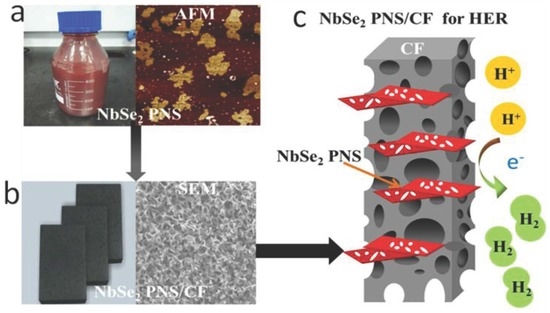
Active Pore-Edge Engineering of Single-Layer Niobium Diselenide Porous Nanosheets Electrode for Hydrogen Evolution
Abstract
1. Introduction
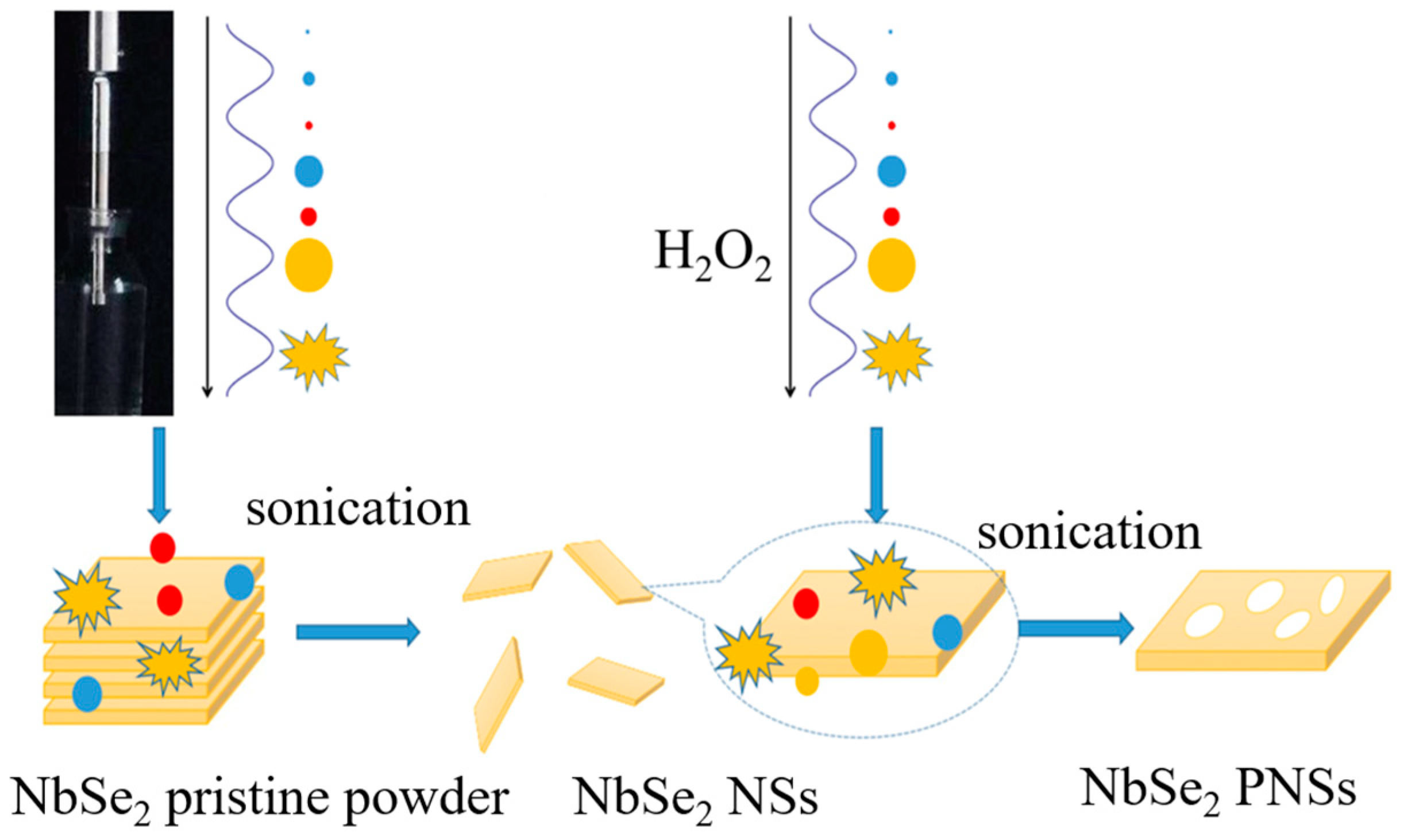
2. Materials and Methods
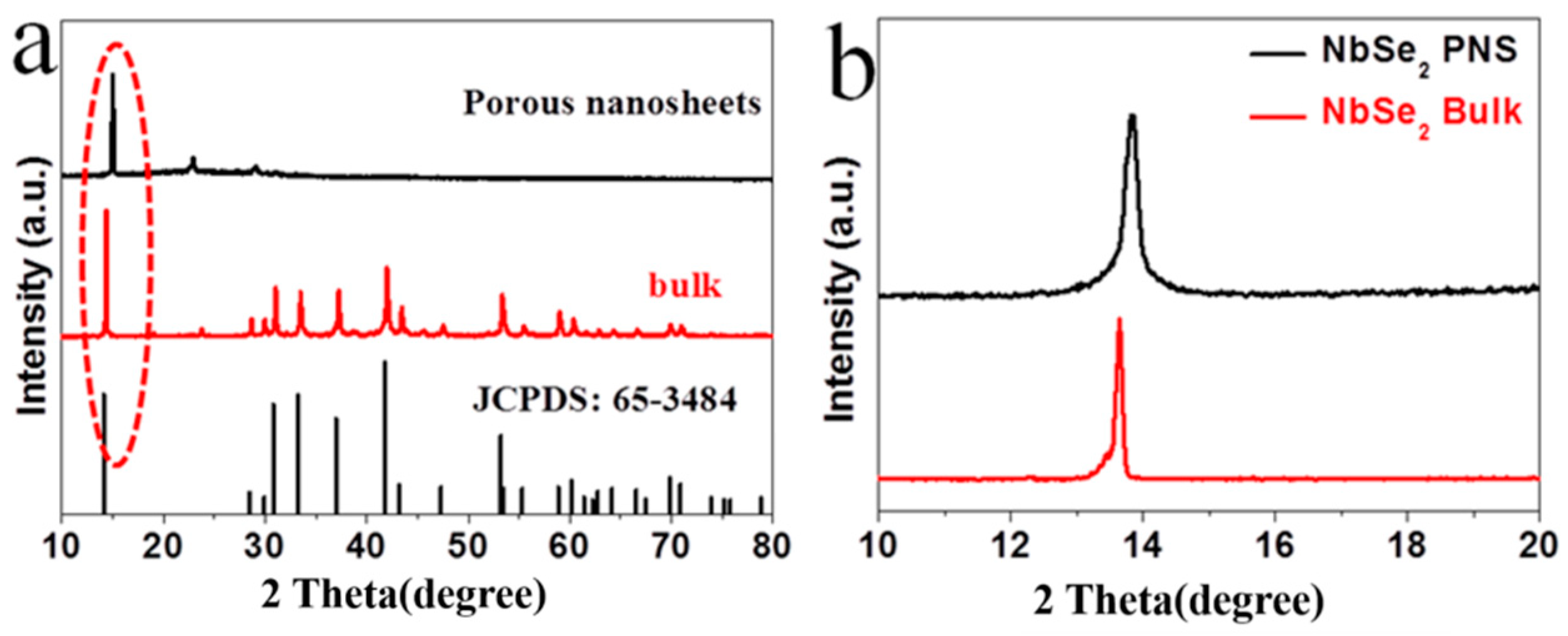
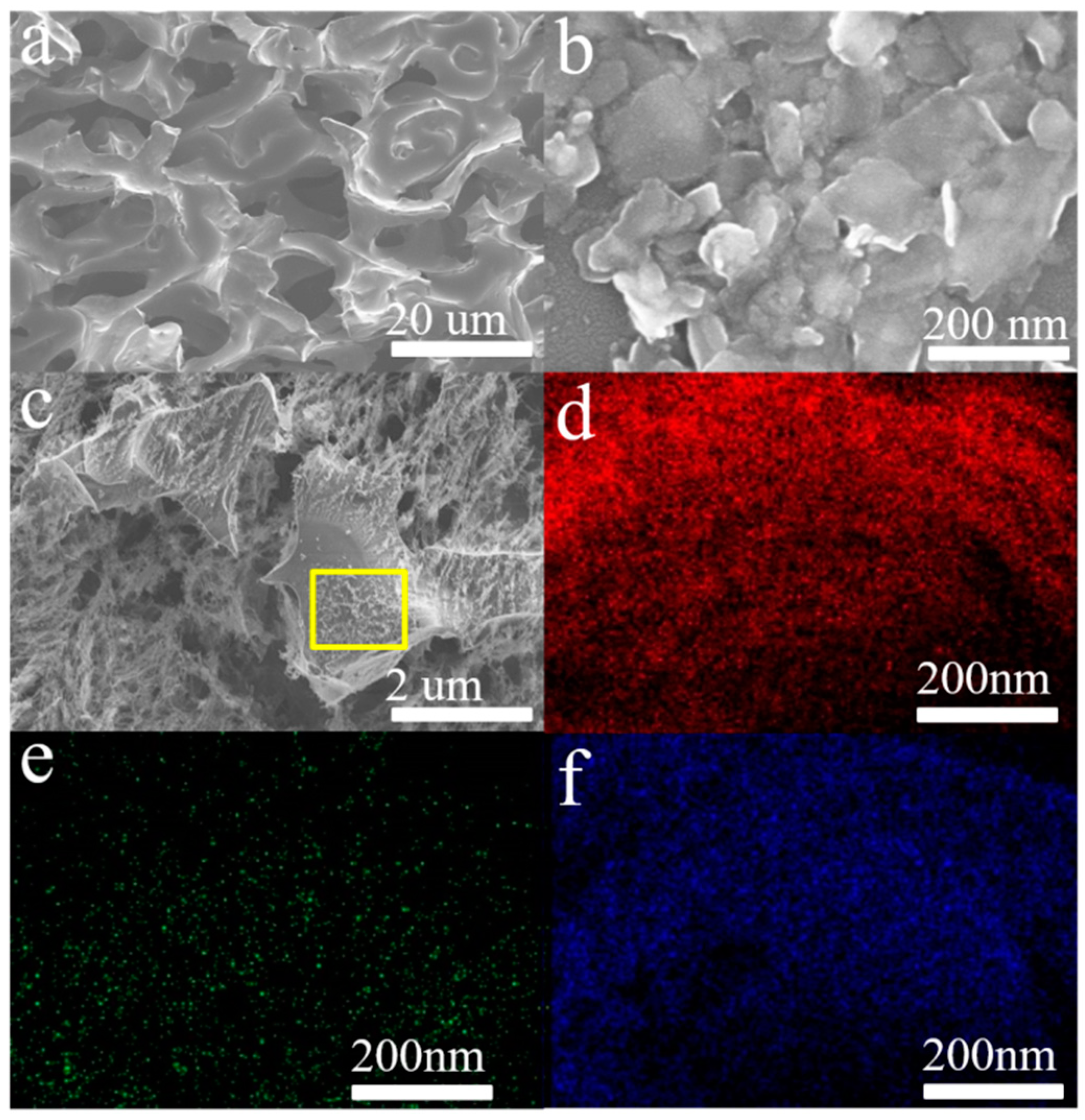
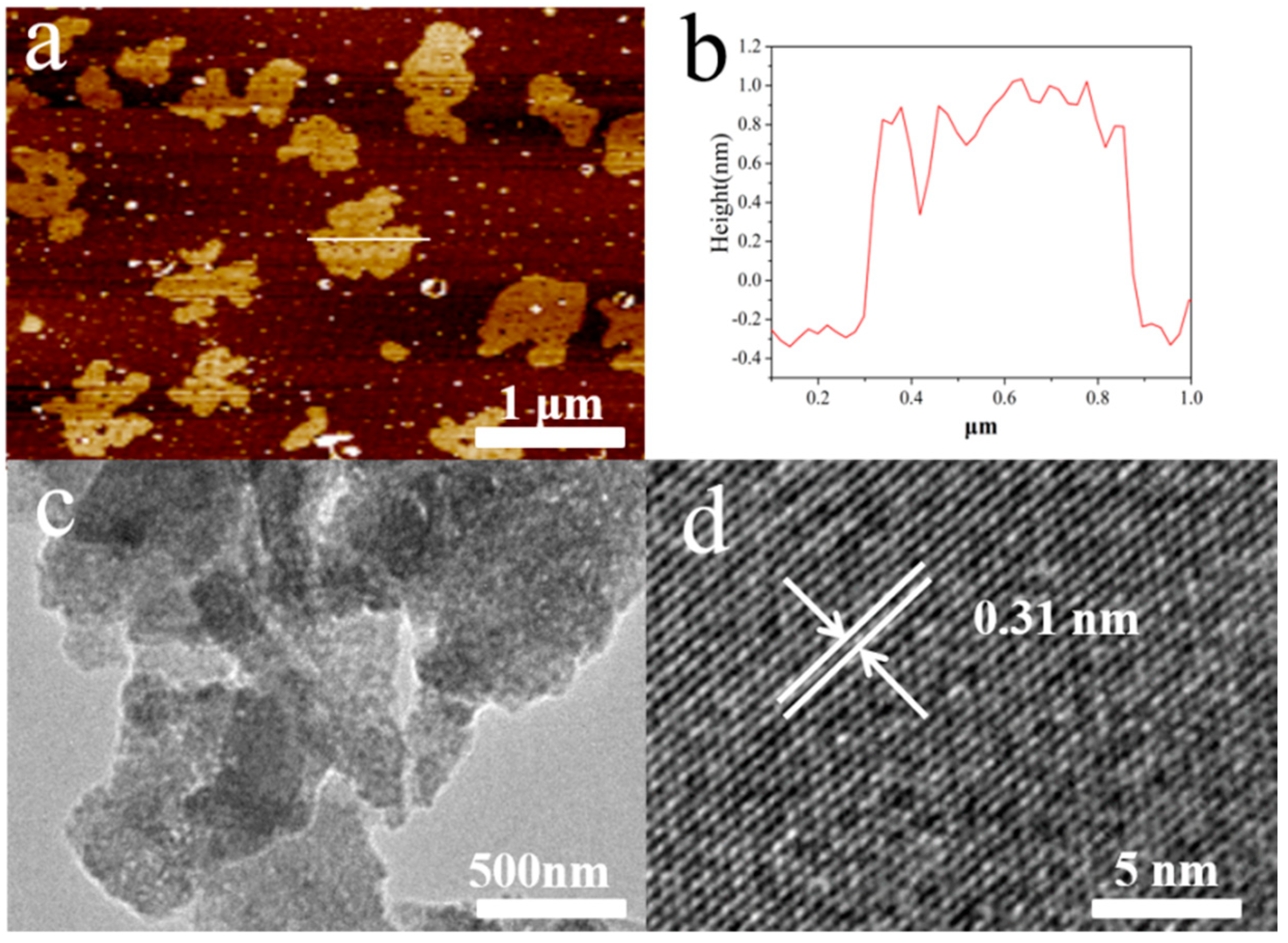
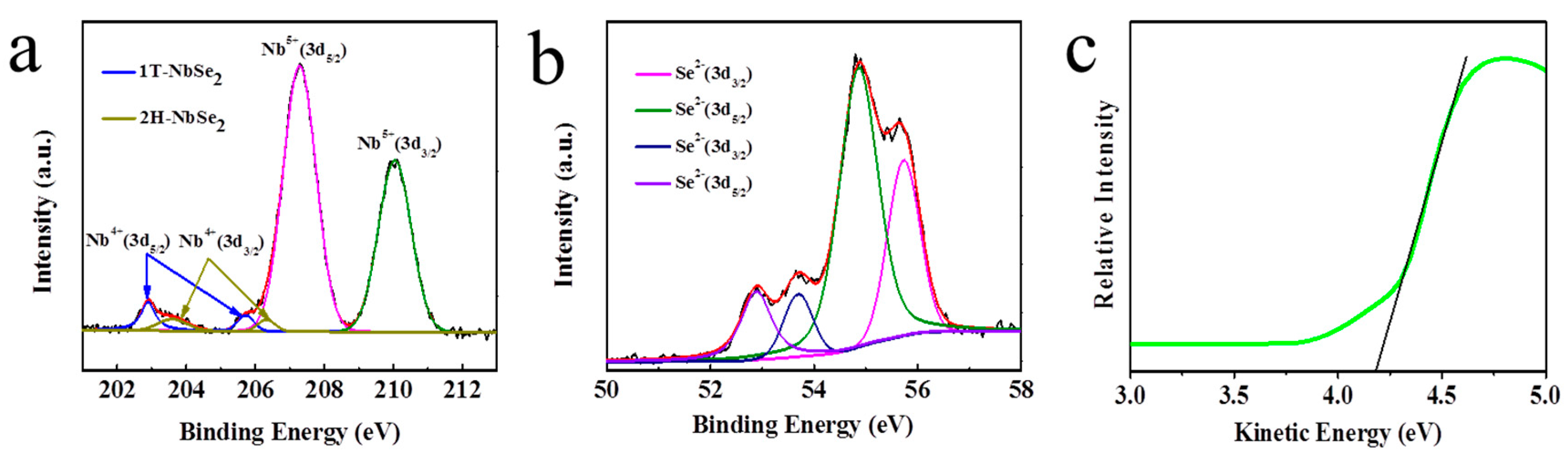
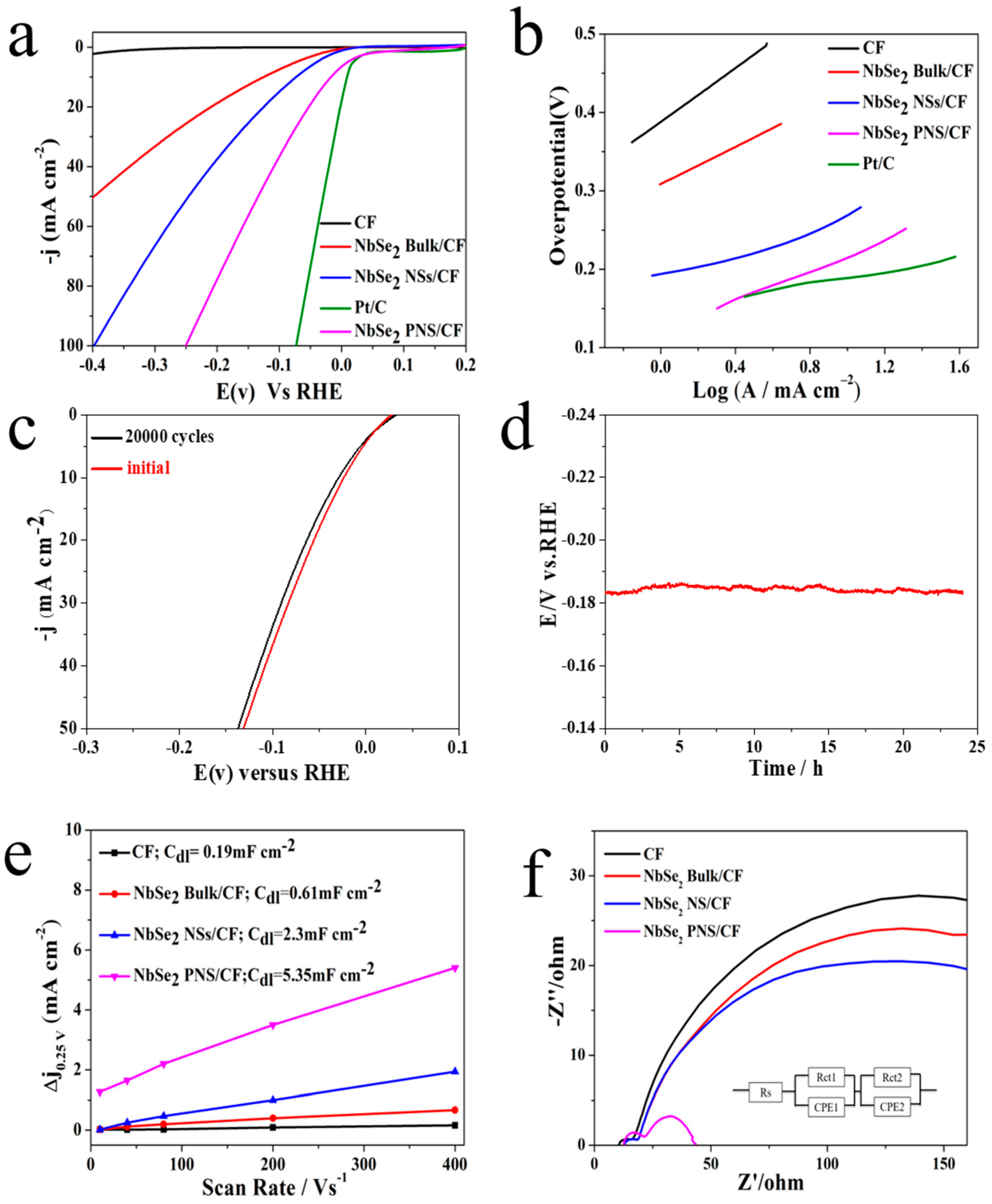
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from Catalytic Reforming of Biomass-derived Hydrocarbons in Liquid Water. Nature 2002, 33, 964–967. [Google Scholar] [CrossRef]
- Wang, X.; Kolen’ko, Y.; Bao, X.; Kovnir, K.; Liu, L. One-Step Synthesis of Self-Supported Nickel Phosphide Nanosheet Array Cathodes for Efficient Electrocatalytic Hydrogen Generation. Angew. Chem. Int. Ed. 2015, 54, 8188–8192. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen-and Hydrogen-involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiong, D.; Petrovykh, D.; Liu, L. Bifunctional Nickel Phosphide Nanocatalysts Supported on Carbon Fiber Paper for Highly Efficient and Stable Overall Water Splitting. Adv. Fun. Mater. 2016, 26, 4067–4077. