Performance of Polyester-Based Electrospun Scaffolds under In Vitro Hydrolytic Conditions: From Short-Term to Long-Term Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffold Preparation
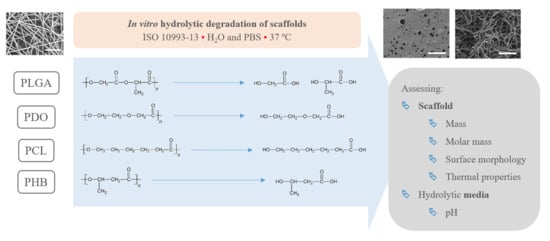
2.3. In Vitro Degradation Methodology
2.4. Scaffold Characterisation
2.4.1. Size Exclusion Chromatography (SEC)
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. Field Emission Scanning Electron Microscopy (FE-SEM)
3. Results
3.1. Initial Properties and Morphology of the Scaffolds
3.2. Hydrolytic in Vitro Degradation
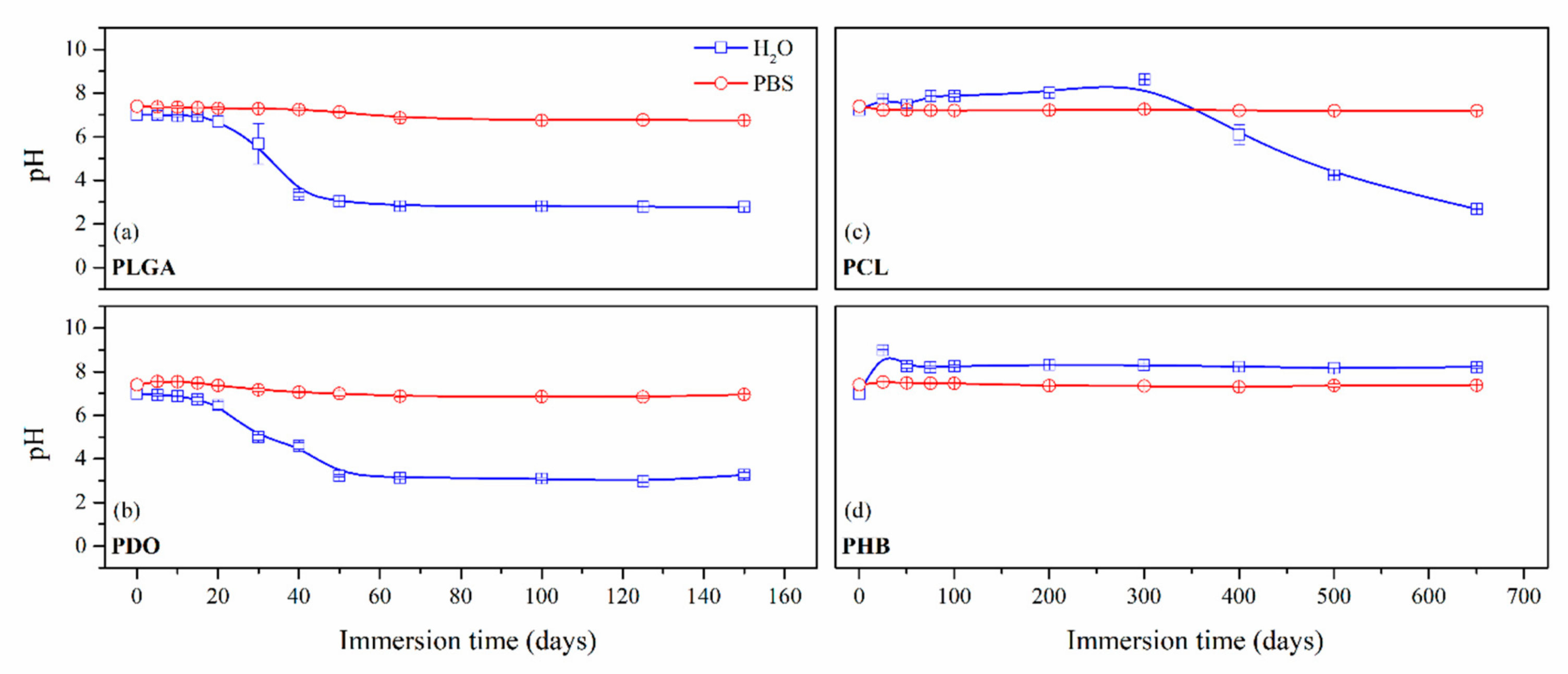
3.2.1. On-line Monitoring: Changes in the Media
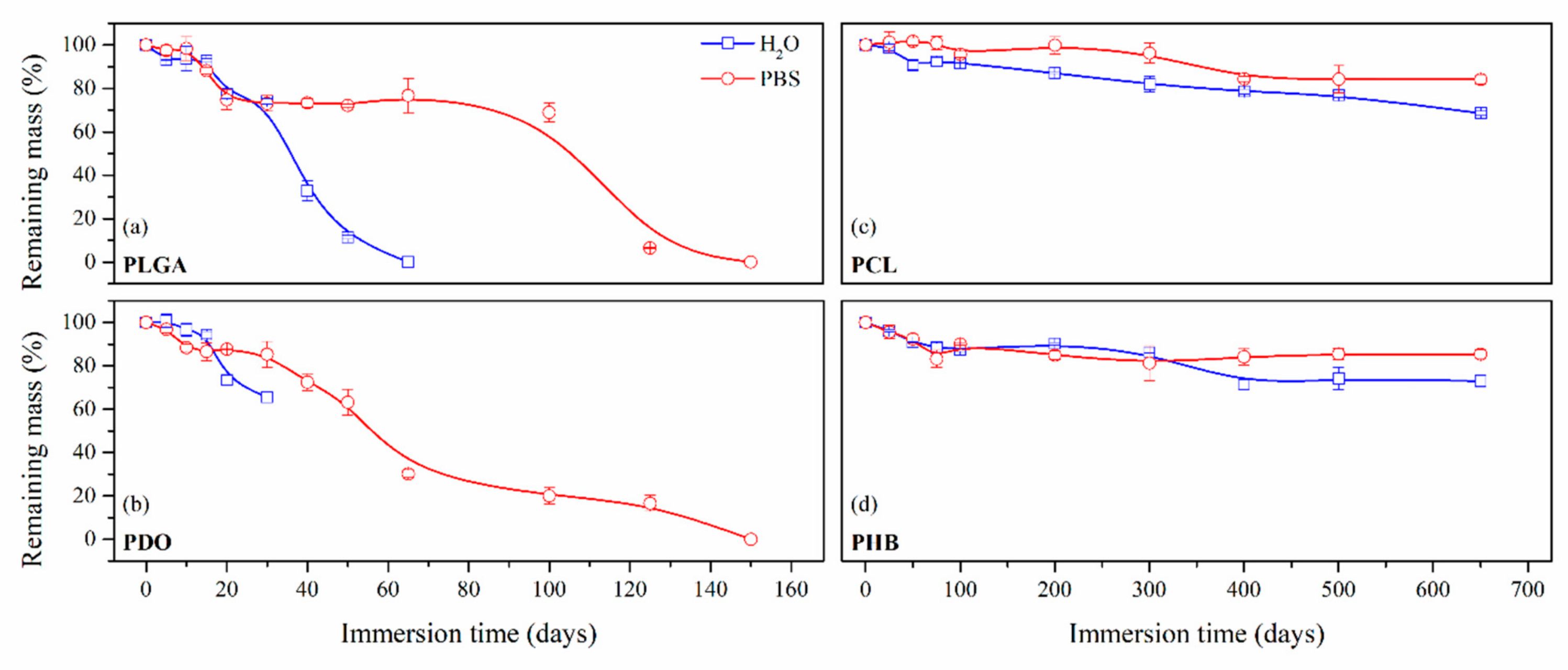
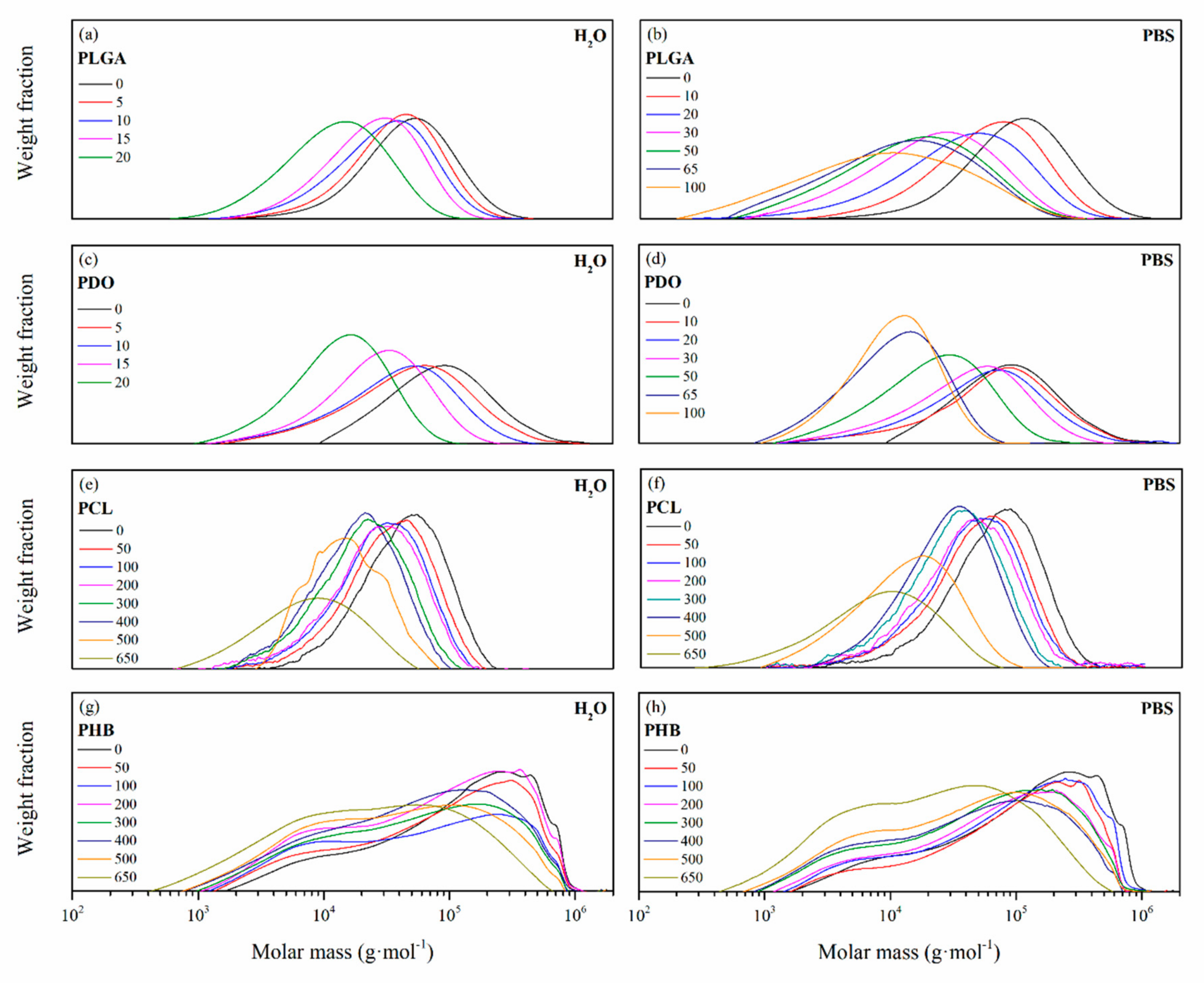
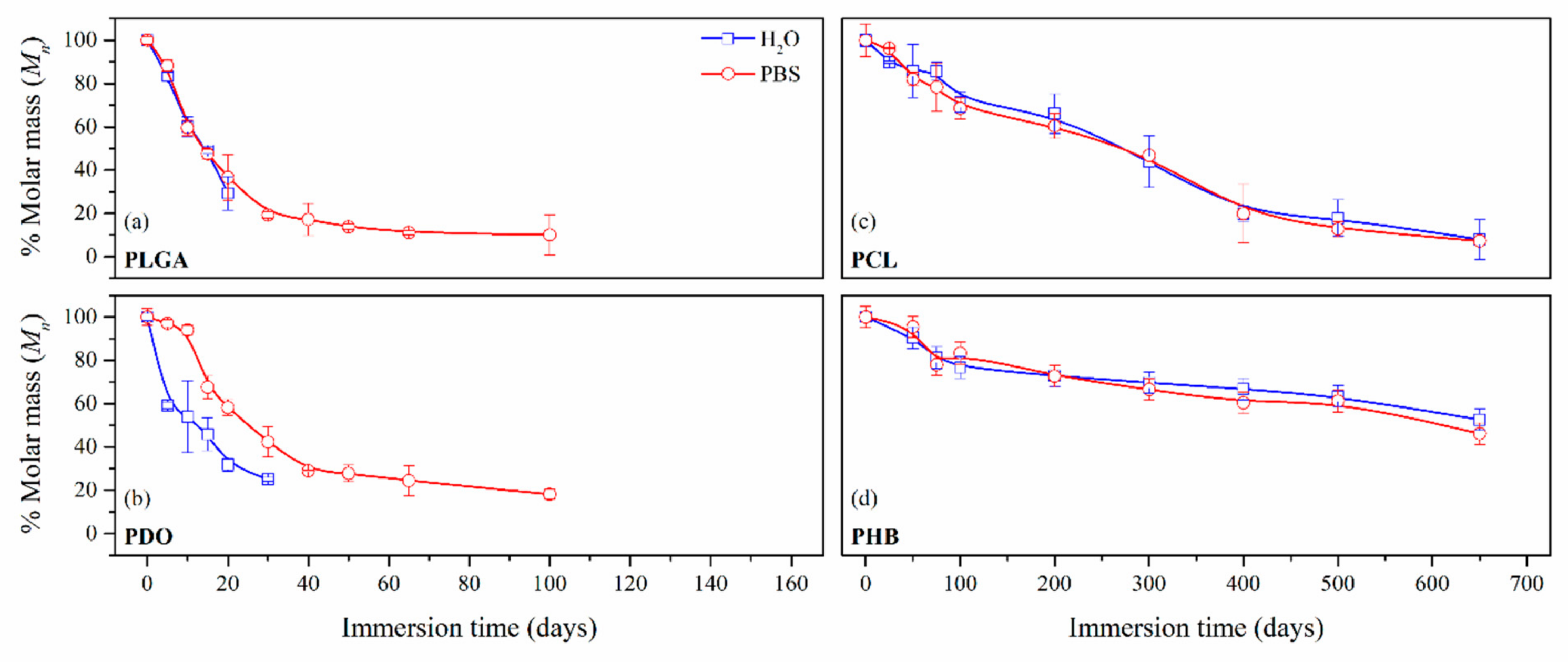
3.2.2. Impact on the Scaffolds Mass and Molar Mass
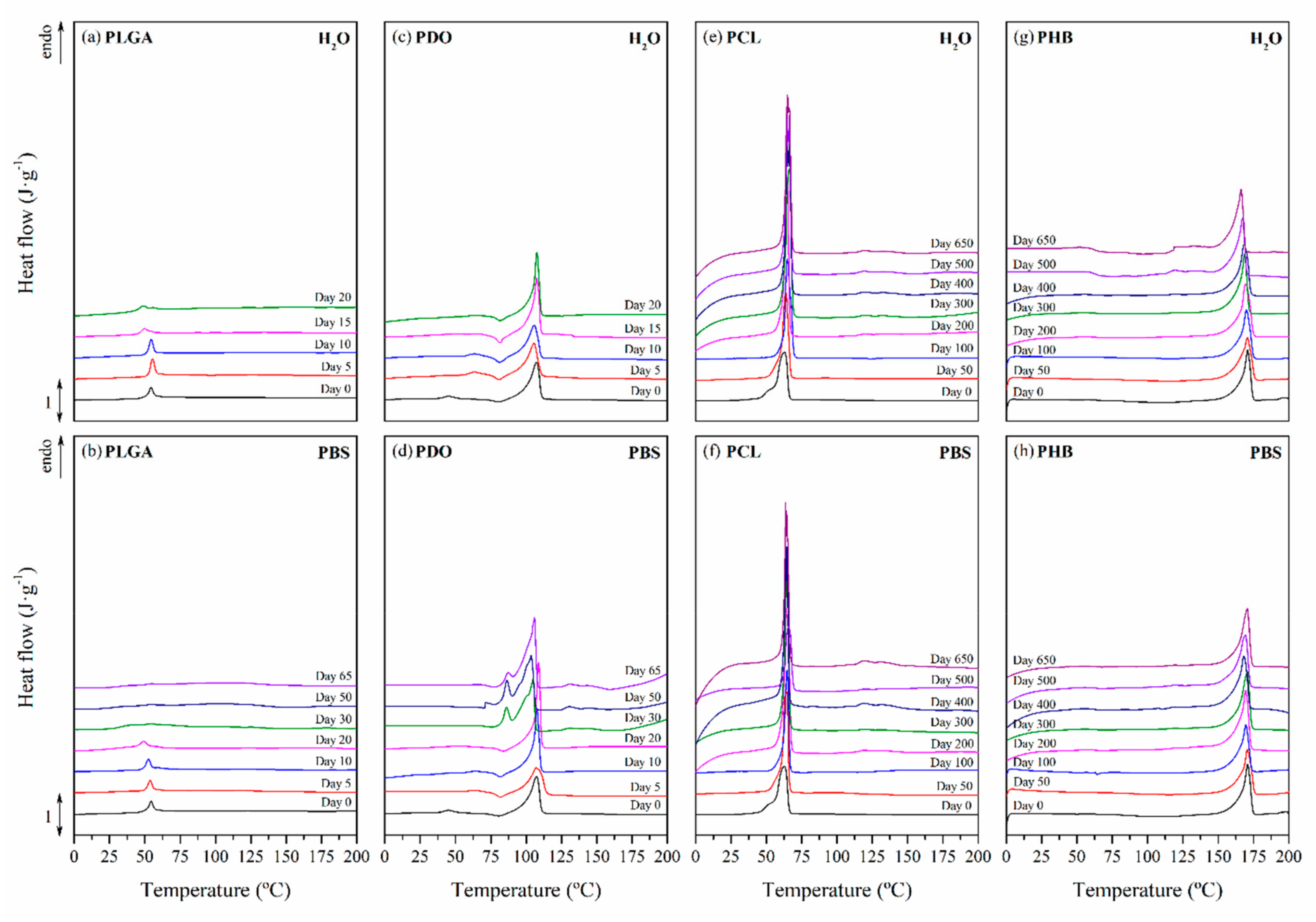
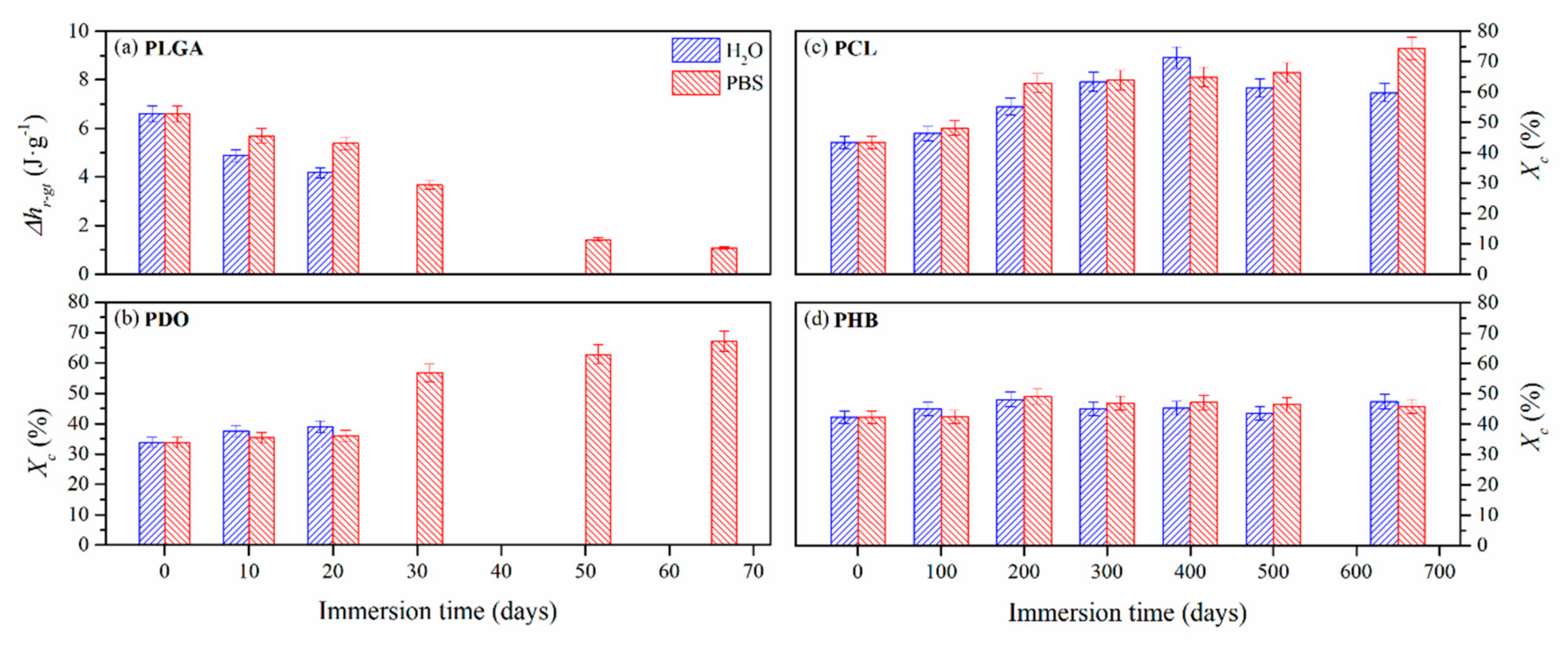
3.2.3. Impact on the Scaffolds Crystallinity
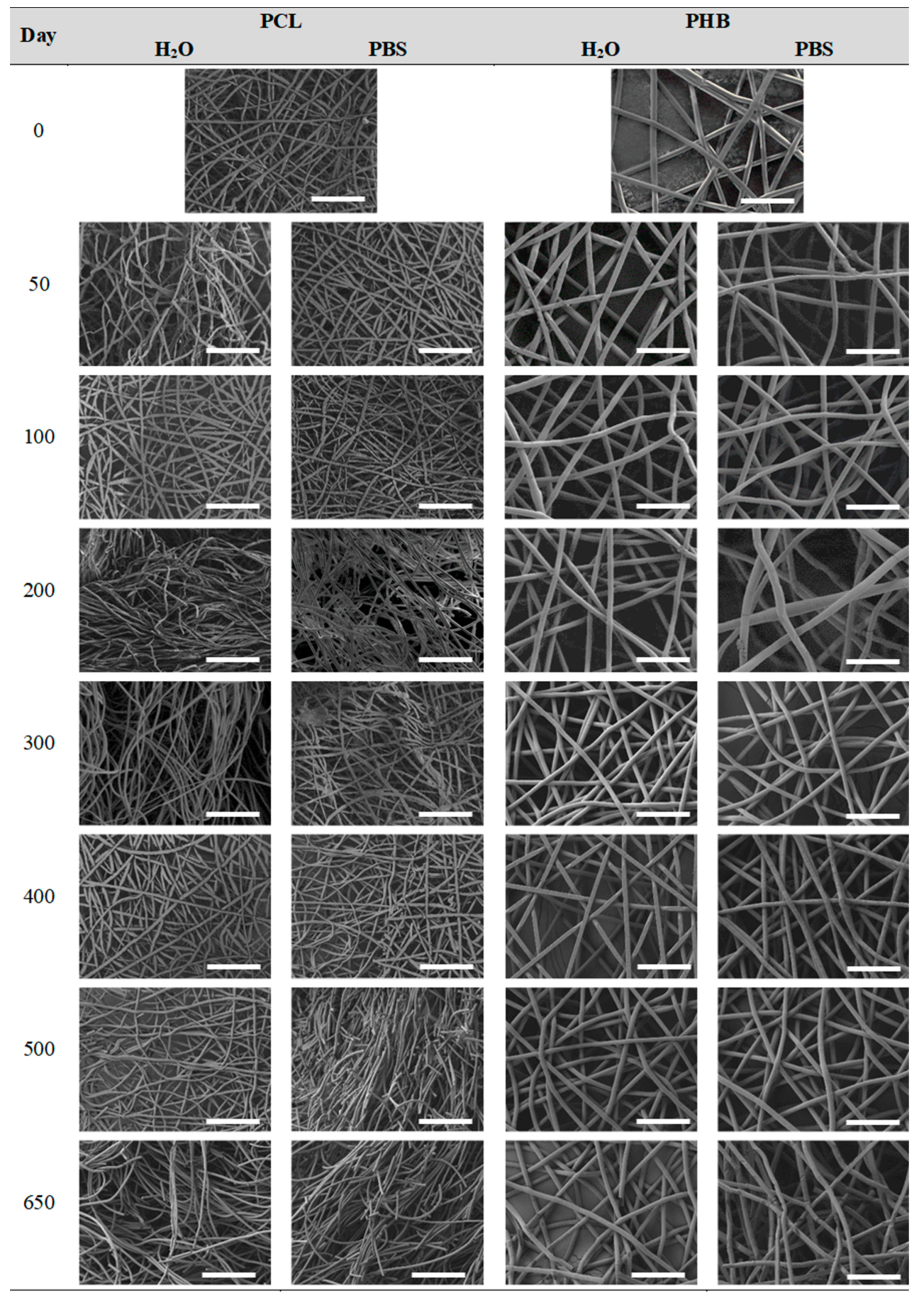
3.2.4. Impact on the Scaffolds Surface Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press, Elsevier: Cambridge, MA, USA, 2013; ISBN 9780123746269. [Google Scholar]
- Li, W.-J.; Cooper, J.A.; Mauck, R.L.; Tuan, R.S. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Dash, M.; Chiellini, E. Biodegradable Polymers for Biomedical Applications. Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 545–604. ISBN 9781612095349. [Google Scholar]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell. Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Gil-Castell, O.; Badia, J.D.D.; Ribes-Greus, A. Tailored electrospun nanofibrous polycaprolactone/gelatin scaffolds into an acid hydrolytic solvent system. Eur. Polym. J. 2018, 101, 273–281. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; Van Den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Gil-Castell, O.; Badia, J.D.; Ontoria-Oviedo, I.; Castellano, D.; Marco, B.; Rabal, A.; Bou, J.J.; Serra, A.; Monreal, L.; Blanes, M.; et al. In vitro validation of biomedical polyester-based scaffolds: Poly(lactide-co-glycolide) as model-case. Polym. Test. 2018, 66, 256–267. [Google Scholar]
- Pereira, R.F.; Bártolo, P.J. Degradation Behavior of Biopolymer-based Membranes for Skin Tissue Regeneration. Procedia Eng. 2013, 59, 285–291. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Biocompatible Nanofiber Matrices for the Engineering of a Dermal Substitute for Skin Regeneration. Tissue Eng. 2005, 11, 847–854. [Google Scholar] [CrossRef]
