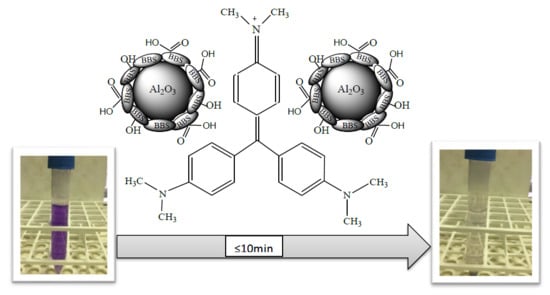
An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Hybrid Materials
2.3. Characterization Methods
2.4. Adsorption Procedures
2.4.1. Analytical Instruments
2.4.2. Kinetic of CV Adsorption
2.4.3. CV Adsorption Study and Model Application
Freundlich Model
Langmuir model
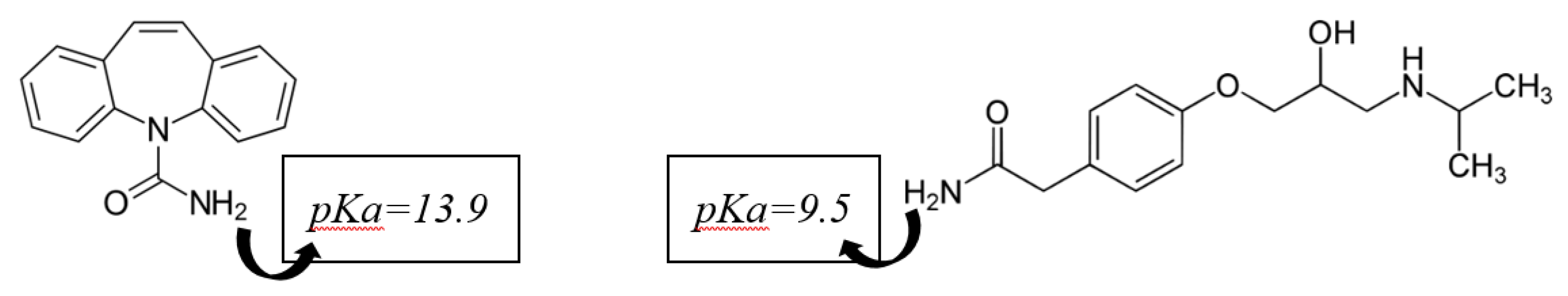
2.4.4. Contaminants of Emerging Concern (CECs) Adsorption Study
3. Results and Discussion
3.1. Materials Characterization
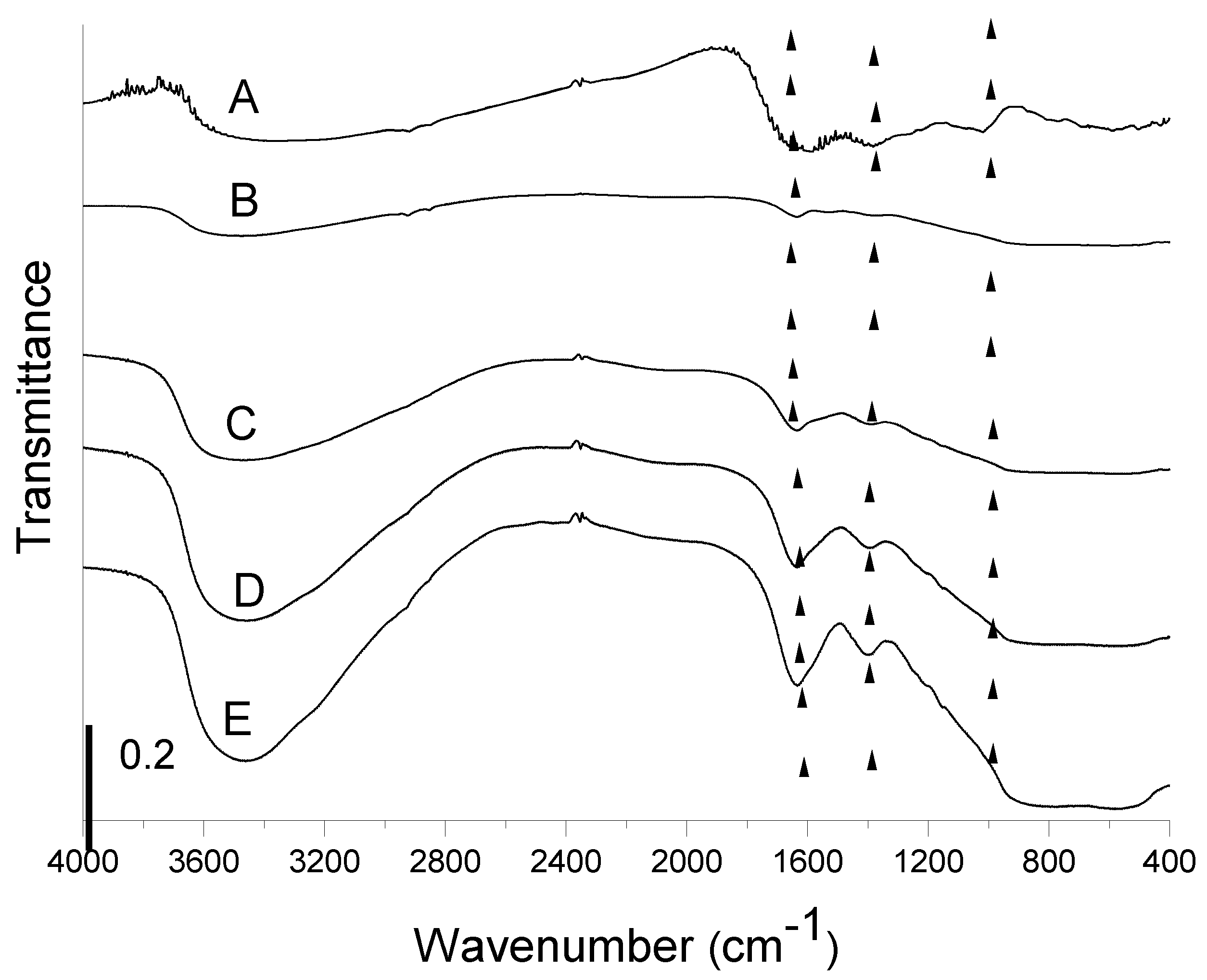
3.1.1. FTIR Spectroscopy
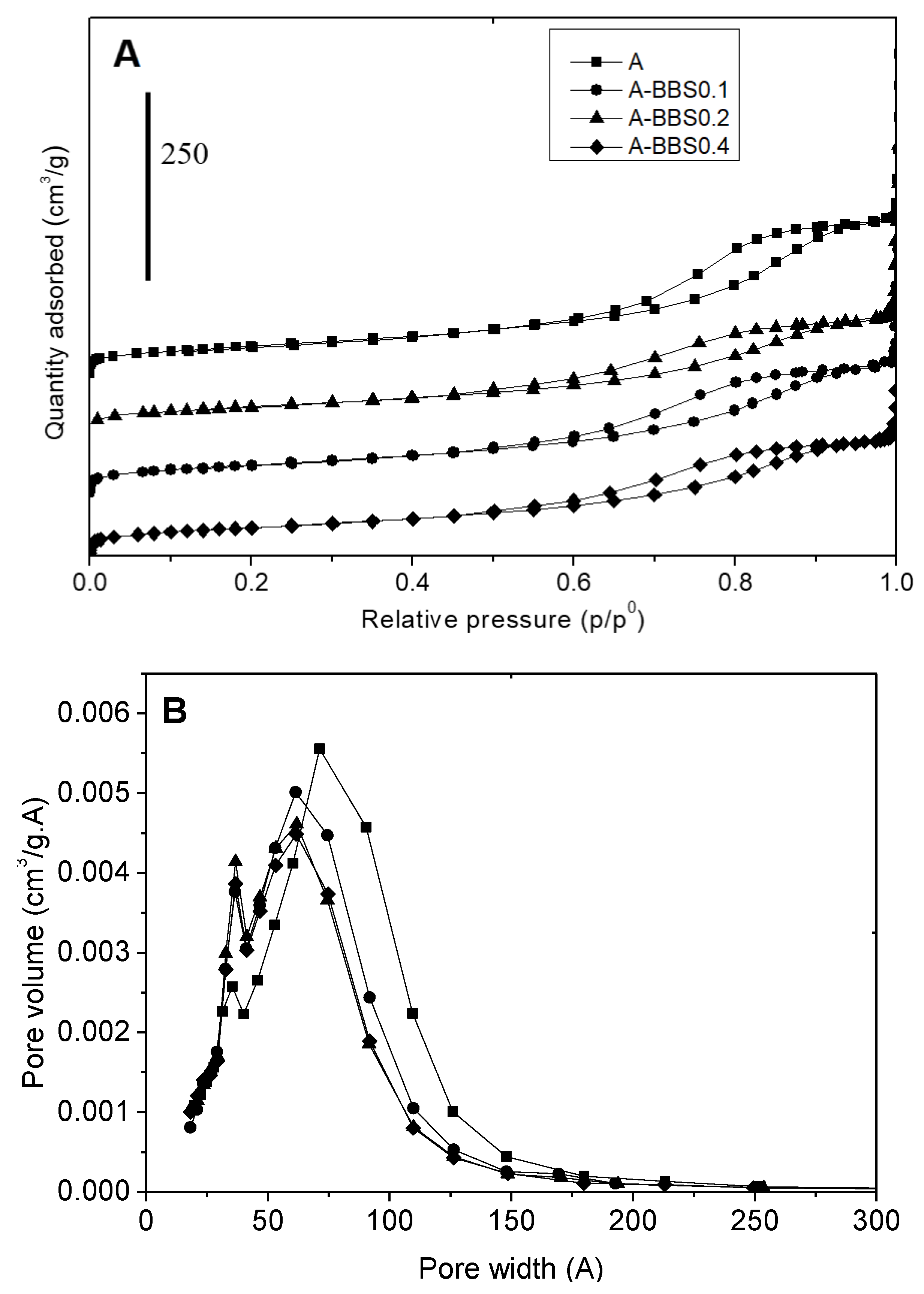
3.1.2. Gas-Volumetric N2 Adsorption at 77K
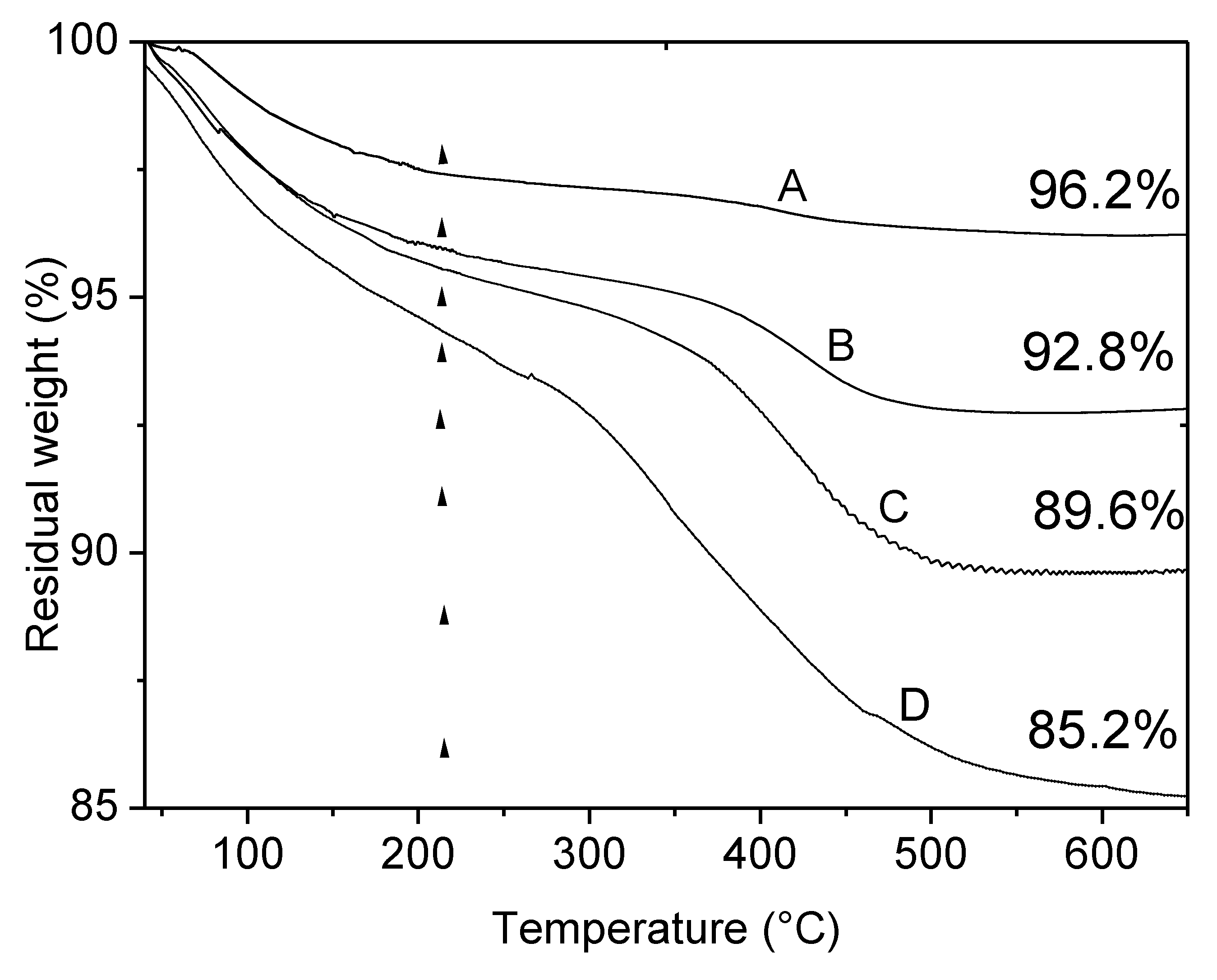
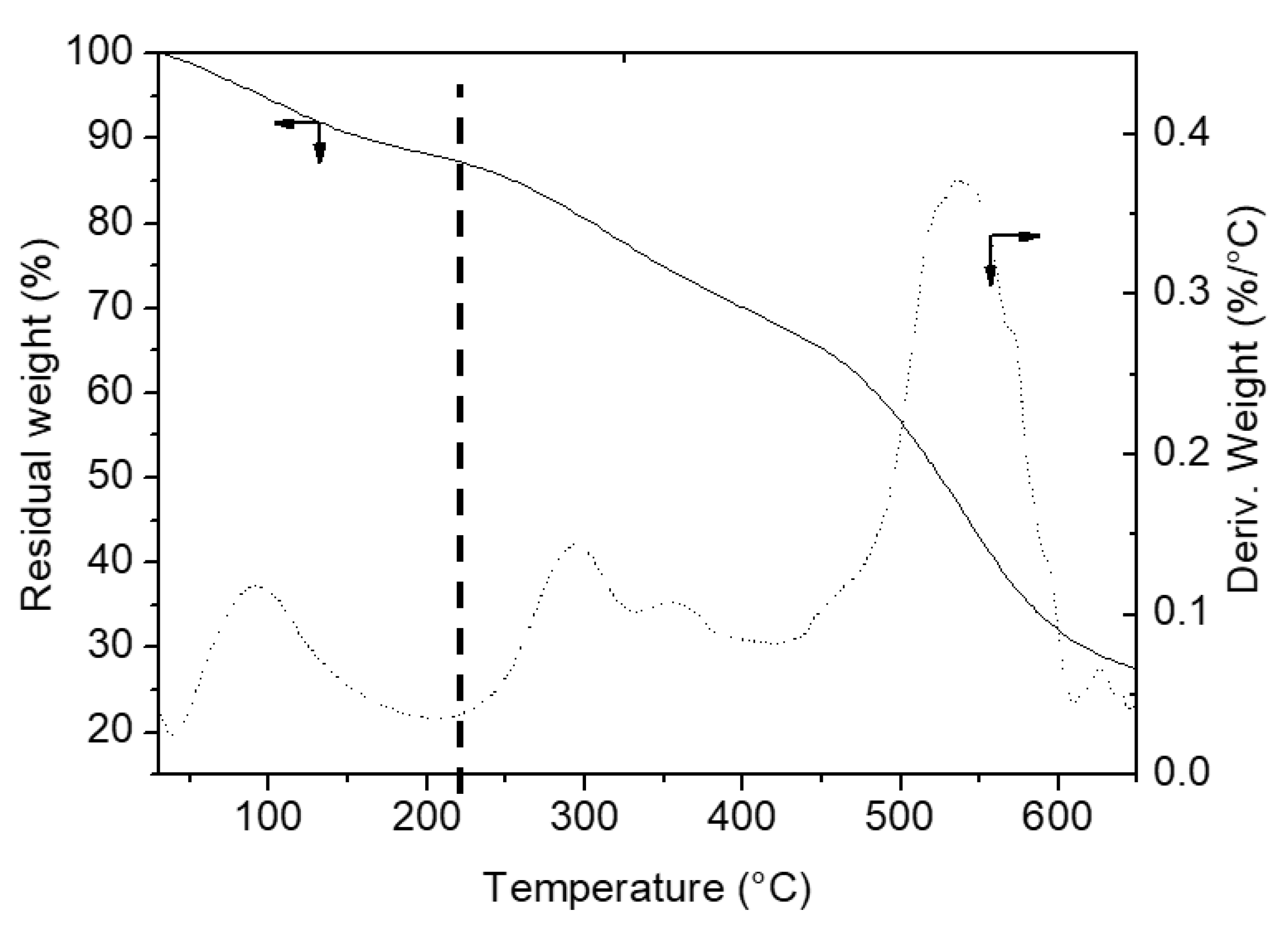
3.1.3. TGA
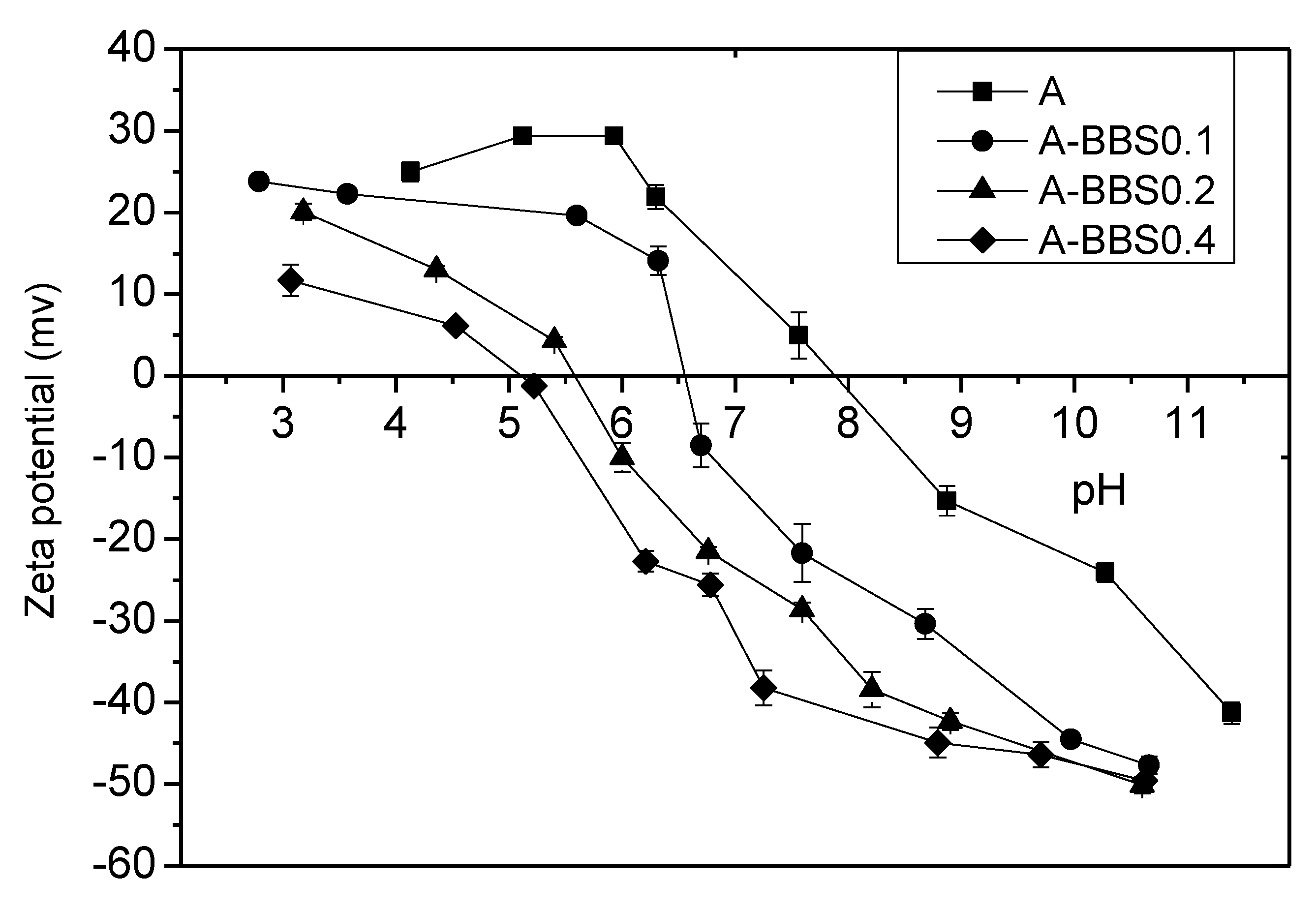
3.1.4. Zeta Potential
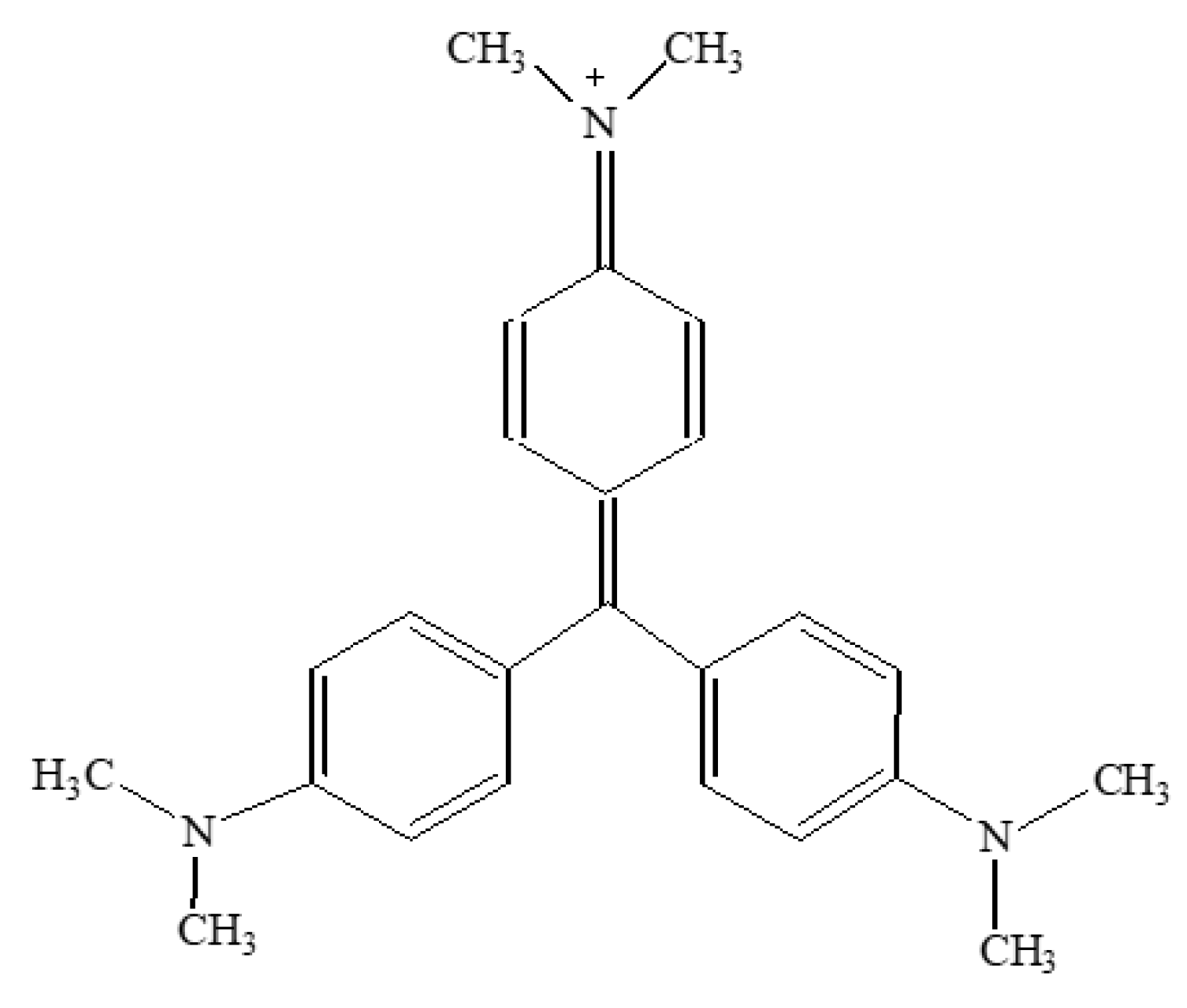
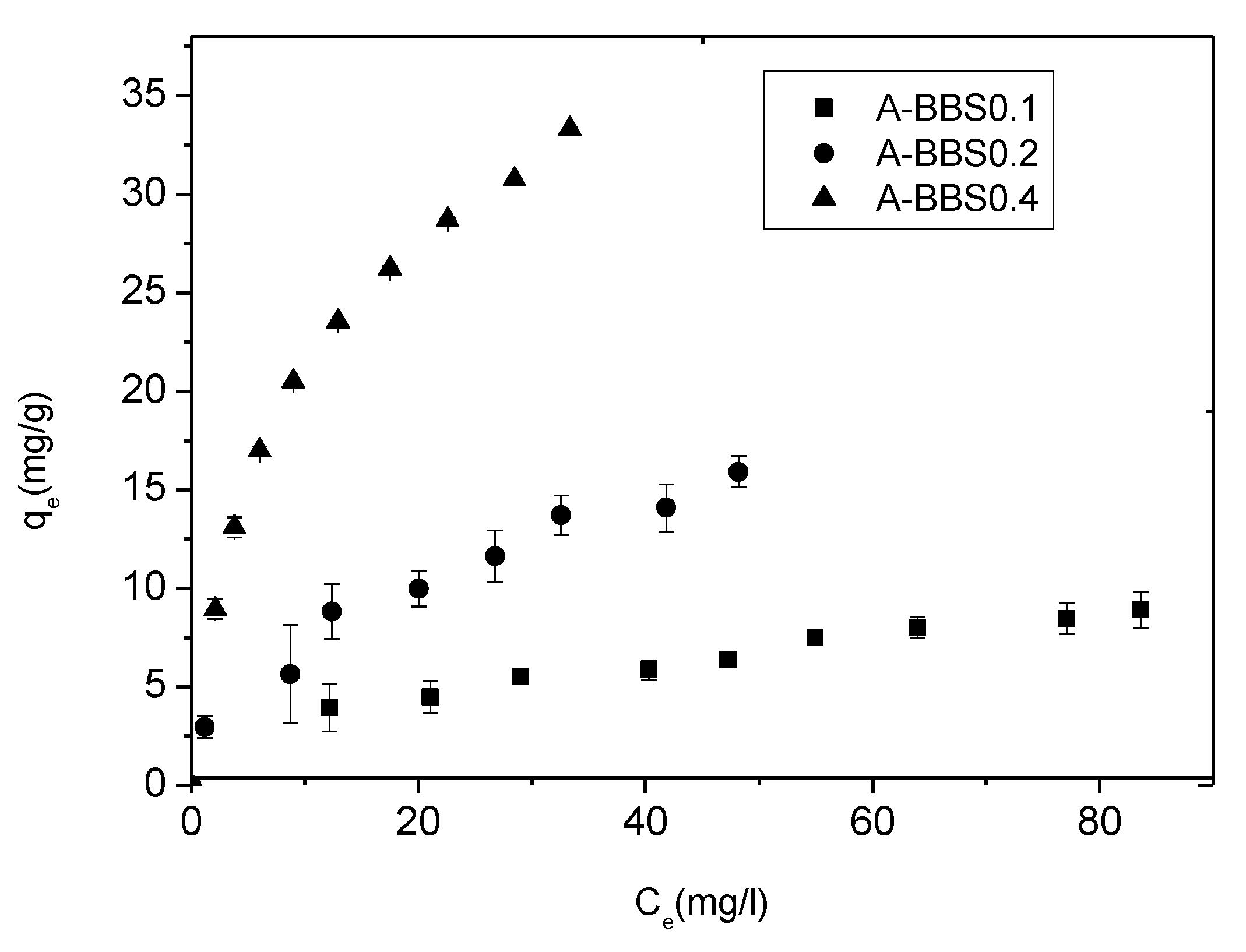
3.2. Removal of Crystal Violet
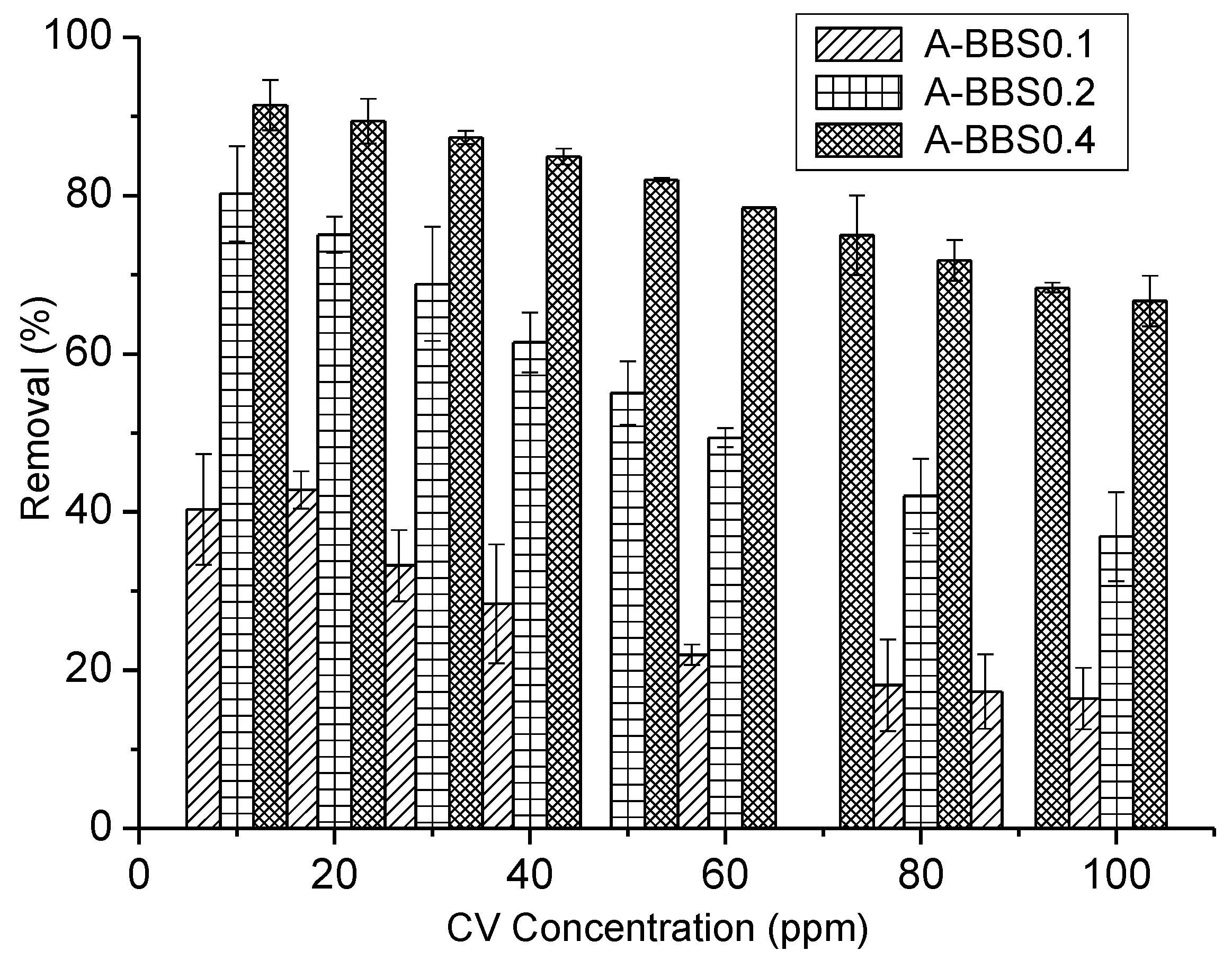
3.2.1. Effect of CV Concentration
3.2.2. Adsorption Isotherms and Model Application
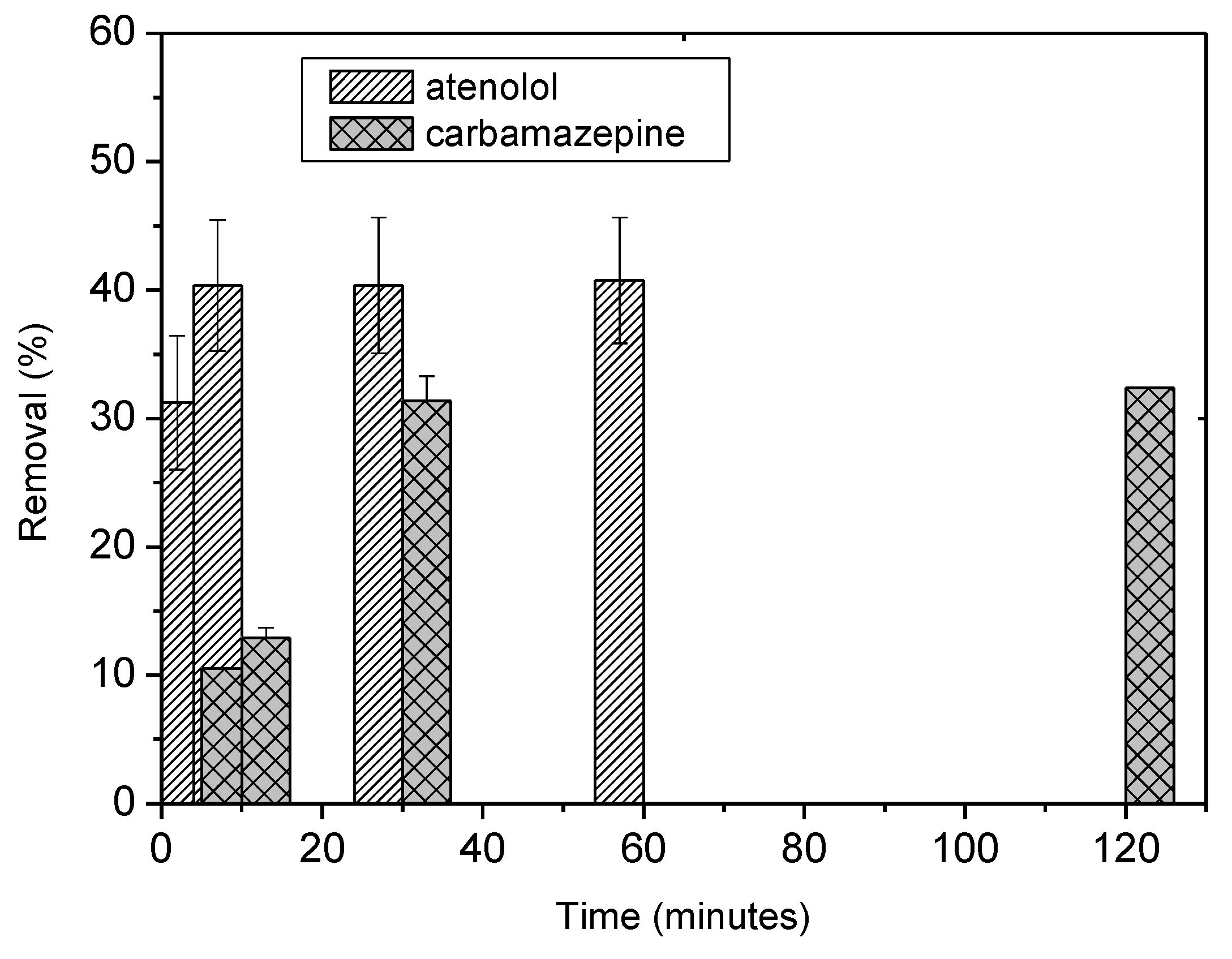
4. Removal of CECs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, R.; Shen, B.; Li, C.; Zheng, C.; Hou, X. Integrating photochemical vapor generation with photo-oxidation trapping for effective mercury removal from polluted water and its on-line monitoring. Microchem. J. 2016, 129, 98–103. [Google Scholar] [CrossRef]
- Farsi, A.; Malvache, C.; De Bartolis, O.; Magnacca, G.; Kristensen, P.K.; Christensen, M.L.; Boffa, V. Design and fabrication of silica-based nanofiltration membranes for water desalination and detoxification. Microporous Mesoporous Mater. 2017, 237, 117–126. [Google Scholar] [CrossRef]
- Calza, P.; Zacchigna, D.; Laurenti, E. Degradation of orange dyes and carbamazepine by soybean peroxidase immobilized on silica monoliths and titanium dioxide. Environ. Sci. Pollut. 2016, 23, 23742–23749. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, C.; Chen, S.; Mei, R.; Wang, X.; Cao, J.; Ma, L.; Zhou, B.; Wei, Q.; Ouyang, G.; et al. Chemosphere Ultrasound enhanced electrochemical oxidation of Alizarin Red S on boron doped diamond (BDD) anode: Effect of degradation process parameters. Chemosphere 2018, 209, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Gautam, R.K.; Banerjee, S.; Rawat, V.; Chattopadhyaya, M.; Chattoapadhyaya, M. Synthesis and characterization of a novel SnFe2O4 @activated carbon magnetic nanocomposite and its effectiveness in the removal of crystal violet from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 2281–2291. [Google Scholar] [CrossRef]
