Ta2O5/rGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
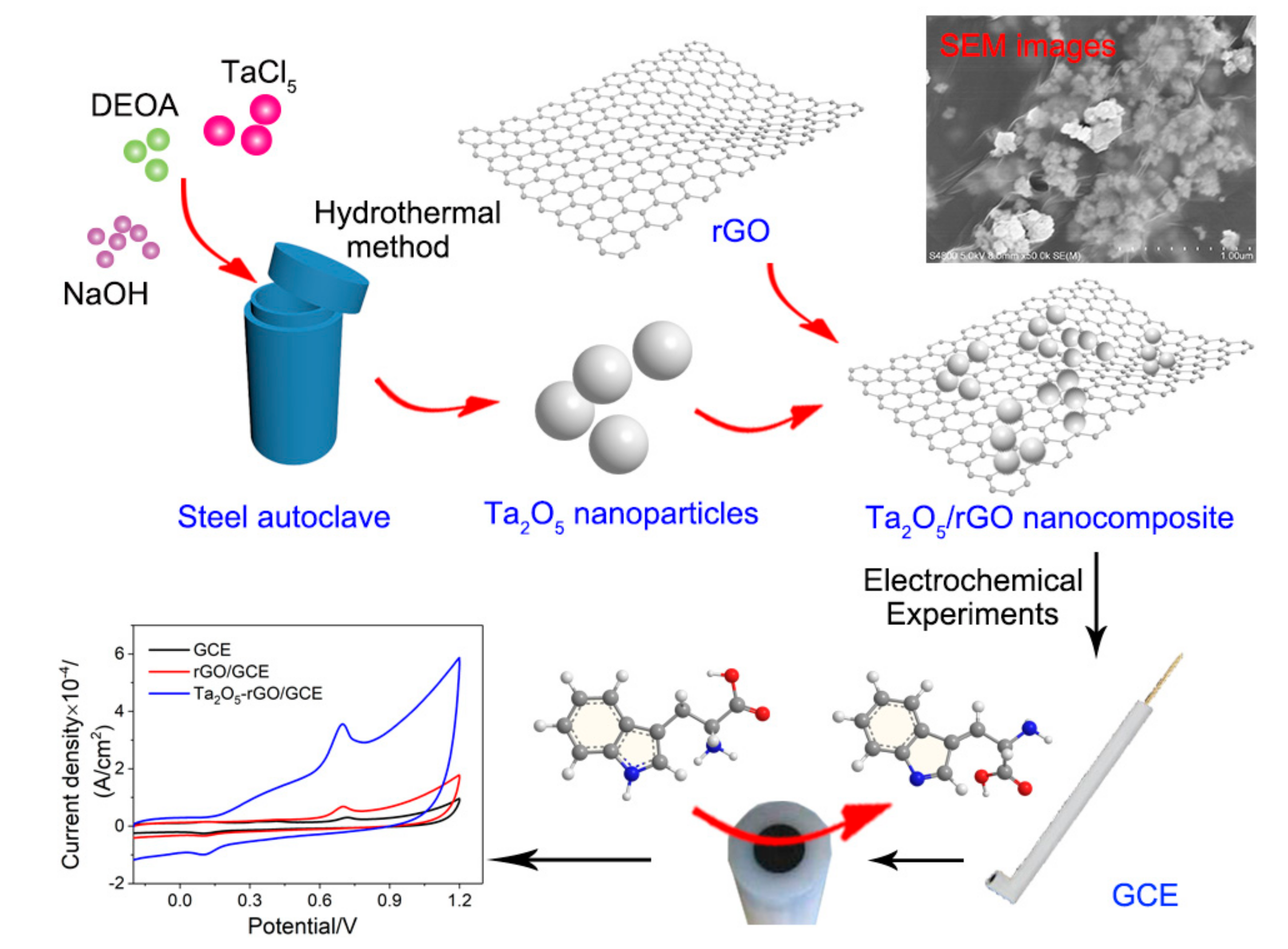
2.2. Preparation of Ta2O5 Nanoparticles
2.3. Synthesis of Ta2O5/GO Composites
2.4. Fabrication of Ta2O5-GO-Modified GCE
2.5. Characterization
2.6. Electrochemical Experiments
2.7. Detection of Tryptophan in Human Serum
3. Results and Discussion
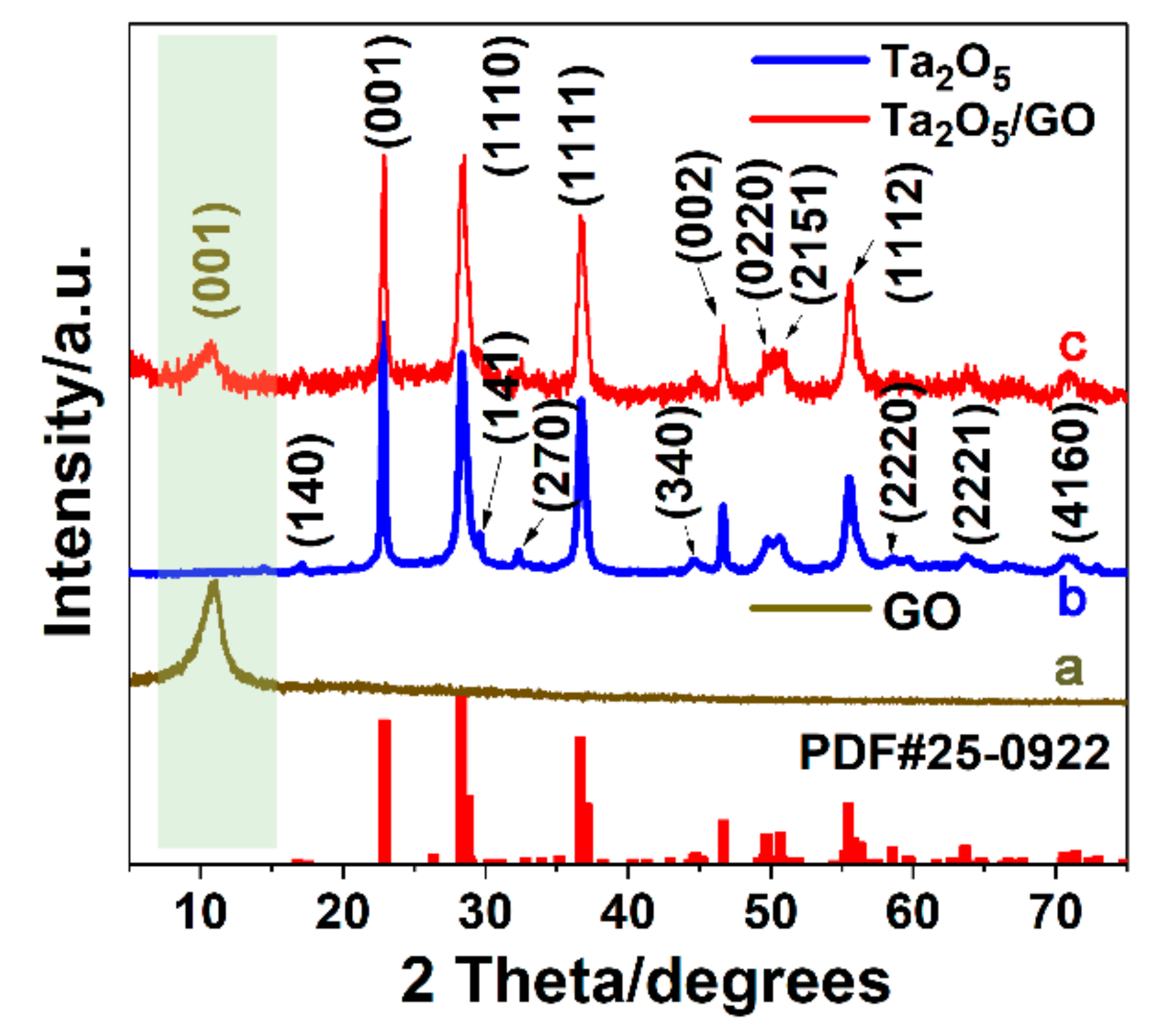
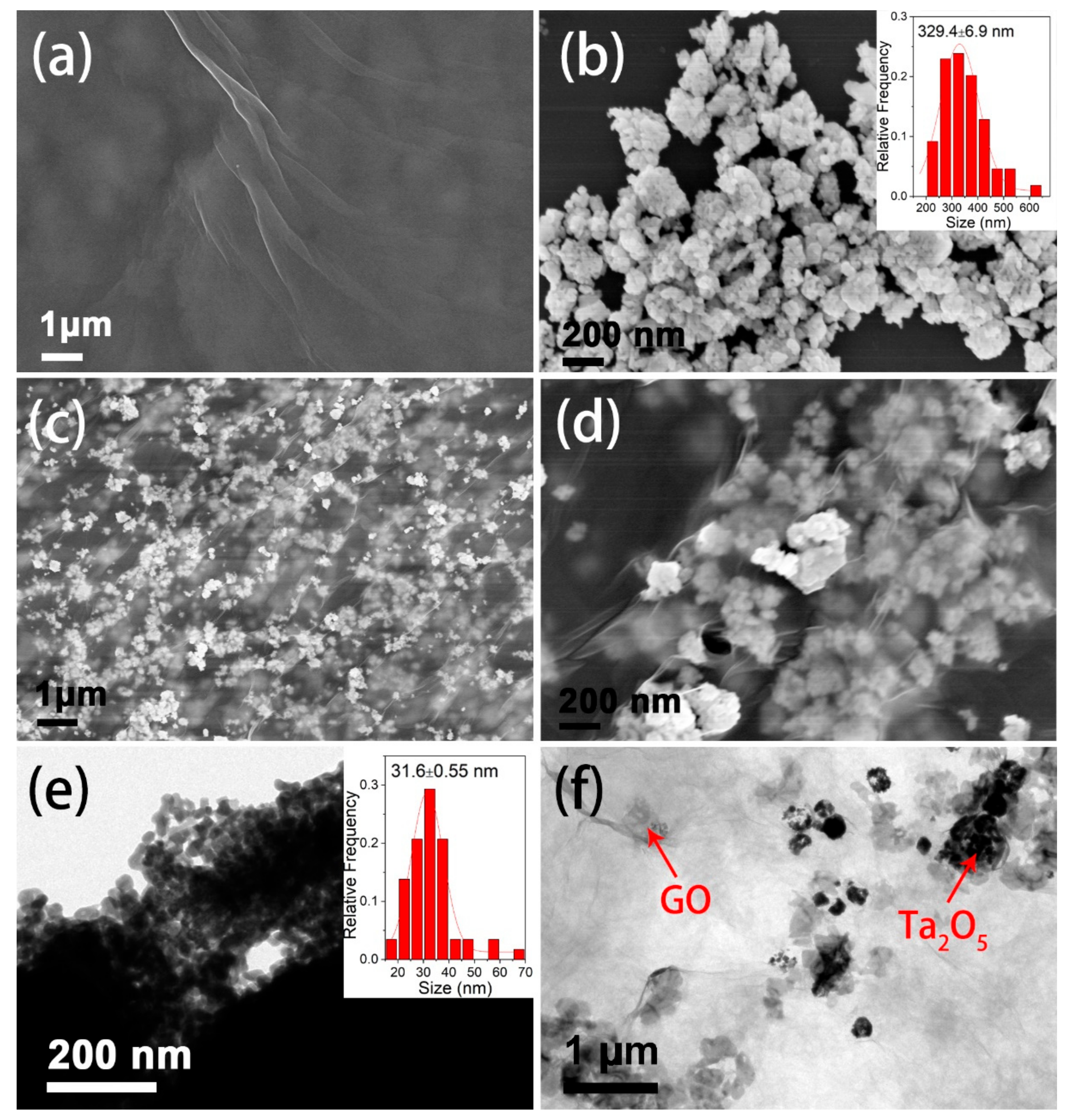
3.1. Structural and Morphologic Characterization of Ta2O5 and Ta2O5-GO
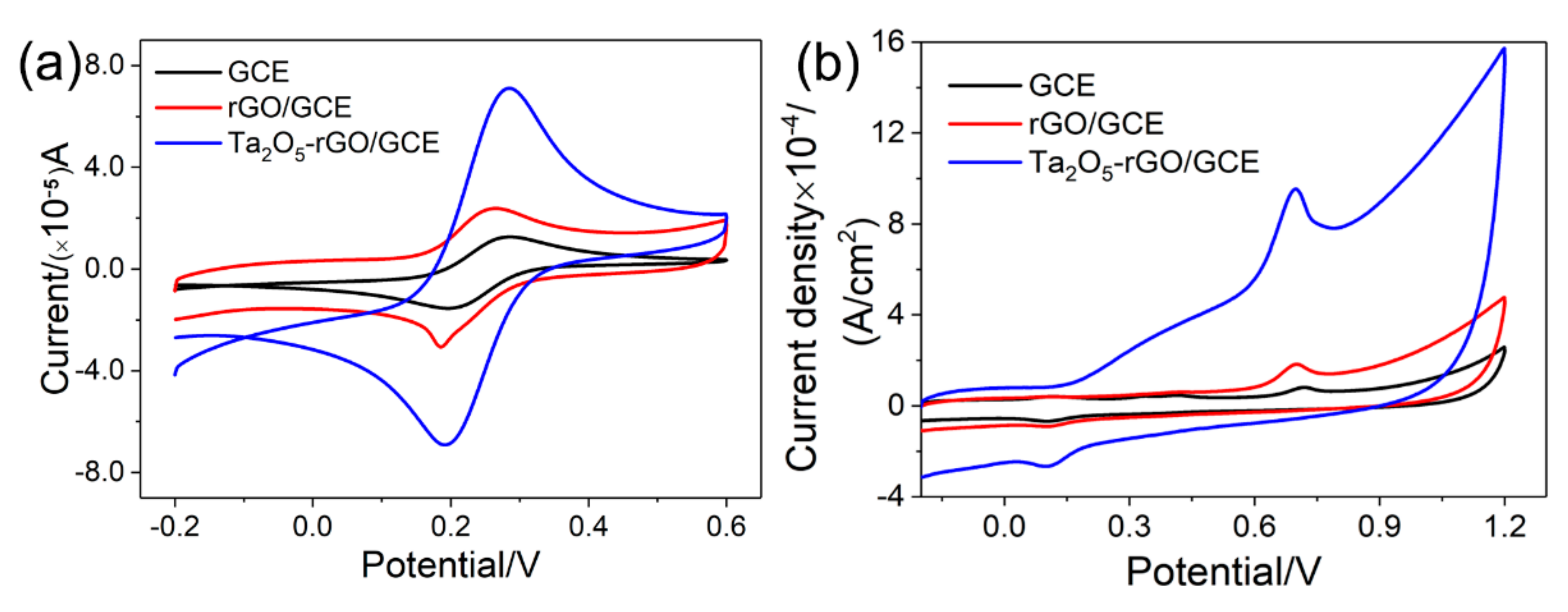
3.2. Electrochemical Activity Area of Ta2O5-rGO-GCE Nanocomposites
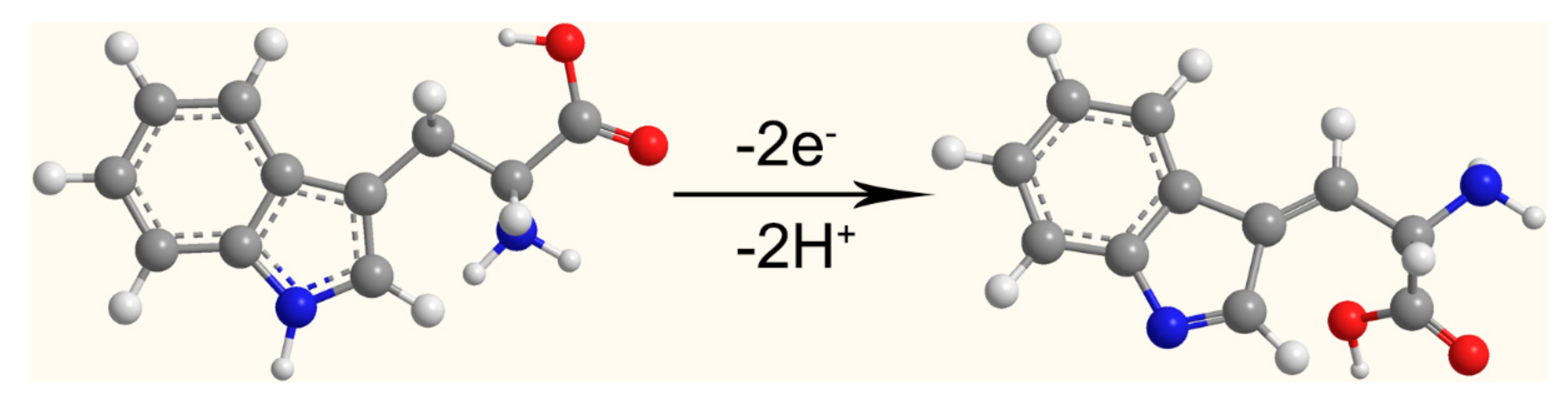
3.3. Electrochemical Behaviors of Tryptophan on Different Electrodes
3.4. Optimizations of Detection Conditions for Tryptophan
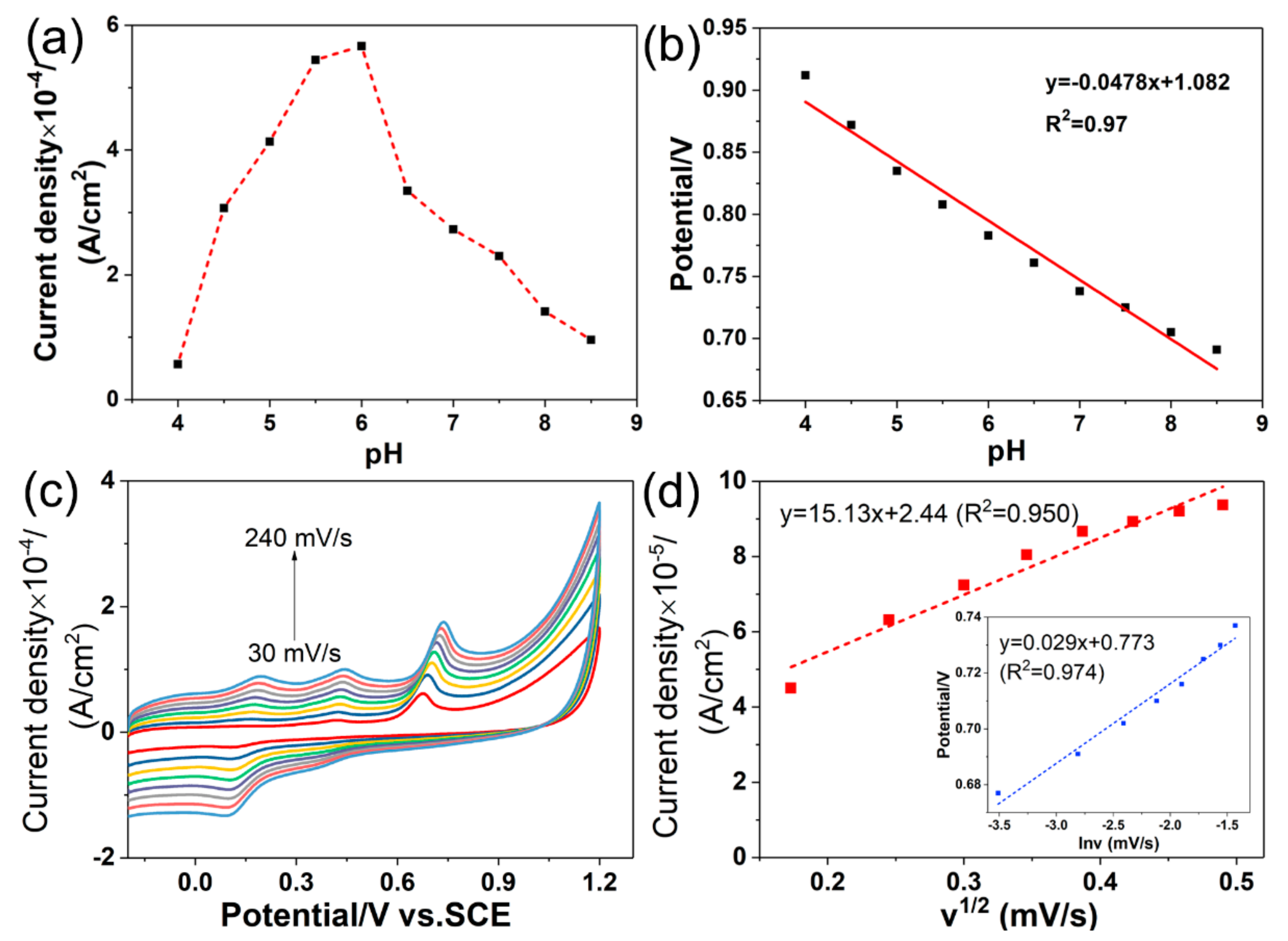
3.4.1. Influence of the pH
3.4.2. Effect of the Scan Rate
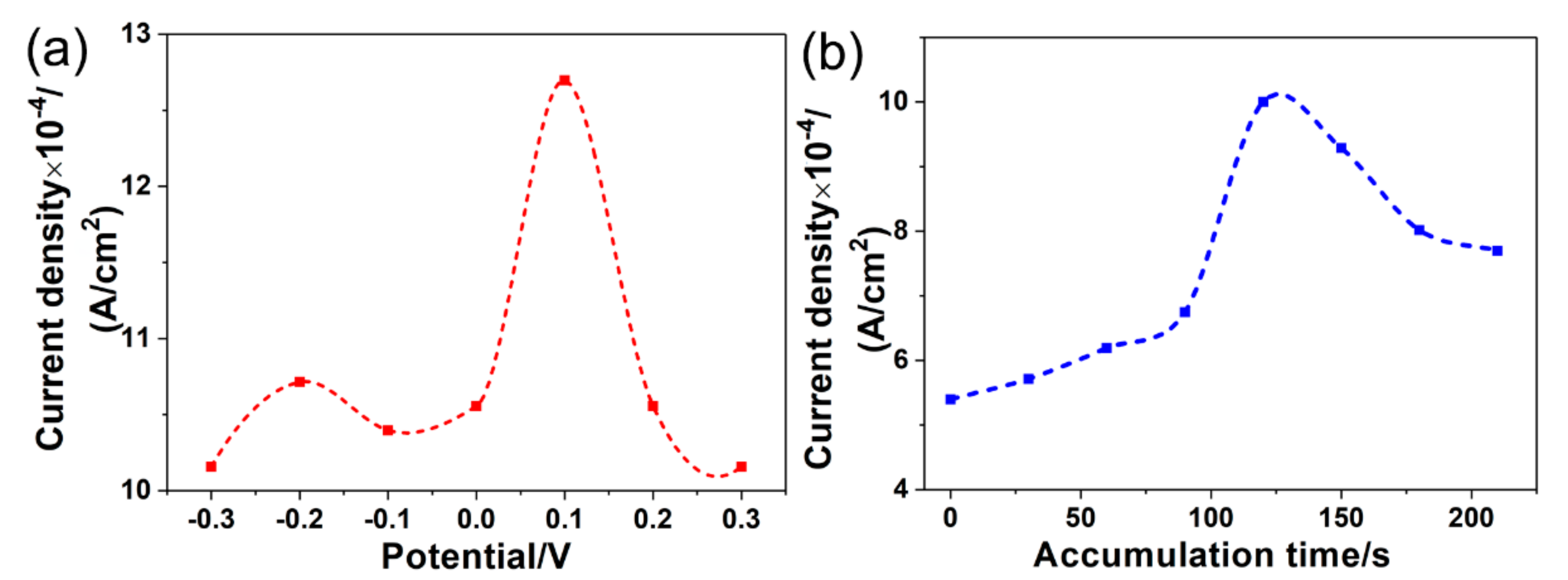
3.4.3. Effect of the Accumulation Conditions
3.5. Stability of the Detection