- Castellano, D.; Blanes, M.; Marco, B.; Cerrada, I.; Ruiz-Saurí, A.; Pelacho, B.; Araña, M.; Montero, J.A.; Cambra, V.; Prosper, F.; et al. A Comparison of Electrospun Polymers Reveals Poly(3-Hydroxybutyrate) Fiber as a Superior Scaffold for Cardiac Repair. Stem Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lee, A.-R.; Lin, W.-H.; Lin, C.-W.; Wu, Y.-K.; Tsai, W.-B. Electrospun PLGA Fibers Incorporated with Functionalized Biomolecules for Cardiac Tissue Engineering. Tissue Eng. Part A 2014, 20, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Maulik, N. Development of next generation cardiovascular therapeutics through bio-assisted nanotechnology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2072–2083. [Google Scholar] [CrossRef]
- Yildirimer, L.; Seifalian, A.M. Three-dimensional biomaterial degradation—Material choice, design and extrinsic factor considerations. Biotechnol. Adv. 2014, 32, 984–999. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials {&} scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Badia, J.D.; Gil-Castell, O.; Ribes-Greus, A. Long-term properties and end-of-life of polymers from renewable resources. Polym. Degrad. Stab. 2017, 137, 35–57. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lee, S.-Y.; Horng, S.; Guy, L.-G.; Yu, T.-B. In vitro and in vivo degradation of microfiber bioresorbable coronary scaffold. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1842–1850. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Albertsson, A.-C.; Karlsson, S. Weight losses and molecular weight changes correlated with the evolution of hydroxyacids in simulated in vivo degradation of homo- and copolymers of PLA and PGA. Polym. Degrad. Stab. 1996, 52, 283–291. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Sabino, M.A.; González, S.; Márquez, L.; Feijoo, J.L. Study of the hydrolytic degradation of polydioxanone PPDX. Polym. Degrad. Stab. 2000, 69, 209–216. [Google Scholar] [CrossRef]
- Fouad, H.; Elsarnagawy, T.; Almajhdi, F.N.; Khalil, K.A. Preparation and In Vitro Thermo-Mechanical Characterization of Electrospun PLGA Nanofibers for Soft and Hard Tissue Replacement. Int. J. Electrochem. Sci. 2013, 8, 2293–2304. [Google Scholar]