- Li, S. Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly(acrylic acid-acrylamide-methacrylate) and amylose. Bioresour. Technol. 2010, 101, 2197–2202. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.; Motlagh, M.M.; Salahshour, S. Synthesis of ZnO/CuO nanocomposite immobilized on γ-Al2O3 and application for removal of methyl orange. Appl. Surf. Sci. 2016, 384, 237–243. [Google Scholar] [CrossRef]
- Brião, G.V.; Jahn, S.L.; Foletto, E.L.; Dotto, G.L. Adsorption of crystal violet dye onto a mesoporous ZSM-5 zeolite synthetized using chitin as template. J. Colloid Interface Sci. 2017, 508, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Lee, D.S. Effect of ZnO nanoparticles on biodegradation and biotransformation of co-substrate and sulphonated azo dye in anaerobic biological sulfate reduction processes. Int. Biodeterior. Biodegrad. 2016, 109, 150–156. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Xu, M.; Zhu, C.; Fang, W.; Wei, Y. Electrocatalytic degradation of methylene blue on Co doped Ti/TiO2 nanotube/PbO2 anodes prepared by pulse electrodeposition. J. Electroanal. Chem. 2015, 759, 158–166. [Google Scholar] [CrossRef]
- Muthuraman, G.; Teng, T.T.; Leh, C.P.; Norli, I. Extraction and recovery of methylene blue from industrial wastewater using benzoic acid as an extractant. J. Hazard. Mater. 2009, 163, 363–369. [Google Scholar] [CrossRef]
- Aguilar, Z.G.; Brillas, E.; Salazar, M.; Nava, J.L.; Sirés, I.; Sadornil, I.S. Evidence of Fenton-like reaction with active chlorine during the electrocatalytic oxidation of Acid Yellow 36 azo dye with Ir-Sn-Sb oxide anode in the presence of iron ion. Appl. Catal. B Environ. 2017, 206, 44–52. [Google Scholar] [CrossRef]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalin. Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef]
- Marković, D.D.; Lekić, B.M.; Rajaković-Ognjanović, V.N.; Onjia, A.E.; Rajaković, L.V. A New Approach in Regression Analysis for Modeling Adsorption Isotherms. Sci. World J. 2014, 2014, 930879. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef]
- Nisticò, R.; Magnacca, G.; Jadhav, S.A.; Scalarone, D.; Sidorenko, A.S. Polystyrene-block-poly(ethylene oxide) copolymers as templates for stacked, spherical large-mesopore silica coatings: Dependence of silica pore size on the PS/PEO ratio. Beilstein J. Nanotechnol. 2016, 7, 1454–1460. [Google Scholar] [CrossRef]
