Study of Magnetic Properties of Fe100-xNix Nanostructures Using the Mössbauer Spectroscopy Method
Abstract
:1. Introduction
2. Materials and Methods
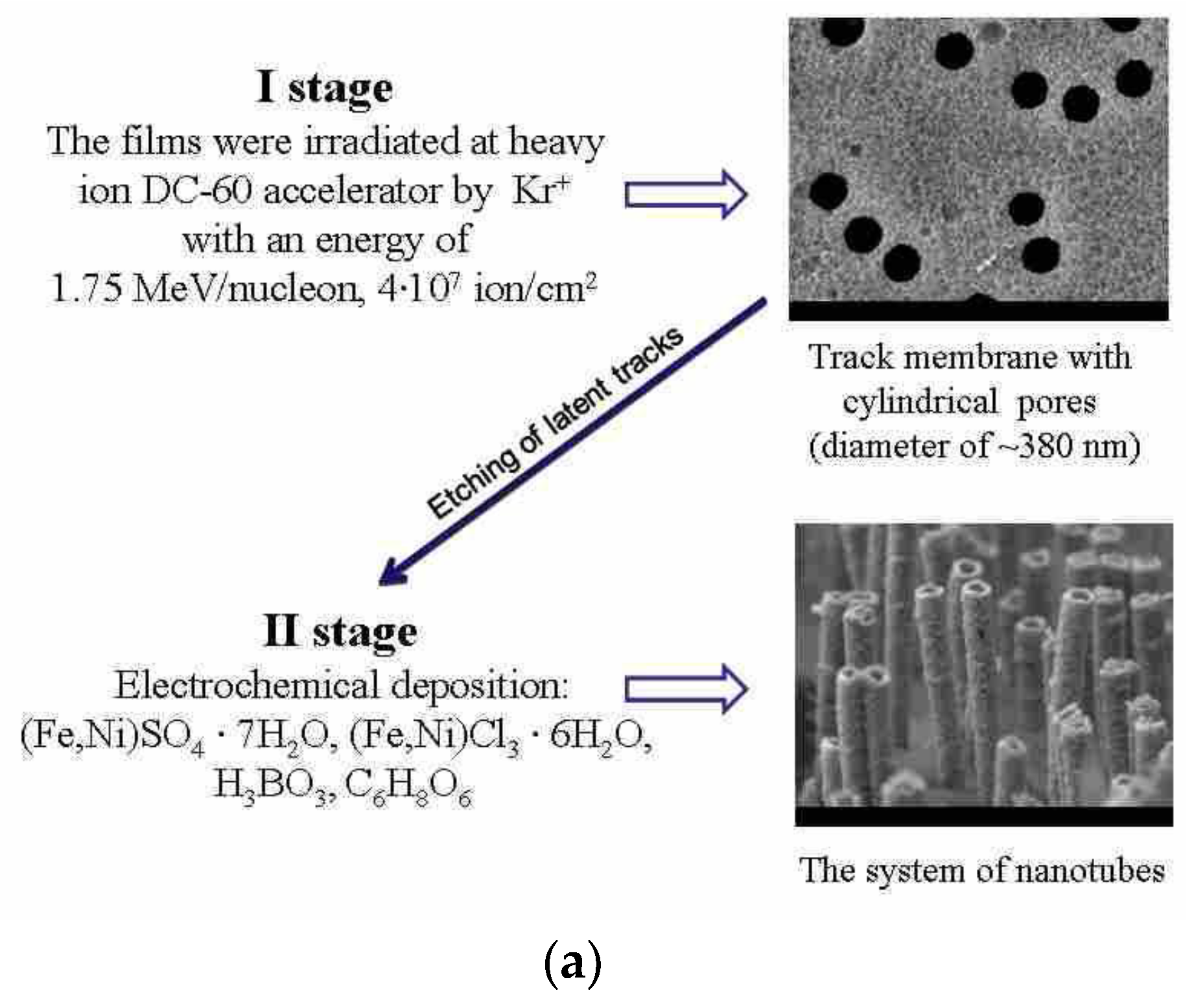
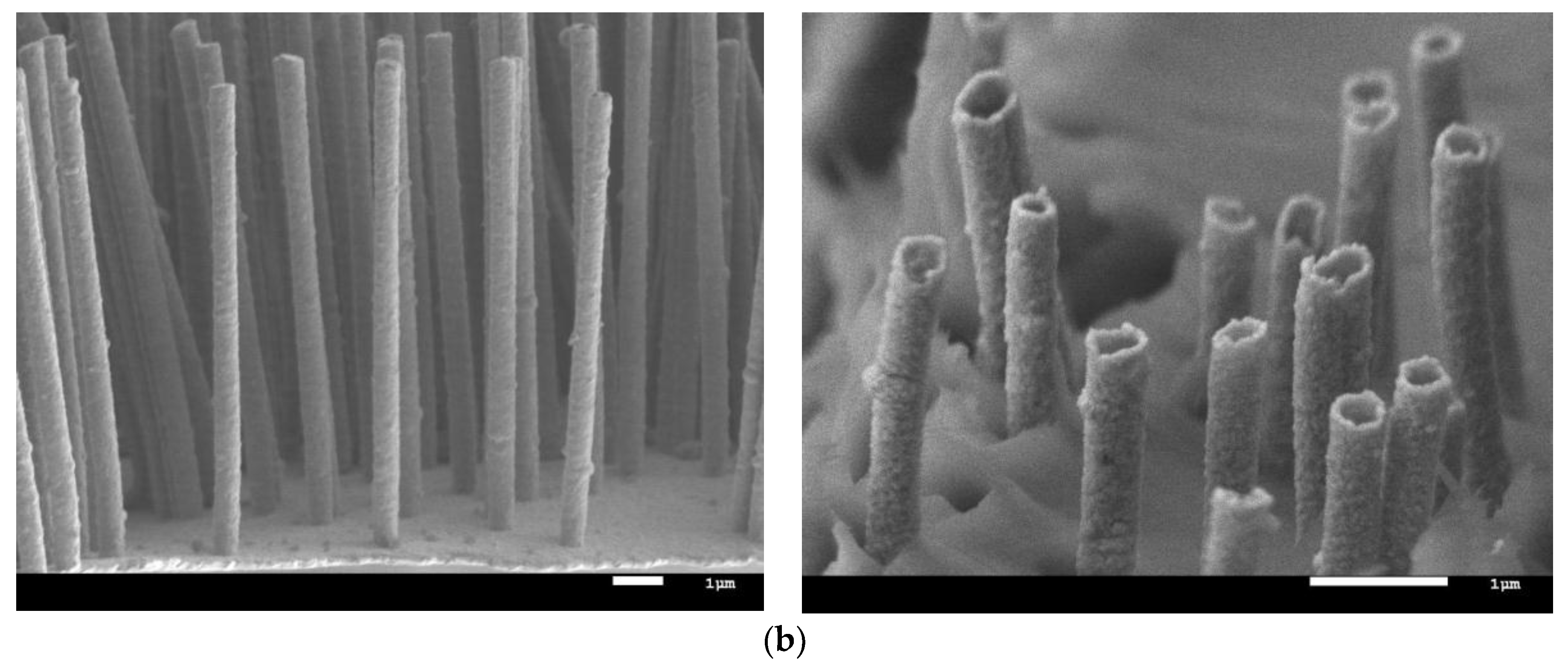
2.1. Obtaining Fe–Ni Nanostructures
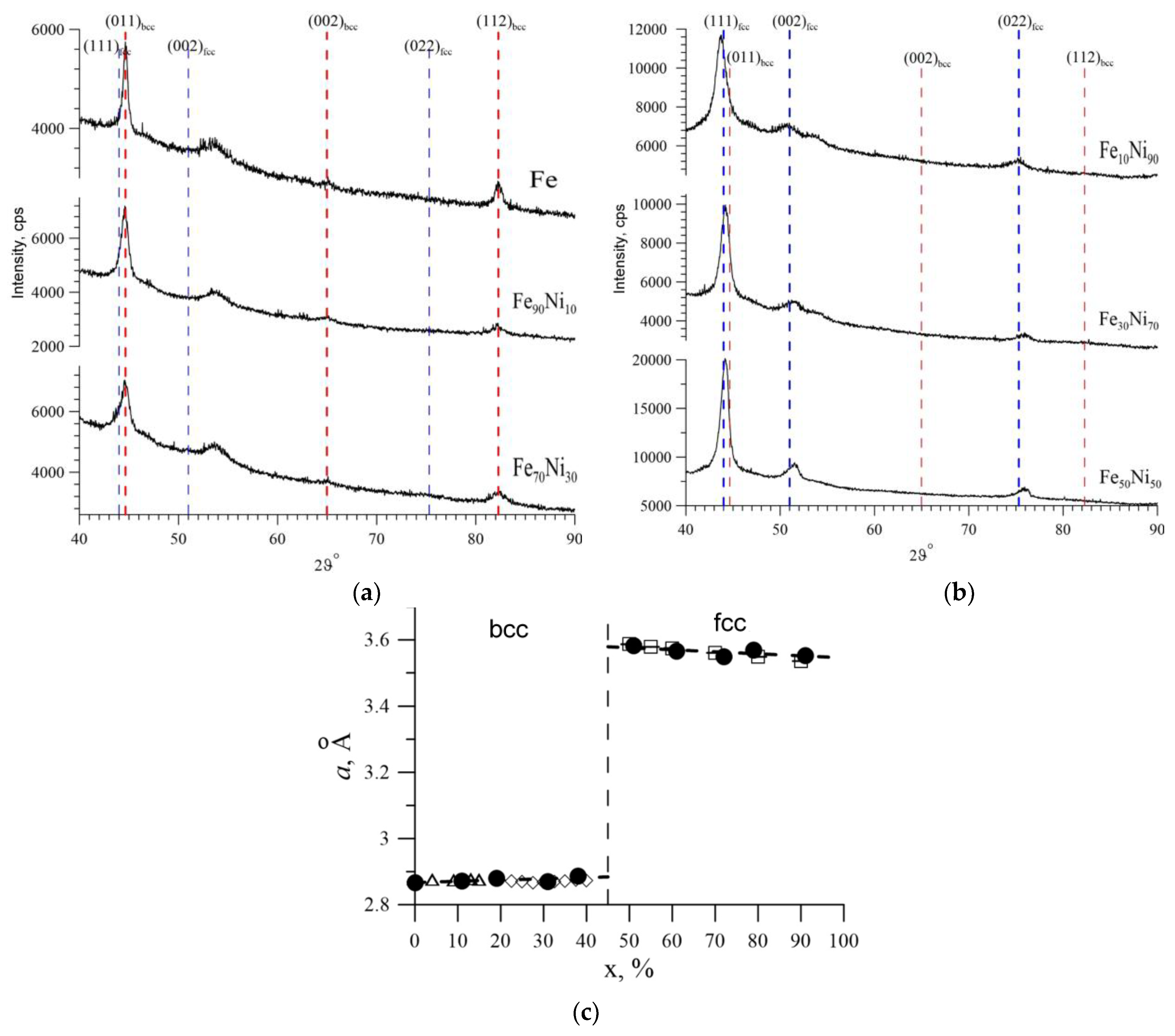
2.2. The Study of Structural Characteristics and Elemental Composition
2.3. Methods of Characterization
3. Results
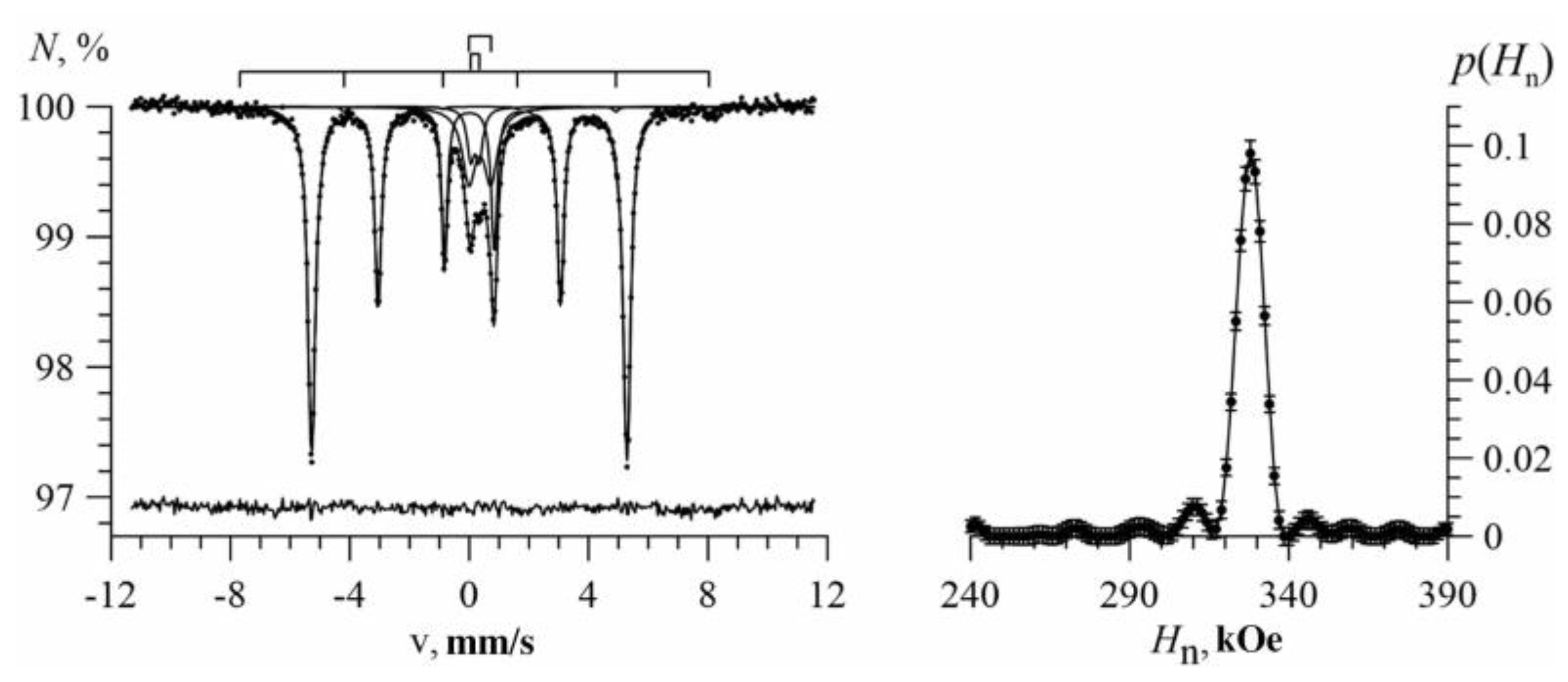
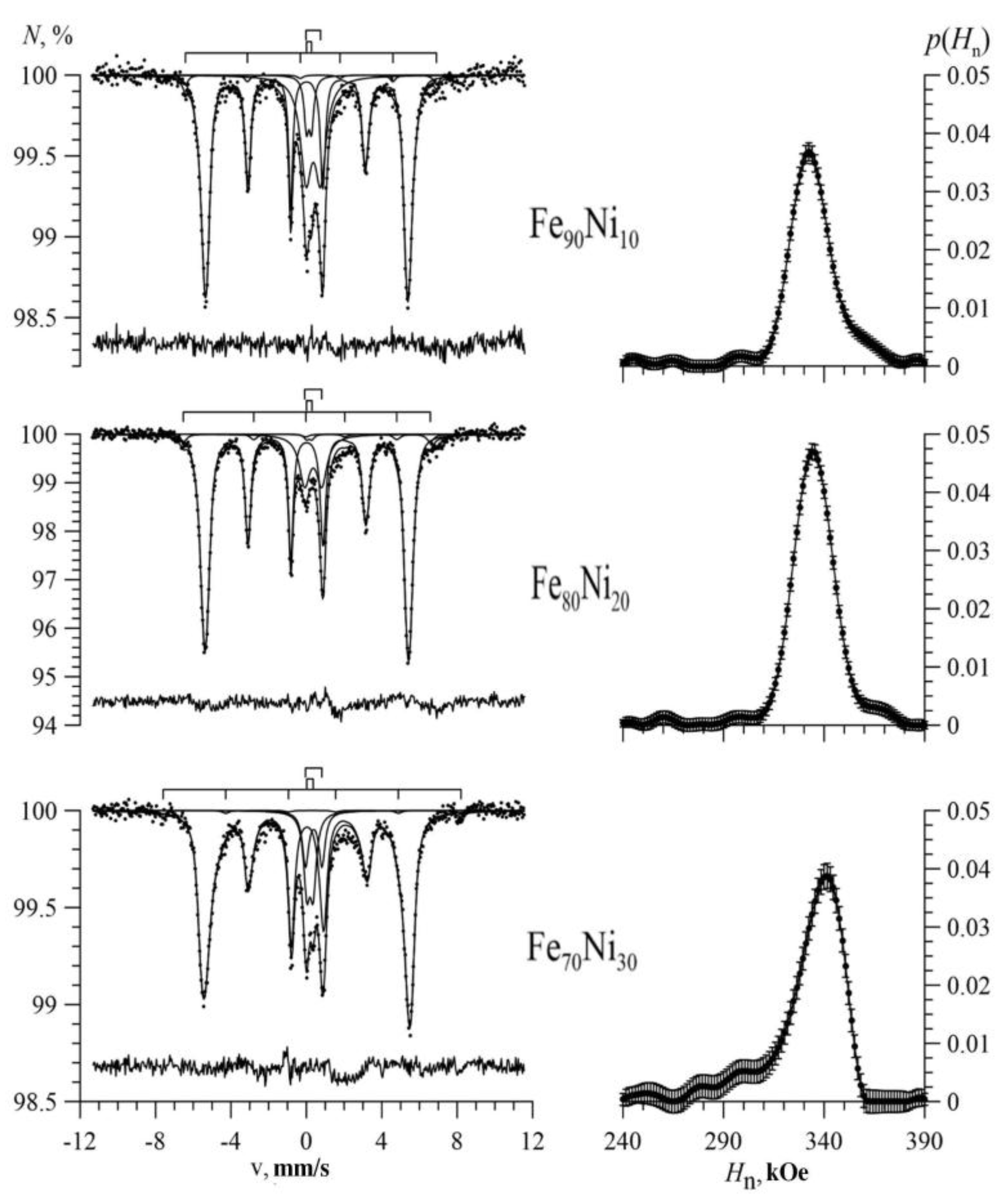
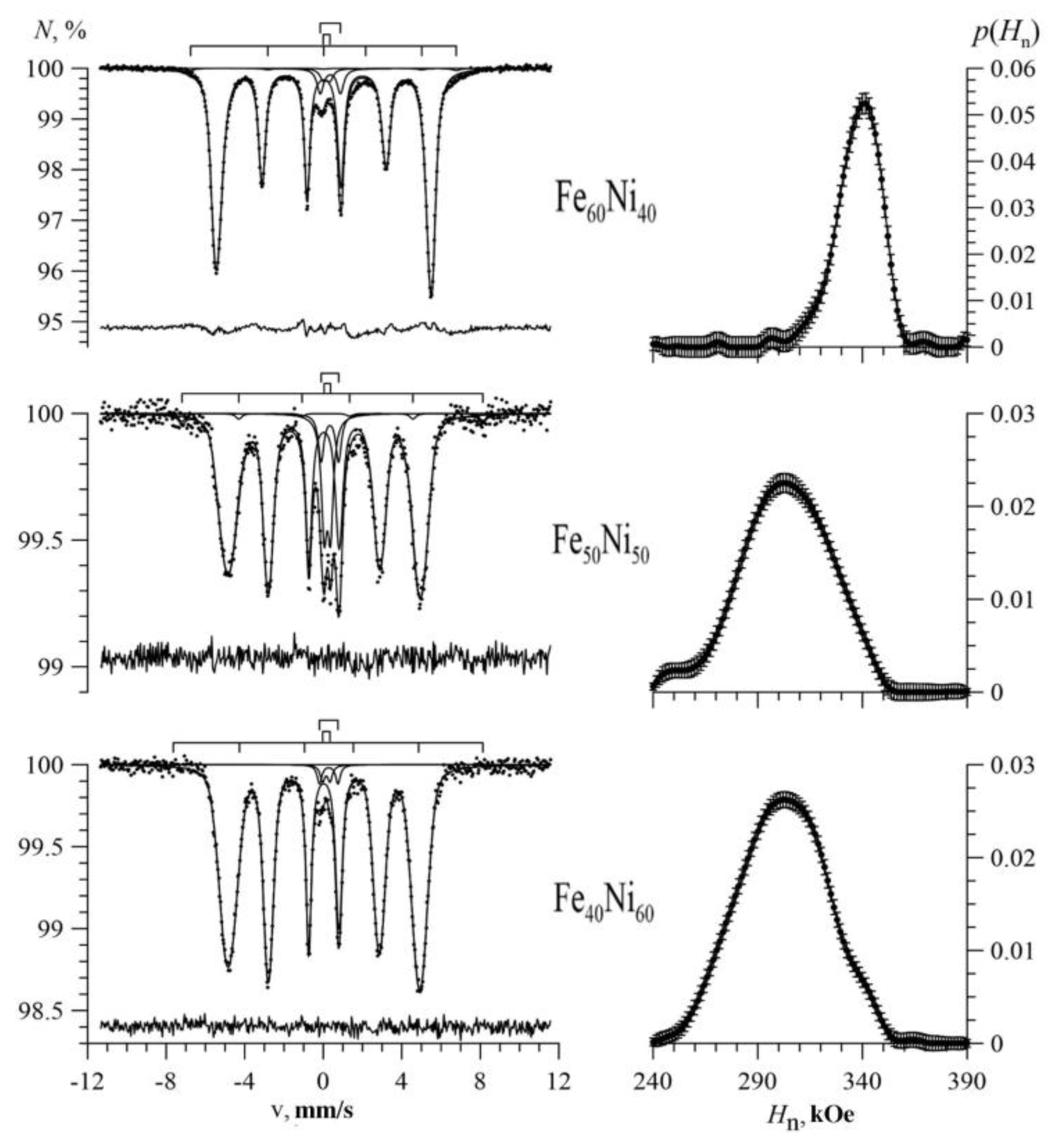
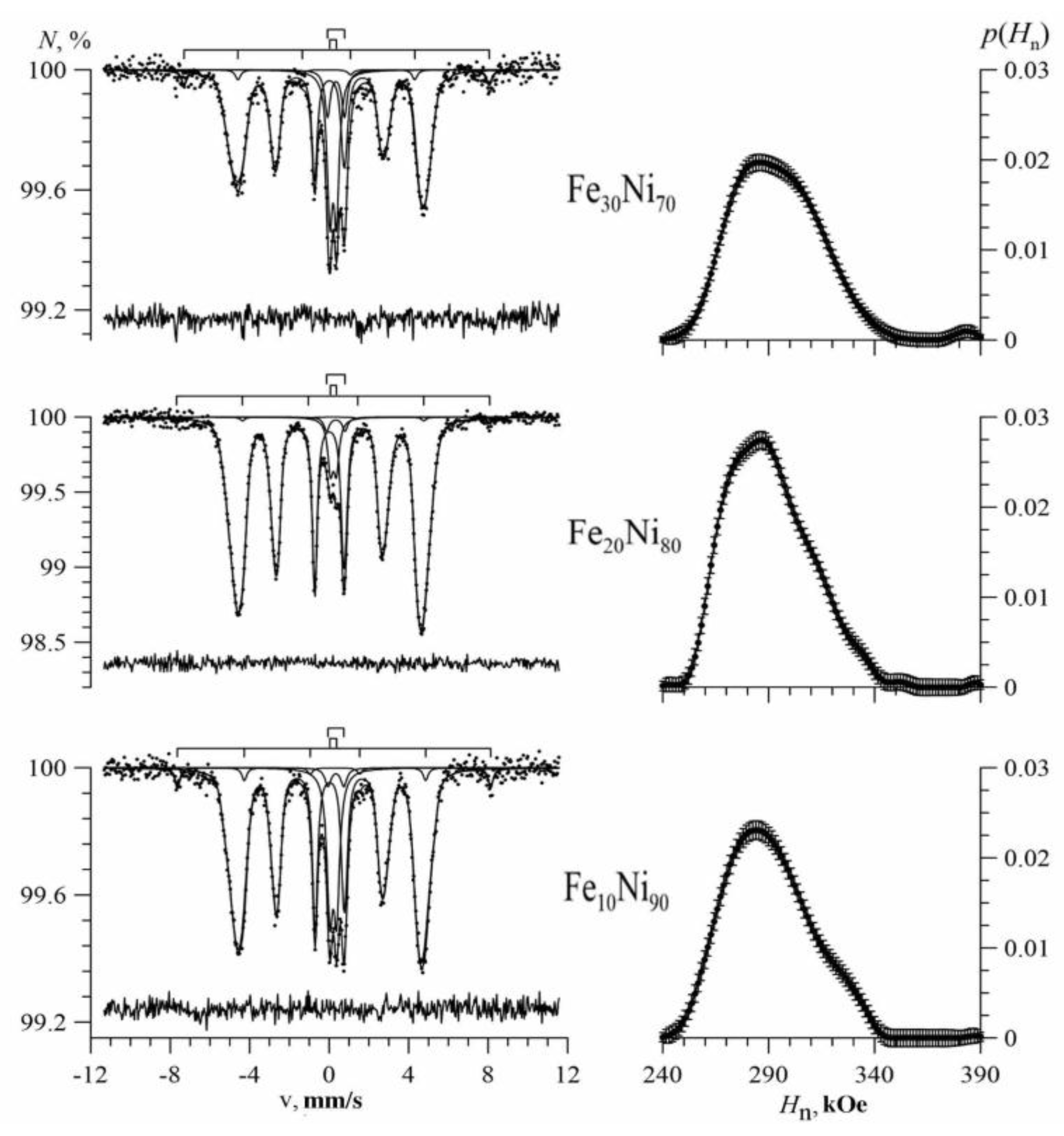
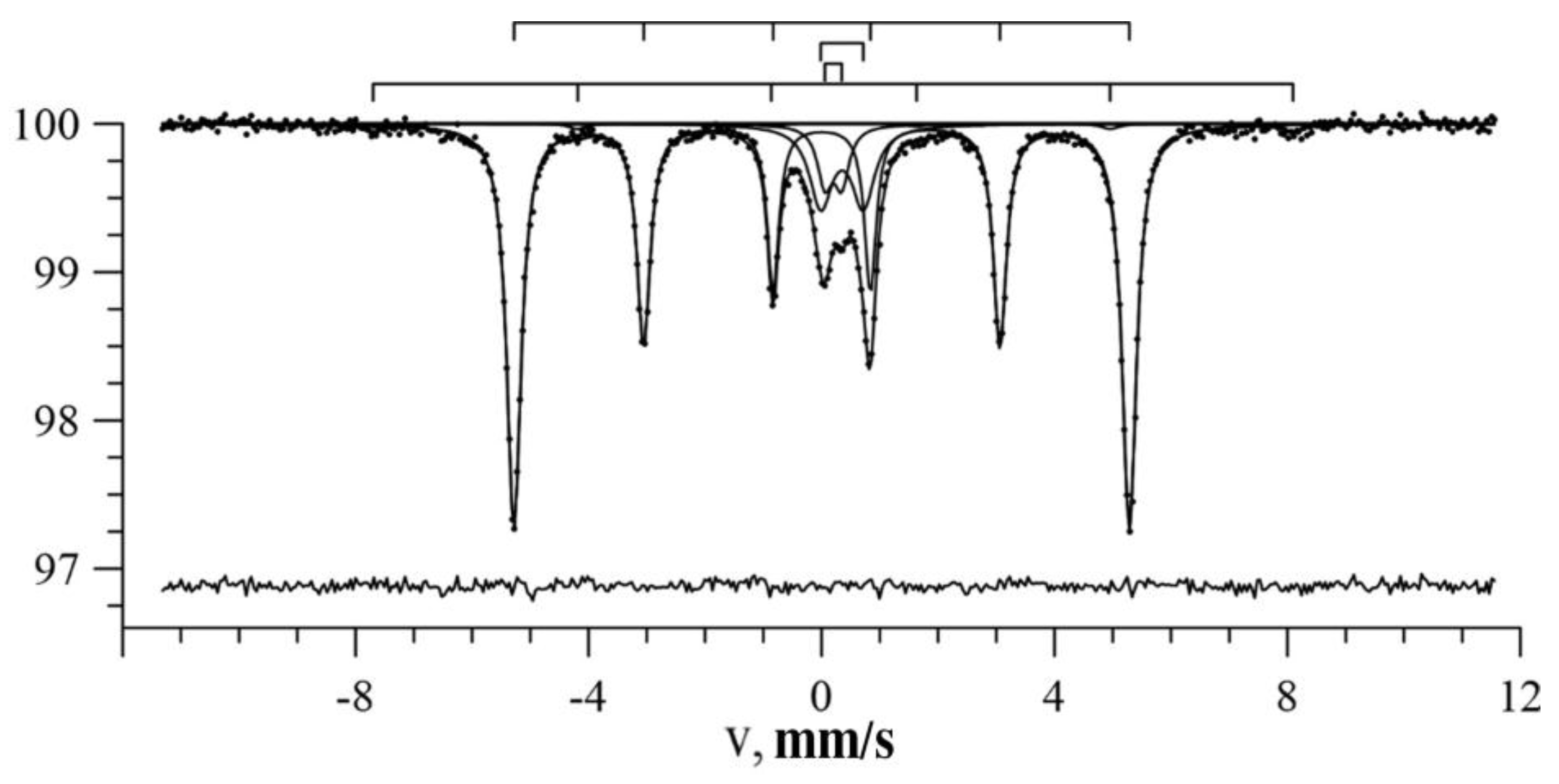
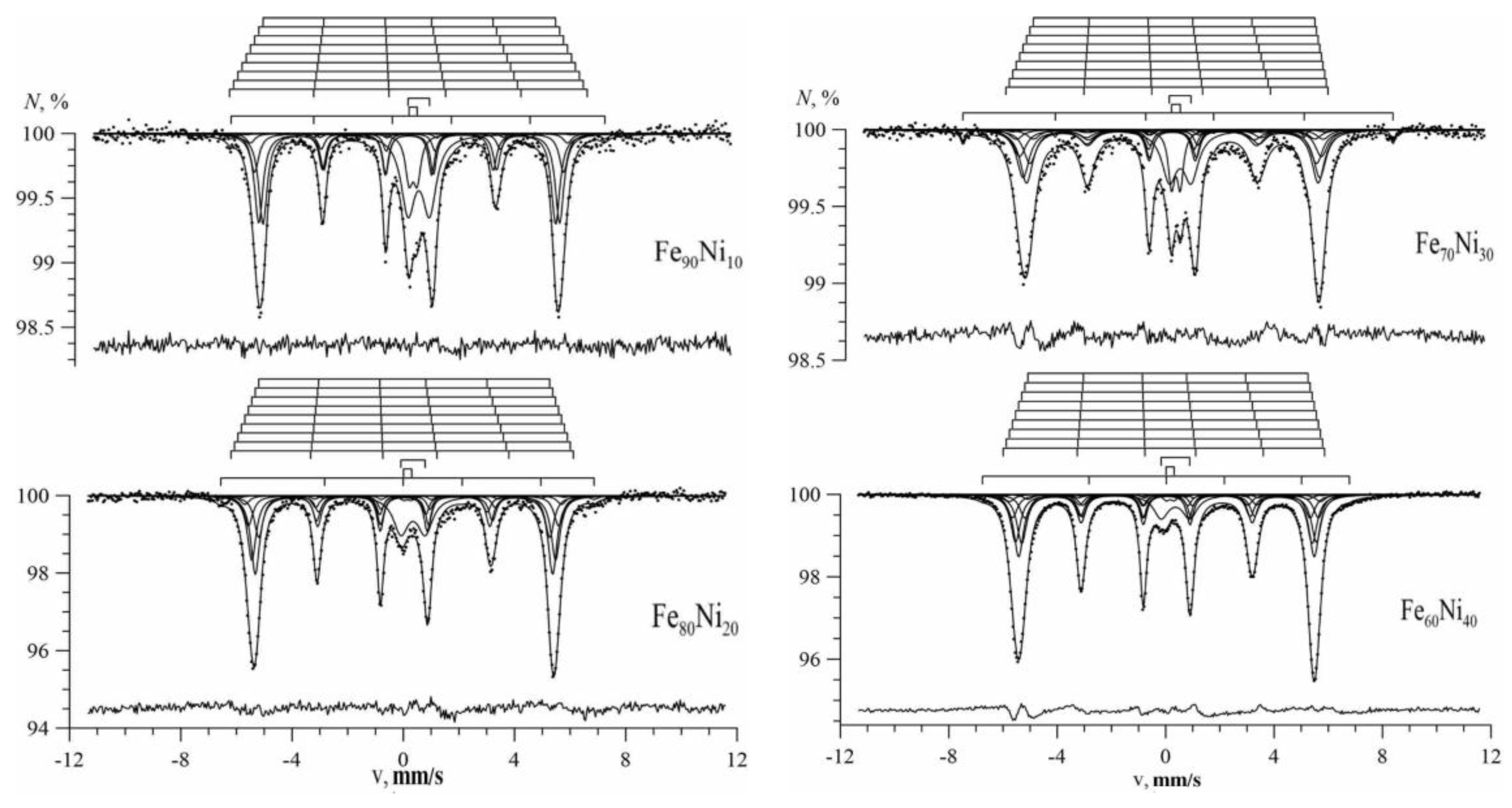
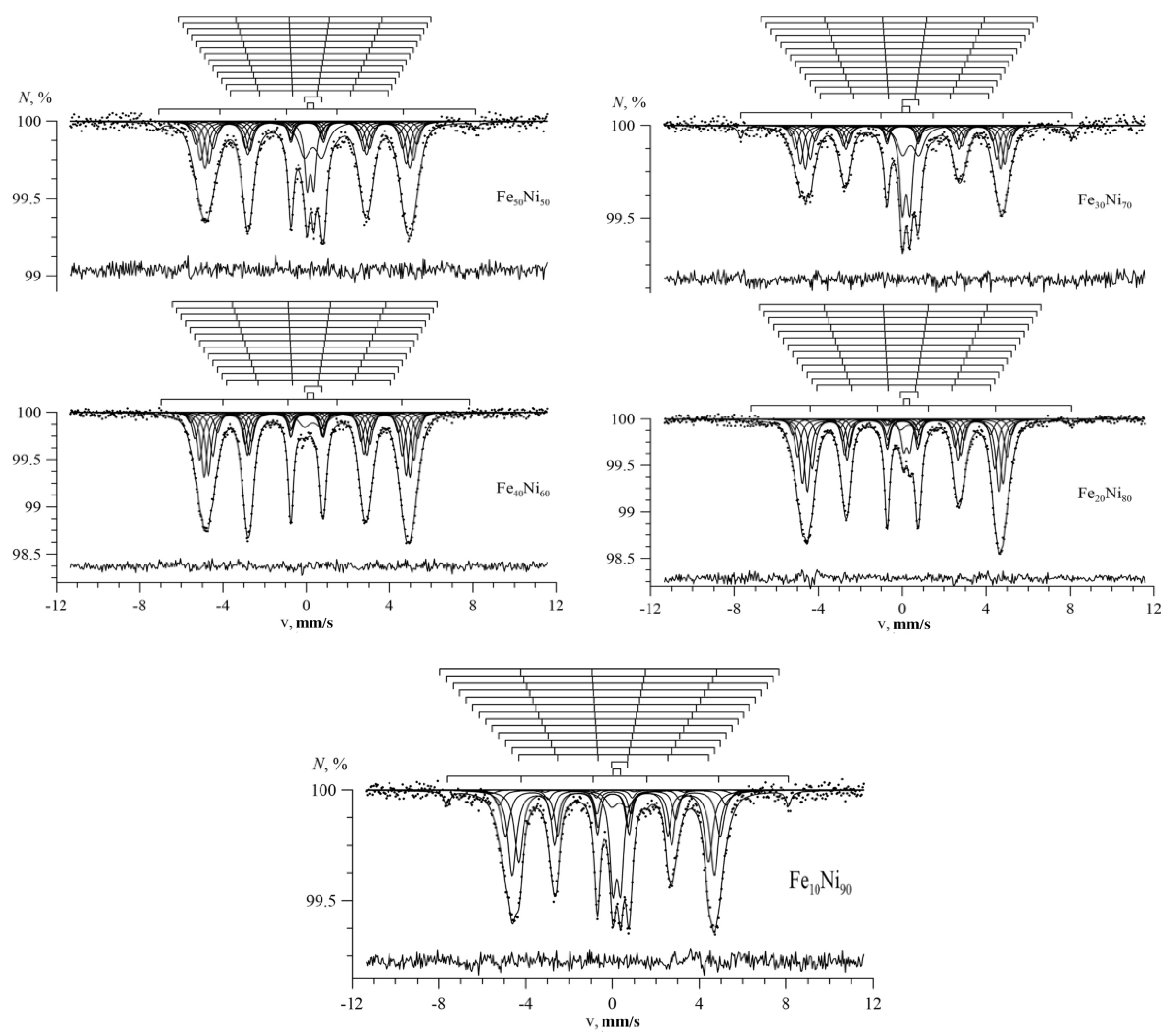
3.1. Results of Restoring the Distribution of Ultrathin Parameters of the Mössbauer Spectra
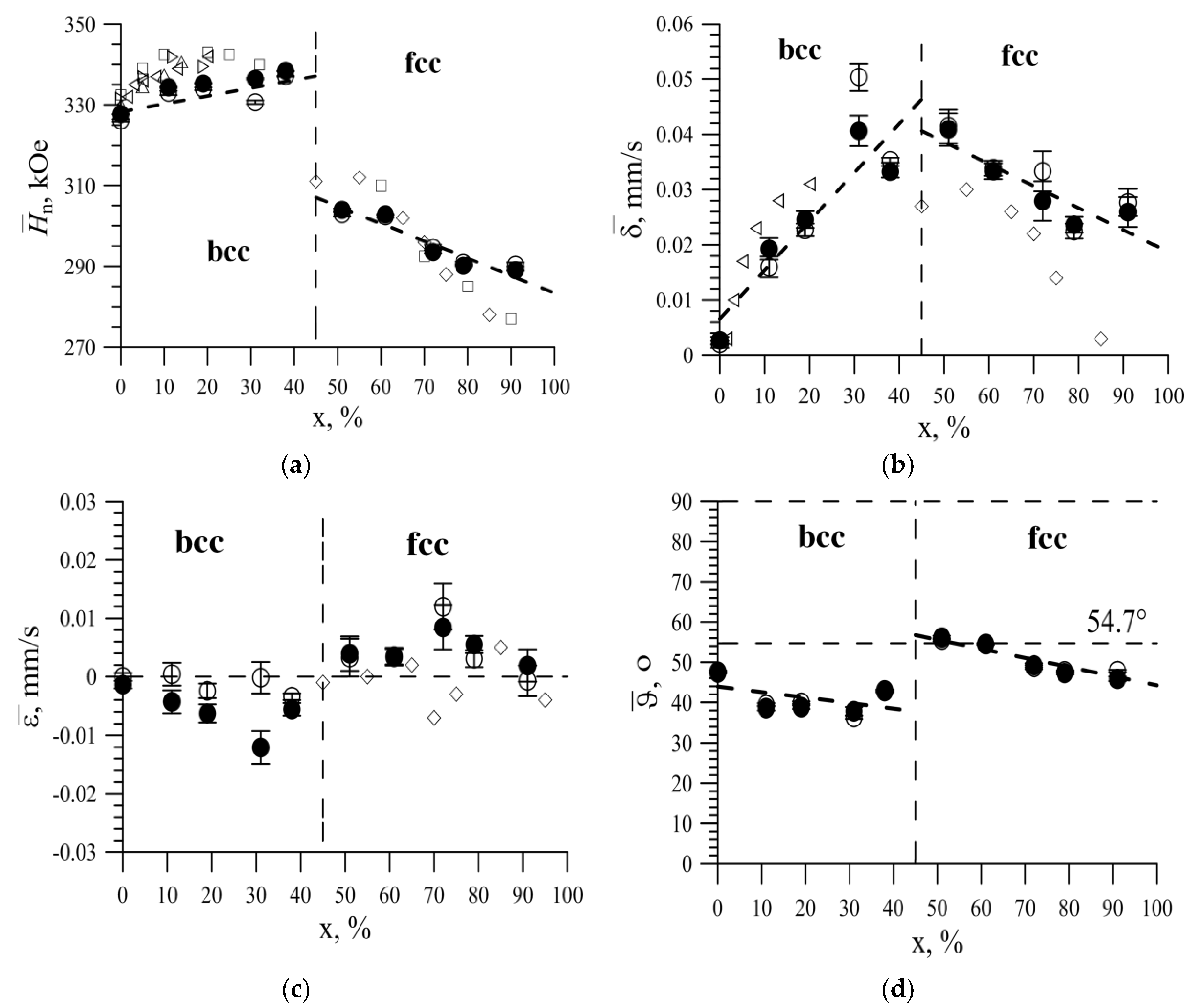
3.2. Results of Model Interpretation of Mössbauer Spectra
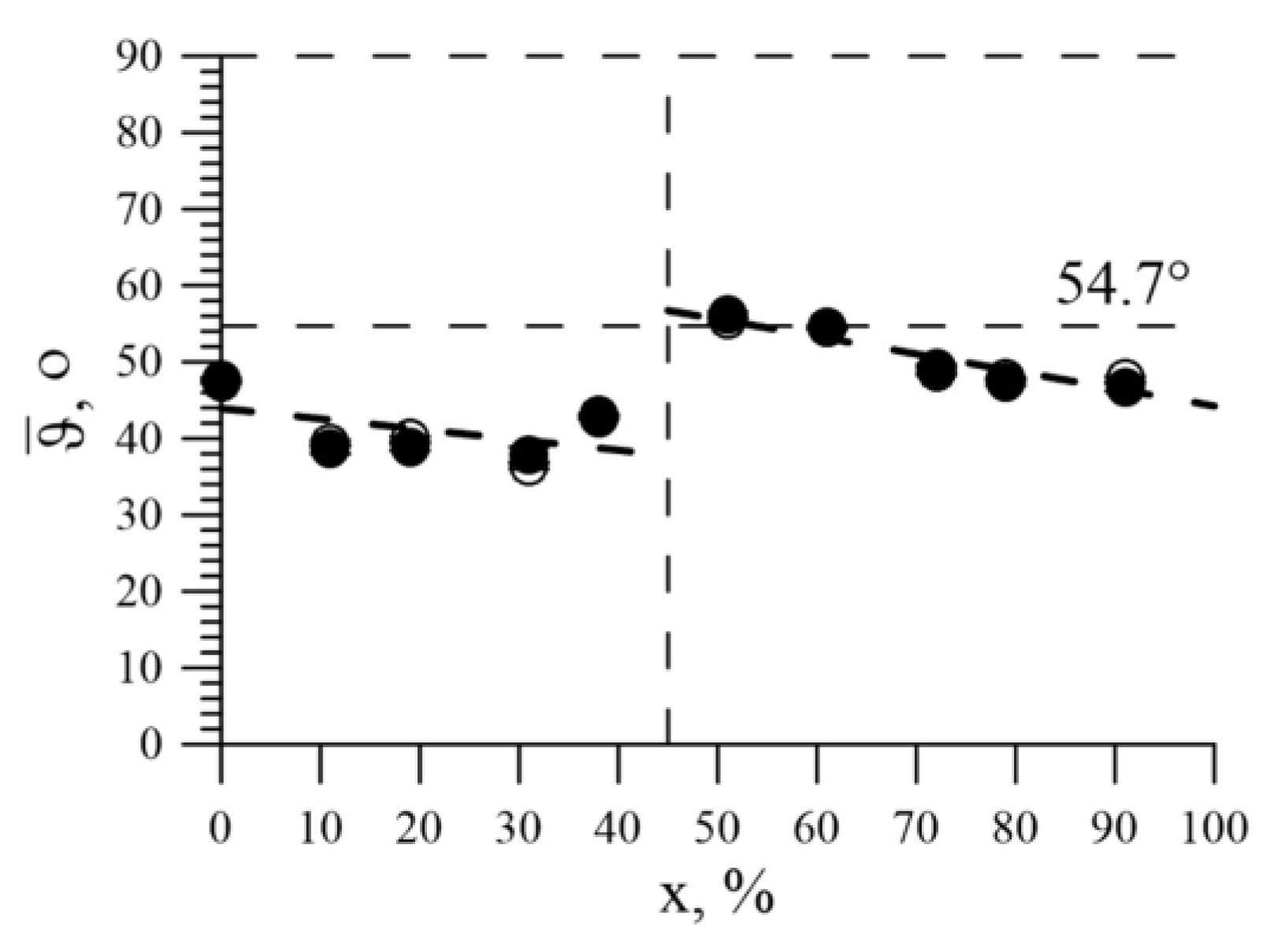
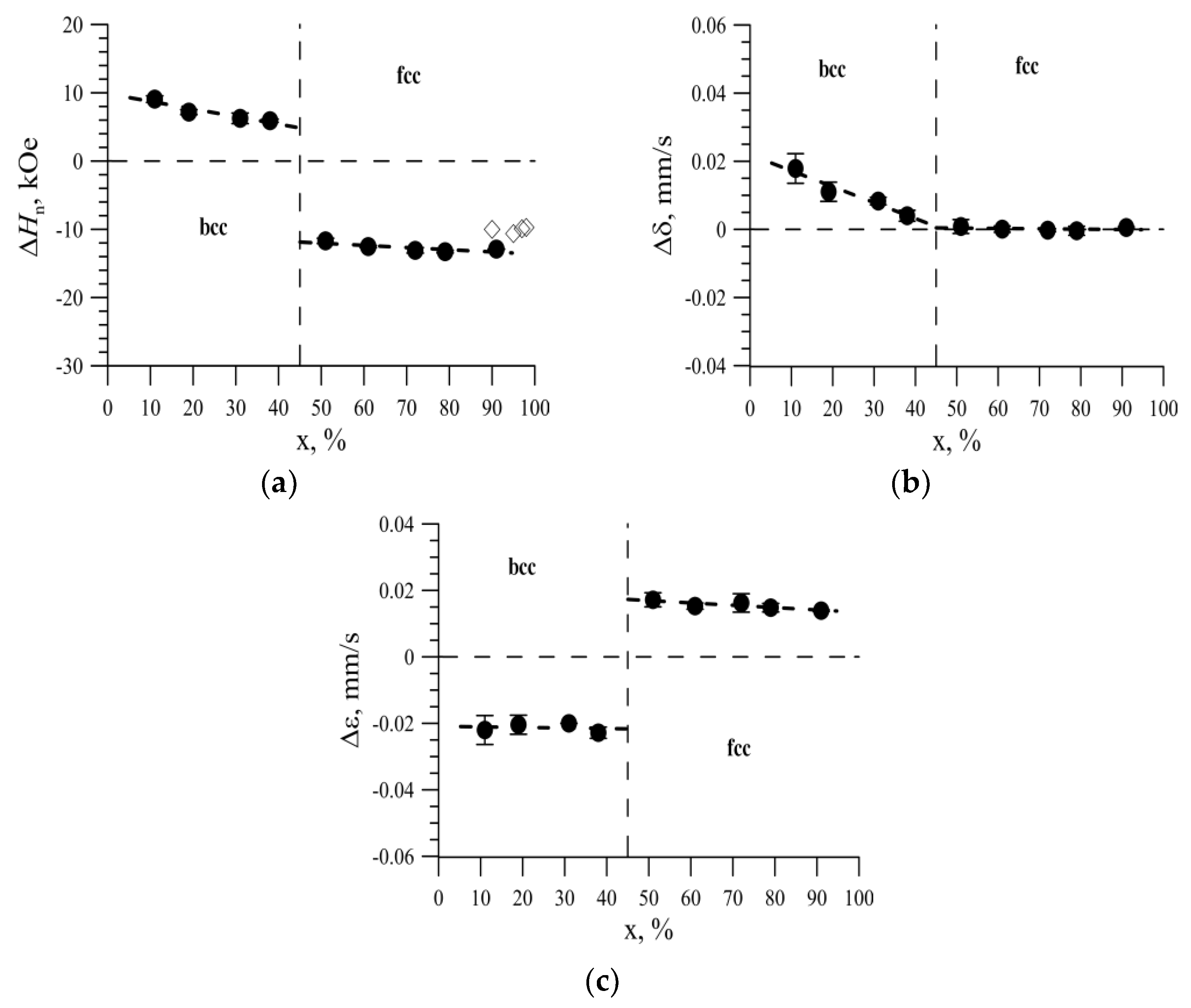
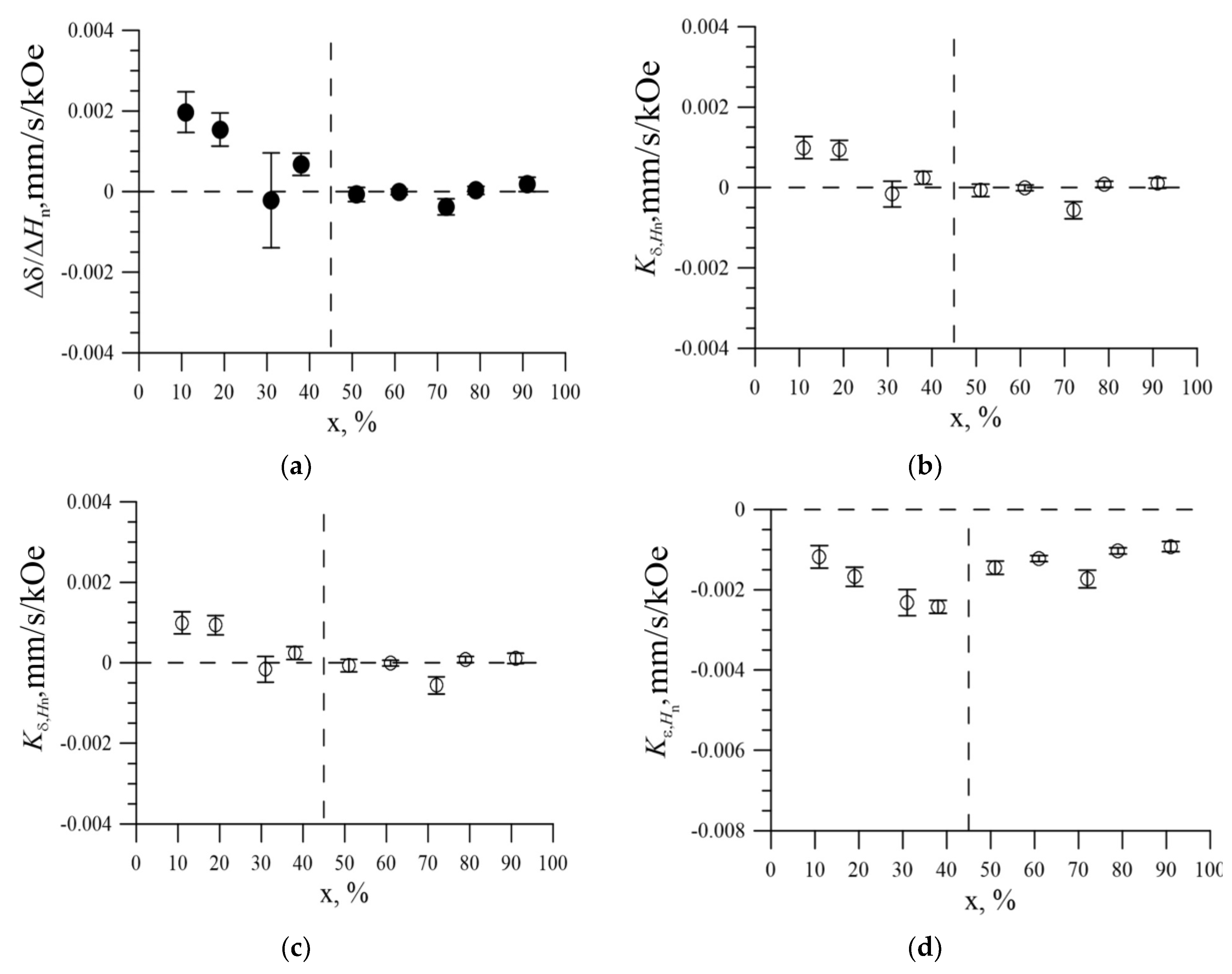
3.3. The Effect of the Replacement of Iron Atoms on Nickel Atoms in the Immediate Environment of Iron Atoms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.; Duan, X.; Wei, Q.; Lieber, C.M. Directed assembly of one-dimensional nanostructures into functional networks. Science 2001, 291, 630–633. [Google Scholar] [CrossRef]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nanosci. Technol. 2010, 67–75. [Google Scholar]
