The Preparation and Catalytic Properties of Nanoporous Pt/CeO2 Composites with Nanorod Framework Structures
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Pt/CeO2 Catalysts
2.2. Material Characterization
2.3. Evaluation of the CO Oxidation Performance
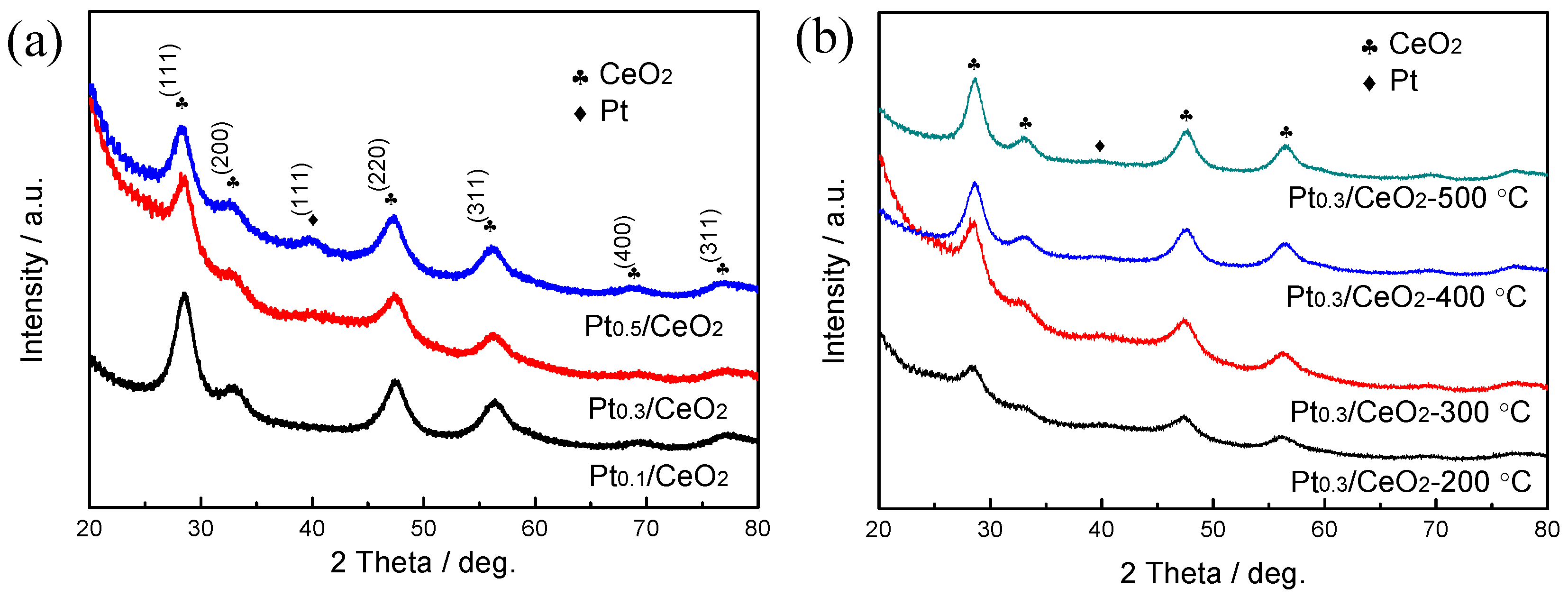
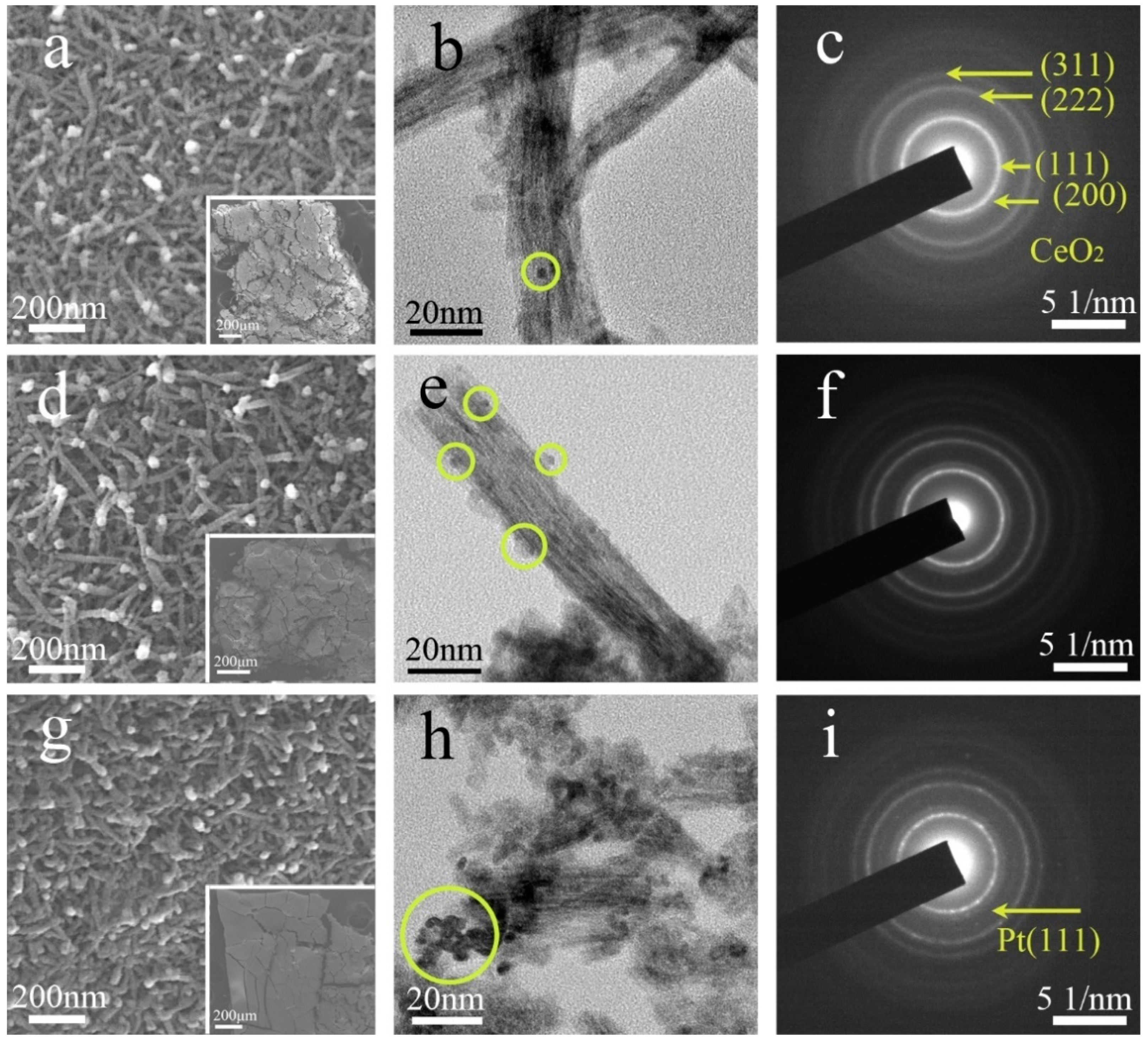
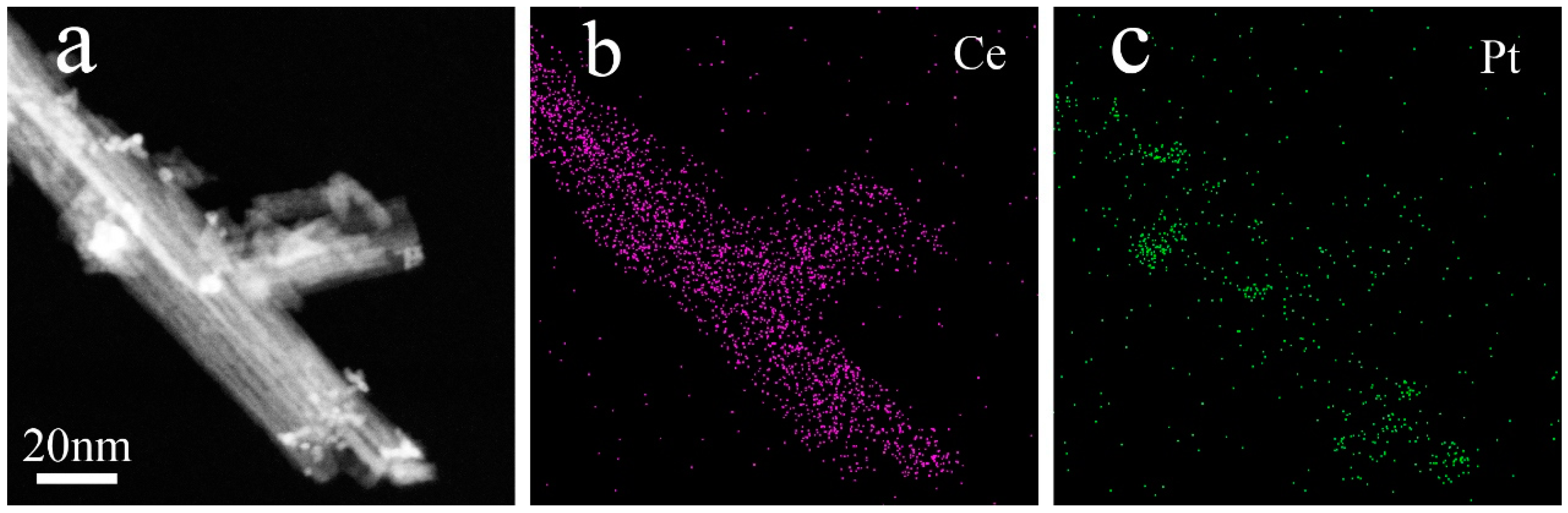
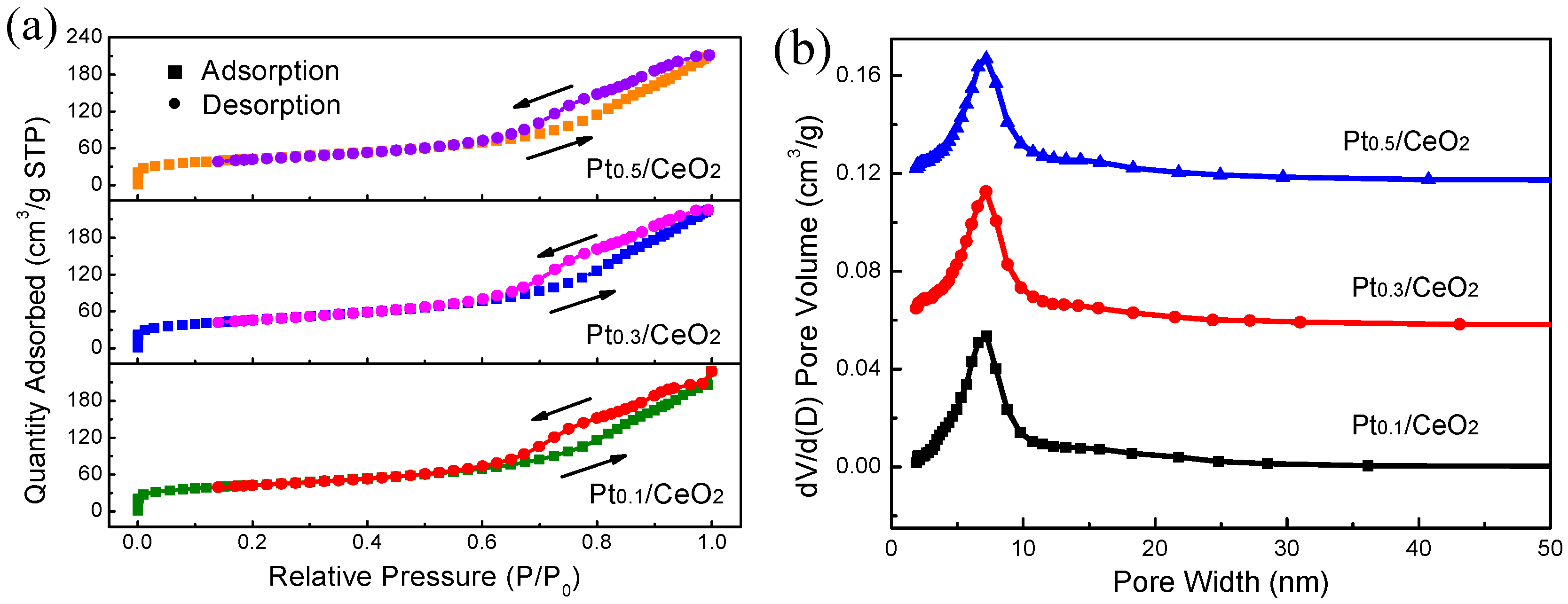
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Min, B.K.; Friend, C.M. Heterogeneous Gold-Based Catalysis for Green Chemistry: Low-Temperature CO Oxidation and Propene Oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef]
- Zhang, J.S.; Di, L.B.; Yu, F.; Duan, D.Z.; Zhang, X.L. Atmospheric-Pressure Cold Plasma Activating Au/P25 for CO Oxidation: Effect ofWorking Gas. Nanomaterials 2018, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, J.; Huang, J.; Xu, T.; Zhu, J.; Zhu, Y.; Li, C. Atomically dispersed Au catalysts supported on CeO2 foam: Controllable synthesis and CO oxidation reaction mechanism. Nanoscale 2017, 9, 16817–16825. [Google Scholar] [CrossRef] [PubMed]
- Najafishirtari, S.; Guardia, P.; Scarpellini, A.; Prato, M.; Marras, S.; Manna, L.; Colombo, M. The effect of Au domain size on the CO oxidation catalytic activity of colloidal Au-FeOx dumbbell-like heterodimers. J. Catal. 