- Molinari, A.; Magnacca, G.; Papazzoni, G.; Maldotti, A. HydrophobicW10O324−/silica photocatalyst for toluene oxidation in water system. Appl. Catal. B Environ. 2013, 138, 446–452. [Google Scholar] [CrossRef]
- Ghaemi, N.; Daraei, P. Enhancement in copper ion removal by PPy@Al2O3 polymeric nanocomposite membrane. J. Ind. Eng. Chem. 2016, 40, 26–33. [Google Scholar] [CrossRef]
- Ghanizadeh, S.; Bao, X.; Vaidhyanathan, B.; Binner, J. Synthesis of nano α-alumina powders using hydrothermal and precipitation routes: A comparative study. Ceram. Int. 2014, 40, 1311–1319. [Google Scholar] [CrossRef]
- Deravanesiyan, M.; Beheshti, M.; Malekpour, A. Alumina nanoparticles immobilization onto the NaX zeolite and the removal of Cr (III) and Co (II) ions from aqueous solutions. J. Ind. Eng. Chem. 2015, 21, 580–586. [Google Scholar] [CrossRef]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Nisticò, R.; Barrasso, M.; Le Roux, G.A.C.; Seckler, M.M.; Sousa, W.; Malandrino, M.; Magnacca, G.; Le Roux, P.G.A.C. Biopolymers from Composted Biowaste as Stabilizers for the Synthesis of Spherical and Homogeneously Sized Silver Nanoparticles for Textile Applications on Natural Fibers. ChemPhysChem 2015, 16, 3902–3909. [Google Scholar] [CrossRef]
- Tummino, M.L.; Agostini, S.; Avetta, P.; Magnacca, G.; Prevot, A.B.; Testa, M.L.; Deganello, F.; Montoneri, E. Synthesis, characterization and environmental application of silica grafted photoactive substances isolated from urban biowaste. RSC Adv. 2015, 5, 47920–47927. [Google Scholar]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. 2 Surfactants and their applications. Annu. Rep. Sect. C (Phys. Chem.) 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Magnacca, G.; Laurenti, E.; Vigna, E.; Franzoso, F.; Tomasso, L.; Montoneri, E.; Boffa, V. Refuse derived bio-organics and immobilized soybean peroxidase for green chemical technology. Process. Biochem. 2012, 47, 2025–2031. [Google Scholar] [CrossRef]
- Avetta, P.; Prevot, A.B.; Montoneri, E.; Bella, F.; Laurenti, E.; Arques, A.; Carlos, L. Waste Cleaning Waste: Photodegradation of Monochlorophenols in the Presence of Waste-Derived Photosensitizer. ACS Sustain. Chem. Eng. 2013, 1, 1545–1550. [Google Scholar] [CrossRef]
