Orange/Red Photoluminescence Enhancement Upon SF6 Plasma Treatment of Vertically Aligned ZnO Nanorods
Abstract
1. Introduction
2. Experimental Procedure
2.1. Synthesis of ZnO Nanorods
2.2. Ar/SF6 Plasma Treatment
2.3. Nanostructures Characterization
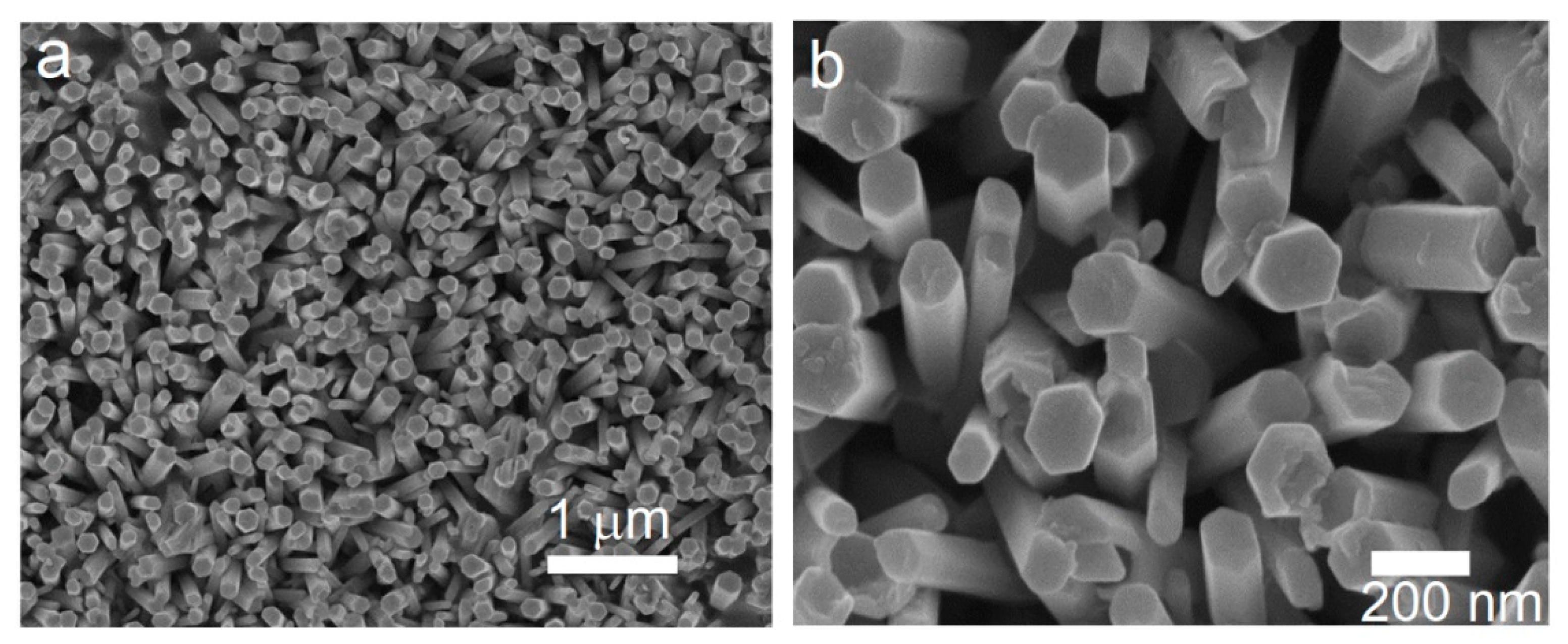
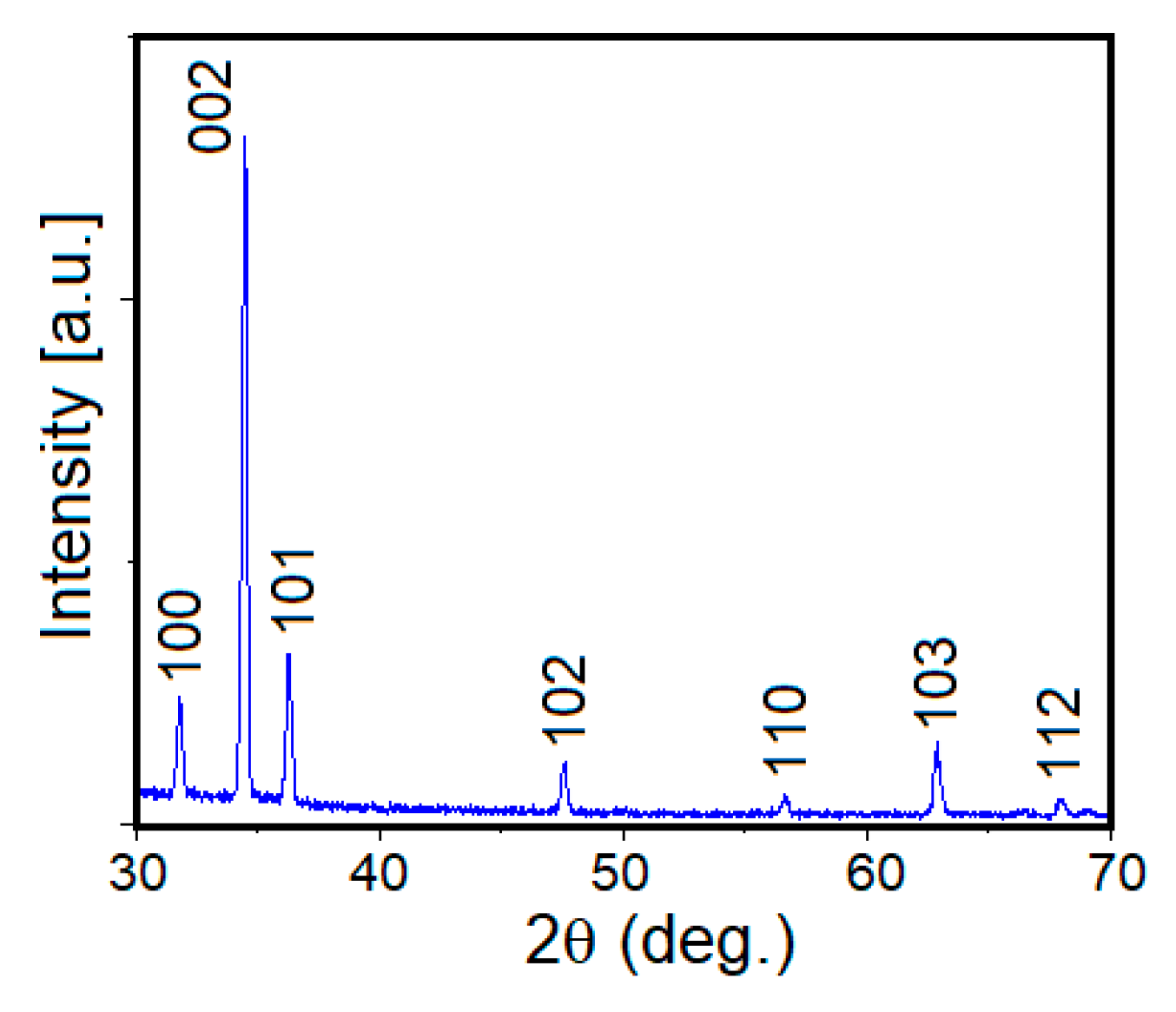
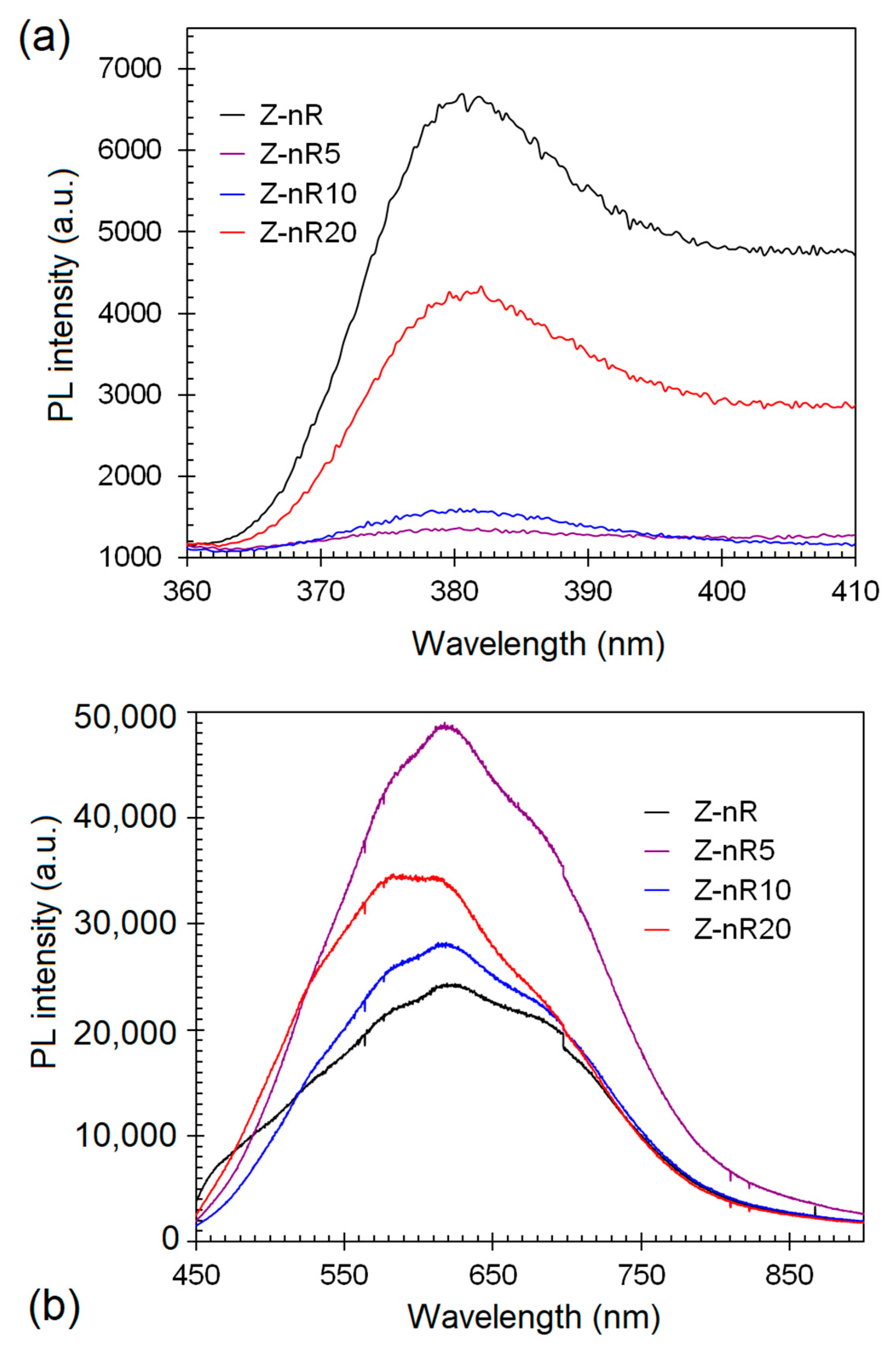
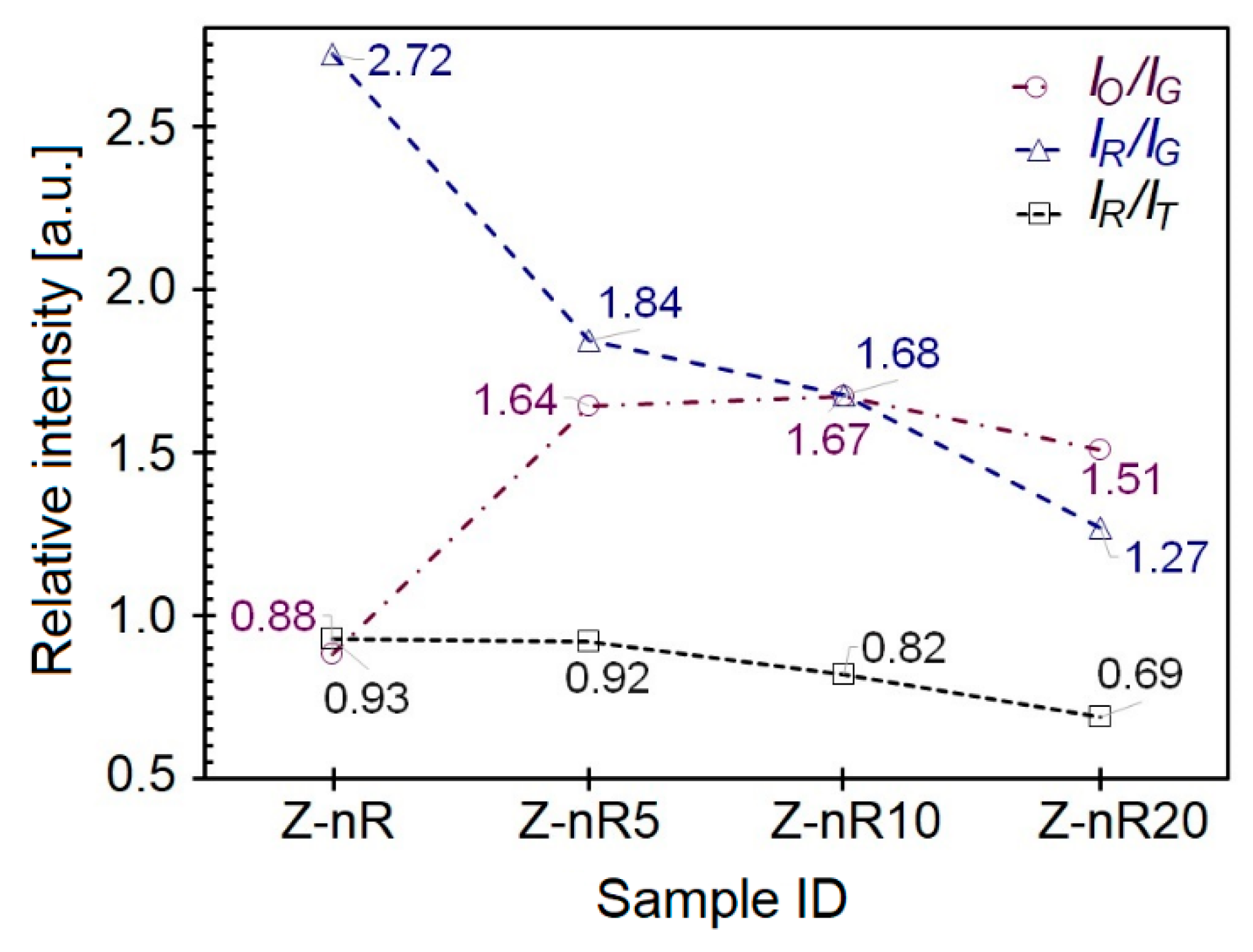
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zang, Z.; Zeng, X.; Du, J.; Wang, M.; Tang, X. Femtosecond laser direct writing of microholes on roughened ZnO for output power enhancement of InGaN light-emitting diodes. Opt. Lett. 2016, 41, 3463–3466. [Google Scholar] [CrossRef]
- Zang, Z. Efficiency enhancement of ZnO/Cu2O solar cells with well oriented and micrometer grain sized Cu2O films. Appl. Phys. Lett. 2018, 112, 042106. [Google Scholar] [CrossRef]
- Achour, A.; Soussou, M.A.; Ait Aissa, K.; Islam, M.; Barreau, N.; Faulques, E.; Le Brizoual, L.; Djouadi, M.A.; Boujtita, M. Nanostructuration and band gap emission enhancement of ZnO film via electrochemical anodization. Thin Solid Films 2014, 571, 168–174. [Google Scholar] [CrossRef]
- Li, C.; Zang, Z.; Han, C.; Hu, Z.; Tang, X.; Du, J.; Leng, Y.; Sun, K. Highly compact CsPbBr3 perovskite thin films decorated by ZnO nanoparticles for enhanced random lasing. Nano Energy 2017, 40, 195–202. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Zhang, H.; Jin, L.; Fang, X.; Shi, L.; Xu, L.; Ma, X.; Zou, Y.; Yin, J. Ultraviolet-enhanced electroluminescence from individual ZnO microwire/p-Si light-emitting diode by reverse tunneling effect. J. Mater. Chem. C 2017, 5, 6640–6646. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Young, S.-J.; Liu, Y.-H. Field emission properties of Al-doped ZnO nanosheet based on field emitter device with UV exposure. RSC Adv. 2017, 7, 14219–14223. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Ma, J.; Liu, Z.; Ma, L.; Lei, S.; Fang, J.; Long, C. Pt-decorated zinc oxide nanorod arrays with graphitic carbon nitride nanosheets for highly efficient dual-functional gas sensing. J. Hazard. Mater. 