- Beenakker, C.W.J.; van Houten, H. Quantum transport in semiconductor nanostructures. Solid State Phys. 1991, 44, 1–228. [Google Scholar]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Lou, X.W.; Archer, L.A.; Yang, Z. Hollow micro-/nanostructures: Synthesis and applications. Adv. Mater. 2008, 20, 3987–4019. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys.-Condensed Mat. 2004, 16, R829. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Xia, Y. Metal nanostructures with hollow interiors. Adv. Mater. 2003, 15, 641–646. [Google Scholar] [CrossRef]
- Majumdar, A. Thermoelectricity in semiconductor nanostructures. Science 2004, 303, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, J.; Lofland, S.E.; Viehland, D. Multiferroic batio3-cofe2o4 nanostructures. Science 2004, 303, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R. Template synthesis of electronically conductive polymer nanostructures. Account. Chem. Res. 1995, 28, 61–68. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.Y.; Au, L.; Hartland, G.V.; Li, X.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.I.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, properties, and applications of hollow micro-/nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; de Moura, A.F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral inorganic nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef]
- Yu, X.Y.; Yu, L.; Lou, X.W. Metal sulfide hollow nanostructures for electrochemical energy storage. Adv. Energy Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef]
- Wen, F.; Dillen, D.C.; Kim, K.; Tutuc, E. Shell morphology and Raman spectra of epitaxial Ge−SixGe1−x and Si−SixGe1−x core-shell nanowires. J. Appl. Phys. 2017, 121, 234302. [Google Scholar] [CrossRef]
- Song, Y.P.; Hu, Y.W. Quantum interference in InAs/InAlAs core-shell nanowires. Appl. Phys. Lett. 2018, 113, 143104. [Google Scholar] [CrossRef]
- Ziegler, J.; Kozlovsky, R.; Gorini, C.; Liu, M.H.; Weishäupl, S.; Maier, H.; Dvoretsky, S.A. Probing spin helical surface states in topological HgTe nanowires. Phys. Rev. B 2018, 97, 035157. [Google Scholar] [CrossRef]
- Beeler, M.; Lim, C.B.; Hille, P.; Bleuse, J.; Schörmann, J.; De La Mata, M.; Monroy, E. Long-lived excitons in GaN/AlN nanowire heterostructures. Phys. Rev. B 2015, 91, 205440. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.; Zhang, W.; Pu, J.; Wang, Z. Homogeneous core–shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- Chen, G.; Ågren, H.; Ohulchanskyy, T.Y.; Prasad, P.N. Light upconverting core–shell nanostructures: Nanophotonic control for emerging applications. Chem. Soc. Rev. 2015, 44, 1680–1713. [Google Scholar] [CrossRef]
- Qi, J.; Lai, X.; Wang, J.; Tang, H.; Ren, H.; Yang, Y.; Su, Z. Multi-shelled hollow micro-/nanostructures. Chem. Soc. Rev. 2015, 44, 6749–6773. [Google Scholar] [CrossRef]