- Musmarra, D.; Prisciandaro, M.; Capocelli, M.; Karatza, D.; Iovino, P.; Canzano, S.; Lancia, A. Ultrasonics Sonochemistry Degradation of ibuprofen by hydrodynamic cavitation: Reaction pathways and effect of operational parameters. Ultrason. Sonochem. 2016, 29, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, T.; Bertolini, T.C.R.; Izidoro, J.C.; Magdalena, C.P.; Fungaro, D.A. Adsorption of Crystal Violet Dye from Aqueous Solution onto Zeolites from Coal Fly and Bottom Ashes. Orbit. Electron. J. Chem. 2013, 5, 179–191. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Das, P. Optimization of crystal violet dye removal using novel soil-silver nanocomposite as nanoadsorbent using response surface methodology. J. Environ. Chem. Eng. 2014, 2, 708–714. [Google Scholar] [CrossRef]
- Porkodi, K.; Kumar, K.V. Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: Eosin yellow, malachite green and crystal violet single component systems. J. Hazard. Mater. 2007, 143, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R. Studies on Adsorption of Crystal Violet Dye from Aqueous Solution onto Coniferous Pinus Bark Powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Pan, S.; Saha, S. Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chem. Eng. J. 2013, 217, 426–434. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Subburaam, C. Liquid phase adsorption of Crystal violet onto activated carbons derived from male flowers of coconut tree. J. Hazard. Mater. 2006, 136, 800–808. [Google Scholar] [CrossRef]
- Hamidzadeh, S.; Torabbeigi, M.; Shahtaheri, S.J. Removal of crystal violet from water by magnetically modified activated carbon and nanomagnetic iron oxide. J. Environ. Health Sci. Eng. 2015, 13, 8. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Eren, E. Investigation of a basic dye removal from aqueous solution onto chemically modified Unye bentonite. J. Hazard. Mater. 2009, 166, 88–93. [Google Scholar] [CrossRef]
- Lee, C.-K.; Liu, S.-S.; Juang, L.-C.; Wang, C.-C.; Lin, K.-S.; Lyu, M.-D. Application of MCM-41 for dyes removal from wastewater. J. Hazard. Mater. 2007, 147, 997–1005. [Google Scholar] [CrossRef]
- Chakraborty, S.; De, S.; Dasgupta, S.; Basu, J.K. Adsorption study for the removal of a basic dye: Experimental and modeling. Chemosphere 2005, 58, 1079–1086. [Google Scholar] [CrossRef]
- Hemmati, F.; Norouzbeigi, R.; Sarbisheh, F.; Shayesteh, H. Malachite green removal using modified sphagnum peat moss as a low-cost biosorbent: Kinetic, equilibrium and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 58, 482–489. [Google Scholar] [CrossRef]