2018, 341, 102–111. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc oxide nanostructure-modified textile and its application to biosensing, photocatalysis, and as antibacterial material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, U.; Kim, D.K.; Islam, M.; Bhatti, A.S. Removal of micrometer size morphological defects and enhancement of UV emission by thermal treatment of Ga-doped ZnO nanostructures. PLoS ONE 2014, 9, e86418. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Islam, M.; Achour, A.; Hayat, A.; Ahsan, B.; Rasheed, H.; Salam, S.; Mujahid, M. Solution processing of cadmium sulfide buffer layer and aluminum-doped zinc oxide window layer for thin films solar cells. Surf. Rev. Lett. 2014, 21, 1450059. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Mei, J.; Tian, C.; Liu, H.; Wang, F.; Zhao, D. Surface plasmon-enhanced two-photon excited whispering-gallery modes ultraviolet laser from Zno microwire. AIP Adv. 2017, 7, 115302. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, L.; Wang, C.; Lun, N.; Qi, Y.; Xiang, D. Origin of visible photoluminescence of ZnO quantum dots: Defect-dependent and size-dependent. J. Phys. Chem. C 2010, 114, 9651–9658. [Google Scholar] [CrossRef]
- Li, D.; Leung, Y.H.; Djurišić, A.B.; Liu, Z.T.; Xie, M.H.; Shi, S.L.; Xu, S.J. Different origins of visible luminescence in ZnO nanostructures fabricated by the chemical and evaporation methods. Appl. Phys. Lett. 2004, 85, 1601–1603. [Google Scholar] [CrossRef]
- Baral, A.; Khanuja, M.; Islam, S.S.; Sharma, R.; Mehta, B.R. Identification and origin of visible transitions in one dimensional (1D) ZnO nanostructures: Excitation wavelength and morphology dependence study. J. Lumin. 2017, 183, 383–390. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Cui, Y.; Li, X.; Toufiq, A.M.; Lu, Y.; Li, Q. Novel method to enhance the visible emission of ZnO nanostructures. Chem. Phys. Lett. 2014, 614, 53–56. [Google Scholar] [CrossRef]