3.6. Linear Ranges and Detection Limit
3.7. Practical Sample Detections
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Jiang, J.; Xu, Z.; Liu, M.; Tang, S.; Yang, C.; Qian, D. Facile synthesis of Pd−Cu@Cu2O/N-RGO hybrid and its application for electrochemical detection of tryptophan. Electrochim. Acta 2018, 260, 526–535. [Google Scholar] [CrossRef]
- Mukdasai, S.; Poosittisak, S.; Ngeontae, W.; Srijaranai, S. A highly sensitive electrochemical determination of l-tryptophan in the presence of ascorbic acid and uric acid using in situ addition of tetrabutylammonium bromide on the ß-cyclodextrin incorporated multi-walled carbon nanotubes modified electrode. Sens. Actuators B Chem. 2018, 272, 518–525. [Google Scholar] [CrossRef]
- Kumar, J.V.; Karthik, R.; Chen, S.-M.; Marikkani, S.; Elangovan, A.; Muthuraj, V. Green synthesis of a novel flower-like cerium vanadate microstructure for electrochemical detection of tryptophan in food and biological samples. J. Colloid Interface Sci. 2017, 496, 78–86. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Zhou, Z.; Li, G.; Jiang, M.; Zhang, C.; Chen, S. Direct determination of free tryptophan contents in soy sauces and its application as an index of soy sauce adulteration. Food Chem. 2010, 118, 159–162. [Google Scholar] [CrossRef]
- Shin, H.J.; Park, N.H.; Lee, W.; Choi, M.H.; Chung, B.C.; Hong, J. Metabolic profiling of tyrosine, tryptophan, and glutamate in human urine using gas chromatography–tandem mass spectrometry combined with single SPE cleanup. J. Chromatogr. B 2017, 1051, 97–107. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Bermejo-Gómez, A.; Martín-Matute, B.; Zou, X. A water-stable lanthanide metal-organic framework for fluorimetric detection of ferric ions and tryptophan. Microchim. Acta 2017, 184, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Şahin, Y. A novel approach for the selective determination of tryptophan in blood serum in the presence of tyrosine based on the electrochemical reduction of oxidation product of tryptophan formed in situ on graphite electrode. Biosens. Bioelectron. 2012, 31, 26–31. [Google Scholar] [CrossRef]
