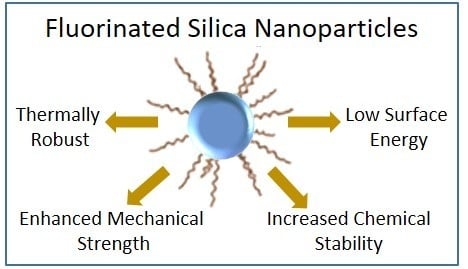
Recent Studies on Fluorinated Silica Nanometer-Sized Particles
Abstract
1. Introduction
2. Synthetic Methods
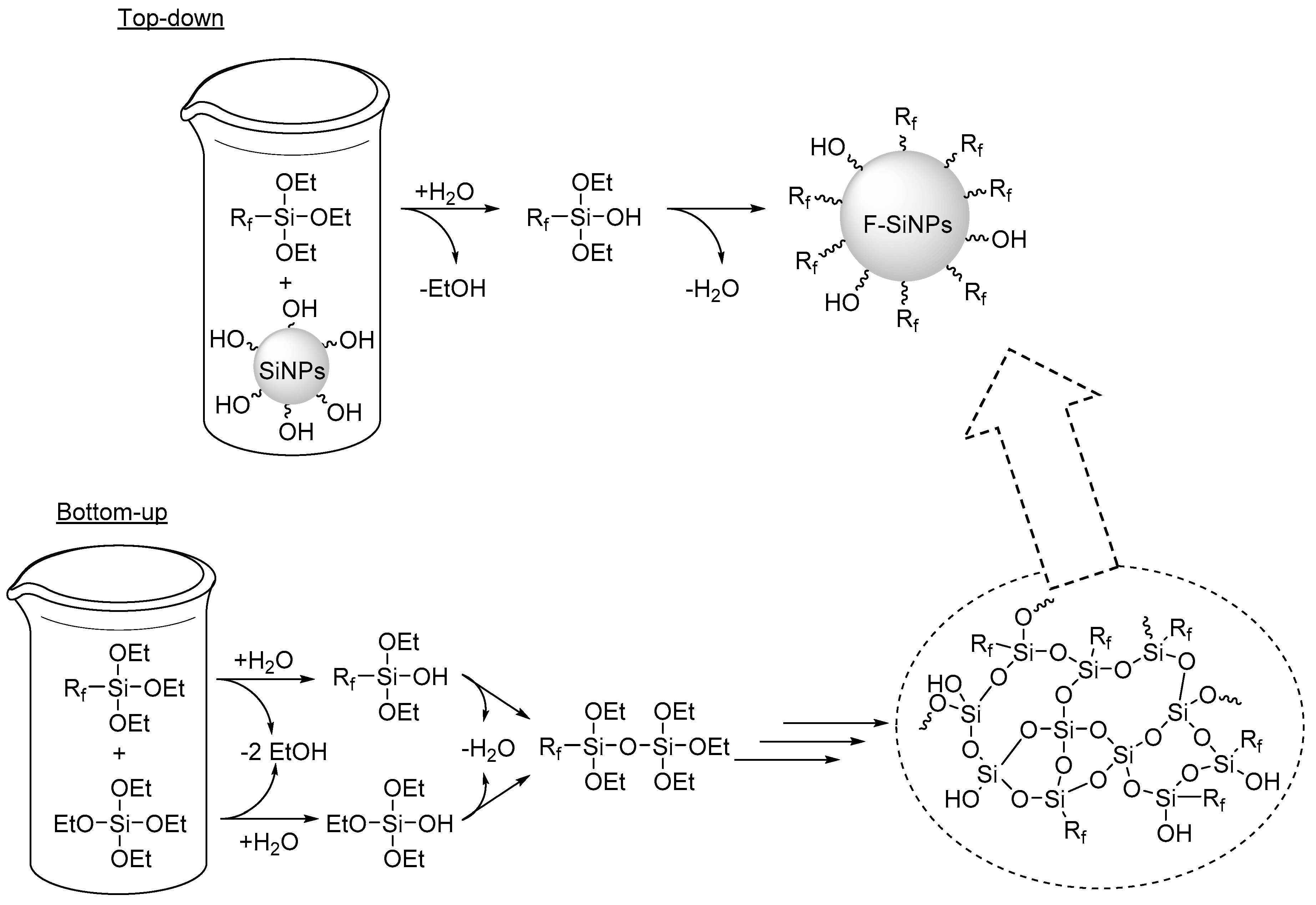
2.1. Sol-gel Methods
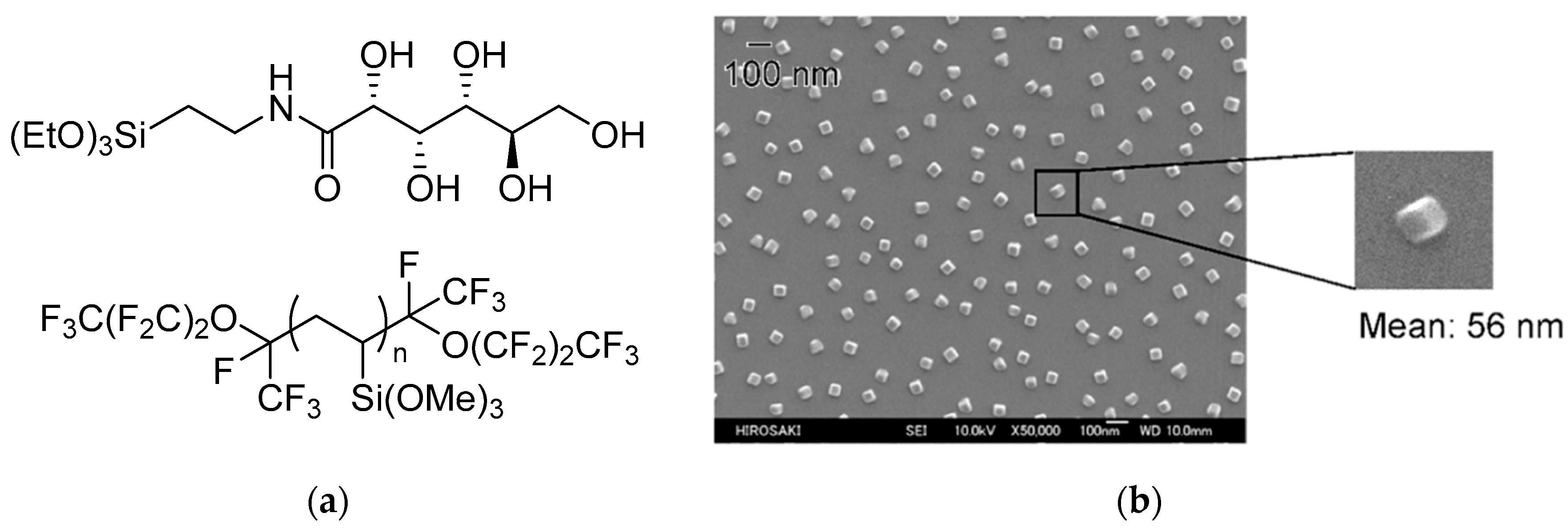
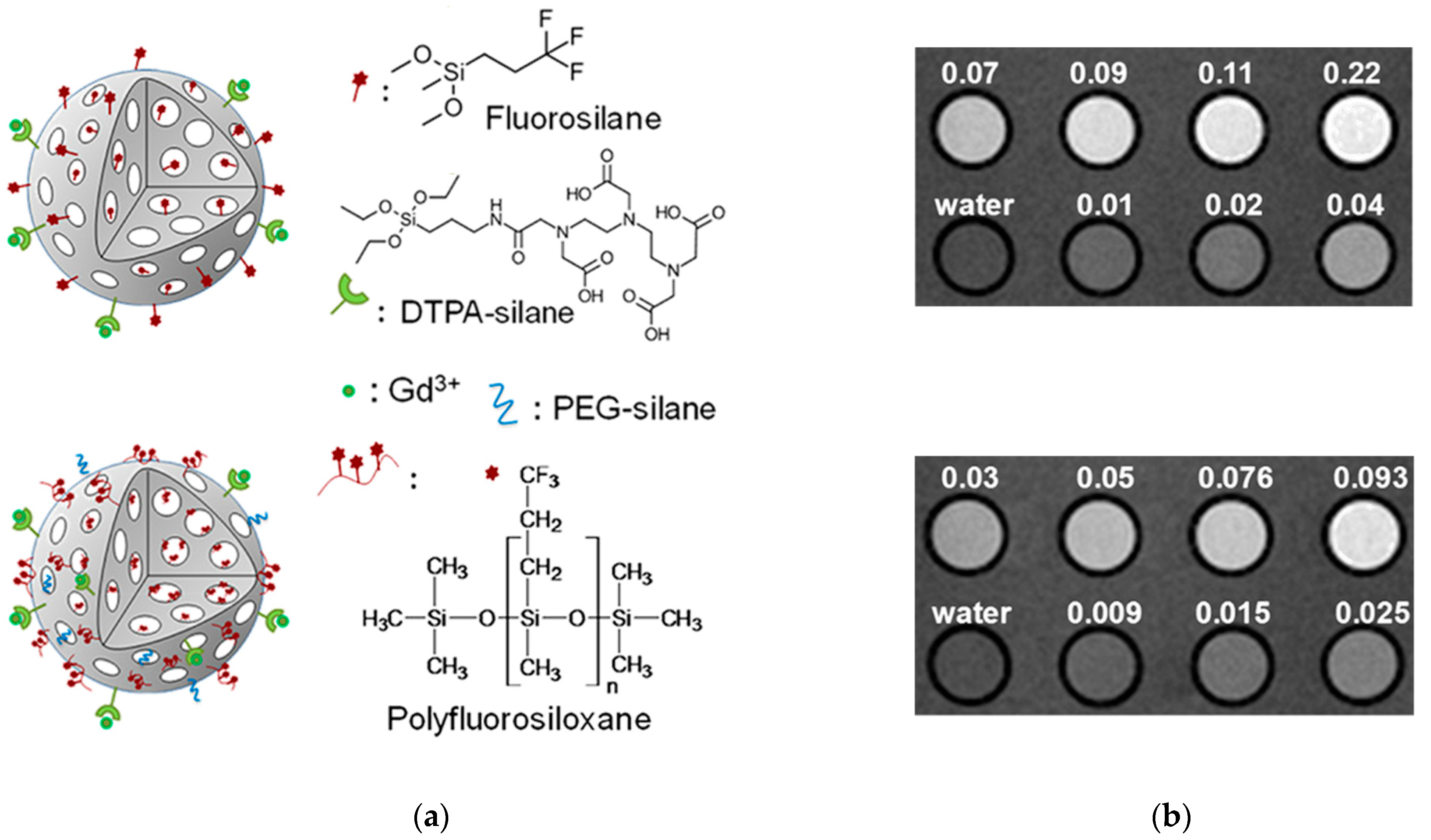
Selected Examples
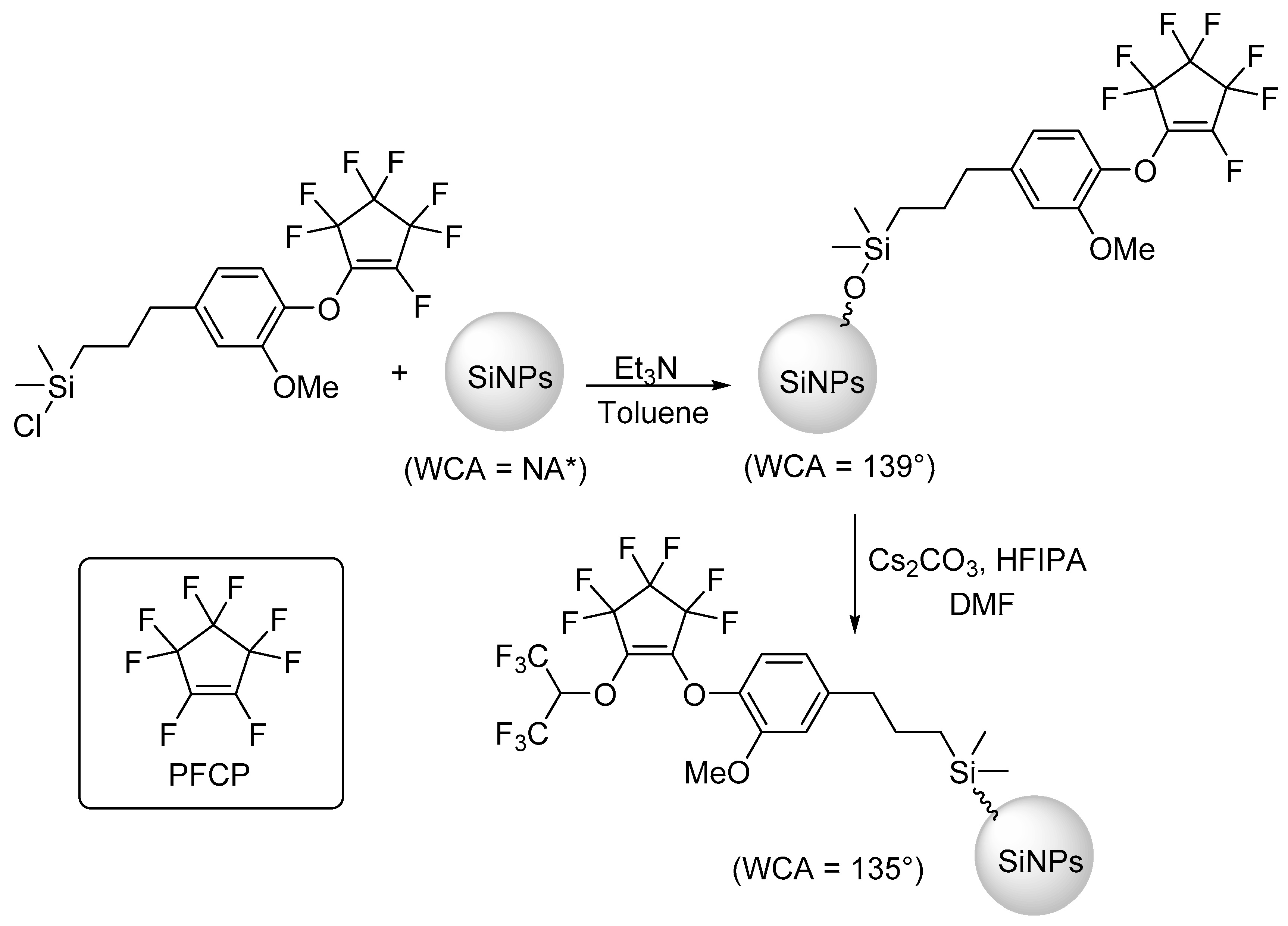
2.2. Additional Synthetic Methods
3. Selected Applications
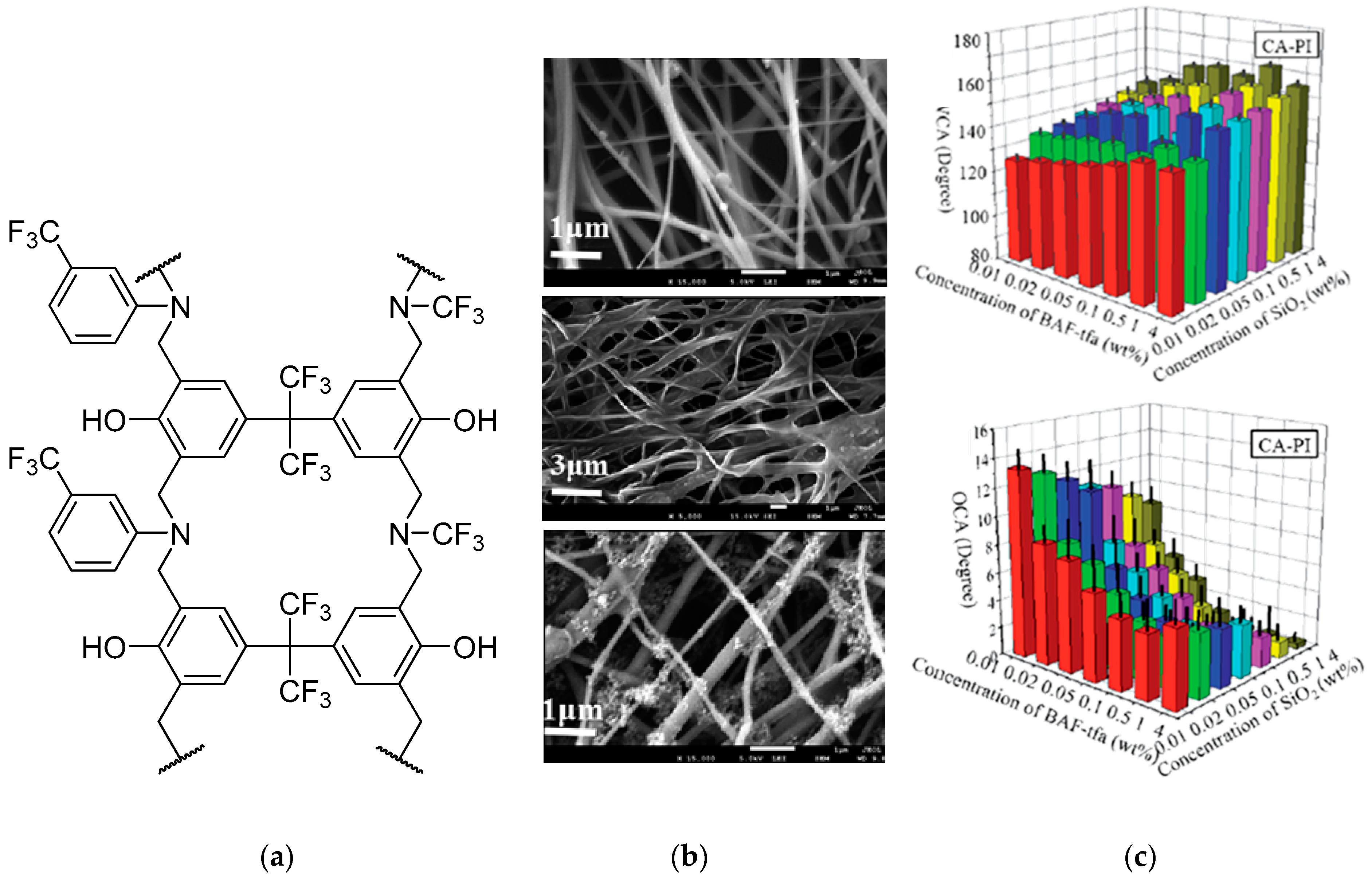
3.1. Advanced Surface Coatings
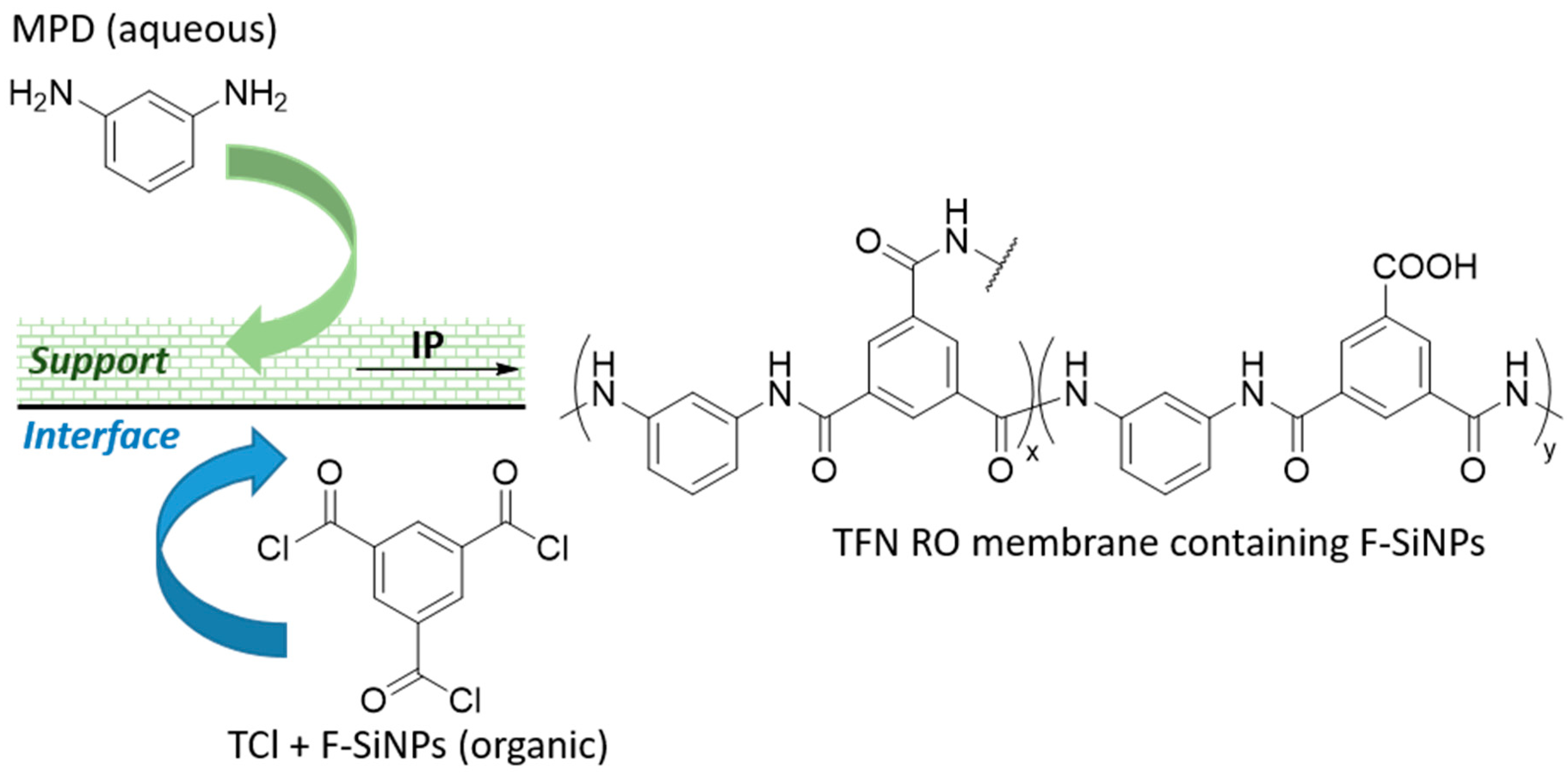
3.2. Membrane Distillation and Separation
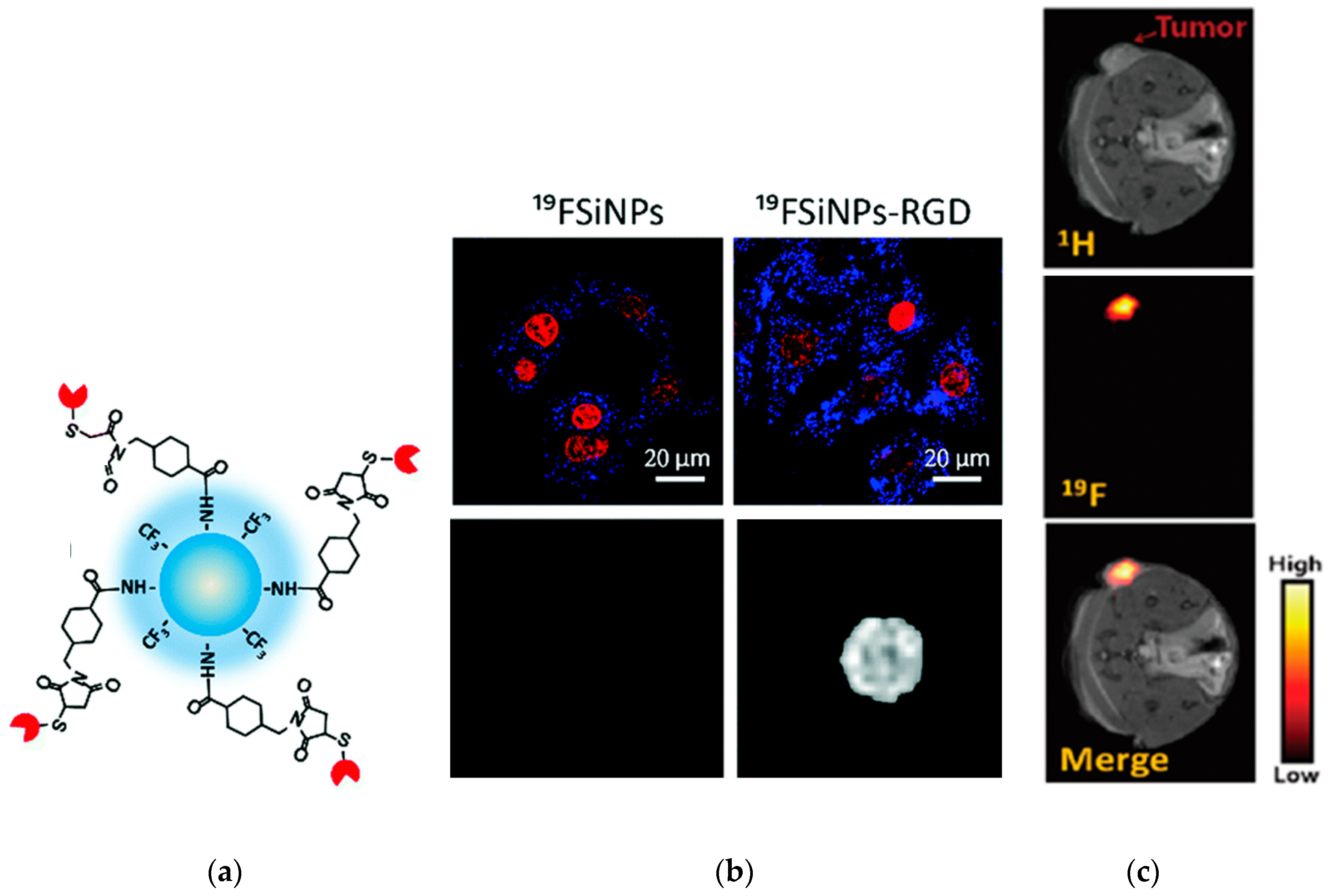
3.3. Biological Probes
4. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Kim, D.N.H.; Kim, C.; Teitell, M.A.; Kim, K.T.; Teitell, M.A.; Zangle, T.A. Soft lithography fabrication of index-matched microfluidic devices for reducing artifacts in fluorescence and quantitative phase imaging. Microfluid Nanofluidics 2018, 22, 2. [Google Scholar] [CrossRef]
- Du, W.; Nyström, A.M.; Zhang, L.; Powell, K.T.; Li, Y.; Cheng, C.; Wickline, S.A.; Wooley, K.L. Amphiphilic Hyperbranched Fluoropolymers as Nanoscopic 19F Magnetic Resonance Imaging Agent Assemblies. Biomacromolecules 2008, 9, 2826–2833. [Google Scholar] [CrossRef]
- Babudri, F.; Farinola, G.M.; Naso, F.; Ragni, R. Fluorinated organic materials for electronic and optoelectronic applications: the role of the fluorine atom. Chem. Commun. 2007, 1003–1022. [Google Scholar] [CrossRef]
- Groult, H.; Tressaud, A. Use of inorganic fluorinated materials in lithium batteries and in energy conversion systems. Chem. Commun. 2018, 54, 11375–11382. [Google Scholar] [CrossRef]