- You, Y.; Min, B.-M.; Lee, S.J.; Lee, T.S.; Park, W.H. In vitro degradation behavior of electrospun polyglycolide, polylactide, and poly(lactide-co-glycolide). J. Appl. Polym. Sci. 2005, 95, 193–200. [Google Scholar] [CrossRef]
- Abhari, R.E.; Mouthuy, P.A.; Zargar, N.; Brown, C.; Carr, A. Effect of annealing on the mechanical properties and the degradation of electrospun polydioxanone filaments. J. Mech. Behav. Biomed. Mater. 2017, 67, 127–134. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Downes, S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Boskhomodgiev, A.P.; Iordanskii, A.L.; Bonartseva, G.A.; Rebrov, A.V.; Makhina, T.K.; Myshkina, V.L.; Yakovlev, S.A.; Filatova, E.A.; Ivanov, E.A.; et al. Hydrolytic Degradation of Poly(3-hydroxybutyrate), Polylactide and their Derivatives: Kinetics, Crystallinity, and Surface Morphology. Mol. Cryst. Liq. Cryst. 2012, 556, 288–300. [Google Scholar]
- International Organization for Standardization. ISO 3696:1987. Water for Analytical Laboratory Use-Specification and Test Methods; Technical Committee ISO/TC 47: Geneva, Switzerland, 1987. [Google Scholar]
- International Organization for Standardization. ISO 10993-13. Biological Evaluation of Medical Devices; Technical Committee ISO/TC 47: Geneva, Switzerland, 2010. [Google Scholar]
- Ishikiriyama, K.; Pyda, M.; Zhang, G.; Forschner, T.; Grebowicz, J.; Wunderlich, B. Heat capacity of poly-p-dioxanone. J. Macromol. Sci. Part B 1998, 37, 27–44. [Google Scholar]
- Mark, J.E. Properties of Polymers Handbook; Springer: Berlin, Germany, 2007. [Google Scholar]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L. The effect of crystalline morphology on the degradation of polycaprolactone in a solution of phosphate buffer and lipase. Polym. Adv. Technol. 2008, 19, 1901–1906. [Google Scholar]
- Cai, H.; Dave, V.; Gross, R.A.; McCarthy, S.P. Effects of physical aging, crystallinity, and orientation on the enzymatic degradation of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2701–2708. [Google Scholar] [CrossRef]
- Yoon, D.M.; Fisher, J.P. Natural and Synthetic Polymeric Scaffolds. In Biomedical Materials; Narayan, R., Ed.; Springer: Berlin, Germany, 2009; pp. 415–442. ISBN 9780387848716. [Google Scholar]