- Jadwisienczak, W.M.; Lozykowski, H.J.; Xu, A.; Pate, B. Visible emission from ZnO doped with rare-earth ions. J. Electron. Mater. 2002, 31, 776–784. [Google Scholar] [CrossRef]
- Jung, Y.-I.; Noh, B.-Y.; Lee, Y.-S.; Baek, S.-H.; Kim, J.H.; Park, I.-K. Visible emission from Ce-doped ZnO nanorods grown by hydrothermal method without a post thermal annealing process. Nanoscale Res. Lett. 2012, 7, 43. [Google Scholar] [CrossRef]
- Reish, M.E.; Zhang, Z.; Ma, S.; Harrison, I.; Everitt, H.O. How annealing and charge scavengers affect visible emission from ZnO nanocrystals. J. Phys. Chem. C 2016, 120, 5108–5113. [Google Scholar] [CrossRef]
- Gwon, M.; Lee, E.; Kim, D.-W.; Yee, K.-J.; Lee, M.J.; Kim, Y.S. Surface-plasmon-enhanced visible-light emission of ZnO/Ag grating structures. Opt. Express 2011, 19, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Gogurla, N.; Bayan, S.; Chakrabarty, P.; Ray, S.K. Plasmon mediated enhancement of visible light emission of Au-ZnO nanocomposites. J. Lumin. 2018, 194, 15–21. [Google Scholar] [CrossRef]
- Tian, J.-L.; Zhang, H.-Y.; Wang, G.-G.; Wang, X.-Z.; Sun, R.; Jin, L.; Han, J.-C. Influence of film thickness and annealing temperature on the structural and optical properties of ZnO thin films on Si (100) substrates grown by atomic layer deposition. Superlattices Microstruct. 2015, 83, 719–729. [Google Scholar] [CrossRef]
- Manzano, C.V.; Alegre, D.; Caballero-Calero, O.; Alén, B.; Martín-González, M.S. Synthesis and luminescence properties of electrodeposited ZnO films. J. Appl. Phys. 2011, 110, 043538. [Google Scholar] [CrossRef]
- Ton-That, C.; Weston, L.; Phillips, M.R. Characteristics of point defects in the green luminescence from Zn- and O-rich ZnO. Phys. Rev. B 2012, 86, 115205. [Google Scholar] [CrossRef]
- Brahma, S.; Khatei, J.; Sunkara, S.; Lo, K.Y.; Shivashankar, S.A. Self-assembled ZnO nanoparticles on ZnO microsheet: Ultrafast synthesis and tunable photoluminescence properties. J. Phys. D Appl. Phys. 2015, 48, 225305. [Google Scholar] [CrossRef]