- Liao, M.H.; Wu, K.Y.; Chen, D.H. Fast adsorption of crystal violet on polyacrylic acid-bound magnetic nanoparticles. Sep. Sci. Technol. 2004, 39, 1563–1575. [Google Scholar] [CrossRef]
- Sarma, G.K.; Gupta, S.S.; Bhattacharyya, K.G. Adsorption of Crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J. Environ. Manag. 2016, 171, 1–10. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmad, R. Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 2011, 265, 112–118. [Google Scholar] [CrossRef]
- Qiao, H.; Zhou, Y.; Yu, F.; Wang, E.; Min, Y.; Huang, Q.; Pang, L.; Ma, T. Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 2015, 141, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, S.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Mango stone biocomposite preparation and application for crystal violet adsorption: A mechanistic study. Microporous Mesoporous Mater. 2017, 239, 180–189. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Adsorption of crystal violet onto functionalised multi-walled carbon nanotubes: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, X.; Chen, X. Synthesis and utilization of a novel carbon nanotubes supported nanocables for the adsorption of dyes from aqueous solutions. J. Solid State Chem. 2015, 229, 342–349. [Google Scholar] [CrossRef]
- Anirudhan, T.; Suchithra, P.; Radhakrishnan, P. Synthesis and characterization of humic acid immobilized-polymer/bentonite composites and their ability to adsorb basic dyes from aqueous solutions. Appl. Clay Sci. 2009, 43, 336–342. [Google Scholar] [CrossRef]
- Alhendawi, H.M.H.; Brunet, E.; Payán, E.R.; Juanes, O.; Ubis, J.C.R.; Al-Asqalany, M. Surfactant-assisted intercalation of crystal violet in layered γ-zirconium phosphate. Dye uptake from aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 387–396. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Min, Y.; Ma, T.; Zhang, M.; Huang, Q.; Niu, J. Removal of Crystal Violet by a Novel Cellulose-Based Adsorbent: Comparison with Native Cellulose. Ind. Eng. Chem. 2014, 53, 5498–5506. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Bagtash, M.; Shariatmanesh, T. Simultaneous removal of binary mixture of Brilliant Green and Crystal Violet using derivative spectrophotometric determination, multivariate optimization and adsorption characterization of dyes on surfactant modified nano-γ-alumina. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1016–1028. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Removal of triphenylmethane dyes by calcium carbonate–lentinan hierarchical mesoporous hybrid materials. Chem. Eng. J. 2015, 273, 371–380. [Google Scholar] [CrossRef]