- Deng, K.-Q.; Zhou, J.-H.; Li, X.-F. Direct electrochemical reduction of graphene oxide and its application to determination of L-tryptophan and L-tyrosine. Colloids Surf. B Biointerfaces 2013, 101, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Yeon, S.-H.; Huh, Y.S.; Han, Y.-K. Electrochemical determination of tryptophan using a glassy carbon electrode modified with flower-like structured nanocomposite consisting of reduced graphene oxide and SnO2. Sens. Actuators B Chem. 2017, 239, 1221–1230. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-Reduced Graphene Nanocomposite Modified Electrodes towards Ultrasensitive Dopamine Detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B Biointerfaces 2018, 172, 565–572. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. A promising sensing platform toward dopamine using MnO2 nanowires/electro-reduced graphene oxide composites. Electrochim. Acta 2019, 296, 683–692. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; Deng, P.; Chen, D. Facile electrochemical sensor for nanomolar rutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials 2019, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and selective detection of tartrazine based on TiO2-electrochemically reduced graphene oxide composite-modified electrodes. Sensors 2018, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wu, Y.; Kai, S.; Li, G.; Ye, B. A newly competitive electrochemical sensor for sensitive determination of chrysin based on electrochemically activated Ta2O5 particles modified carbon paste electrode. Electroanalysis 2017, 29, 835–842. [Google Scholar] [CrossRef]
- Xie, Z.; Li, G.; Fu, Y.; Sun, M.; Ye, B. Sensitive, simultaneous determination of chrysin and baicalein based on Ta2O5-chitosan composite modified carbon paste electrode. Talanta 2017, 165, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Akimov, A.V.; Asahi, R.; Jinnouchi, R.; Prezhdo, O.V. What