Facile Synthesis and Optical Properties of CsPbX3/ZIF-8 Composites for Wide-Color-Gamut Display
Abstract
:1. Introduction
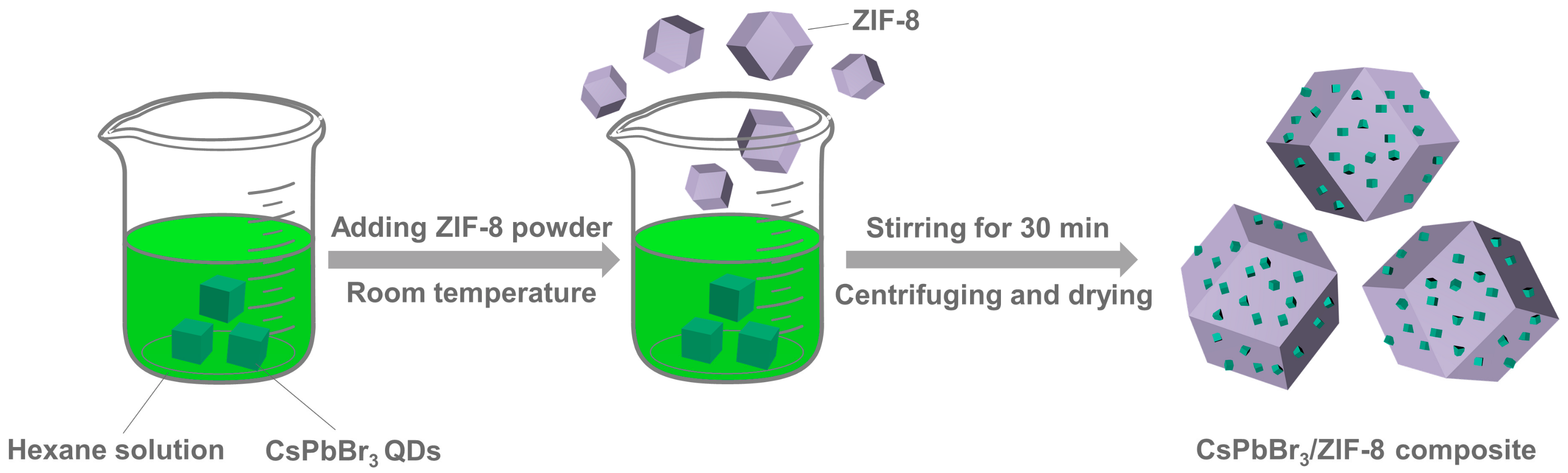
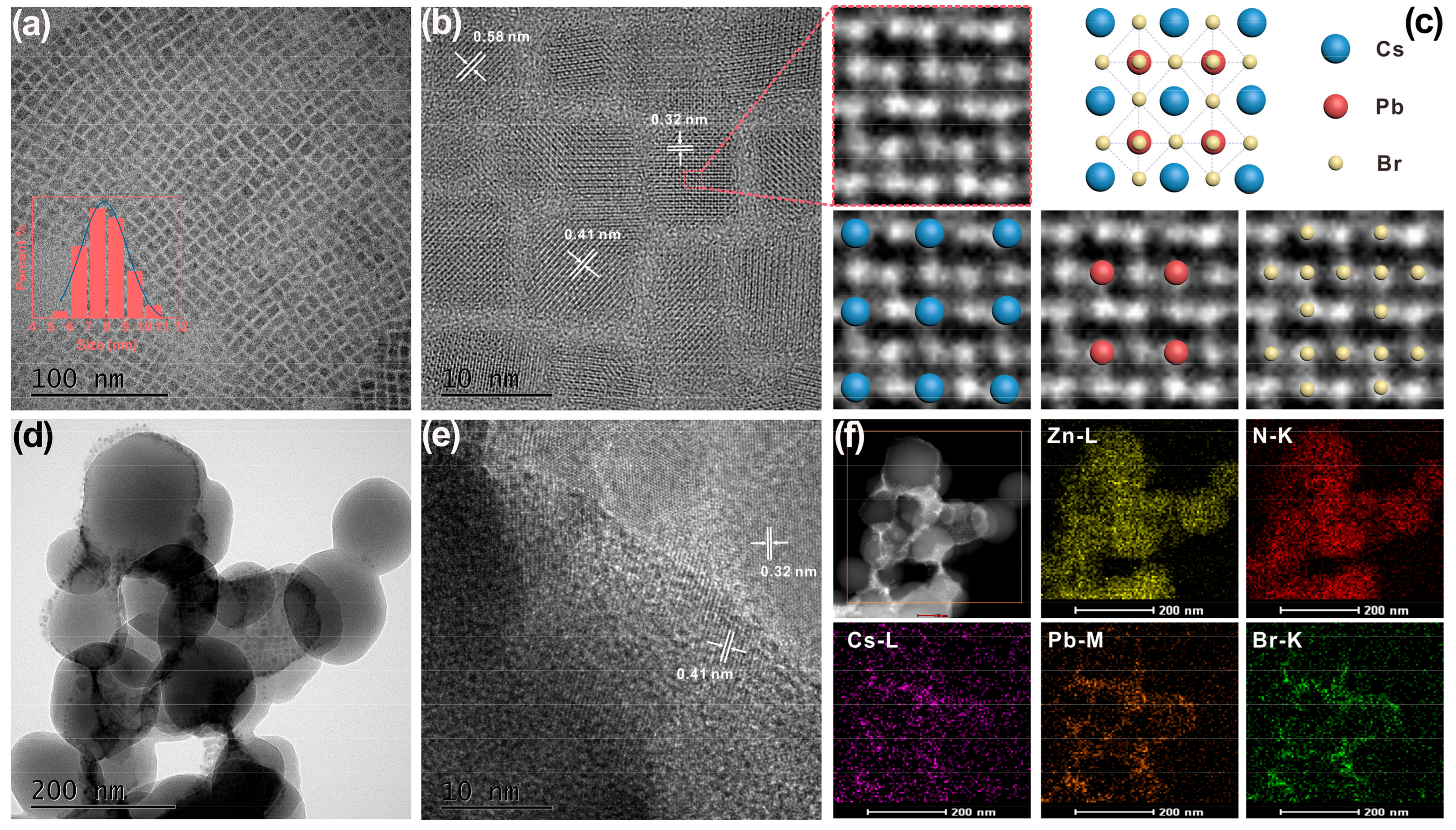
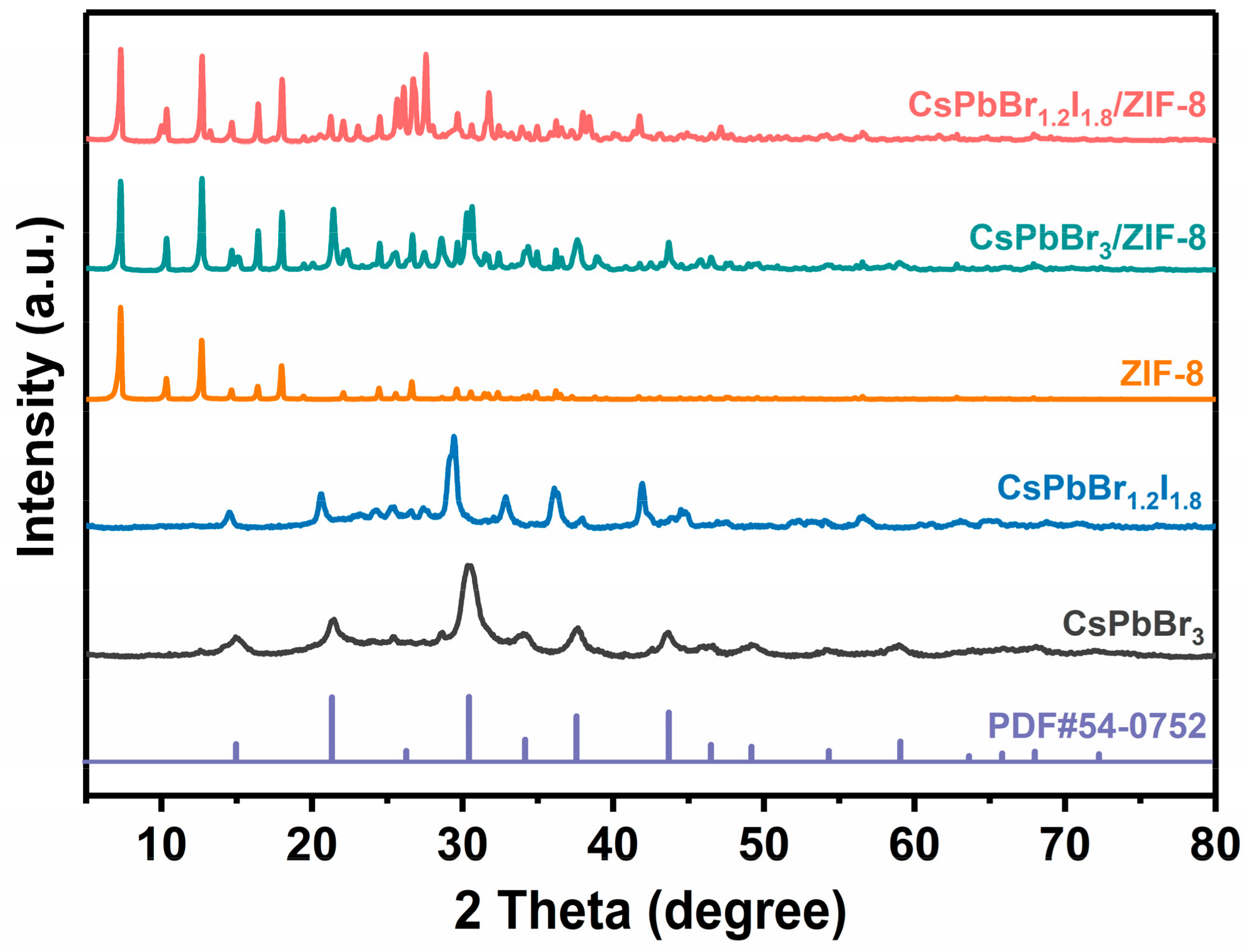
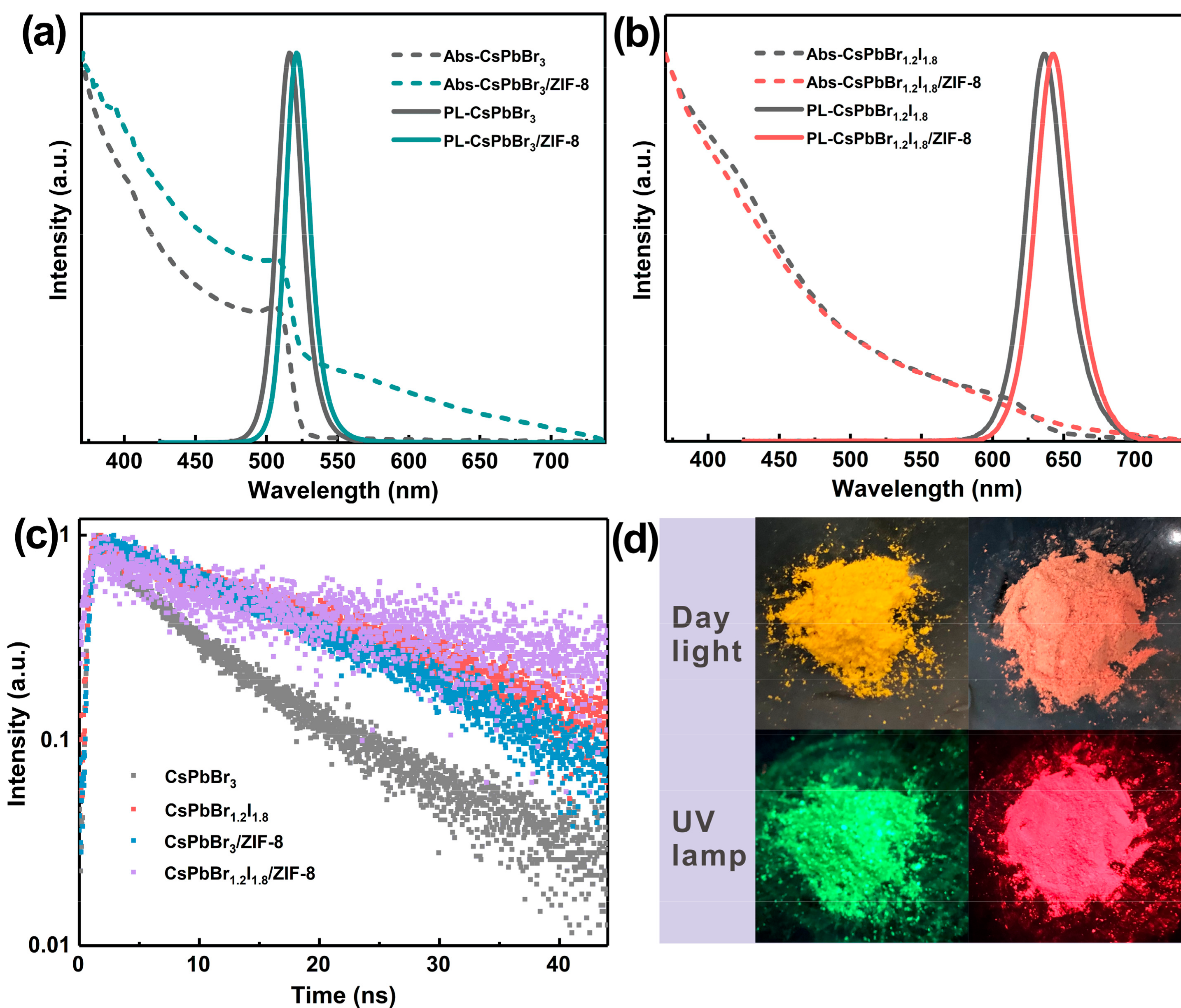
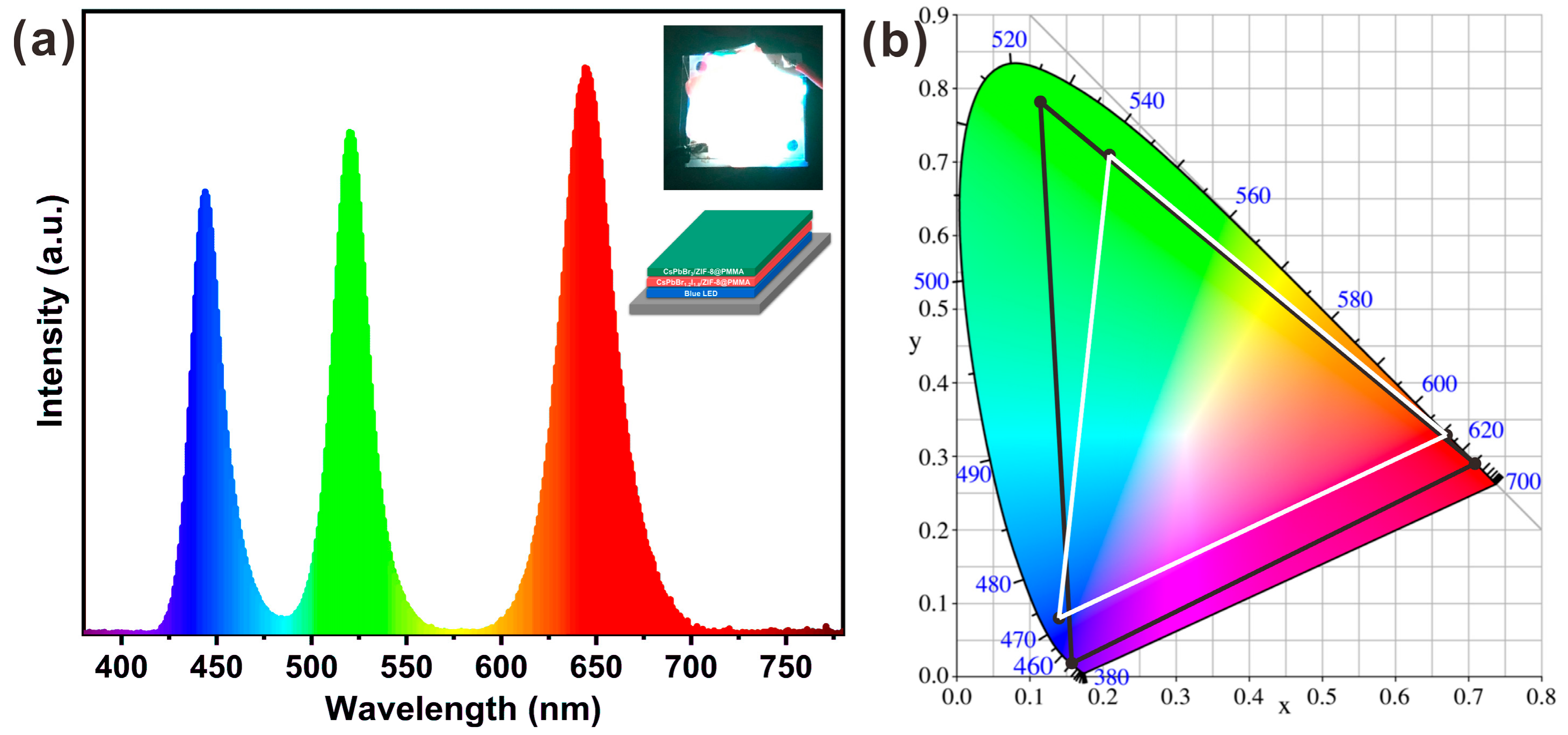
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Preparation of Cs-Oleate Solution
4.3. Synthesis of CsPbX3 QDs
4.4. Synthesis of CsPbX3/ZIF-8 Composites
4.5. Stability Test
4.6. Fabrication of the CsPbX3/ZIF-8@PMMA Films
4.7. Fabrication of a Remote-Type White LED
4.8. Characterization
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Lu, Z.; Zhang, X.; Zhang, Y.; Ma, H.; Ji, Y.; Xu, X.; Yu, L.; Xu, J.; Chen, K. Low power consumption red light-emitting diodes based on inorganic perovskite quantum dots under an alternating current driving mode. Nanomaterials 2018, 8, 974. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Gandini, M.; Di Stasio, F.; Rastogi, P.; Palazon, F.; Bertoni, G.; Ball, J.M.; Prato, M.; Petrozza, A.; Manna, L. Strongly emissive perovskite nanocrystal inks for high-voltage solar cells. Nat. Energy 2017, 2, 16194. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Sun, H.; Zeng, H. Amino-mediated anchoring perovskite quantum dots for stable and low-threshold random lasing. Adv. Mater. 2017, 29, 1701185. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Lim, D.H.; Kim, B.; Lee, S.H.; Lee, M.S.; Lee, J.S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Liu, X.; Zhang, W.; Liu, R.; Zheng, L.; Guo, R.; Tian, P. High-bandwidth white-light system combining a micro-LED with perovskite quantum dots for visible light communication. ACS Appl. Mater. Interfaces 2018, 10, 5641–5648. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Wang, B.; Zhu, N.; Zhang, C.; Kong, L.; Zhang, Q.; Shan, A.; Li, L. Morphology evolution and degradation of CsPbBr3 nanocrystals under blue light-emitting diode illumination. ACS Appl. Mater. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, L.; Huang, S.; Li, L. Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica/alumina monolith. Angew. Chem. Int. Ed. 2017, 56, 8134–8138. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Kong, L.; Zhu, N.; Shan, A.; Li, L. Enhancing the stability of CH3NH3PbBr3 quantum dots by embedding in silica spheres derived from tetramethyl orthosilicate in ”waterless” toluene. J. Am. Chem. Soc. 2016, 138, 5749–5752. [Google Scholar] [CrossRef]
- Cai, J.; Gu, K.; Zhu, Y.; Zhu, J.; Wang, Y.; Shen, J.; Trinchi, A.; Li, C.; Wei, G. Highly stable CsPbBr3@SiO2 nanocomposites prepared via confined condensation for use as a luminescent ink. Chem. Commun. 2018, 54, 8064–8067. [Google Scholar] [CrossRef]
- Zhong, Q.; Cao, M.; Hu, H.; Yang, D.; Chen, M.; Li, P.; Wu, L.; Zhang, Q. One-pot synthesis of highly stable CsPbBr3@SiO2 core-shell nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef]