2016, 338, 115–123. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Huang, X.; Li, G. Fabrication of Ag-CeO2 core-shell nanospheres with enhanced catalytic performance due to strengthening of the interfacial interactions. J. Mater. Chem. 2012, 22, 10480–10487. [Google Scholar] [CrossRef]
- Bera, P.; Patil, K.C.; Jayaram, V.; Subbanna, G.N.; Hegde, M.S. Ionic Dispersion of Pt and Pd on CeO2 by Combustion Method: Effect of Metal-Ceria Interaction on Catalytic Activities for NO Reduction and CO and Hydrocarbon Oxidation. J. Catal. 2000, 196, 293–301. [Google Scholar] [CrossRef]
- Vogel, D.; Spiel, C.; Suchorski, Y.; Trinchero, A.; Schlögl, R.; Grönbeck, H.; Rupprechter, G. Local Catalytic Ignition during CO Oxidation on Low-Index Pt and Pd Surfaces: A Combined PEEM, MS, and DFT Study. Angew. Chem. Int. Ed. 2012, 51, 10041–10044. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Hu, P.D.; Long, M.C. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, L.; Dou, J.; Andolina, C.M.; Li, Y.; Ma, H.; House, S.D.; Zhang, X.; Yang, J.; Tao, F.F. Transition of surface phase of cobalt oxide during CO oxidation. Phys. Chem. Chem. Phys. 2018, 20, 6440–6449. [Google Scholar] [CrossRef] [PubMed]