- Cao, B.; Cai, W.; Zeng, H. Temperature-dependent shifts of three emission bands for ZnO nanoneedle arrays. Appl. Phys. Lett. 2006, 88, 161101. [Google Scholar] [CrossRef]
- Liu, M.; Kitai, A.H.; Mascher, P. Point defects and luminescence centres in zinc oxide and zinc oxide doped with manganese. J. Lumin. 1992, 54, 35–42. [Google Scholar] [CrossRef]
- Chua, S.J.; Loh, K.P.; Fitzgerald, E. The effect of post-annealing treatment on photoluminescence of ZnO nanorods prepared by hydrothermal synthesis. J. Cryst. Growth 2006, 287, 157–161. [Google Scholar]
- Chen, C.; He, H.; Lu, Y.; Wu, K.; Ye, Z. Surface Passivation Effect on the photoluminescence of ZnO nanorods. ACS Appl. Mater. Interfaces 2013, 5, 6354–6359. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, E.; Zeniou, A.; Tsikritzis, D.; Soultati, A.; Sakellis, I.; Gardelis, S.; Papadopoulos, T.A.; Briscoe, J.; Palilis, L.C.; Kennou, S.; et al. Surface passivation effect by fluorine plasma treatment on ZnO for efficiency and lifetime improvement of inverted polymer solar cells. J. Mater. Chem. A 2016, 4, 11844–11858. [Google Scholar] [CrossRef]
- Prucnal, S.; Gao, K.; Zhou, S.; Wu, J.; Cai, H.; Gordan, O.D.; Zahn, D.R.T.; Larkin, G.; Helm, M.; Skorupa, W. Optoelectronic properties of ZnO film on silicon after SF6 plasma treatment and milliseconds annealing. Appl. Phys. Lett. 2014, 105, 221903. [Google Scholar] [CrossRef]
- Lee, S.; Peng, J.W.; Liu, C.S. Photoluminescence and SERS investigation of plasma-treated ZnO nanorods. Appl. Surf. Sci. 2013, 285, 748–754. [Google Scholar] [CrossRef]
- Baratto, C.; Comini, E.; Ferroni, M.; Faglia, G.; Sberveglieri, G. Plasma-induced enhancement of UV photoluminescence in ZnO nanowires. CrystEngComm 2013, 15, 7981–7986. [Google Scholar] [CrossRef]