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the Electrochemistry of the Hydrogen-Evolution Reaction Through Combining Experiment and Theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in Activity for the Water Electrolyser Reactions on 3d M (Ni,Co,Fe,Mn) Hydr(oxy)oxide Catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Gao, M.R.; Liang, J.X.; Zheng, Y.R.; Xu, Y.F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.H. An Efficient Molybdenum Disulfide/Cobalt Diselenide Hybrid Catalyst for Electrochemical Hydrogen Generation. Nat. Commun. 2015, 6, 5982–5984. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hessel, C.M.; Wang, J.; Yang, N.; Yu, R.; Zhao, H.; Wang, D. Two-Dimensional Carbon Leading to New Photoconversion Processes. Chem. Soc. Rev. 2014, 43, 4281–4299. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, Q.; Li, J.; Tang, B.; Zhang, Z.; Wang, X. Three-Dimensional Well-Mixed/Highly-Densed NiS-CoS Nanorod Arrays: An Efficient and Stable Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reactions. Electrochim. Acta 2018, 260, 82–91. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, J.; Zheng, Z.; Li, L.; Yu, L.; Wang, C.; Yang, G. A Floating Sheet for Efficient Photocatalytic Water Splitting. Adv. Energy Mater. 2016, 6, 1600510–1600516. [Google Scholar] [CrossRef]