- Martinelli, C.; Cardone, A.; Farinola, G.M.; Pinto, V. Synthetic Aspects and Electro-Optical Properties of Fluorinated Arylenevinylenes for Luminescence and Photovoltaics. Materials 2013, 6, 1205–1236. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.; Griffini, G.; Levi, M.; Turri, S. Novel conductive nanocomposites from perfluoropolyether waterborne polyurethanes and carbon nanotubes. Polym. Adv. Technol. 2014, 25, 1082–1088. [Google Scholar] [CrossRef]
- Sun, J.-P.; Blaskovits, J.T.; Bura, T.; Beaupre, S.; Leclerc, M.; Hill, I.G. Photovoltaic device performance of highly regioregular fluorinated poly(3-hexylthiophene). Org. Electron. 2017, 50, 115–120. [Google Scholar] [CrossRef]
- Yao, C.; Peng, C.; Yang, Y.; Li, L.; Bo, M.; Wang, J. Elucidating the key role of fluorine in improving the charge mobility of electron acceptors for non-fullerene organic solar cells by multiscale simulations. J. Mater. Chem. C 2018, 6, 4912–4918. [Google Scholar] [CrossRef]
- Haranahalli, K.; Honda, T.; Ojima, I. Recent progress in the strategic incorporation of fluorine into medicinally active compounds. J. Fluorine Chem. 2019, 217, 29–40. [Google Scholar] [CrossRef]
- Wang, B.-C.; Wang, L.-J.; Jiang, B.; Wang, S.-Y.; Wu, N.; Li, X.-Q.; Shi, D.-Y. Application of Fluorine in Drug Design During 2010–2015 Years: A Mini-Review. Mini Rev. Med. Chem. 2017, 17, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Riess, J.G. Highly fluorinated systems for oxygen transport, diagnosis and drug delivery. Colloids Surf. A 1994, 84, 33–48. [Google Scholar] [CrossRef]
- El-Maiss, J.; Darmanin, T.; Guittard, F. Branched versus linear perfluorocarbon chains in the formation of superhydrophobic electrodeposited films with low bioaccumulative potential. J. Mater. Sci. 2014, 49, 7760–7769. [Google Scholar] [CrossRef]
- Castner, D.G.; Grainger, D.W. Fluorinated Surfaces, Coatings, and Films; American Chemical Society: Washington, DC, USA, 2001; Volume 787, 260p. [Google Scholar]