- Vey, E.; Roger, C.; Meehan, L.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation mechanism of poly(lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution. Polym. Degrad. Stab. 2008, 93, 1869–1876. [Google Scholar]
- Díaz, E.; Sandonis, I.; Valle, M.B. In Vitro Degradation of Poly(caprolactone)/nHA Composites. J. Nanomater. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Höglund, A.; Hakkarainen, M.; Albertsson, A. Degradation Profile of Poly(ϵ-caprolactone)–the Influence of Macroscopic and Macromolecular Biomaterial Design. J. Macromol. Sci. Part A 2007, 44, 1041–1046. [Google Scholar] [CrossRef]
- Laeger, T.; Metges, C.C.; Kuhla, B. Role of β-hydroxybutyric acid in the central regulation of energy balance. Appetite 2010, 54, 450–455. [Google Scholar] [CrossRef]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 4905–4916. [Google Scholar] [CrossRef]
- Kim, K.; Yu, M.; Zong, X.; Chiu, J.; Fang, D.; Seo, Y.-S.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 2003, 24, 4977–4985. [Google Scholar] [CrossRef]
- Zong, X.; Ran, S.; Kim, K.S.; Fang, D.; Hsiao, B.S.; Chu, B. Structure and morphology changes during in vitro degradation of electrospun poly(glycolide-co-lactide) nanofiber membrane. Biomacromolecules 2003, 4, 416–423. [Google Scholar] [CrossRef]
- Sabino, M.A.; Albuerne, J.; Müller, A.; Brisson, J.; Prud’homme, R.E. Influence of in vitro hydrolytic degradation on the morphology and crystallization behavior of poly(p-dioxanone). Biomacromolecules 2004, 5, 358–370. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Chanprateep, S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010, 110, 621–632. [Google Scholar] [CrossRef]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, Q.; Yan, N.; Deng, X.; Yang, X. In vitro hydrolytic and enzymatic degradation of nestlike-patterned electrospun poly(D,L-lactide-co-glycolide) scaffolds. J. Biomed. Mater. Res. Part A 2010, 95A, 755–765. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone microparticles and their biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Badia, J.D.; Vilaplana, F.; Karlsson, S.; Ribes-Greus, A. Thermal analysis as a quality tool for assessing the influence of thermo-mechanical degradation on recycled poly(ethylene terephthalate). Polym. Test. 2009, 28, 169–175. [Google Scholar] [CrossRef]