- Montoneri, E.; Boffa, V.; Savarino, P.; Perrone, D.G.; Musso, G.; Mendichi, R.; Chierotti, M.R.; Gobetto, R. Biosurfactants from Urban Green Waste. ChemSusChem 2009, 2, 239–247. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- García, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Franco, A.M.M.; Luque, R. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron-Benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef]
- Esfandian, H.; Samadi-Maybodi, A.; Parvini, M.; Khoshandam, B. Development of a novel method for the removal of diazinon pesticide from aqueous solution and modeling by artificial neural networks (ANN). J. Ind. Eng. Chem. 2015, 35, 295–308. [Google Scholar] [CrossRef]
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Prevot, A.B.; Magnacca, G.; Carlos, L.; Mártire, D.O. Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Padovano, E.; Giancotti, V.; Baiocchi, C. Identification of the unknown transformation products derived from clarithromycin and carbamazepine using liquid chromatography/high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1687–1704. [Google Scholar] [CrossRef]
- Punyapalakul, P.; Sitthisorn, T. Removal of ciprofloxazin and carbamazepine by adsorption on functionalized mesoporous silicates. World Acad. Sci. Eng. Technol. 2010, 45, 546–550. [Google Scholar]
- Akpinar, I.; Yazaydin, A.O. Rapid and Efficient Removal of Carbamazepine from Water by UiO-67. Ind. Eng. Chem. Res. 2017, 56, 15122–15130. [Google Scholar] [CrossRef]
- Park, J.; Hwa, K.; Lee, E.; Lee, S.; Cho, J. Sorption of pharmaceuticals to soil organic matter in a constructed wetland by electrostatic interaction. Sci. Total Environ. 2018, 635, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Calisto, V.; Esteves, V.I. Adsorption of the antiepileptic carbamazepine onto agricultural soils. J. Environ. Monit. 2012, 14, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Mallek, M.; Chtourou, M.; Portillo, M.; Monclús, H.; Walha, K.; Ben Salah, A.; Salvado, V. Granulated cork as biosorbent for the removal of phenol derivatives and emerging contaminants. J. Environ. Manag. 2018, 223, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of atenolol by adsorption—Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Koltsakidou, A.; Nanaki, S.G.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of beta-blockers from aqueous media by adsorption onto graphene oxide. Sci. Total Environ. 2015, 537, 411–420. [Google Scholar] [CrossRef]