- Jennings, G.K.; Escobar, C.A. Partially fluorinated coatings by surface-initiated ring-opening metathesis polymerization. In Functional Polymer Coatings: Principles, Methods, and Applications; Wu, L., Baghdachi, J., Eds.; Wiley: New York, NY, USA, 2015; pp. 239–249. [Google Scholar]
- Wan, Y.; Yu, Y.; Cao, L.; Zhang, M.; Gao, J.; Qi, C. Corrosion and tribological performance of PTFE-coated electroless nickel boron coatings. Surf. Coat. Technol. 2016, 307, 316–323. [Google Scholar] [CrossRef]
- Pauling, L. Nature of the chemical bond. IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 1932, 54, 3570–3582. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Champagne, P.A.; Desroches, J.; Paquin, J.-F. Organic Fluorine as a Hydrogen-Bond Acceptor: Recent Examples and Applications. Synthesis 2015, 47, 306–322. [Google Scholar]
- Grainger, D.W.; Stewart, C.W. Fluorinated Coatings and Films: Motivation and Significance. In Fluorinated Surfaces, Coatings, and Films; American Chemical Society: Washington, DC, USA, 2001; Volume 787, pp. 1–14. [Google Scholar]
- Krick, B.A.; Pitenis, A.A.; Harris, K.L.; Junk, C.P.; Sawyer, W.G.; Brown, S.C.; Rosenfeld, H.D.; Kasprzak, D.J.; Johnson, R.S.; Chan, C.D.; et al. Ultralow wear fluoropolymer composites: Nanoscale functionality from microscale fillers. Tribol. Int. 2016, 95, 245–255. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Bayer, I.S.; Tiwari, M.K.; Megaridis, C.M. Novel Fluoropolymer Blends for the Fabrication of Sprayable Multifunctional Superhydrophobic Nanostructured Composites. Ind. Eng. Chem. Res. 2011, 50, 11117–11123. [Google Scholar] [CrossRef]
- Clark, M.D.; Walker, L.S.; Hadjiev, V.G.; Khabashesku, V.; Corral, E.L.; Krishnamoorti, R. Fast sol-gel preparation of silicon carbide-silicon oxycarbide nanocomposites. J. Am. Ceram. Soc. 2011, 94, 4444–4452. [Google Scholar] [CrossRef]