- Santonja-Blasco, L.; Moriana, R.; Badía, J.D.; Ribes-Greus, A. Thermal analysis applied to the characterization of degradation in soil of polylactide: I. Calorimetric and viscoelastic analyses. Polym. Degrad. Stab. 2010, 95, 2185–2191. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Material valorisation of amorphous polylactide. Influence of thermo-mechanical degradation on the morphology, segmental dynamics, thermal and mechanical performance. Polym. Degrad. Stab. 2012, 97, 670–678. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Kittikorn, T.; Strömberg, E.; Ek, M.; Karlsson, S.; Ribes-Greus, A.; Strömberg, E.; Ek, M.; Karlsson, S.; et al. Impact of hydrothermal ageing on the thermal stability, morphology and viscoelastic performance of PLA/sisal biocomposites. Polym. Degrad. Stab. 2016, 132, 87–96. [Google Scholar] [CrossRef]
- Badia, J.D.; Kittikorn, T.; Strömberg, E.; Santonja-Blasco, L.; Martínez-Felipe, A.; Ribes-Greus, A.; Ek, M.; Karlsson, S. Water absorption and hydrothermal performance of PHBV/sisal biocomposites. Polym. Degrad. Stab. 2014, 108, 166–174. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Kittikorn, T.; Strömberg, E.; Martínez-Felipe, A.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Hydrothermal ageing of polylactide/sisal biocomposites. Studies of water absorption behaviour and Physico-Chemical performance. Polym. Degrad. Stab. 2014, 108, 212–222. [Google Scholar] [CrossRef]
- Badia, J.D.; Ribes-Greus, A. Mechanical recycling of polylactide, upgrading trends and combination of valorization techniques. Eur. Polym. J. 2016, 84, 22–39. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Ribes-Greus, A. Suitability of blends from virgin and reprocessed polylactide: Performance and energy valorization kinetics. J. Renew. Mater. 2018, 6, 370–382. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Kittikorn, T.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Relevant factors for the eco-design of polylactide/sisal biocomposites to control biodegradation in soil in an end-of-life scenario. Polym. Degrad. Stab. 2017, 143, 9–19. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Lauritzen, J.I. Crystallization of Bulk Polymers with Chain Folding: Theory of Growth of Lamellar Spherulites. J. Res. Natl. Bur. Stand. A. Phys. Chem. 1961, 65, 1961. [Google Scholar] [CrossRef]