makes the photocatalytic CO2 reduction on N-doped Ta2O5 efficient: Insights from nonadiabatic molecular dynamics. J. Am. Chem. Soc. 2015, 137, 11517–11525. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Imai, J.; Shiraishi, Y.; Tanaka, S.; Ichikawa, S.; Hirai, T. Photocatalytic dehalogenation of aromatic halides on Ta2O5-supported Pt–Pd bimetallic alloy nanoparticles activated by visible light. ACS Catal. 2017, 7, 5194–5201. [Google Scholar] [CrossRef]
- Gurung, K.; Ncibi, M.C.; Shestakova, M.; Sillanpää, M. Removal of carbamazepine from MBR effluent by electrochemical oxidation (EO) using a Ti/Ta2O5-SnO2 electrode. Appl. Catal. B Environ. 2018, 221, 329–338. [Google Scholar] [CrossRef]
- Anandan, S.; Pugazhenthiran, N.; Selvamani, T.; Hsieh, S.-H.; Lee, G.-J.; Wu, J.J. Investigation on photocatalytic potential of Au–Ta2O5 semiconductor nanoparticle by degrading Methyl Orange in aqueous solution by illuminating with visible light. Catal. Sci. Technol. 2012, 2, 2502–2507. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; He, Q.; Tian, Q.; Wu, W.; Xiao, X.; Jiang, C. Catalytic Application and Mechanism Studies of Argentic Chloride Coupled Ag/Au Hollow Heterostructures: Considering the Interface Between Ag/Au Bimetals. Nanoscale Res. Lett. 2019, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D Flowerlike α-Fe2O3@TiO2 Core–Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Ye, D.; Luo, L.; Ding, Y.; Liu, B.; Liu, X. Fabrication of Co3O4 nanoparticles-decorated graphene composite for determination of L-tryptophan. Analyst 2012, 137, 2840–2845. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Wurtman, R. Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science 1971, 173, 149–152. [Google Scholar] [CrossRef] [PubMed]


| Sample | Detected Amount (μmol/L) | RSD% | Added Amount (μmol/L) | Total Found Amount (μmol/L) | RSD% | Recovery |
|---|---|---|---|---|---|---|
| 1# | 36.6 | 1.34 | 20 | 56.7 | 1.6 | 101% |