- Liu, X.; Yue, D.; Yang, C.; Li, N.; Gao, S.; Liu, Y.; Mo, G.; Wu, Z.; Yin, J.; Su, B.; et al. Fluorinated carbon nanofiber/polyimide composites: Electrical, mechanical, and hydrophobic properties. Surf. Coat. Technol. 2019. Ahead of Print. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, M.; Luo, W.; Yu, J.; Ding, B. Thermally induced chemical crosslinking reinforced fluorinated polyurethane/polyacrylonitrile/polyvinyl butyral nanofibers for waterproof-breathable application. RSC Adv. 2016, 6, 29629–29637. [Google Scholar] [CrossRef]
- Banerjee, D.A.; Kessman, A.J.; Cairns, D.R.; Sierros, K.A. Tribology of silica nanoparticle-reinforced, hydrophobic sol-gel composite coatings. Surf. Coat. Technol. 2014, 260, 214–219. [Google Scholar] [CrossRef]
- Corriu, R.J.P. Ceramics and Nanostructures from Molecular Precursors. Angew. Chem. Int. Ed. 2000, 39, 1376–1398. [Google Scholar] [CrossRef]
- Bernardo, E.; Fiocco, L.; Parcianello, G.; Storti, E.; Colombo, P. Advanced Ceramics from Preceramic Polymers Modified at the Nano-Scale: A Review. Materials 2014, 7, 1927. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Dorigato, A.; Dzenis, Y.; Pegoretti, A. Filler aggregation as a reinforcement mechanism in polymer nanocomposites. Mech. Mater. 2013, 61, 79–90. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
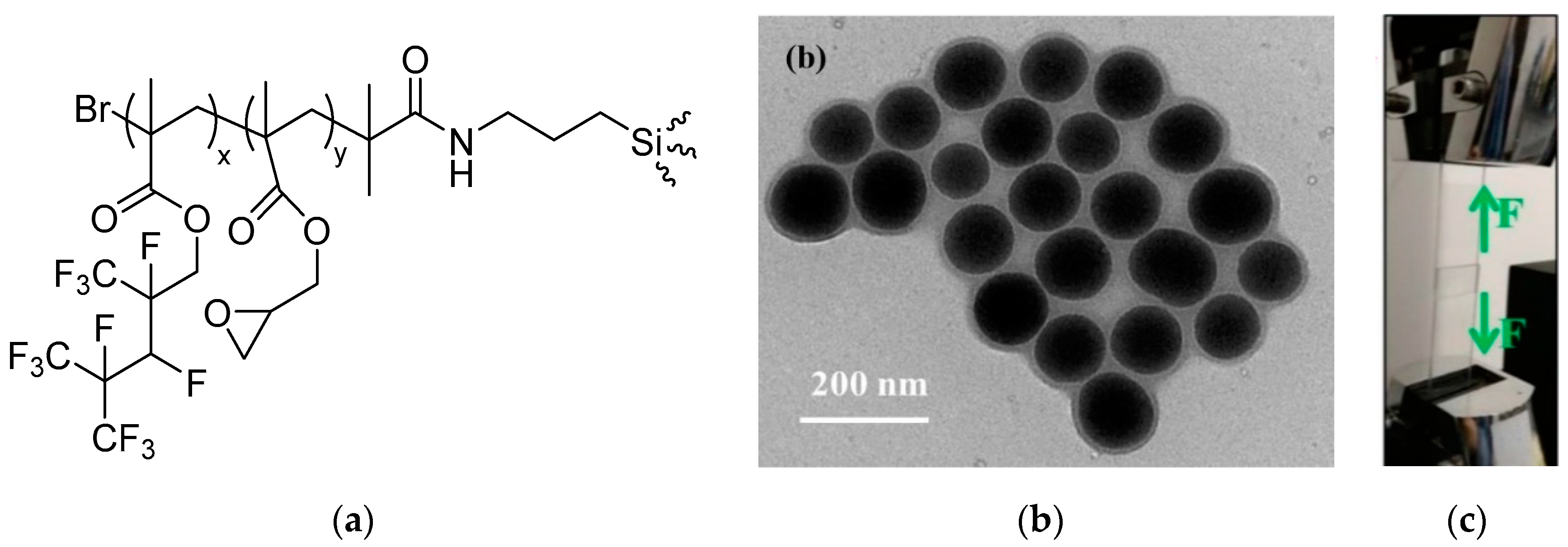
- Jennings, A.R.; Budy, S.M.; Thrasher, C.J.; Iacono, S.T. Synthesis of fluorinated silica nanoparticles containing latent reactive groups for post-synthetic modification and for tunable surface energy. Nanoscale 2016, 8, 16212–16220. [Google Scholar] [CrossRef]
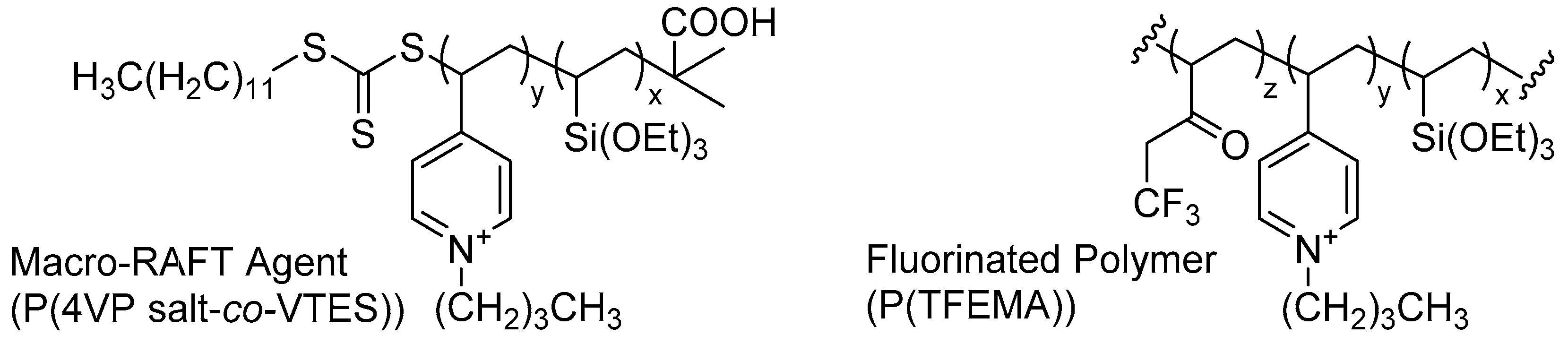
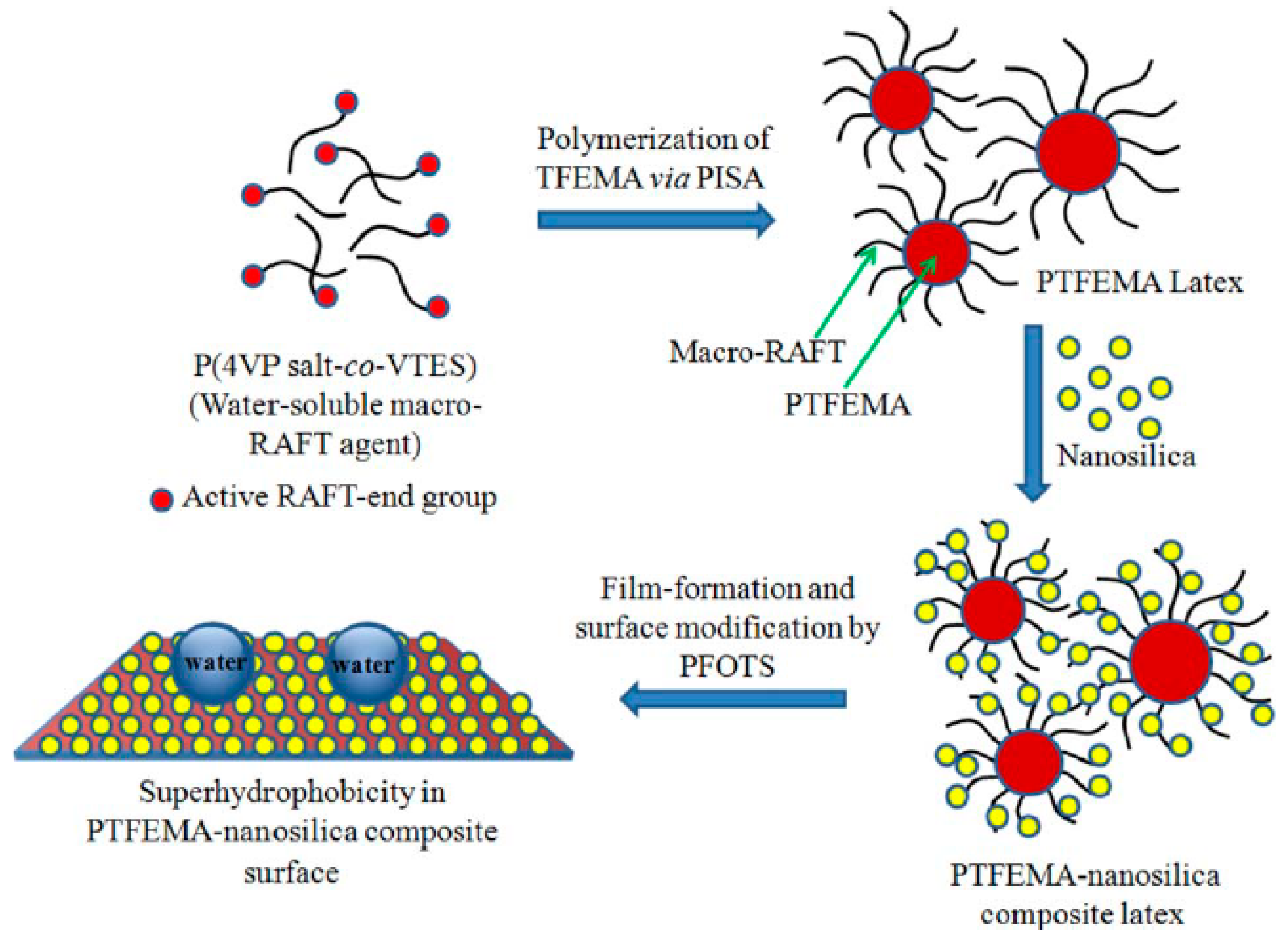