- Lauritzen, J.I.; Hoffman, J.D. Formation of Polymer Crystals with Folded Chains from Dilute Solution. J. Chem. Phys. 1959, 31, 1680–1681. [Google Scholar] [CrossRef]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Ping Ooi, C.; Cameron, R.E. The hydrolytic degradation of polydioxanone (PDSII) sutures. Part I: Morphological aspects. J. Biomed. Mater. Res. 2002, 63, 280–290. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Effect of the dissolution time into an acid hydrolytic solvent to tailor electrospun nanofibrous polycaprolactone scaffolds. Eur. Polym. J. 2017, 87, 174–187. [Google Scholar] [CrossRef]
- Sultana, N.; Khan, T.H. In Vitro Degradation of PHBV Scaffolds and nHA/PHBV Composite Scaffolds Containing Hydroxyapatite Nanoparticles for Bone Tissue Engineering. J. Nanomater. 2012, 2012, 1–12. [Google Scholar]

| Polymer (type) | Concentration (% wt) | Solvent (type) | Feed Rate (mL·h−1) | Voltage (kV) | Distance (cm) |
|---|---|---|---|---|---|
| PCL | 18 | DMF/CHCl3 1:8 | 4 | 7.32/−5.54 | 25 |
| PDO | 8 | HFIP | 1 | 3.85/−3.35 | 21 |
| PHB | 23 | CHCl3 | 1 | 7.80/−1.93 | 20 |
| PLGA | 30 | DMF | 1 | 9.15/−8.80 | 20 |
| Polymer | Constitutional Repetitive Unit (CRU) | Surface Morphology (500×) | Fibre Diameter Distribution | Average Diameter | ρ | ρs |
|---|---|---|---|---|---|---|
| (µm) | (g·cm−3) | (g·m−2) | ||||
| PLGA | 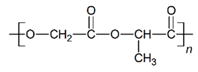 | 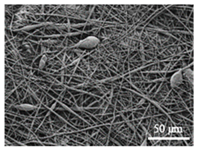 | 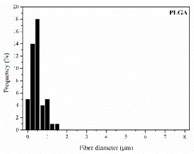 | 0.61 | 1.25 | 2.60 |
| PCL | 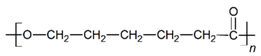 | 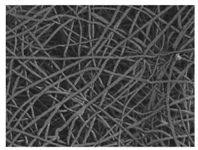 | 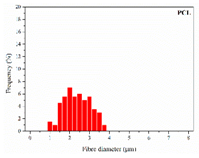 | 2.45 | 1.14 | 2.66 |
| PDO | 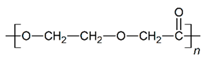 | 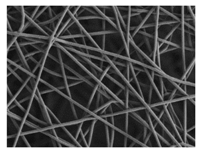 | 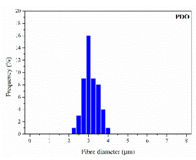 | 3.21 | 1.18 | 2.62 |
| PHB | 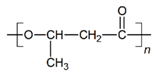 |  | 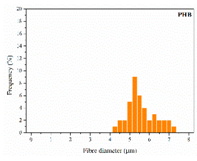 | 5.78 | 1.25 | 2.04 |
| Immersion time (Days) | PLGA | PDO | Immersion Time (Days) | PCL | PHB | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (g·mol−1) | Mn (g·mol−1) | ||||||||
| H2O | PBS | H2O | PBS | H2O | PBS | H2O | PBS | ||
| 0 | 43,290 | 52,000 | 0 | 42,030 | 20,450 | ||||
| 5 | 36,080 | 38,210 | 30,800 | 50,450 | 50 | 36,080 | 34,500 | 18,490 | 19,510 |