- Bi, W.; Li, X.; Zhang, L.; Jin, T.; Zhang, L.; Zhang, Q.; Luo, Y.; Wu, C.; Xie, Y. Molecular Co-Catalyst Accelerating Hole Transfer for Enhanced Photocatalytic H2 Evolution. Nat. Commun. 2015, 6, 8647–8651. [Google Scholar] [CrossRef]
- Ma, Z.; Meng, H.; Wang, M.; Tang, B.; Li, J.; Wang, X. Porous Ni-Mo-S Nanowire Network Film Electrode as High-Efficiency Bifunctional Electrocatalyst for Overall Water Splitting. ChemElectroChem 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Ma, Z.; Li, R.; Wang, M.; Meng, H.; Zhang, F.; Bao, X.; Tang, B.; Wang, X. Self-Supported Porous Ni-Fe-P Composite as An Efficient Electrocatalyst for Hydrogen Evolution Reaction in Both Acidic and Alkaline Medium. Electrochim. Acta 2016, 219, 194–203. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Ma, G.; He, Y.; Wang, M.; Zhu, F.; Tang, B.; Wang, X. An Efficient Route for Catalytic Activity Promotion via Hybrid Electrodepositional Modification on Commercial Nickel Foam for Hydrogen Evolution Reaction in Alkaline Water Electrolysis. Appl. Surf. Sci. 2014, 313, 512–523. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G. Enhanced Catalytic Activity in Strained Chemically Exfoliated WS2 Nanosheets for Hydrogen Evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef]