- Chakrabarty, A.; Ponnupandian, S.; Kang, N.-G.; Mays, J.W.; Singha, N.K. Designing superhydrophobic surface based on fluoropolymer–silica nanocomposite via RAFT-mediated polymerization-induced self-assembly. J. Polym. Sci. Part A 2018, 56, 266–275. [Google Scholar] [CrossRef]
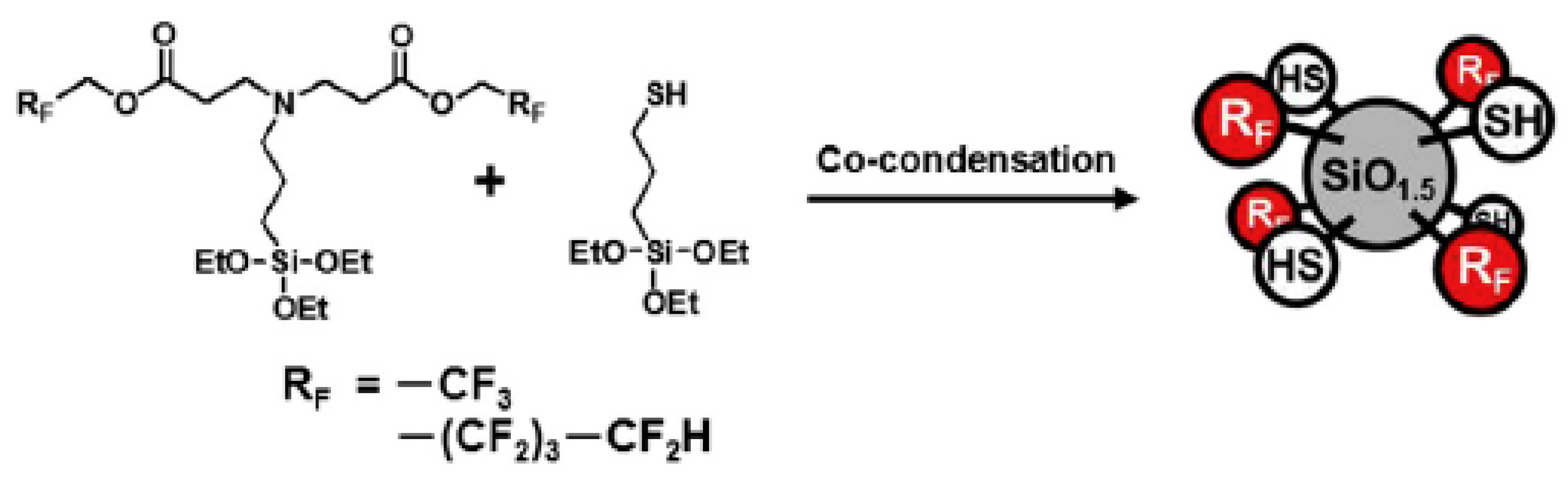
- Kimura, T.; Sobu, S.; Nakabayashi, K.; Mori, H. Dual-functional fluorinated-thiolated silsesquioxane nanoparticles for UV nanoimprinting via thiol-ene chemistry. Polymer 2017, 122, 60–70. [Google Scholar] [CrossRef]
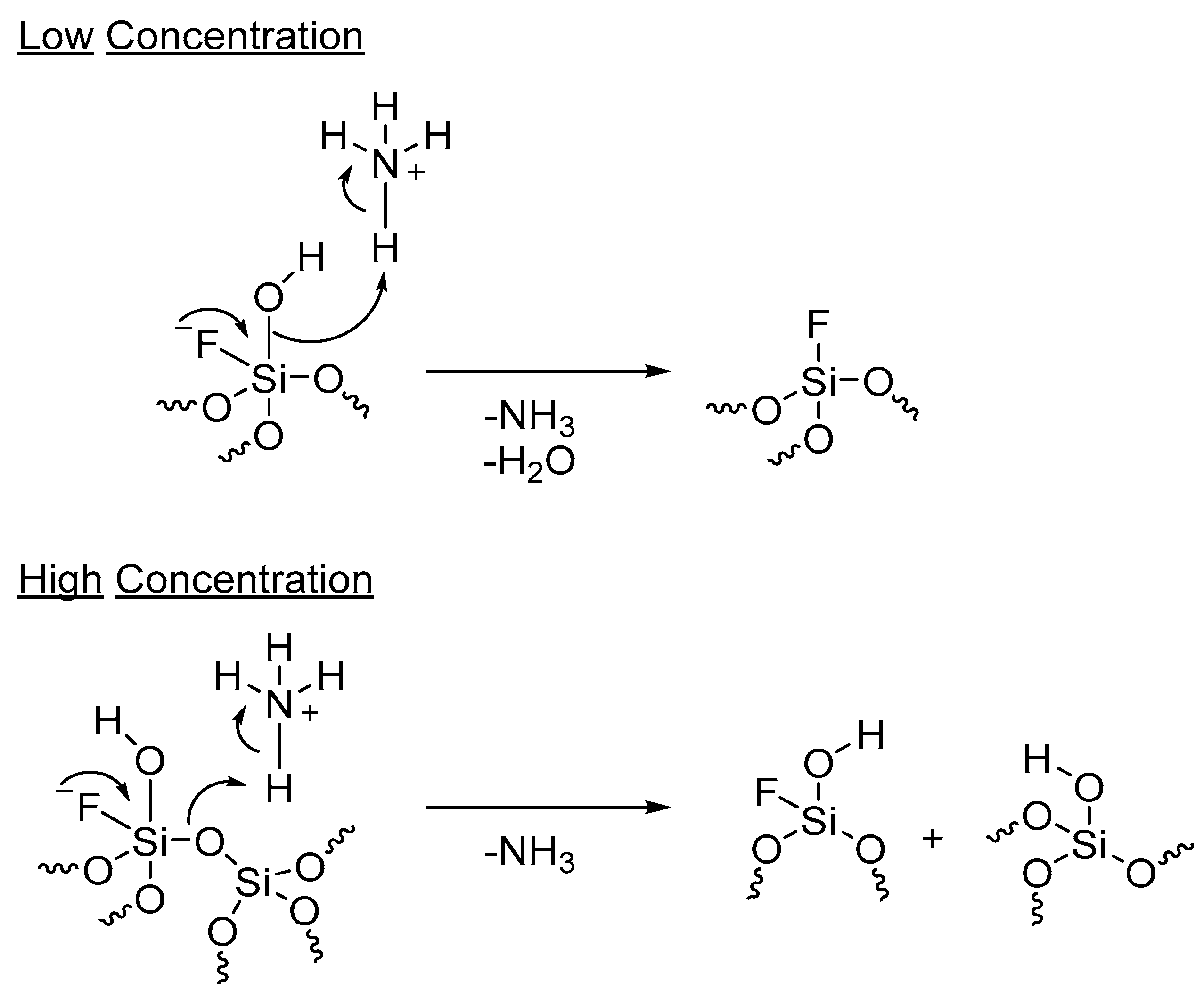
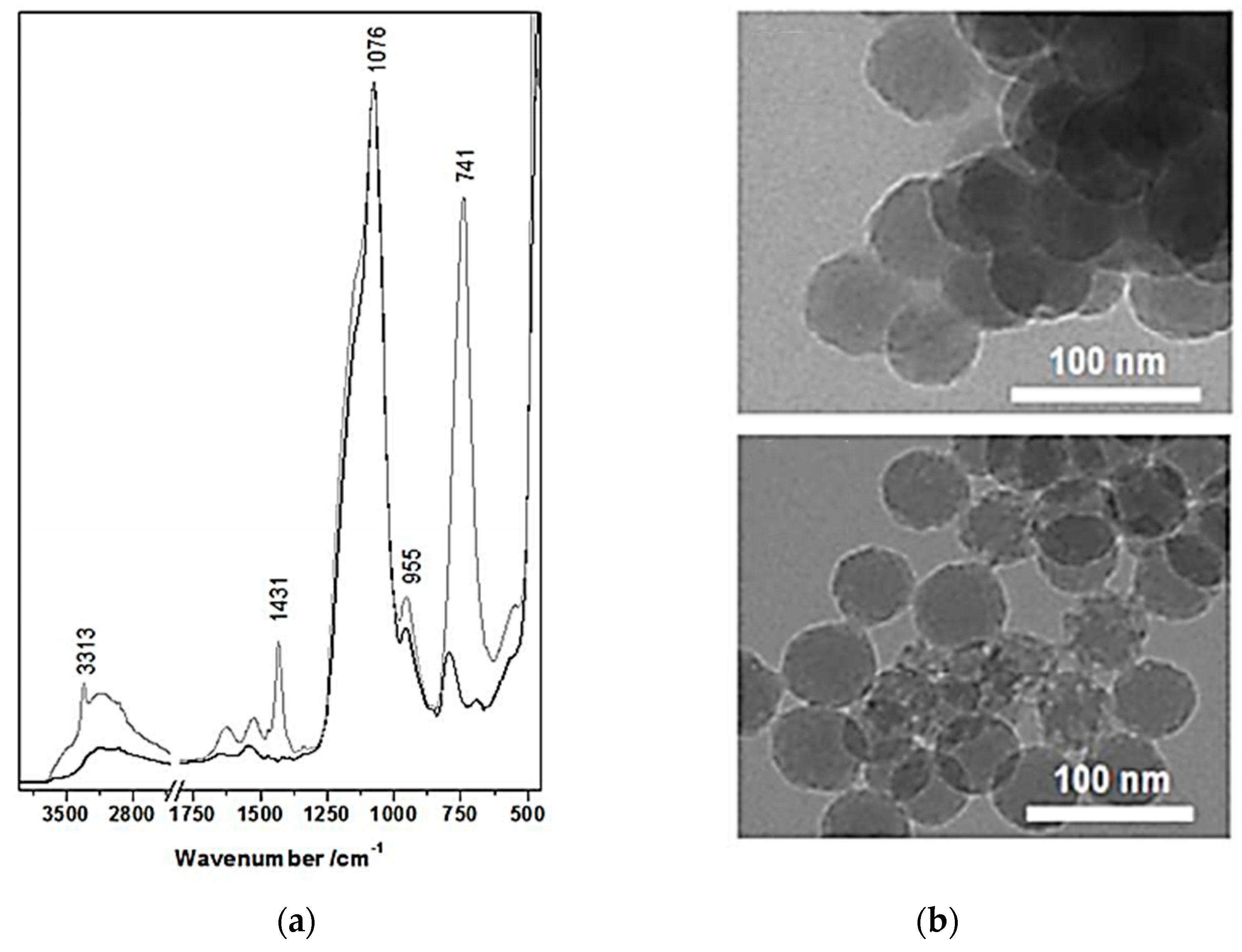
- Hartmeyer, G.; Marichal, C.; Lebeau, B.; Caullet, P.; Hernandez, J. Fluorination of Silica Nanoparticles by Aqueous NH4F Solutions. J. Phys. Chem. C 2007, 111, 6634–6644. [Google Scholar] [CrossRef]
- Lopes, L.M.F.; Ilharco, L.M. Hydrofluoric acid-induced fluorination and formation of silica nanocapsules for 19F magnetic resonance imaging. RSC Adv. 2014, 4, 16931–16934. [Google Scholar] [CrossRef]
- Kota, A.K.; Choi, W.; Tuteja, A. Superomniphobic surfaces: Design and durability. MRS Bull. 2013, 38, 383–390. [Google Scholar] [CrossRef]
- Kim, D.S.; Suh, A.; Yang, S.; Yoon, D.K. Grooving of nanoparticles using sublimable liquid crystal for transparent omniphobic surface. J. Coll. Interf. Sci. 2018, 513, 585–591. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, P.; Yu, M.; Li, G. Fabrication and performance of a novel 3D superhydrophobic material based on a loofah sponge from plant. Mater. Lett. 2018, 230, 219–223. [Google Scholar] [CrossRef]
- Kota, A.K.; Li, Y.; Mabry, J.M.; Tuteja, A. Hierarchically Structured Superoleophobic Surfaces with Ultralow Contact Angle Hysteresis. Adv. Mater. 2012, 24, 5838–5843. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Li, Y.; Zhang, J.; Ding, Y. The surface properties and corrosion resistance of fluorinated polyurethane coatings. J. Fluorine Chem. 2015, 176, 14–19. [Google Scholar] [CrossRef]