- Zhou, D.; Cai, L.H.; Wen, F.S.; Li, F.S. Template synthesis and magnetic behavior of FeNi alloy nanotube arrays. Chin. J. Chem. Phys. 2007, 20, 821. [Google Scholar] [CrossRef]
- Xue, S.; Li, M.; Wang, Y.; Xu, X. Electrochemically synthesized binary alloy FeNi nanorod and nanotube arrays in polycarbonate membranes. Thin Solid Films 2009, 517, 5922–5926. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wu, T.; Li, Z.; Zhang, Z.; Sun, H. Comparative study in fabrication and magnetic properties of FeNi alloy nanowires and nanotubes. J. Magnet. Magnet. Mater. 2013, 331, 162–167. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, F.; Xiang, J.; Hu, W.; Yuan, S.; Liu, Z. Microwave absorption properties of multiwalled carbon nanotube/FeNi nanopowders as light-weight microwave absorbers. J. Magnet. Magnet. Mater. 2013, 343, 281–285. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, L.; Hui, D.; Chipara, M. Microstructure, electrical conductivity and microwave absorption properties of γ-FeNi decorated carbon nanotube composites. Compos. Part B-Eng. 2016, 87, 256–262. [Google Scholar] [CrossRef]
- Al Salmi, W.; Gyawali, P.; Dahal, B.; Pegg, I.L.; Philip, J. Core–shell FeNi–NixFe3−xO4 nanowires. J. Vacuum Sci. Technol. B 2015, 33, 040604. [Google Scholar] [CrossRef]
- Ramazani, A.; Asgari, V.; Montazer, A.H.; Kashi, M.A. Tuning magnetic fingerprints of FeNi nanowire arrays by varying length and diameter. Curr. Appl. Phys. 2015, 15, 819–828. [Google Scholar] [CrossRef]