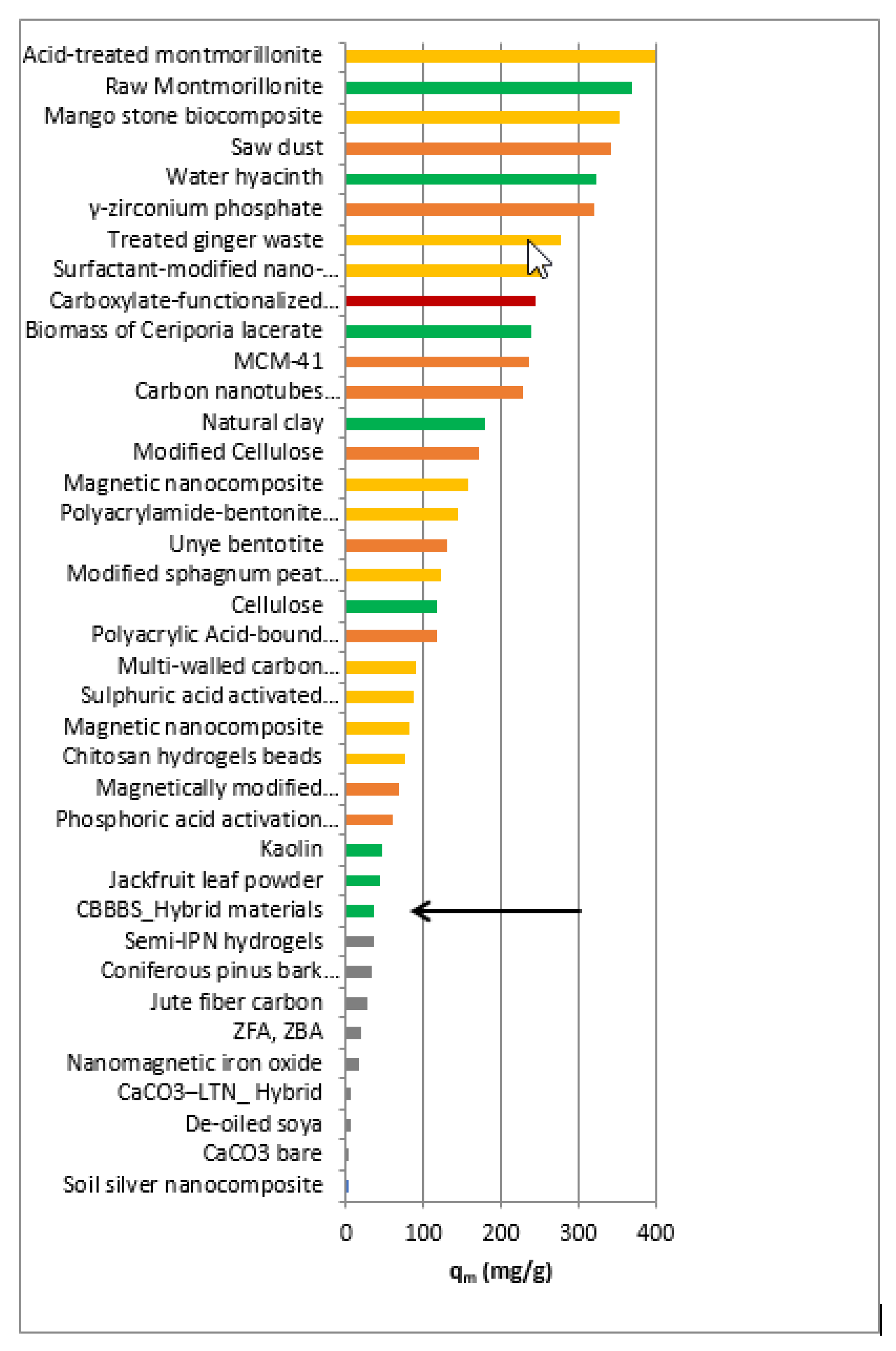
| Adsorbents | qm (Max Capacity) (mg/g) | Ref. | Temperature °C | pH |
|---|---|---|---|---|
| Zeolite from fly ash | 19.6 | [29] | 25 | 5 |
| Acid treated zeolite | 17.7 | [29] | 25 | 5 |
| Magnetic nanocomposite | 158.73 | [6] | 50 | 6.5 |
| Soil silver nanocomposite | 1.923 | [30] | 30 | 4.68 |
| Jute fiber carbon | 27.99 | [31] | 30 | 9 |
| Semi-IPN hydrogels | 35.09 | [7] | 25 | 7.4 |
| Coniferous pinus bark powder (CPBP) | 32.78 | [32] | 30 | 8 |
| Chitosan hydrogels beads | 76.9 | [33] | 30 | 7 |
| Phosphoric acid activation carbon (PAAC) | 60.42 | [34] | 28 | 6 |
| Sulphuric acid activated carbon (SAAC) | 85.84 | [34] | 28 | 6.04 |
| Magnetically modified activated carbon | 67.1 | [35] | 20 | 9 |
| Nanomagnetic iron oxide | 16.5 | [35] | 20–40 | 9 |
| Magnetic nanocomposite | 81.7 | [36] | (10–50) | 8.5 |
| Unye bentonite | 131 | [37] | 22 | 6.5 |
| MCM-41 | 236.64 | [38] | 25 | 4 |
| Saw dust | 341 | [39] | (15–50) | neutral pH |
| Modified sphagnum peat moss | 121.95 | [40] | 20 | 6.5 |
| Polyacrylic Acid-bound magnetic nano particles | 116 | [41] | 25 | 6 |
| Acid-treated montmorillonite | 400.0 | [42] | 30 | 5.9 |
| Treated ginger waste | 277.7 | [43] | (30–50) | 6.2 |
| Carboxylate-functionalized cellulose nanocrystals | 243.9 | [44] | 30 | 6 |
| Mango stone biocomposite | 352.79 | [45] | 33 | 8 |
| Multi-walled carbon nanotubes | 90.52 | [46] | 25 | 6–8 |
| Carbon nanotubes supported nanocables | 228.30 | [47] | (25–45) | 9 |
| Polyacrylamide-bentonite composite | 144.60 | [48] | 30 | 6 |
| γ-zirconium phosphate | 320.20* | [49] | 25 | 9 |
| Modified Cellulose | 169.9–218.8 | [50] | 50 | 9 |
| Surfactant-modified nano-alumina | 254.3 | [51] | 25 | 4 |
| CaCO3–LTN Hybrids | 6.34 * | [52] | 25 | 6 |
| CaCO3 bare | 3.35 | [52] | 25 | 6 |
| De-oiled soya | 5 | [16] | 30 | 8 |
| Materials | BET Specific Surface Area (m2/g) | BJH Pore Volume (cm3/g) |
|---|---|---|
| A | 186 | 0.42 |
| A-BBS0.1 | 181 | 0.33 |
| A-BBS0.2 | 172 | 0.30 |
| A-BBS0.4 | 165 | 0.29 |
| Materials | 40–220 °C (Adsorbed Water %) | 220–650 °C (Organic Content and OH Groups Eliminated %) | OH Groups Eliminated (%) | Measured Organic Content (%) ± 0.1 |