- Lykakia, M.; Pachatouridou, E.; Carabineiroc, S.A.C.; Iliopouloub, E.; Andriopouloud, C.; Kallithrakas-Kontose, N.; Soghomon, B.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Singhania, N.; Anumol, E.A.; Ravishankar, N.; Madras, G. Influence of CeO2 morphology on the catalytic activity of CeO2-Pt hybrids for CO oxidation. Dalton Trans. 2013, 42, 15343. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support Morphology-Dependent Catalytic Activity of Pd/CeO2 for Formaldehyde Oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Fu, X.P.; Yu, W.Z.; Wang, W.W.; Jia, C.J.; Du, P.P.; Si, R.; Wang, Y.H.; Li, L.D.; Zhou, L.; et al. Pt-Embedded CuOx-CeO2 Multicore-Shell Composites: Interfacial Redox Reaction-Directed Synthesis and Composition-Dependent Performance for CO Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 34172–34183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Wang, J.; Wang, H.; Li, H.; Wang, J.; Shen, M. Effect of active oxygen on the performance of Pt/CeO2 catalysts for CO oxidation. J. Rare Earths 2018, 36, 257–264. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef]
- Lin, K.S.; Chowdhury, S. Synthesis, characterization, and application of 1-D cerium oxide nanomaterials: A review. Int. J. Mol. Sci. 2010, 11, 3226–3251. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L.; Fang, J. Template-Free Synthesis, Controlled Conversion, and CO Oxidation Properties of CeO2 Nanorods, Nanotubes, Nanowires, and Nanocubes. Eur. J. Inorg. Chem. 2008, 2008, 2429–2436. [Google Scholar] [CrossRef]