- Achour, H.; Achour, A.; Solaymani, S.; Islam, M.; Vizireanu, S.; Arman, A.; Ahmadpourian, A.; Dinescu, G. Plasma surface functionalization of boron nitride nano-sheets. Diam. Relat. Mater. 2017, 77, 110–115. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Vizireanu, S.; Ionita, M.D.; Dinescu, G.; Enculescu, I.; Baibarac, M.; Baltog, I. Post-synthesis carbon nanowalls transformation under hydrogen, oxygen, nitrogen, tetrafluoroethane and sulfur hexafluoride plasma treatments. Plasma Process. Polym. 2012, 9, 363–370. [Google Scholar] [CrossRef]
- Lee, Y.; Zhang, Y.; Ng, S.L.G.; Kartawidjaja, F.C.; Wang, J. Hydrothermal growth of vertical ZnO nanorods. J. Am. Ceram. Soc. 2009, 92, 1940–1945. [Google Scholar] [CrossRef]
- Riaz, A.; Ashraf, A.; Taimoor, H.; Javed, S.; Akram, M.A.; Islam, M.; Mujahid, M.; Ahmad, I.; Saeed, K. Photocatalytic and Photostability Behavior of Ag- and/or Al-Doped ZnO Films in Methylene Blue and Rhodamine B under UV-C Irradiation. Coatings 2019, 9, 202. [Google Scholar] [CrossRef]
- Cao, X.L.; Zeng, H.B.; Wang, M.; Xu, X.J.; Fang, M.; Ji, S.L.; Zhang, L.D. Large scale fabrication of quasi-aligned ZnO stacking nanoplates. J. Phys. Chem. C 2008, 112, 5267–5270. [Google Scholar] [CrossRef]
- Lepot, N.; van Bael, M.K.; van den Rul, H.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Synthesis of ZnO nanorods from aqueous solution. Mater. Lett. 2007, 61, 2624–2627. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Tan, D.H.; Montano, M.; Goldberger, J.; Somorja, G.; Yang, P. General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett. 2005, 5, 1231–1236. [Google Scholar] [CrossRef]
- Zad, A.K.; Majid, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Ilican, S.; Caglary, Y.; Caglar, M.; Demirci, B. Polycrystalline indium-doped ZnO thin films: Preparation and characterization. J. Optoelectron. Adv. Mater. 2008, 10, 2592–2598. [Google Scholar]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Pomar, F.S.; Martínez, E.; Meléndrez, M.F.; Tijerina, E.P. Growth of vertically aligned ZnO nanorods using textured ZnO films. Nanoscale Res. Lett. 2011, 6, 524. [Google Scholar] [CrossRef]