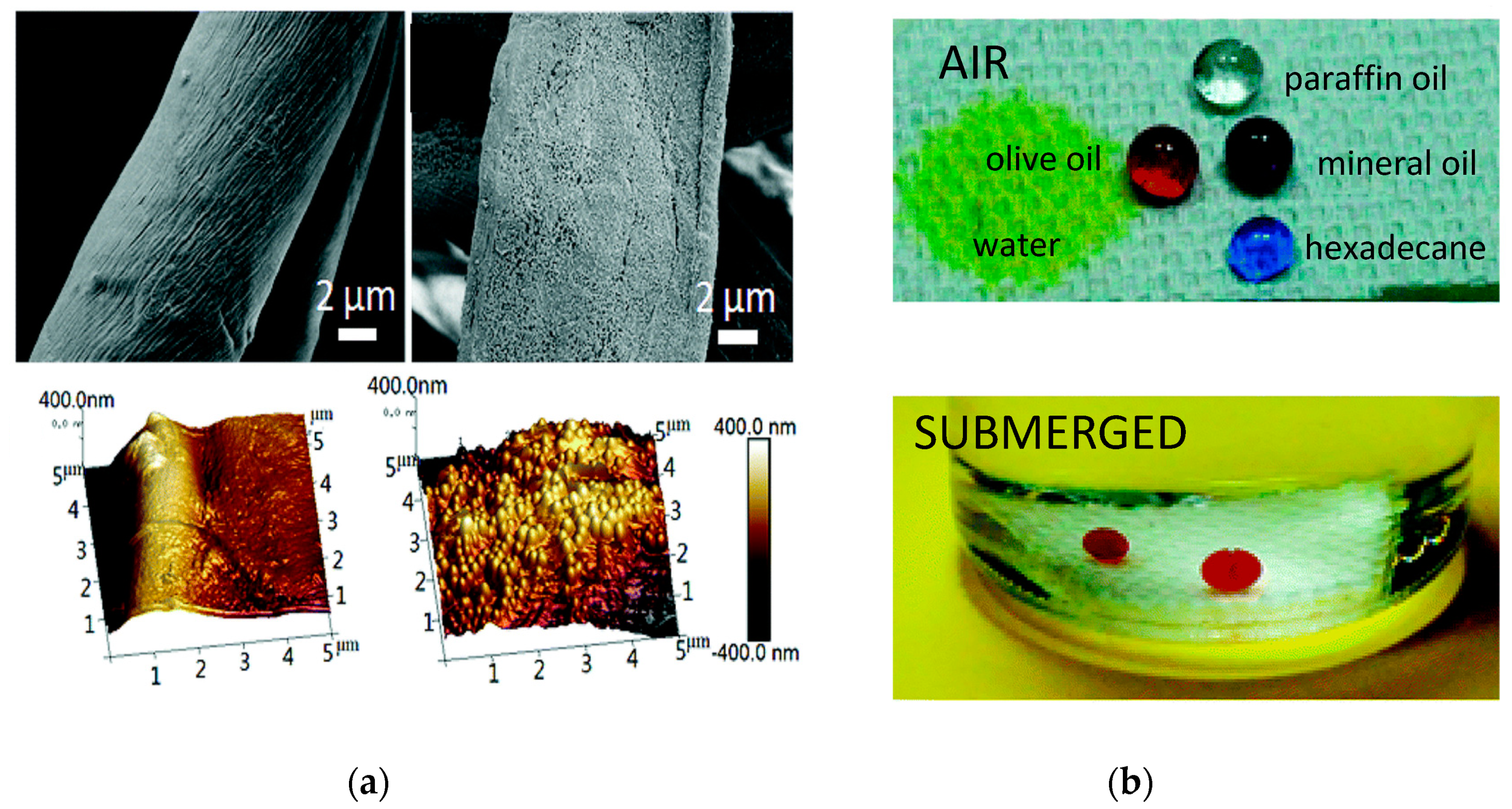
- Cai, Y.; Li, J.; Yi, L.; Yan, X.; Li, J. Fabricating superhydrophobic and oleophobic surface with silica nanoparticles modified by silanes and environment-friendly fluorinated chemicals. Appl. Surf. Sci. 2018, 450, 102–111. [Google Scholar] [CrossRef]
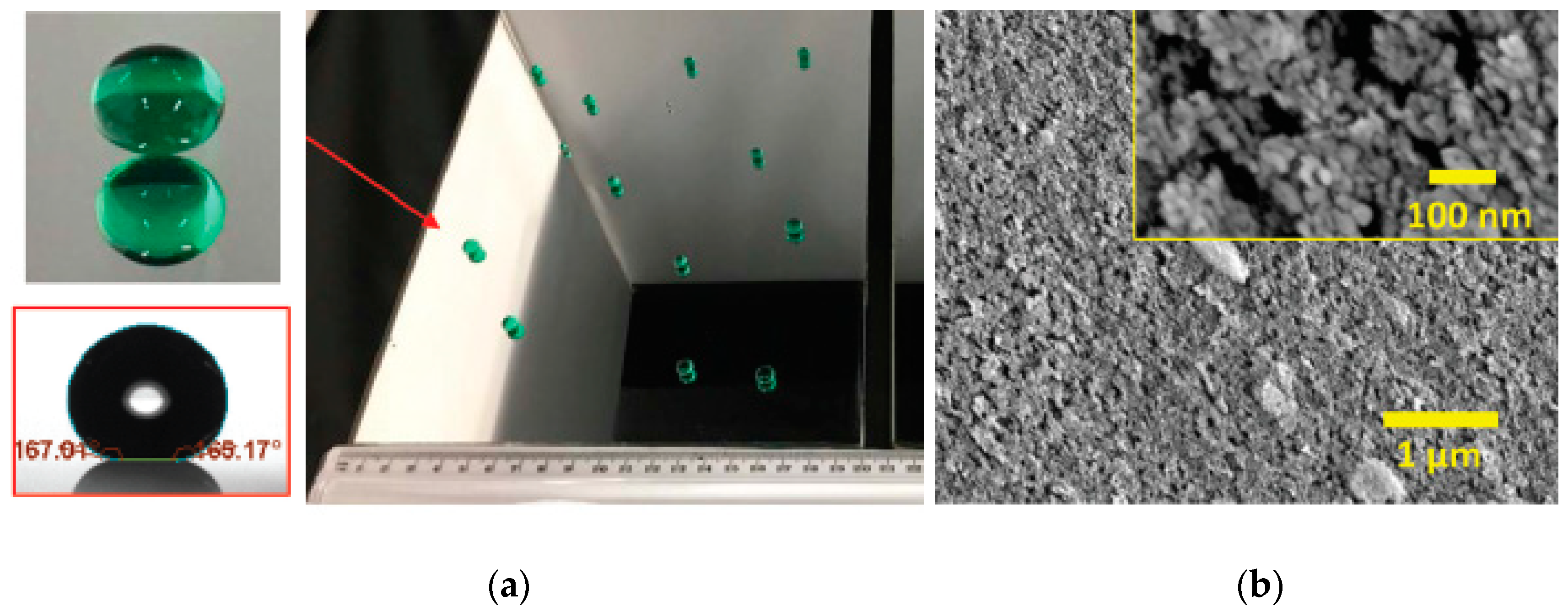
- Torun, I.; Onses, M.S. Robust superhydrophobicity on paper: Protection of spray-coated nanoparticles against mechanical wear by the microstructure of paper. Surf. Coat. Tech. 2017, 319, 301–308. [Google Scholar] [CrossRef]
- Wang, J.; He, L.; Pan, A.; Zhao, Y. Hydrophobic and Durable Adhesive Coatings Fabricated from Fluorinated Glycidyl Copolymers Grafted on SiO2 Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 617–626. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Yang, W.; Niu, H.; Wei, X.; Fu, S.; Liu, S.; Shao, H.; Lin, T. Durable superoleophobic–superhydrophilic fabrics with high anti-oil-fouling property. RSC Adv. 2018, 8, 26939–26947. [Google Scholar] [CrossRef]
- Jang, G.G.; Smith, D.B.; List, F.A.; Lee, D.F.; Ievlev, A.V.; Collins, L.; Park, J.; Polizos, G. The anti-soiling performance of highly reflective superhydrophobic nanoparticle-textured mirrors. Nanoscale 2018, 10, 14600–14612. [Google Scholar] [CrossRef]
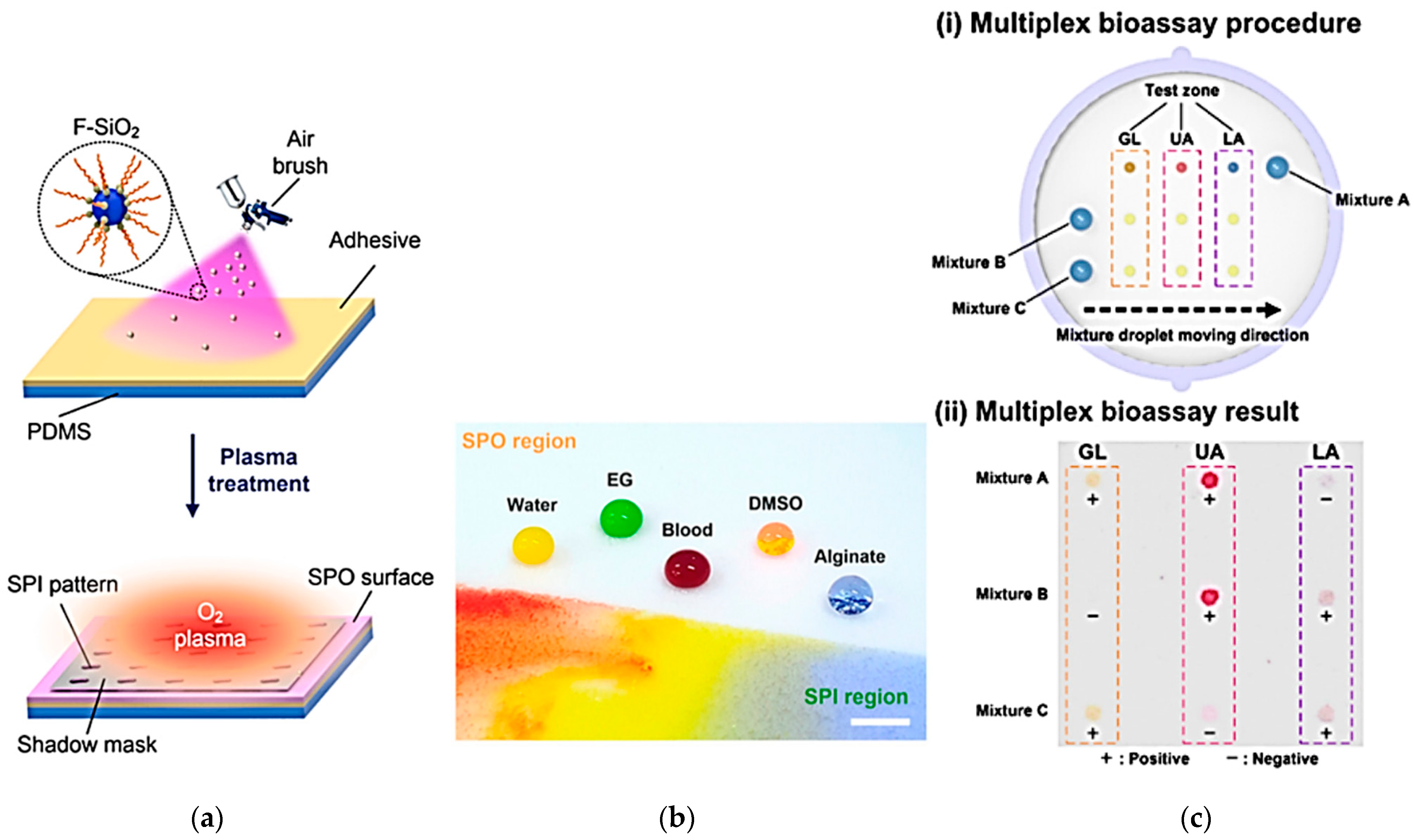
- Han, H.; Lee, J.S.; Kim, H.; Shin, S.; Lee, J.; Kim, J.; Hou, X.; Cho, S.-W.; Seo, J.; Lee, T. Single-Droplet Multiplex Bioassay on a Robust and Stretchable Extreme Wetting Substrate through Vacuum-Based Droplet Manipulation. ACS Nano 2018, 12, 932–941. [Google Scholar] [CrossRef]