- Tana; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef]
- Bera, P.; Gayen, A.; Hegde, M.S.; Lalla, N.P.; Spadaro, L.; Frusteri, F.; Arena, F. Promoting Effect of CeO2 in Combustion Synthesized Pt/ CeO2 Catalyst for CO Oxidation. J. Phys. Chem. B 2003, 107, 6122–6130. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Song, S.; Zhang, H. Pt@ CeO2 multicore@shell self-assembled nanospheres: Clean synthesis, structure optimization, and catalytic applications. J. Am. Chem. Soc. 2013, 135, 15864–15872. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, L.; Jia, C.J.; Yan, C.H. Pt-embedded-CeO2 hollow spheres for enhancing CO oxidation performance. Mater. Chem. Front. 2017, 1, 1754–1763. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Liu, J.; Xu, C.; Jiang, G.; Duan, A. Design and synthesis of 3D ordered macroporous CeO2-supported Pt@CeO2 core-shell nanoparticle materials for enhanced catalytic activity of soot oxidation. Small 2013, 9, 3957–3963. [Google Scholar] [CrossRef]
- Cao, F.X.; Zhang, S.; Gao, W.; Cao, T.; Qu, Y.Q. Facile synthesis of highly-dispersed Pt/CeO2 by a spontaneous surface redox chemical reaction for CO oxidation. Catal. Sci. Technol. 2018, 8, 3233–3237. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Kou, T.; Si, C.; Pinto, J.; Ma, C.; Zhang, Z. Dealloying assisted high-yield growth of surfactant-free <110> highly active Cu-doped CeO2 nanowires for low-temperature CO oxidation. Nanoscale 2017, 9, 8007–8014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, G.; Yang, S.; Song, X.; Sun, Z. Nanoporous CuO ribbons modified by Au nanoparticles through chemical dealloying and calcination for CO oxidation. Microporous Mesoporous Mater. 2016, 226, 61–70. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Shi, W.; Wei, C.; Song, X.; Yang, S.; Sun, Z. Baize-like CeO2 and NiO/CeO2 nanorod catalysts prepared by dealloying for CO oxidation. Nanotechnology 2017, 28, 045602. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, D.; Li, G.; Feng, W.; Yang, S.; Sun, Z. Monolithic Au/CeO2 nanorod framework catalyst prepared by dealloying for low-temperature CO oxidation. Nanotechnology 2018, 29, 095606. [Google Scholar] [CrossRef]
- Wang, H.; Liang, M.; Duan, D.; Shi, W.; Song, Y.; Sun, Z. Rose-like Ni3S4 as battery-type electrode for hybrid supercapacitor with excellent charge storage performance. Chem. Eng. J. 2018, 350, 523–533. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, H.; Qin, Z.; Wang, G.; Wang, J. Effects of CO2 on the stability of Pd/CeO2–TiO2 catalyst for low-temperature CO oxidation. Catal. Commun. 2009, 10, 737–740. [Google Scholar] [CrossRef]