- Macaluso, R.; Mosca, M.; Calì, C.; di Franco, F.; Santamaria, M.; di Quarto, F.; Reverchon, J.-L. Erroneous p-type assignment by Hall effect measurements in annealed ZnO films grown on InP substrate. J. Appl. Phys. 2013, 113, 164508. [Google Scholar] [CrossRef]
- Børseth, T.M.; Svensson, B.G.; Kuznetsov, A.Y.; Klason, P.; Zhao, Q.X.; Willander, M. Identification of oxygen and zinc vacancy optical signals in ZnO. Appl. Phys. Lett. 2006, 89, 262112. [Google Scholar] [CrossRef]
- Lozada, E.V.; González, G.M.C.; Torchynska, T. Photoluminescence emission and structure diversity in ZnO:Ag nanorods. J. Phys. Conf. Ser. 2015, 582, 012031. [Google Scholar] [CrossRef]
- Kumar, V.; Swartn, H.C.; Ntwaeaborwa, O.M.; Kroon, R.E.; Terblans, J.J.; Shaat, S.K.K.; Yousif, A.; Duvenhage, M.M. Origin of the red emission in zinc oxide nanophosphors. Mater. Lett. 2013, 101, 57–60. [Google Scholar] [CrossRef]
- Jiang, S.; Ren, Z.; Gong, S.; Yin, S.; Yu, Y.; Li, X.; Xu, G.; Shen, G.; Han, G. Tunable photoluminescence properties of well-aligned ZnO nanorod array by oxygen plasma post-treatment. Appl. Surf. Sci. 2014, 289, 252–256. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Leem, J.-Y. Optical and electrical properties of F-doped ZnO thin films grown on muscovite mica substrates and their optical constants. J. Nanosci. Nanotechnol. 2017, 17, 5693–5696. [Google Scholar] [CrossRef]
- Papari, G.P.; Silvestri, B.; Vitiello, G.; de Stefano, L.; Rea, I.; Luciani, G.; Aronne, A.; Andreone, A. Morphological, structural, and charge transfer properties of F-doped ZnO: A spectroscopic investigation. J. Phys. Chem. C 2017, 121, 16012–16020. [Google Scholar] [CrossRef]
- Xu, H.Y.; Liu, Y.C.; Ma, J.G.; Luo, Y.M.; Lu, Y.M.; Shen, D.Z.; Zhang, J.Y.; Fan, X.W.; Mu, R. Photoluminescence of F-passivated ZnO nanocrystalline films made from thermally oxidized ZnF2 films. J. Phys. Condens. Matter 2004, 16, 5143–5150. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kang, K.-M.; Park, H.H. Anion-controlled passivation effect of the atomic layer deposited ZnO films by F substitution to O-related defects on the electronic band structure for transparent contact layer of solar cell applications. Sol. Energy Mater. Sol. Cells 2015, 132, 403–409. [Google Scholar] [CrossRef]