- Wu, H. Synthesis and magnetic properties of size-controlled FeNi alloy nanoparticles attached on multiwalled carbon nanotubes. J. Phys. Chem. Solids 2010, 71, 290–295. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.X.; Guo, A.B.; Guan, H.Y.; Wang, Z.; Wang, K. Magnetic properties and thermodynamics in a metallic nanotube. J. Magnet. Magnet. Mater. 2014, 355, 309–318. [Google Scholar] [CrossRef]
- Liang, J.Y.; Zou, C.L.; Jiang, W.; Li, X.X. Ferrimagnetic behaviors in a double-wall cubic metal nanotube. Physica E 2015, 69, 81–88. [Google Scholar] [CrossRef]
- Kaniukov, E.; Shumskaya, A.; Yakimchuk, D. FeNi nanotubes: Perspective tool for targeted delivery. Appl. Nanosci. 2018, 1–10. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Price, R.R.; Lvov, Y.M. Halloysite nanotubes as biomimetic nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, J.E.; Perez, J.F.; Solorza, O.; Garcia, A. Al2O3 + FeCo and Al2O3 + FeNi coatings for multiwalled carbon nanotubes growth. Diam. Relat. Mater. 2015, 53, 18–22. [Google Scholar] [CrossRef]
- Zubar, T.I.; Panina, L.V.; Kovaleva, N.N. Anomalies in growth of electrodeposited Ni–Fe nanogranular films. CrystEngComm 2018, 20, 2306–2315. [Google Scholar] [CrossRef]
- An, X.; Shin, D.; Ocon, J.D.; Lee, J.K.; Son, Y.I.; Lee, J. Electrocatalytic oxygen evolution reaction at a FeNi composite on a carbon nanofiber matrix in alkaline media. Chin. J. Catal. 2014, 35, 891–895. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zhanbotin, A.; Zdorovets, M.; Manakova, I.; Ozernoy, A.; Kadyrzhanov, K.; Rusakov, V. Study of Ni/Fe nanotube properties. Nucl. Instrum. Methods B 2015, 365, 663–667. [Google Scholar] [CrossRef]