- Voiry, D.; Fullon, R.; Yang, J.; De, C.C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M.J.; Batson, P.E.; Gupta, G. The Role of Electronic Coupling Between Substrate and 2D MoS2 Nanosheets in Electrocatalytic Production of Hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef]
- Zhao, B.; Huang, J.; Fu, Q.; Yang, L.; Zhang, J.; Xiang, B. MoS2/NbSe2 Hybrid Nanobelts for Enhanced Hydrogen Evolution. J. Electrochem. Soc. 2016, 163, 384–387. [Google Scholar] [CrossRef]
- Xi, X.; Zhao, L.; Wang, Z.; Berger, H.; Forró, L.; Shan, J.; Mak, K.F. Strongly Enhanced Charge-Density-Wave Order in Monolayer NbSe2. Nat. Nanotech. 2015, 10, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Ambrosi, A.; Lazar, P.; Sofer, Z.; Pumera, M. Electrocatalysis of Layered Group 5 Metallic Transition Metal Dichalcogenides (MX2, M = V, Nb, and Ta; X = S, Se, and Te). J. Mater. Chem. A 2016, 4, 14241. [Google Scholar] [CrossRef]
- Tang, H.; Cao, K.; Wu, Q.; Li, C.; Yang, X.; Yan, X. Synthesis and Tribological Properties of Copper Matrix Solid Self-Lubricant Composites Reinforced with NbSe2 Nanoparticles. Cryst. Res. Technol. 2011, 46, 195–200. [Google Scholar] [CrossRef]
- Sipos, B.; Kusmartseva, A.F.; Akrap, A.; Berger, H.; Forro, L.; Tutis, E. From Mott State to Superconductivity in 1T-TaS2. Nat. Mater. 2008, 7, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Calandra, M.; Mazin, I.I.; Mauri, F. Effect of Dimensionality on the Charge-Density-Wave in Few-Layers 2H-NbSe2. Phys. Rev. B 2009, 80, 241108. [Google Scholar] [CrossRef]