- Chen, W.; Hao, J.; Hu, W.; Zang, Z.; Tang, X.; Fang, L.; Niu, T.; Zhou, M. Enhanced stability and tunable photoluminescence in perovskite CsPbX3/ZnS quantum dot heterostructure. Small 2017, 13, 1604085. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhong, Q.; Yang, D.; Chen, M.; Hu, H.; Pan, Q.; Liu, H.; Cao, M.; Xu, Y.; Sun, B.; et al. Improving the stability and size tunability of cesium lead halide perovskite nanocrystals using trioctylphosphine oxide as the capping ligand. Langmuir 2017, 33, 12689–12696. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.; Yang, X.; Lou, S.; Huang, J.; Liu, Y.; Yu, J.; Li, H.; Wong, K.L.; Wang, C.; Wang, J. Highly stable CsPbBr3 quantum dots coated with alkyl phosphate for white light-emitting diodes. Nanoscale 2017, 9, 15286–15290. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiao, H.; Xie, Z.; Liang, S.; Liang, S.; Cai, X.; Huang, S.; Al Kheraif, A.A.; Jang, H.S.; Cheng, Z.; et al. Highly luminescent lead halide perovskite quantum dots in hierarchical CaF2 matrices with enhanced stability as phosphors for white light-emitting diodes. Adv. Opt. Mater. 2018, 6, 1701343. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q.; Xu, Z.; Li, H.; Zheng, L.; Fu, H. Embedding perovskite nanocrystals into a polymer matrix for tunable luminescence probes in cell imaging. Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Piao, X.; Tian, P.; Fang, M.; An, X.; Luo, C.; Qi, R.; Chen, Y.; Peng, H. Organometal halide perovskite nanocrystals embedded in silicone resins with bright luminescence and ultrastability. J. Mater. Chem. C 2017, 5, 12044–12049. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, S.Y.; Tang, A.C.; Singh, B.P.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Mesoporous silica particles integrated with all-inorganic CsPbBr3 perovskite quantum-dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Rabouw, F.T.; Yang, X.F.; Huang, X.Y.; Jing, X.P.; Ye, S.; Zhang, Q.Y. Facile two-step synthesis of all-inorganic perovskite CsPbX3 (X = Cl, Br, and I) zeolite-Y composite phosphors for potential backlight display application. Adv. Funct. Mater. 2017, 27, 1704371. [Google Scholar] [CrossRef]
- James, S.L. Metal–organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal–organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Song, T.; Yu, J.; Yang, Y.; Wang, Z.; Qian, G. Dye encapsulated metal-organic framework for warm-white LED with high color-rendering index. Adv. Funct. Mater. 2015, 25, 4796–4802. [Google Scholar] [CrossRef]
- Wen, Y.; Sheng, T.; Zhu, X.; Zhuo, C.; Su, S.; Li, H.; Hu, S.; Zhu, Q.L.; Wu, X. Introduction of red-green-blue fluorescent dyes into a metal–organic framework for tunable white light emission. Adv. Mater. 2017, 29, 1700778. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, T.; An, J.; Li, X.; Zhang, L.; Li, L.; Li, G.; Wu, X.; Su, Z.; Wang, C. Carbon nanodots@zeolitic imidazolate framework-8 nanoparticles for simultaneous pH-responsive drug delivery and fluorescence imaging. CrystEngComm 2014, 16, 3259–3263. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and applications of metal-organic framework-quantum dot (QD at MOF) composites. Coord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef]
- Kumar, R.; Jayaramulu, K.; Maji, T.K.; Rao, C.N.R. Hybrid nanocomposites of ZIF-8 with graphene oxide exhibiting tunable morphology, significant CO2 uptake and other novel properties. Chem. Commun. 2013, 49, 4947–4949. [Google Scholar] [CrossRef]
- Lu, G.; Farha, O.K.; Zhang, W.; Huo, F.; Hupp, J.T. Engineering ZIF-8 thin films for hybrid MOF-based devices. Adv. Mater. 2012, 24, 3970–3974. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Li, X.; Yang, Y.; Yang, Q.; Li, C. Assembly of ZIF nanostructures around free Pt nanoparticles: Efficient size-selective catalysts for hydrogenation of alkenes under mild conditions. Chem. Commun. 2013, 49, 3330–3332. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Wang, H.; Zhan, Q.; Liu, Q.; Xia, Z. Postsynthetic surface trap removal of CsPbX3 (X=Cl, Br, or I) quantum dots via a ZnX2/Hexane solution toward an enhanced luminescence quantum yield. Chem. Mater. 2018, 30, 8546–8554. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, C.; Bai, X.; Zhang, X.; Wang, Y.; Wang, Y.; Song, H.; Yu, W.W. Efficient and stable CsPb(Br/I)3@anthracene composites for white light-emitting devices. ACS Appl. Mater. Interfaces 2018, 10, 16768–16775. [Google Scholar] [CrossRef] [PubMed]
- Moreels, I.; Rainò, G.; Gomes, R.; Hens, Z.; Stöferle, T.; Mahrt, R.F. Band-edge exciton fine structure of small, nearly spherical colloidal CdSe/ZnS quantum dots. ACS Nano 2011, 5, 8033–8039. [Google Scholar] [CrossRef] [PubMed]
- Chirvony, V.S.; González-Carrero, S.; Suárez, I.; Galian, R.E.; Sessolo, M.; Bolink, H.J.; Martínez-Pastor, J.P.; Pérez-Prieto, J. Delayed Luminescence in Lead Halide Perovskite Nanocrystals. J. Phys. Chem. C 2017, 121, 13381–13390. [Google Scholar] [CrossRef]
- Jang, E.; Jun, S.; Jang, H.; Lim, J.; Kim, B.; Kim, Y. White-light-emitting diodes with quantum dot color converters for display backlights. Adv. Mater. 2010, 22, 3076–3080. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.C.; Tang, A.C.; Lin, S.Y.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Robust and stable narrow-band green emitter: An option for advanced wide-color-gamut backlight display. Chem. Mater. 2016, 28, 8493–8497. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, G.; Yin, Y.; Miao, J.; Li, K.; Wang, C.; Xu, X.; Shen, C.; Meng, H. Aluminum-doped cesium lead bromide perovskite nanocrystals with stable blue photoluminescence used for display backlight. Adv. Sci. 2017, 4, 1700335. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Han, J.S.; Kim, W.; Jang, H.S. Facile synthesis of thermally stable CsPbBr3 perovskite quantum dot-inorganic SiO2 composites and their application to white light-emitting diodes with wide color gamut. Dyes Pigments 2018, 149, 246–252. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, S.; Yang, B.; Wei, X.; Dai, H.; Chen, Z.; Cui, Z.; Zhang, G.; Xie, F.; Zhang, W.; Guo, R. Facile Synthesis and Optical Properties of CsPbX3/ZIF-8 Composites for Wide-Color-Gamut Display. Nanomaterials 2019, 9, 832. https://doi.org/10.3390/nano9060832
Mei S, Yang B, Wei X, Dai H, Chen Z, Cui Z, Zhang G, Xie F, Zhang W, Guo R. Facile Synthesis and Optical Properties of CsPbX3/ZIF-8 Composites for Wide-Color-Gamut Display. Nanomaterials. 2019; 9(6):832. https://doi.org/10.3390/nano9060832
Chicago/Turabian StyleMei, Shiliang, Bobo Yang, Xian Wei, Hanqing Dai, Zhihao Chen, Zhongjie Cui, Guilin Zhang, Fengxian Xie, Wanlu Zhang, and Ruiqian Guo. 2019. "Facile Synthesis and Optical Properties of CsPbX3/ZIF-8 Composites for Wide-Color-Gamut Display" Nanomaterials 9, no. 6: 832. https://doi.org/10.3390/nano9060832
APA StyleMei, S., Yang, B., Wei, X., Dai, H., Chen, Z., Cui, Z., Zhang, G., Xie, F., Zhang, W., & Guo, R. (2019). Facile Synthesis and Optical Properties of CsPbX3/ZIF-8 Composites for Wide-Color-Gamut Display. Nanomaterials, 9(6), 832. https://doi.org/10.3390/nano9060832