|---|---|---|---|---|
| A | 2.8 | - | 1.1 | - |
| A-BBS0.1 | 4.4 | 2.7 | 1.1 | 1.6 |
| A-BBS0.2 | 4.9 | 5.4 | 1.1 | 4.3 |
| A-BBS0.4 | 6.5 | 8.2 | 1.1 | 7.1 |
| Model | A-BBS0.1 | A-BBS0.2 | A-BBS0.4 | ||||
|---|---|---|---|---|---|---|---|
| Value | S.E. | Value | S.E. | Value | S.E | ||
| Langmuir | qm (mg/g) | 8.92 | 0.59 | 21.38 | 2.50 | 35.09 | 0.61 |
| KL (mg/L) | 0.065 | 0.020 | 0.087 | 0.048 | 0.09 | 0.090 | |
| r2 | 0.975 | - | 0.977 | - | 0.992 | - | |
| RL | 0.898 | 0.861 | 0.763 | ||||
| Freundlich | KF (mg/g) (L/mg) | 14.08 | 5.58 | 28.547 | 6.75 | 67.54 | 8.455 |
| n | 2.52 | 0.65 | 2.170 | 0.203 | 2.084 | 0.164 | |
| r2 | 0.995 | - | 0.94 | - | 0.978 | - |
| Model | Carbamazepine | Atenolol | |||
|---|---|---|---|---|---|
| Value | S.E. | Value | S.E. | ||
| Langmuir | qm (mg/g) | 3.0628 | 0.06 | 3.26 | 0.145 |
| KL (mg/L) | 0.119 | 0.057 | 0.11 | 0.008 | |
| r2 | 0.917 | - | 0.904 | - | |
| Freundlich | KF (mg/g) (L/mg) | 0.405 | 0.07 | 4.43 | 0.13 |
| n | 1.526 | 0.35 | 0.77 | 0.006 | |
| r2 | 0.905 | - | 0.906 | - |
| Adsorbents | qm (mg/g) | Ref. | CEC | Temperature (°C) | pH |
|---|---|---|---|---|---|
| A-BBS0.4 | 3.26 3.06 | This study | Atenolol Carbamazepine | 20 | 6.5 |
| Agricultural soils | 0.01 | [62] | Carbamazepine | 25 | 9.2 |
| Functionalized silica | 0.04 | [63] | Carbamazepine | 25 | 7 |
| Granulated cork | 1.84 | [64] | Carbamazepine | 20±2 | 4.6 |
| Activated carbon | 18.8 | [65] | Atenolol | 25 | 6 |
| Graphene oxide | 95 | [66] | Atenolol | 25 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadraei, R.; Paganini, M.C.; Calza, P.; Magnacca, G. An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants. Nanomaterials 2019, 9, 731. https://doi.org/10.3390/nano9050731
Sadraei R, Paganini MC, Calza P, Magnacca G. An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants. Nanomaterials. 2019; 9(5):731. https://doi.org/10.3390/nano9050731
Chicago/Turabian StyleSadraei, Razieh, Maria Cristina Paganini, Paola Calza, and Giuliana Magnacca. 2019. "An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants" Nanomaterials 9, no. 5: 731. https://doi.org/10.3390/nano9050731
APA StyleSadraei, R., Paganini, M. C., Calza, P., & Magnacca, G. (2019). An Easy Synthesis for Preparing Bio-Based Hybrid Adsorbent Useful for Fast Adsorption of Polar Pollutants. Nanomaterials, 9(5), 731. https://doi.org/10.3390/nano9050731