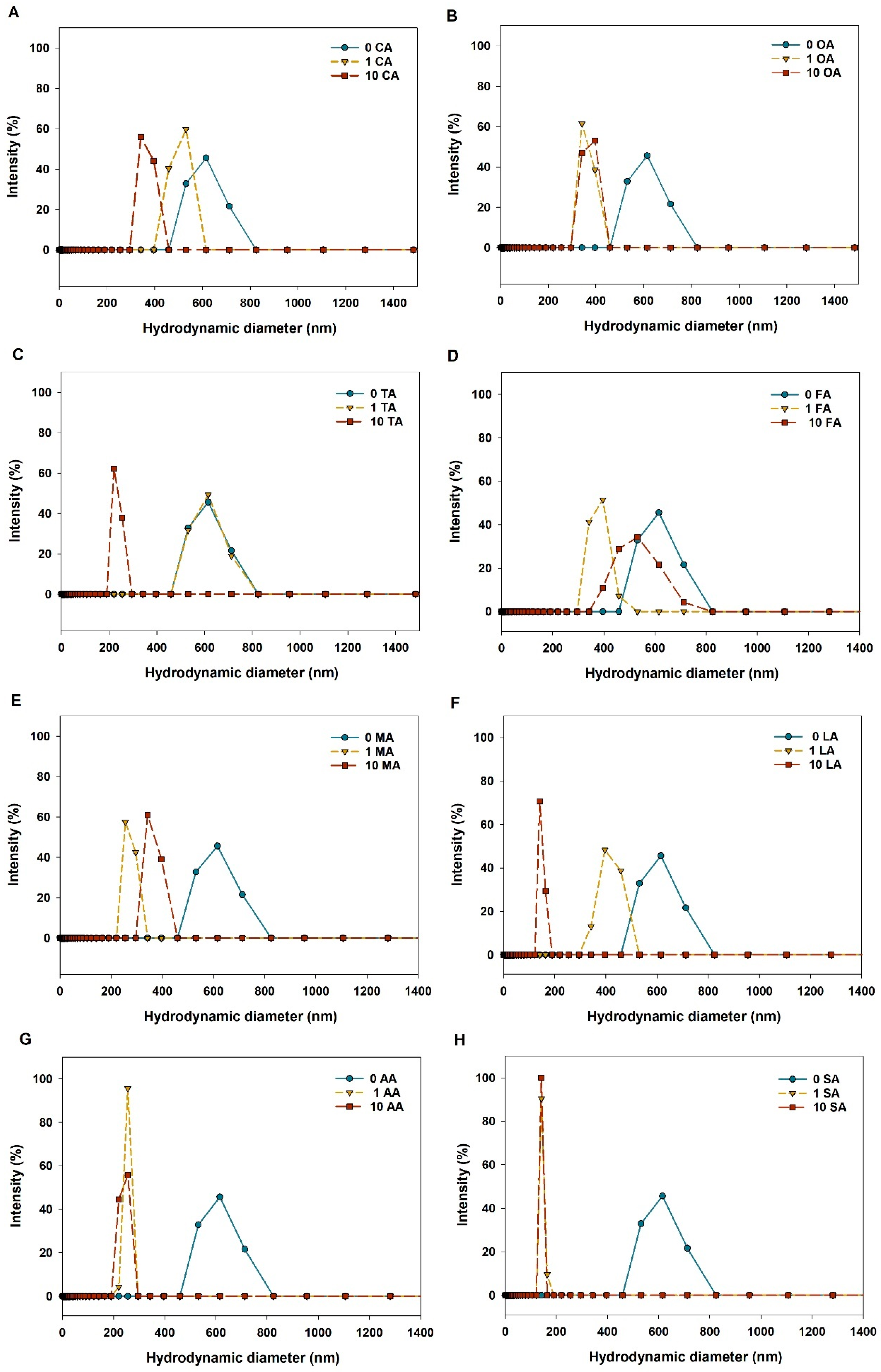
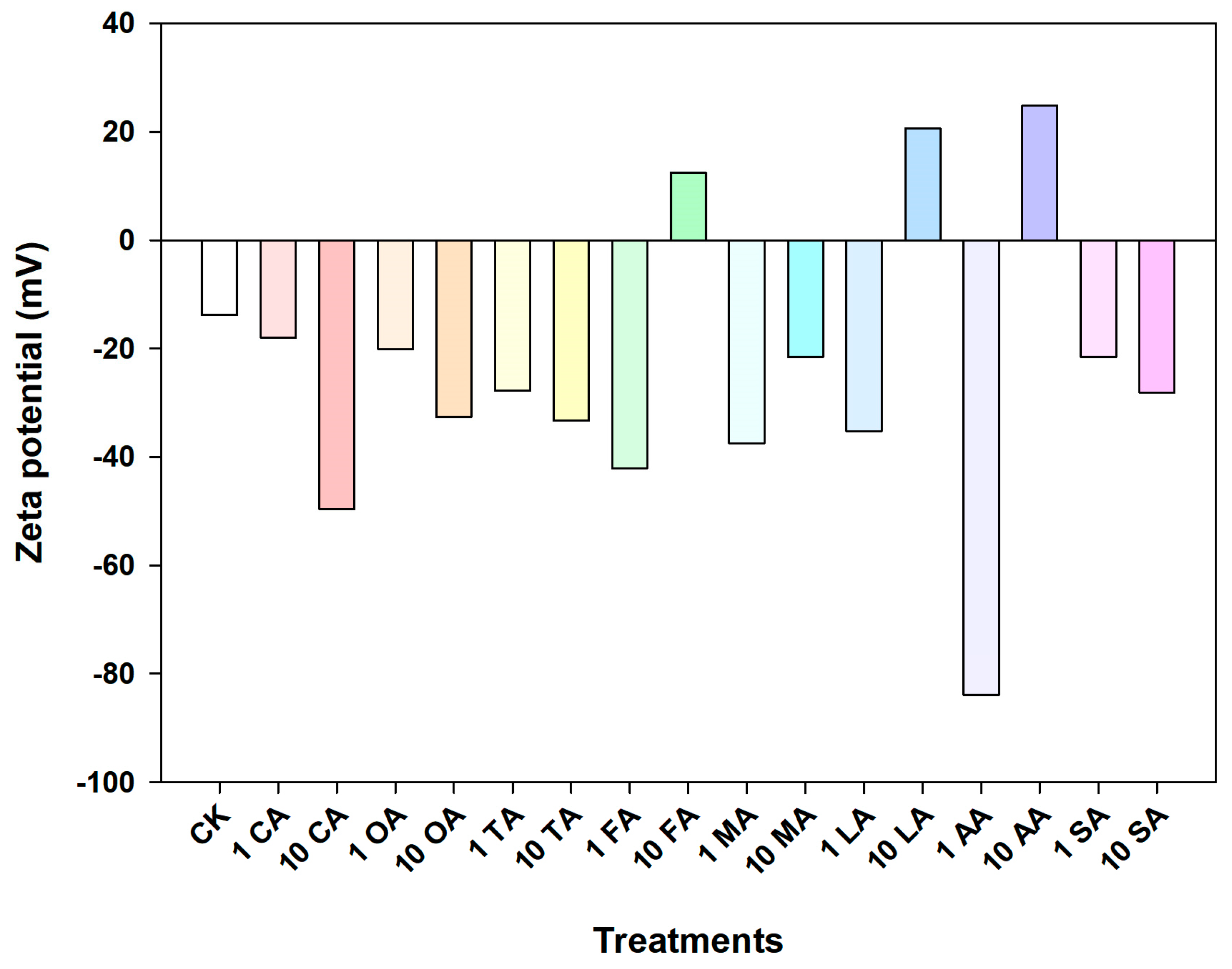
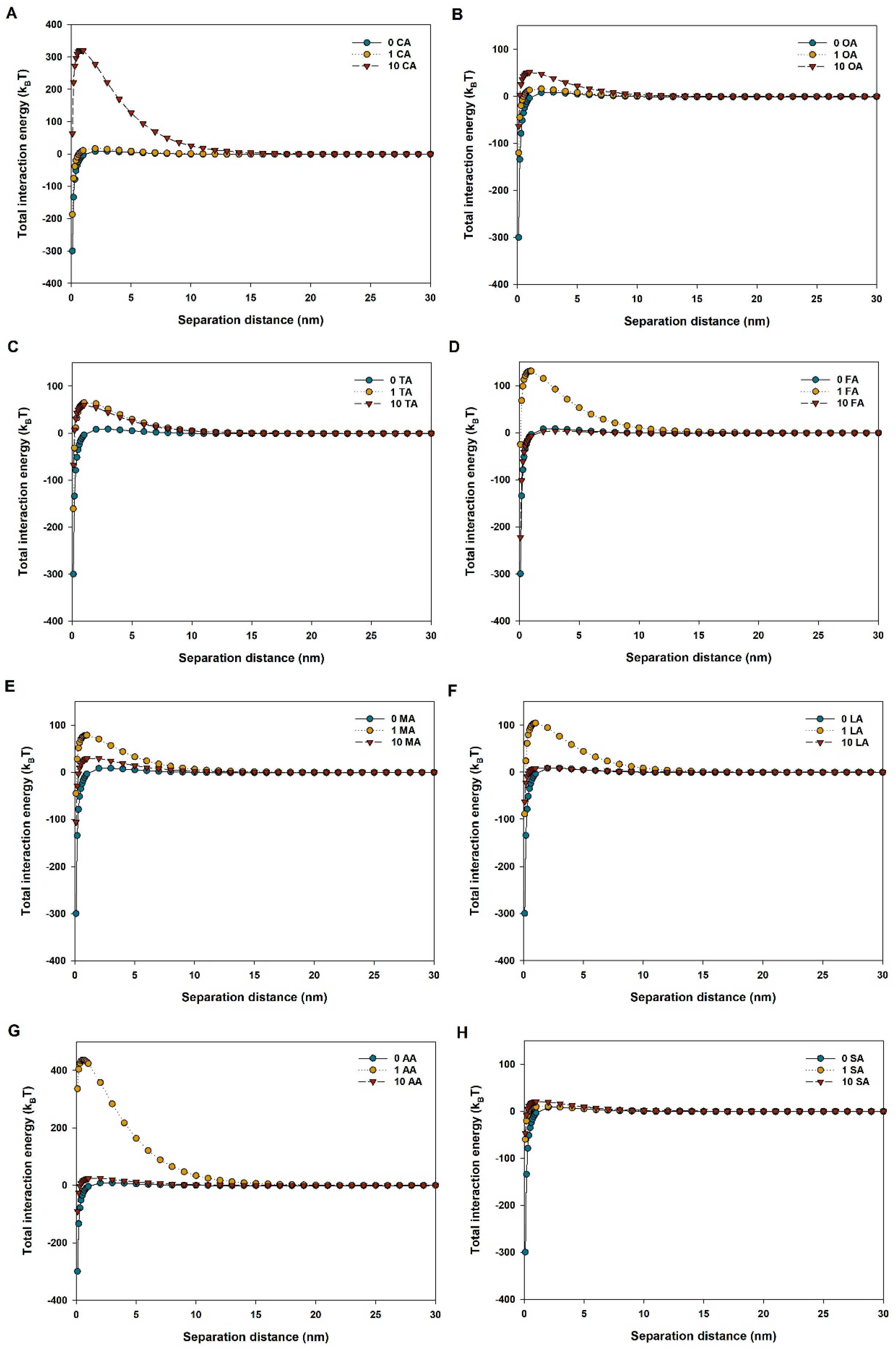
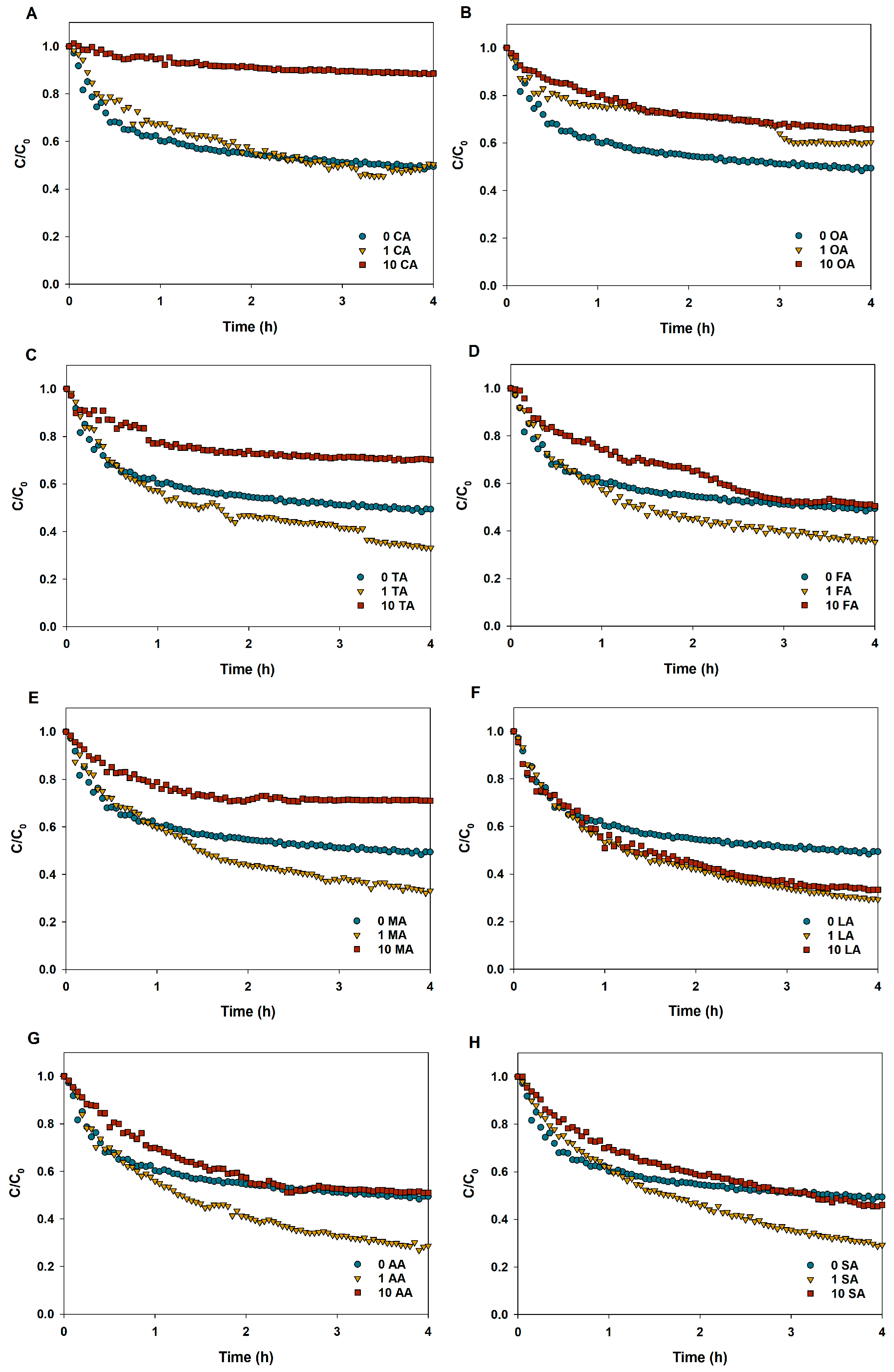

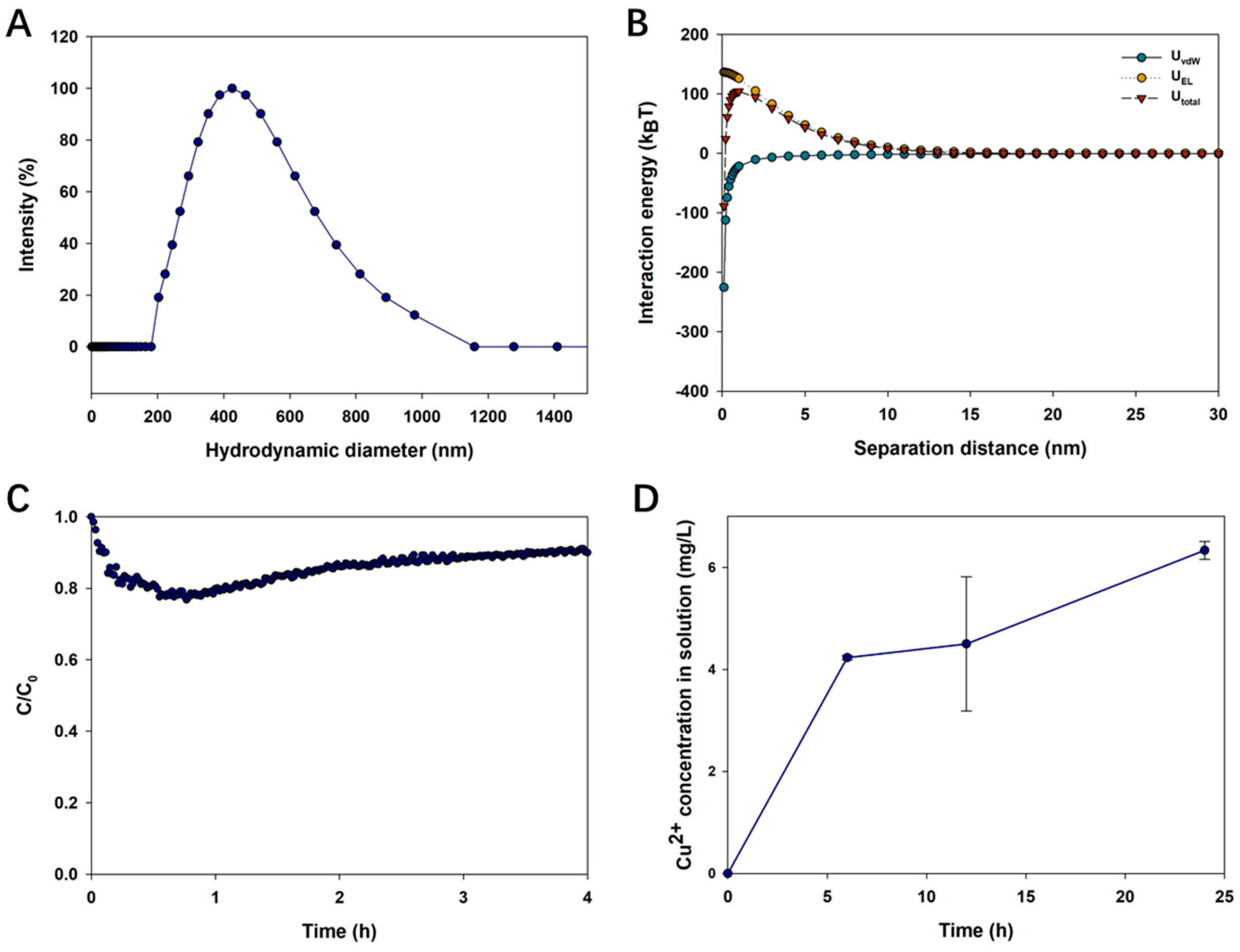
| LMWOAs | Concentration (mg/L) | Sedimentation Rate (h−1) | R2 |
|---|---|---|---|
| Control | 0 | 0.121 | 0.7669 |
| Citric acid | 1 | 0.164 | 0.8888 |
| 10 | 0.028 | 0.8755 | |
| Oxalic acid | 1 | 0.098 | 0.9076 |
| 10 | 0.088 | 0.8932 | |
| Tartaric acid | 1 | 0.223 | 0.9060 |