- Barakat, N.A.; Akhtar, M.S.; Mohamed, I.M.; Dakka, Y.A.; Hamdan, R.; El-Deen, A.G.; Al-Meer, S. Effective and stable FeNi@ N-doped graphene counter electrode for enhanced performance dye sensitized solar cells. Mater. Lett. 2017, 191, 80–84. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.B.; Fultz, B. Two-phase coexistence in Fe–Ni alloys synthesized by ball milling. J. Appl. Phys. 1996, 79, 3946. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.B.; Fultz, B. Two-phase coexistence in Fe–Cu alloys synthesized by ball milling. Acta Mater. 1998, 46, 2937–2946. [Google Scholar] [CrossRef]
- Valderruten, J.F.; Perez Alcazar, G.A.; Greneche, J.M. Mossbauer and X-ray study of mechanically alloyed Fe–Ni alloys around the Invar composition. J. Phys.-Condens. Matter. 2008, 20, 485204:1. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Rusakov, V.S.; Kozlovskiy, A.L.; Zdorovets, M.V.; Kaniukov, E.Y.E.; Shumskaya, A.E.; Kenzhina, I.E.; Fadeev, M.S. Structural and Magnetic Studies of Fe100-X Cox Nanotubes Obtained by Template Method. Prog. Electromagnet. Res. 2018, 82, 77–88. [Google Scholar] [CrossRef]
- Matsnev, M.E.; Rusakov, V.S. Study of spatial spin-modulated structures by Mössbauer spectroscopy using SpectrRelax. AIP Conf. Proceed. 2014, 1622, 40–49. [Google Scholar] [Green Version]