- Katayama, S.; Fujii, S.; Saito, T.; Yamazaki, S.; Sawada, H. Preparation of fluoroalkyl end-capped vinyltrimethoxysilane oligomeric silica nanocomposites containing gluconamide units possessing highly oleophobic/superhydrophobic, highly oleophobic/superhydrophilic, and superoleophilic/superhydrophobic characteristics on the modified surfaces. Polymers 2017, 9, 292. [Google Scholar]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membrane Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in Membrane Distillation for Water Desalination and Purification Applications. Water 2013, 5, 94. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Schneider, K.; van Gassel, T.J. Membrandestillation. Chem. Ing. Tech. 1984, 56, 514–521. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Shao, Y.; Zhang, D.; Ruan, X.; Xiao, W.; Li, X.; Wu, X.; Jiang, X. Enhanced performance of superhydrophobic polypropylene membrane with modified antifouling surface for high salinity water treatment. Sep. Purif.Technol. 2019, 214, 11–20. [Google Scholar] [CrossRef]
- Pang, R.; Zhang, K. Fabrication of hydrophobic fluorinated silica-polyamide thin film nanocomposite reverse osmosis membranes with dramatically improved salt rejection. J. Coll. Interf. Sci. 2018, 510, 127–132. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Samal, S.K.; Wang, F.; Gao, B.; Pan, H.; Xu, H.; Yao, J.; Zhan, X.; De Smedt, S.C.; et al. Core–sheath structured electrospun nanofibrous membranes for oil–water separation. RSC Adv. 2016, 6, 41861–41870. [Google Scholar] [CrossRef]
- Tirotta, I.; Dichiarante, V.; Pigliacelli, C.; Cavallo, G.; Terraneo, G.; Bombelli, F.B.; Metrangolo, P.; Resnati, G. 19F Magnetic Resonance Imaging (MRI): From Design of Materials to Clinical Applications. Chem. Rev. 2015, 115, 1106–1129. [Google Scholar] [CrossRef]