| 10 | 0.066 | 0.7624 | |
| Formic acid | 1 | 0.218 | 0.8802 |
| 10 | 0.162 | 0.9537 | |
| Malic acid | 1 | 0.245 | 0.9313 |
| 10 | 0.061 | 0.6581 | |
| Lactic acid | 1 | 0.268 | 0.9349 |
| 10 | 0.238 | 0.9222 | |
| Acetic acid | 1 | 0.284 | 0.9447 |
| 10 | 0.155 | 0.8610 | |
| Succinic acid | 1 | 0.286 | 0.9754 |
| 10 | 0.176 | 0.9536 |
| LMWOAs | Concentration (mg/L) | Dissolution Rate (Cu2+ mg·L−1·h−1) |
|---|---|---|
| Control | 0 | 0.060 |
| Citric acid | 1 | 0.141 |
| 10 | 0.785 | |
| Oxalic acid | 1 | 0.152 |
| 10 | 0.873 | |
| Tartaric acid | 1 | 0.099 |
| 10 | 0.691 | |
| Formic acid | 1 | 0.172 |
| 10 | 1.256 | |
| Malic acid | 1 | 0.115 |
| 10 | 0.652 | |
| Lactic acid | 1 | 0.111 |
| 10 | 0.591 | |
| Acetic acid | 1 | 0.195 |
| 10 | 1.085 | |
| Succinic acid | 1 | 0.143 |
| 10 | 0.714 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Tong, H.; Yuan, P.; Sun, L.; Jiang, L.; Shi, J. Aggregation, Sedimentation, and Dissolution of Copper Oxide Nanoparticles: Influence of Low-Molecular-Weight Organic Acids from Root Exudates. Nanomaterials 2019, 9, 841. https://doi.org/10.3390/nano9060841
Peng C, Tong H, Yuan P, Sun L, Jiang L, Shi J. Aggregation, Sedimentation, and Dissolution of Copper Oxide Nanoparticles: Influence of Low-Molecular-Weight Organic Acids from Root Exudates. Nanomaterials. 2019; 9(6):841. https://doi.org/10.3390/nano9060841
Chicago/Turabian StylePeng, Cheng, Hong Tong, Peng Yuan, Lijuan Sun, Lei Jiang, and Jiyan Shi. 2019. "Aggregation, Sedimentation, and Dissolution of Copper Oxide Nanoparticles: Influence of Low-Molecular-Weight Organic Acids from Root Exudates" Nanomaterials 9, no. 6: 841. https://doi.org/10.3390/nano9060841
APA StylePeng, C., Tong, H., Yuan, P., Sun, L., Jiang, L., & Shi, J. (2019). Aggregation, Sedimentation, and Dissolution of Copper Oxide Nanoparticles: Influence of Low-Molecular-Weight Organic Acids from Root Exudates. Nanomaterials, 9(6), 841. https://doi.org/10.3390/nano9060841