| 2# | 56.3 | 2.82 | 40 | 99 | 1.08 | 106% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Deng, Z.; Wu, Z.; Xie, M.; Tian, Y.; Wu, Y.; Liu, J.; Li, G.; He, Q. Ta2O5/rGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route. Nanomaterials 2019, 9, 811. https://doi.org/10.3390/nano9060811
Zhou S, Deng Z, Wu Z, Xie M, Tian Y, Wu Y, Liu J, Li G, He Q. Ta2O5/rGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route. Nanomaterials. 2019; 9(6):811. https://doi.org/10.3390/nano9060811
Chicago/Turabian StyleZhou, Shun, Zefeng Deng, Zhongkang Wu, Mei Xie, Yaling Tian, Yiyong Wu, Jun Liu, Guangli Li, and Quanguo He. 2019. "Ta2O5/rGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route" Nanomaterials 9, no. 6: 811. https://doi.org/10.3390/nano9060811
APA StyleZhou, S., Deng, Z., Wu, Z., Xie, M., Tian, Y., Wu, Y., Liu, J., Li, G., & He, Q. (2019). Ta2O5/rGO Nanocomposite Modified Electrodes for Detection of Tryptophan through Electrochemical Route. Nanomaterials, 9(6), 811. https://doi.org/10.3390/nano9060811