- Park, S.K.; Chung, D.Y.; Ko, D.; Sung, Y.; Piao, Y. Three-Dimensional Carbon Foam/N-Doped Graphene@MoS2 Hybrid Nanostructures as Effective Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2016, 4, 12720–12725. [Google Scholar] [CrossRef]
- Tang, H.; Dou, K.; Kaun, C.C.; Kuang, Q.; Yang, S. MoSe2 Nanosheets and Their Graphene Hybrids: Synthesis, Characterization and Hydrogen Evolution Reaction Studies. J. Mater. Chem. A 2013, 2, 360–364. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Xu, S.; Kong, D.; Cha, J.J.; Zheng, G.; Hsu, P.C.; Yan, K.; Bradshaw, D.; Prinz, F.B. Electrochemical Tuning of Vertically Aligned MoS2 Nanofilms and Its Application in Improving Hydrogen Evolution Reaction. Proc. Nat. Acad. Sci. USA 2013, 110, 19701–19706. [Google Scholar] [CrossRef]
- Chang, Y.; Wu, F.; Chen, T.; Hsu, C.; Chen, C.; Wiryo, F.; Wei, K.; Chiang, C.; Li, L. Three-Dimensional Molybdenum Sulfide Sponges for Electrocatalytic Water Splitting. Small 2014, 10, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, K.; Hou, D.; Liu, X.; Li, G.; Sang, Y.; Liu, H.; Li, L.; Chen, S. Three-Dimensional Hierarchical Frameworks Based on MoS2 Nanosheets Self-Assembled on Graphene Oxide for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interf. 