| 10 | 26,020 | 25,780 | 28,080 | 48,880 | 100 | 30,010 | 28,820 | 15,700 | 17,040 |
| 20 | 12,690 | 15,850 | 16,620 | 30,260 | 200 | 27,810 | 25,390 | 14,900 | 14,850 |
| 30 | - | 8340 | 13,030 | 21,990 | 300 | 18,470 | 19,670 | 14,250 | 13,610 |
| 50 | - | 5960 | - | 14,460 | 400 | 8350 | 8380 | 13,650 | 12,350 |
| 65 | - | 4800 | - | 12,710 | 500 | 7520 | 5370 | 12,960 | 12,520 |
| 100 | - | 4360 | - | 9450 | 650 | 3340 | 3060 | 10,760 | 9440 |
| Immersion Time (Days) | PLGA | PDO | Immersion Time (Days) | PCL | PHB | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tr-gt (°C) | Tm (°C) | Tm (°C) | Tm (°C) | ||||||
| H2O | PBS | H2O | PBS | H2O | PBS | H2O | PBS | ||
| 0 | 54.5 | 107.9 | 0 | 63.0 | 170.9 | ||||
| 5 | 55.3 | 53.6 | 105.4 | 107.7 | 50 | 63.8 | 64.4 | 170.6 | 170.8 |
| 10 | 54.5 | 52.5 | 105.9 | 107.8 | 100 | 64.8 | 64.5 | 169.8 | 169.5 |
| 20 | 48.6 | 49.3 | 105.2 | 108.4 | 200 | 64.8 | 64.5 | 169.4 | 169.4 |
| 30 | - | 41.1 | - | 104.1 | 300 | 66.0 | 64.1 | 168.5 | 170.6 |
| 50 | - | - | - | 103.5 | 400 | 65.4 | 64.2 | 168.3 | 168.3 |
| 65 | - | - | - | 105.9 | 500 | 64.2 | 64.2 | 167.6 | 169.5 |
| - | - | - | - | - | 650 | 63.8 | 64.4 | 166.3 | 169.2 |
| Immersion Time (Days) | PLGA | PDO | Immersion Time (Days) | PCL | PHB | ||||
|---|---|---|---|---|---|---|---|---|---|
| Diameter (µm) | Diameter (µm) | ||||||||
| H2O | PBS | H2O | PBS | H2O | PBS | H2O | PBS | ||
| 0 | 0.610 | 3.214 | 0 | 2.458 | 5.783 | ||||
| 5 | 2.842 | 1.925 | 3.197 | 3.214 | 50 | 2.634 | 2.238 | 5.409 | 5.120 |
| 10 | 3.261 | 2.062 | 3.191 | 3.021 | 100 | 2.383 | 2.140 | 4.704 | 5.374 |
| 20 | - | 1.869 | 2.988 | 2.921 | 200 | 2.309 | 2.294 | 4.802 | 5.055 |
| 30 | - | 1.919 | 2.720 | 2.749 | 300 | 2.133 | 2.681 | 4.797 | 4.907 |
| 50 | - | 1.905 | - | 2.432 | 400 | 2.644 | 2.187 | 4.918 | 4.942 |
| 65 | - | 3.823 | - | 2.090 | 500 | 2.262 | 2.121 | 4.817 | 5.004 |
| 650 | 2.150 | 2.455 | 4.755 | 4.861 | |||||
| Poly(lactide-co-glycolide) (50:50) (PLGA) | Polydioxanone (PDO) | Polycaprolactone (PCL) | Polyhydroxybutyrate (PHB) | |
|---|---|---|---|---|
| Origin | Synthetic | Synthetic | Synthetic | Natural |
| Microstructure | Amorphous | Semicrystalline | Semicrystalline | Semicrystalline |
| Temperature (37 °C) | Below the Tg (48 to 52 °C) | Above the Tg (−10 to −5 °C) | Above the Tg (−65 to −60 °C) | Above the Tg (0 to 5 °C) |