- Aguilar-Guerrero, V.; Gates, B.C. Kinetics of CO oxidation catalyzed by highly dispersed CeO2-supported gold. J. Catal. 2008, 260, 351–357. [Google Scholar] [CrossRef]
- Ojala, T.; Pietikainen, M.; Harwood, D. A comparative study of texture measures with classification based on feature distributions. Pattern Recoonit. 1996, 29, 51–59. [Google Scholar] [CrossRef]
- Peng, R.; Li, S.; Sun, X.; Ren, Q.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/ CeO2 catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Wang, B.; Chen, B.; Sun, Y.; Xiao, H.; Xu, X.; Fu, M.; Wu, J.; Chen, L.; Ye, D. Effects of dielectric barrier discharge plasma on the catalytic activity of Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 238, 328–338. [Google Scholar] [CrossRef]
- Stadnichenko, A.I.; Muravev, V.V.; Koscheev, S.V.; Zaikovskii, V.I.; Aleksandrov, H.A.; Neyman, K.M.; Boronin, A.I. Study of active surface centers of Pt/CeO2 catalysts prepared using radio-frequency plasma sputtering technique. Surf. Sci. 2019, 679, 237–283. [Google Scholar] [CrossRef]
- Luo, M.F.; Zhong, Y.J.; Yuan, X.X.; Zheng, X.M. TPR and TPD studies of CuO/ CeO2 catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 1997, 162, 121–131. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Qin, Z.; Shan, W.J.; Shen, W.J.; Wang, J.G. Pd/ CeO2-CeO2 catalyst for CO oxidation at low temperature: A TPR study with H2 and CO as reducing agents. J. Catal. 2004, 225, 267–277. [Google Scholar] [CrossRef]