2014, 6, 21534–21540. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.M.; Lodeiro, C.; Capelo-Martínez, J.L. The Power of Ultrasound. In Ultrasound in Chemistry: Analytical Applications; Capelo-Martínez, J.L., Ed.; Wiley: Weinheim, Germany, 2009; Volume 1, pp. 15–16. [Google Scholar]
- Backes, C.; Szydlowska, B.M.; Harvey, A.; Yuan, S.; Vega-Mayoral, V.; Davies, B.R.; Zhao, P.L.; Hanlon, D.; Santos, E.; Katsnelson, M.I. Production of Highly Monolayer Enriched Dispersions of Liquid-Exfoliated Nanosheets by Liquid Cascade Centrifugation. ACS Nano 2016, 10, 1589–1601. [Google Scholar] [CrossRef]
- Khan, U.; Neill, A.O.; Lotya, M.; De, S.; Coleman, J.N. High-Concentration Solvent Exfoliation of Graphene. Small 2010, 6, 864–871. [Google Scholar] [CrossRef]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-Concentration, Surfactant-Stabilized Graphene Dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; Neill, A.O.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G. Large-Scale Exfoliation of Inorganic Layered Compounds in Aqueous Surfactant Solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef]
- Chung, D.Y.; Yoo, J.M.; Park, S.; Jung, G.Y.; Kang, J.S.; Ahn, C.Y.; Kwak, S.K.; Sung, Y. Edge-Terminated MoS2 Nanoassembled Electrocatalyst via In Situ Hybridization with 3D Carbon Network. Small 2018, 14, 1802191. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nam, G.; He, Q.; Wu, X.; Liu, Z.; Gu, L.; Du, Y.; Huang, W.; Zhang, H. High Phase-Purity 1T′-MoS2- and 1T′-MoSe2-Layered Crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef]
- Jin, Y.O.; Ji, H.L.; Han, S.W.; Chae, S.S.; Bae, E.J.; Kang, Y.H.; Choi, W.J.; Song, Y.C.; Lee, J.O.; Hong, K.B. Chemically Exfoliated Transition Metal Dichalcogenide Nanosheet-based Wearable Thermoelectric Generators. Energy Environ. Sci. 2016, 9, 1696–1705. [Google Scholar]
- Lei, Z.; Xu, S.; Wu, P. Ultra-Thin and Porous MoSe2 Nanosheets: Facile Preparation and Enhanced Electrocatalytic Activity Towards Hydrogen Evolution Reaction. Phys. Chem. Chem. Phys. 2016, 18, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Ultrathin Cobalt-Manganese Layered Double Hydroxide is An Efficient Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2014, 136, 16481–16484. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Liu, X.Y.; Yang, G.W. Amorphous Mixed-Metal Hydroxide Nanostructures for Advanced Water Oxidation Catalysts. Nanoscale 2016, 8, 5015–5023. [Google Scholar] [CrossRef]
- Mccrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]

| NbSe2 NSs | NbSe2 PNS | NbSe2 PNS after 50 cycles | NbSe2 PNS after 25 h | |
|---|---|---|---|---|
| Nb 3d | 0.285 | 0.149 | 0.178 | 0.126 |
| Se 3d | 0.644 | 0.374 | 0.339 | 0.174 |
| O 1s | 0.071 | 0.476 | 0.482 | 0.696 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, X.; Liu, Y.; Yang, G. Active Pore-Edge Engineering of Single-Layer Niobium Diselenide Porous Nanosheets Electrode for Hydrogen Evolution. Nanomaterials 2019, 9, 751. https://doi.org/10.3390/nano9050751
Wang J, Liu X, Liu Y, Yang G. Active Pore-Edge Engineering of Single-Layer Niobium Diselenide Porous Nanosheets Electrode for Hydrogen Evolution. Nanomaterials. 2019; 9(5):751. https://doi.org/10.3390/nano9050751
Chicago/Turabian StyleWang, Jianxing, Xinyue Liu, Ying Liu, and Guowei Yang. 2019. "Active Pore-Edge Engineering of Single-Layer Niobium Diselenide Porous Nanosheets Electrode for Hydrogen Evolution" Nanomaterials 9, no. 5: 751. https://doi.org/10.3390/nano9050751
APA StyleWang, J., Liu, X., Liu, Y., & Yang, G. (2019). Active Pore-Edge Engineering of Single-Layer Niobium Diselenide Porous Nanosheets Electrode for Hydrogen Evolution. Nanomaterials, 9(5), 751. https://doi.org/10.3390/nano9050751