- Rusakov, V.S.; Pokatilov, V.S.; Sigov, A.S.; Matsnev, M.E.; Gubaidulina, T.V. Temperature investigations of the spatial spin-modulated structure of multiferroic BiFeO3 by means of Mössbauer spectroscopy. Bull. Russ. Acad. Sci.-Phys. 2015, 79, 708–711. [Google Scholar] [CrossRef]
- Kamali, M.S.; Blixt, A.M.; Häggström, L.; Wäppling, R.; Stanciu, V.; Nordblad, P. Magnetic properties of a Fe0.82Ni0.18/V (001) superlattice studied by Mössbauer spectroscopy. J. Magnet. Magnet. Mater. 2004, 272, 1263–1265. [Google Scholar] [CrossRef]
| Sample | Atomic Ratio, % | ||||
|---|---|---|---|---|---|
| Ni | Fe | Sample | Ni | Fe | |
| Fe100 | 0 | 100 | Fe50Ni50 | 51 ± 4 | 49 ± 3 |
| Fe90Ni10 | 91 ± 4 * | 9 ± 2 | Fe40Ni60 | 38 ± 2 | 62 ± 4 |
| Fe80Ni20 | 79 ± 4 | 21 ± 2 | Fe30Ni70 | 31 ± 3 | 69 ± 3 |
| Fe70Ni30 | 72 ± 4 | 28 ± 3 | Fe20Ni80 | 19 ± 2 | 81 ± 4 |
| Fe60Ni40 | 61 ± 3 | 39 ± 3 | Fe10Ni90 | 11 ± 2 | 89 ± 4 |
| Sample | PseudoVoight Sextet | PseudoVoight Doublet 1 | PseudoVoight Doublet 2 | |||
|---|---|---|---|---|---|---|
| Intensity, % | I2/I1 | Hyperfine Magnetic Field, kOe | Quadrupole Shift, mm/s | Intensity, % | Intensity, % | |
| Fe100 | 79 ± 3 | 0.495 | 327.8 ± 0.2 | −0.001 ± 0.001 | 15 ± 1 | 6 ± 1 |
| Fe90Ni10 | 69 ± 2 | 0.337 | 332.5 ± 0.6 | 0.001 ± 0.003 | 25 ± 1 | 6 ± 1 |
| Fe80Ni20 | 85 ± 3 | 0.346 | 333.4 ± 0.6 | −0.001 ± 0.001 | 14 ± 1 | 1 ± 1 |