- El Hichou, A.; Bougrine, A.; Bubendorff, J.L.; Ebothe, J.; Addou, M.; Troyon, M. Structural, optical and cathodoluminescence characteristics of sprayed undoped and fluorine-doped ZnO thin films. Semicond. Sci. Technol. 2002, 17, 607–613. [Google Scholar] [CrossRef]
- Kumar, P.M.R.; Kartha, C.S.; Vijayakumar, K.P.; Singh, F.; Avasthi, D.K. Effect of fluorine doping on structural, electrical and optical properties of ZnO thin films. Mater. Sci. Eng. B 2005, 117, 307–312. [Google Scholar] [CrossRef]
- Achour, A.; Aissa, K.A.; Mbarek, M.; el Hadj, K.; Ouldhamadouche, N.; Barreau, N.; le Brizoual, L.; Djouadi, M.A. Enhancement of near-band edge photoluminescence of ZnO film buffered with TiN. Thin Solid Films 2013, 583, 71–77. [Google Scholar] [CrossRef]
- Xu, H.Y.; Liu, Y.C.; Mu, R.; Shao, C.L.; Lu, Y.M.; Shen, D.Z.; Fan, X.W. F-doping effects on electrical and optical properties of ZnO nanocrystalline films. Appl. Phys. Lett. 2005, 86, 123107. [Google Scholar] [CrossRef]
- Huang, T.-H.; Yang, P.-K.; Lien, D.-H.; Kang, C.-F.; Tsai, M.-L.; Chueh, Y.-L.; He, J.-H. Resistive memory for harsh electronics: Immunity to surface effect and high corrosion resistance via surface modification. Sci. Rep. 2014, 4, 4402. [Google Scholar] [CrossRef]
- Hsieh, P.T.; Chen, Y.C.; Kao, K.S.; Wang, C.M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Liu, W.; Jiang, G.; Zhu, C. Study of oxygen vacancies influence on the lattice parameter in ZnO thin film. Mater. Lett. 2012, 85, 25–28. [Google Scholar] [CrossRef]
- Chen, Y.; Nayak, J.; Ko, H.U.; Kim, J. Effect of annealing temperature on the characteristics of ZnO thin films. J. Phys. Chem. Solids 2012, 73, 1259–1263. [Google Scholar] [CrossRef]
- Achour, A.; Islam, M.; Solaymani, S.; Vizireanu, S.; Saeed, K.; Dinescu, G. Influence of plasma functionalization treatment and gold nanoparticles on surface chemistry and wettability of reactive-sputtered TiO2 thin films. Appl. Surf. Sci. 2018, 458, 678–685. [Google Scholar] [CrossRef]