- Li, S.; Yuan, Y.; Yang, Y.; Li, C.; McMahon, M.T.; Liu, M.; Chen, S.; Zhou, X. Potential detection of cancer with fluorinated silicon nanoparticles in 19F MR and fluorescence imaging. J. Mater. Chem. B 2018, 6, 4293–4300. [Google Scholar] [CrossRef]
- Bouchoucha, M.; van Heeswijk, R.B.; Gossuin, Y.; Kleitz, F.; Fortin, M.-A. Fluorinated Mesoporous Silica Nanoparticles for Binuclear Probes in 1H and 19F Magnetic Resonance Imaging. Langmuir 2017, 33, 10531–10542. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Peng, F.; Bao, F.; Wang, S.; Ji, X.; Yang, L.; Su, Y.; Lee, S.-T.; He, Y. Large-Scale Aqueous Synthesis of Fluorescent and Biocompatible Silicon Nanoparticles and Their Use as Highly Photostable Biological Probes. J. Am. Chem. Soc. 2013, 135, 8350–8356. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Functional Nanoparticles: Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1–40. [Google Scholar]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chang, V.W.C.; Gin, K.Y.H. Environmental toxicity of PFCs: An enhanced integrated biomarker assessment and structure–activity analysis. Environ. Toxicol. Chem. 2013, 32, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacono, S.T.; Jennings, A.R. Recent Studies on Fluorinated Silica Nanometer-Sized Particles. Nanomaterials 2019, 9, 684. https://doi.org/10.3390/nano9050684
Iacono ST, Jennings AR. Recent Studies on Fluorinated Silica Nanometer-Sized Particles. Nanomaterials. 2019; 9(5):684. https://doi.org/10.3390/nano9050684
Chicago/Turabian StyleIacono, Scott T., and Abby R. Jennings. 2019. "Recent Studies on Fluorinated Silica Nanometer-Sized Particles" Nanomaterials 9, no. 5: 684. https://doi.org/10.3390/nano9050684
APA StyleIacono, S. T., & Jennings, A. R. (2019). Recent Studies on Fluorinated Silica Nanometer-Sized Particles. Nanomaterials, 9(5), 684. https://doi.org/10.3390/nano9050684