| Fe70Ni30 | 82 ± 3 | 0.285 | 341.9 ± 0.6 | −0.03 ± 0.01 | 8 ± 1 | 10 ± 1 |
| Fe60Ni40 | 94 ± 2 | 0.405 | 342.3 ± 1.6 | −0.02 ± 0.01 | 5 ± 1 | 1 ± 1 |
| Fe50Ni50 | 85 ± 3 | 0.689 | 301.5 ± 0.5 | 0.01 ± 0.01 | 5 ± 1 | 10 ± 1 |
| Fe40Ni60 | 97 ± 2 | 0.660 | 301.5 ± 0.3 | 0.01 ± 0.01 | 2 ± 1 | 1 ± 1 |
| Fe30Ni70 | 76 ± 3 | 0.525 | 285.1 ± 0.7 | 0.03 ± 0.01 | 6 ± 1 | 18 ± 1 |
| Fe20Ni80 | 93 ± 3 | 0.504 | 287.7 ± 3.1 | 0.01 ± 0.01 | 2 ± 1 | 5 ± 1 |
| Fe10Ni90 | 83 ± 3 | 0.504 | 283.8 ± 0.5 | 0.01 ± 0.01 | 2 ± 1 | 15 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadyrzhanov, K.K.; Rusakov, V.S.; Fadeev, M.S.; Kiseleva, T.Y.; Kozlovskiy, A.L.; Kenzhina, I.E.; Zdorovets, M.V. Study of Magnetic Properties of Fe100-xNix Nanostructures Using the Mössbauer Spectroscopy Method. Nanomaterials 2019, 9, 757. https://doi.org/10.3390/nano9050757
Kadyrzhanov KK, Rusakov VS, Fadeev MS, Kiseleva TY, Kozlovskiy AL, Kenzhina IE, Zdorovets MV. Study of Magnetic Properties of Fe100-xNix Nanostructures Using the Mössbauer Spectroscopy Method. Nanomaterials. 2019; 9(5):757. https://doi.org/10.3390/nano9050757
Chicago/Turabian StyleKadyrzhanov, Kayrat K., Vyacheslav S. Rusakov, Maxim S. Fadeev, Tatyana Yu. Kiseleva, Artem L. Kozlovskiy, Inesh E. Kenzhina, and Maxim V. Zdorovets. 2019. "Study of Magnetic Properties of Fe100-xNix Nanostructures Using the Mössbauer Spectroscopy Method" Nanomaterials 9, no. 5: 757. https://doi.org/10.3390/nano9050757