- Javed, F.; Javed, S.; Akram, M.A.; Mujahid, M.; Islam, M.; Bhatti, A.S. Surface plasmon mediated optical properties of ZnO/Au/TiO2 nanoheterostructure rod arrays. Mater. Sci. Eng. B 2018, 231, 32–39. [Google Scholar] [CrossRef]


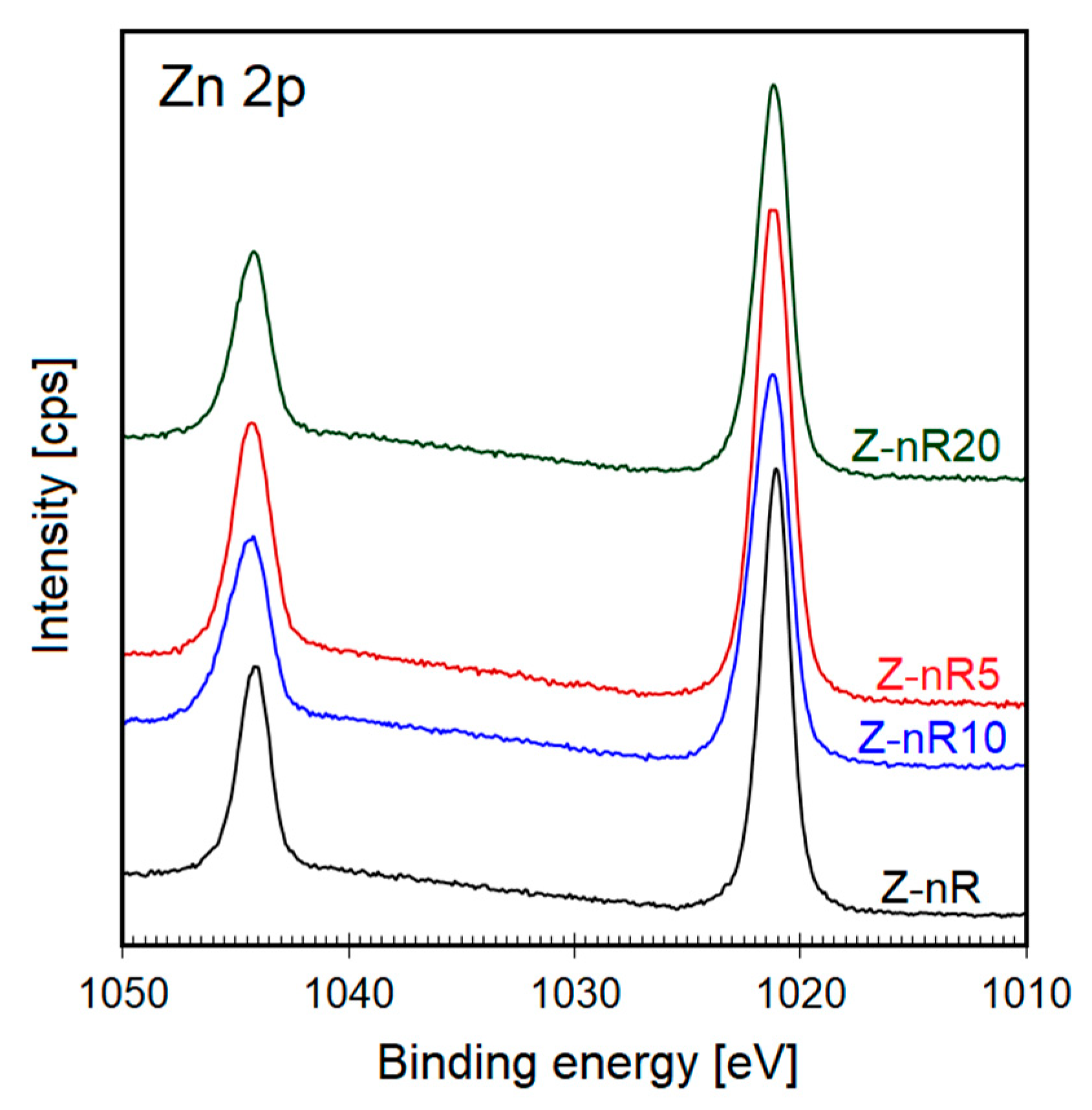
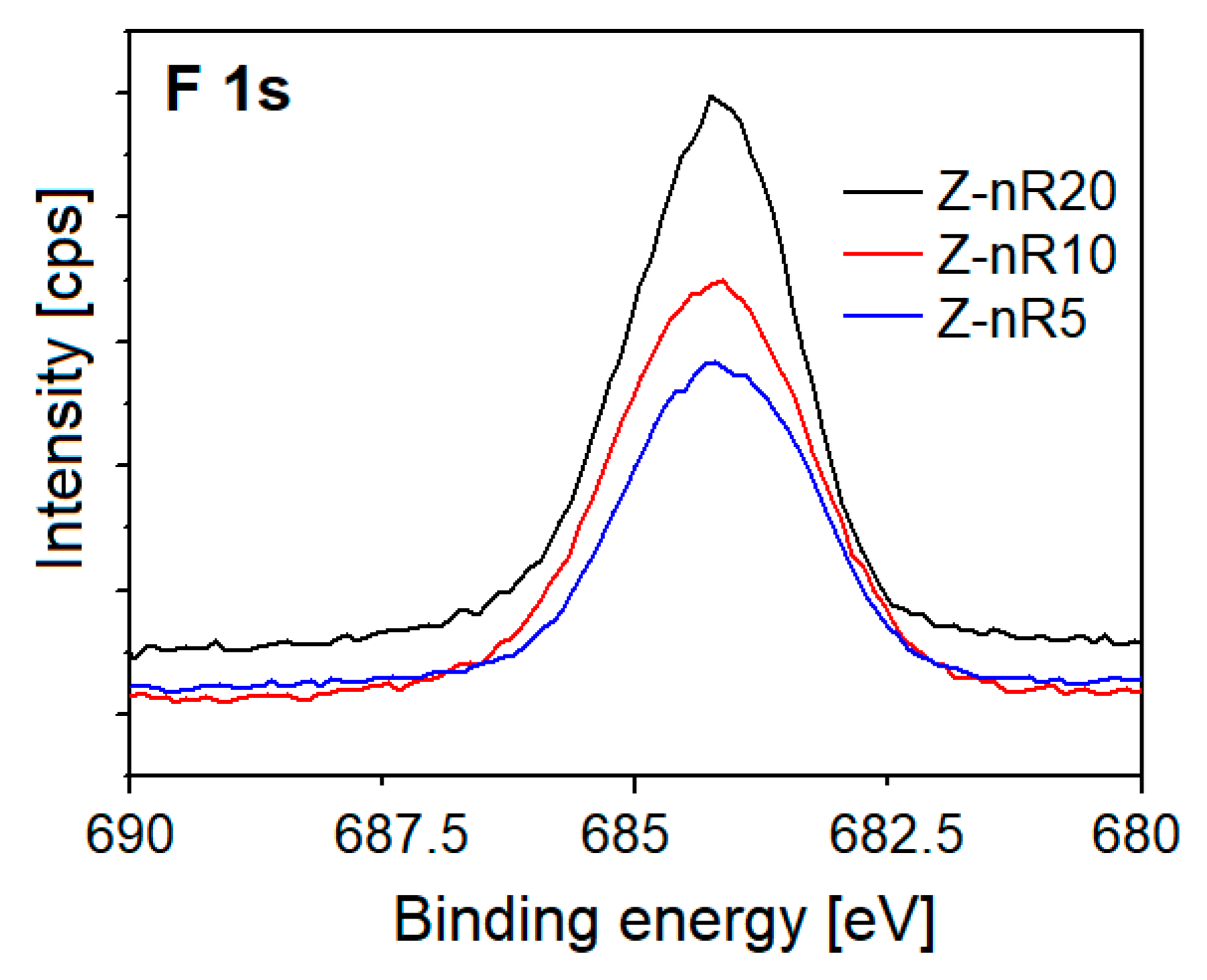

| Sample | O 1s Binding Energy (eV) | Percentage (%) |
|---|---|---|
| Z-nR | OL: 529.8 | 23.8 |
| Oi: 531.0 | 55.1 | |
| OC: 532.1 | 21.1 | |
| Z-nR5 | OL: 529.7 | 35.1 |
| Oi: 531.2 | 54.2 | |
| OC: 532.4 | 10.7 | |
| Z-nR10 | OL: 529.6 | 22.6 |
| Oi: 531.2 | 69.3 | |
| OC: 532.5 | 8.1 | |
| Z-nR20 | OL: 529.5 | 21.7 |
| Oi: 531.1 | 76.2 | |
| OC: 533.4 | 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achour, A.; Islam, M.; Vizireanu, S.; Ahmad, I.; Akram, M.A.; Saeed, K.; Dinescu, G.; Pireaux, J.-J. Orange/Red Photoluminescence Enhancement Upon SF6 Plasma Treatment of Vertically Aligned ZnO Nanorods. Nanomaterials 2019, 9, 794. https://doi.org/10.3390/nano9050794
Achour A, Islam M, Vizireanu S, Ahmad I, Akram MA, Saeed K, Dinescu G, Pireaux J-J. Orange/Red Photoluminescence Enhancement Upon SF6 Plasma Treatment of Vertically Aligned ZnO Nanorods. Nanomaterials. 2019; 9(5):794. https://doi.org/10.3390/nano9050794
Chicago/Turabian StyleAchour, Amine, Mohammad Islam, Sorin Vizireanu, Iftikhar Ahmad, Muhammad Aftab Akram, Khalid Saeed, Gheorghe Dinescu, and Jean-Jacques Pireaux. 2019. "Orange/Red Photoluminescence Enhancement Upon SF6 Plasma Treatment of Vertically Aligned ZnO Nanorods" Nanomaterials 9, no. 5: 794. https://doi.org/10.3390/nano9050794
APA StyleAchour, A., Islam, M., Vizireanu, S., Ahmad, I., Akram, M. A., Saeed, K., Dinescu, G., & Pireaux, J.-J. (2019). Orange/Red Photoluminescence Enhancement Upon SF6 Plasma Treatment of Vertically Aligned ZnO Nanorods. Nanomaterials, 9(5), 794. https://doi.org/10.3390/nano9050794