| Degradation pattern | Random chain scission, increase polydispersity | Preferential long-segment chain scission, reduce polydispersity | Random chain scission, increase polydispersity | Preferential long-segment chain scission, high polydispersity |
| Scaffold degradation mechanism (Adapted from [3]) |  |  |  |  |
| Hydrolytic degradation ultimately reaction | 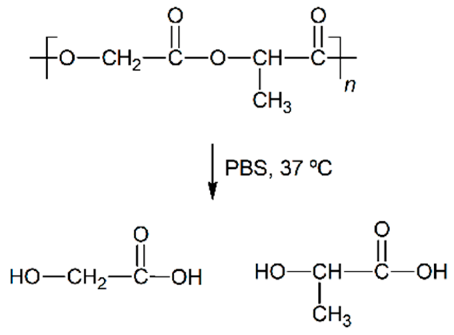 | 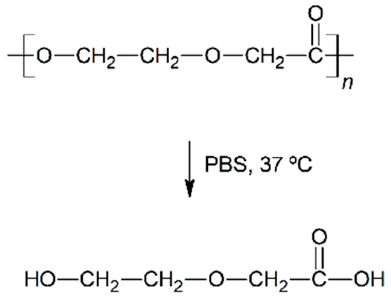 | 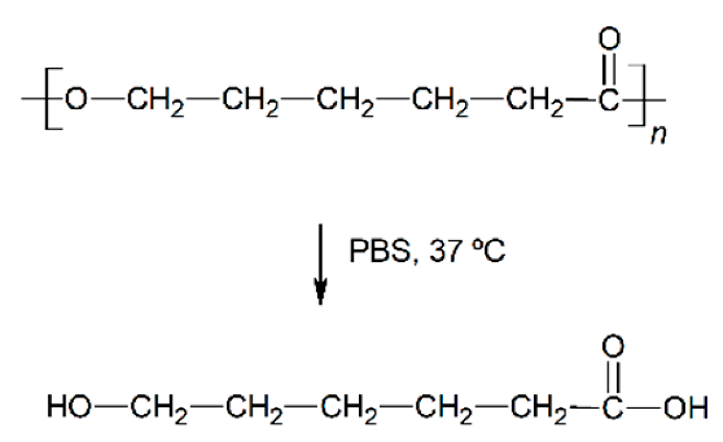 | 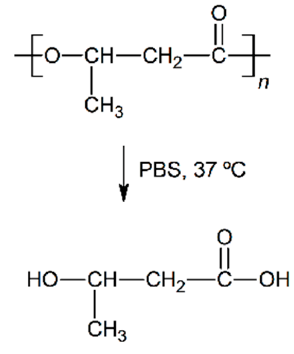 |
| Degradation rate in physiologic conditions (50% Mn decrease) | 15 days | 30 days | 300 days | 650 days |
| Disintegration | 125 days | 125 days | 650 days | 650 days |
| pH reduction (<7) | 65 days | 50 days | No change | No change |
| Changes in the microstructure | Disappearance of structural relaxation and separation of glass transitions of the co-monomers (PLA, PGA) | Crystalline development of lower lamellar thickness first, and then perfection of these structures | Crystalline development of higher lamellar thickness domains | High stability of crystalline domains with a slight decrease of lamellar thickness |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Castell, O.; Badia, J.D.; Bou, J.; Ribes-Greus, A. Performance of Polyester-Based Electrospun Scaffolds under In Vitro Hydrolytic Conditions: From Short-Term to Long-Term Applications. Nanomaterials 2019, 9, 786. https://doi.org/10.3390/nano9050786
Gil-Castell O, Badia JD, Bou J, Ribes-Greus A. Performance of Polyester-Based Electrospun Scaffolds under In Vitro Hydrolytic Conditions: From Short-Term to Long-Term Applications. Nanomaterials. 2019; 9(5):786. https://doi.org/10.3390/nano9050786
Chicago/Turabian StyleGil-Castell, Oscar, José David Badia, Jordi Bou, and Amparo Ribes-Greus. 2019. "Performance of Polyester-Based Electrospun Scaffolds under In Vitro Hydrolytic Conditions: From Short-Term to Long-Term Applications" Nanomaterials 9, no. 5: 786. https://doi.org/10.3390/nano9050786
APA StyleGil-Castell, O., Badia, J. D., Bou, J., & Ribes-Greus, A. (2019). Performance of Polyester-Based Electrospun Scaffolds under In Vitro Hydrolytic Conditions: From Short-Term to Long-Term Applications. Nanomaterials, 9(5), 786. https://doi.org/10.3390/nano9050786