- Si, G.; Yu, J.; Xiao, X.; Guo, X.; Huang, H.; Mao, D.; Lu, G. Boundary role of Nano-Pd catalyst supported on ceria and the approach of promoting the boundary effect. Mol. Catal. 2018, 444, 1–9. [Google Scholar] [CrossRef]
- Chen, G.Z.; Yang, Y.; Guo, Z.; Gao, D.; Zhao, W.; Yan, H.; Wang, W.; Jia, C.J.; Sun, G.X. Thermally stable and highly active Pt/CeO2@SiO2 catalysts with a porous/hollow structure. Catal. Sci. Technol. 2018, 8, 4413–4419. [Google Scholar] [CrossRef]
- Chen, J.; Wanyan, Y.; Zeng, J.; Fang, H.; Li, Z.; Dong, Y.; Qin, R.; Wu, C.; Liu, D.; Wang, M.; et al. Surface Engineering Protocol to Obtain an Atomically Dispersed Pt/ CeO2 Catalyst with High Activity and Stability for CO Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 14054–14062. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Li, T.; Zhao, Z.; Gong, X.Q.; Chen, Y.; Duan, A.; Jiang, G.; Wei, Y. Interfacial Effects of CeO2-Supported Pd Nanorod in Catalytic CO Oxidation: A Theoretical Study. J. Phys. Chem. C 2015, 119, 12923–12934. [Google Scholar] [CrossRef]
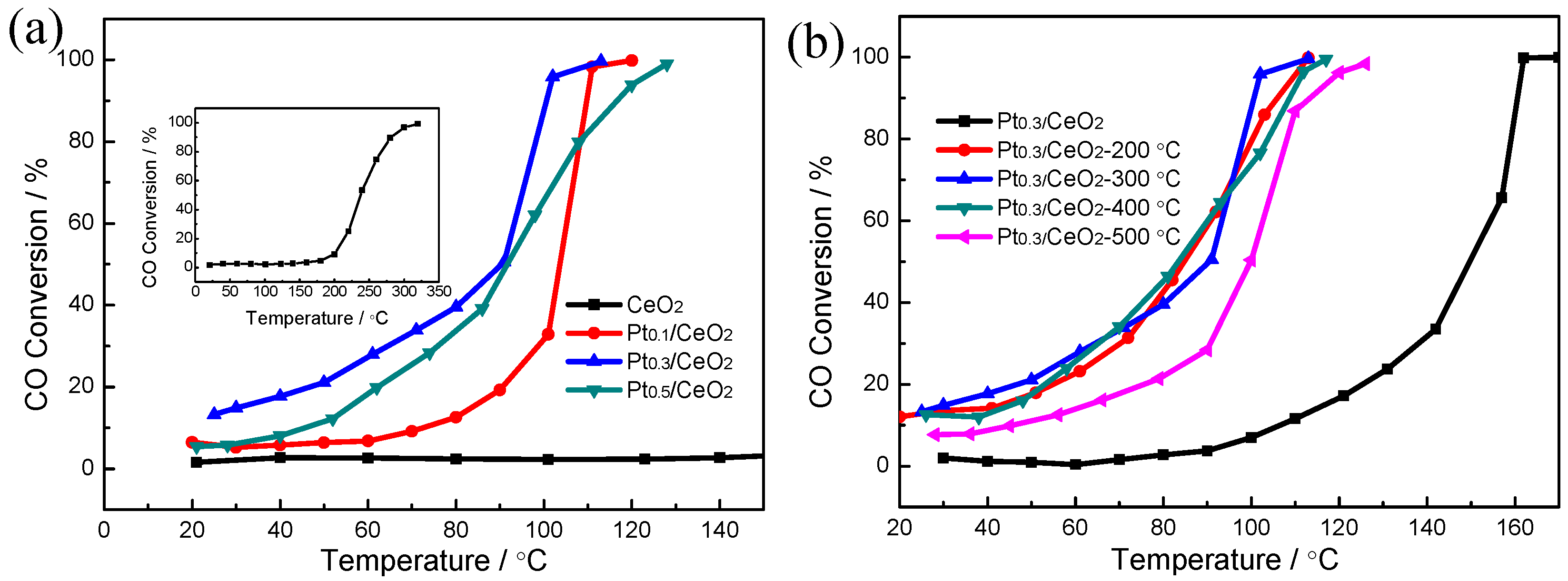
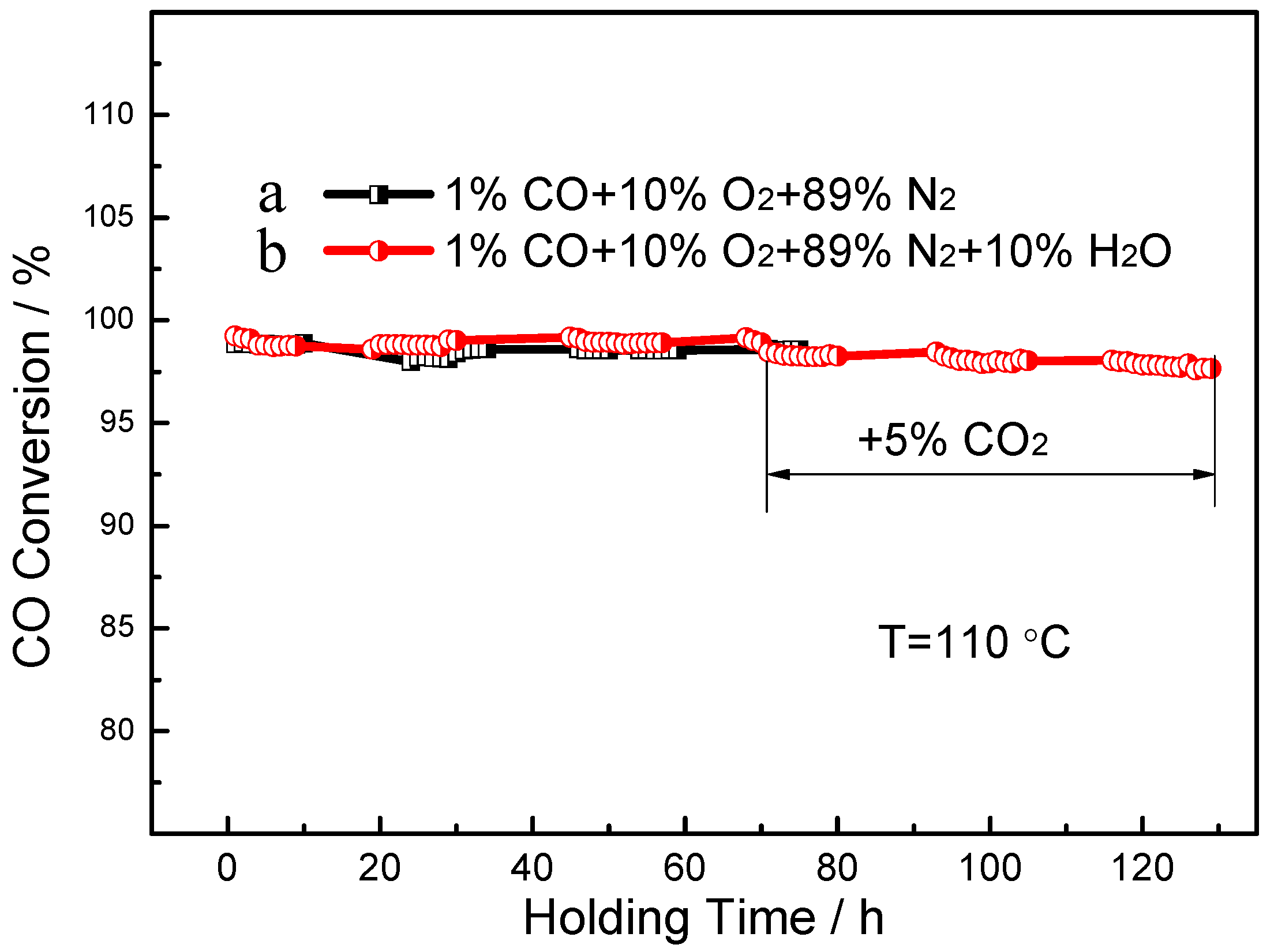
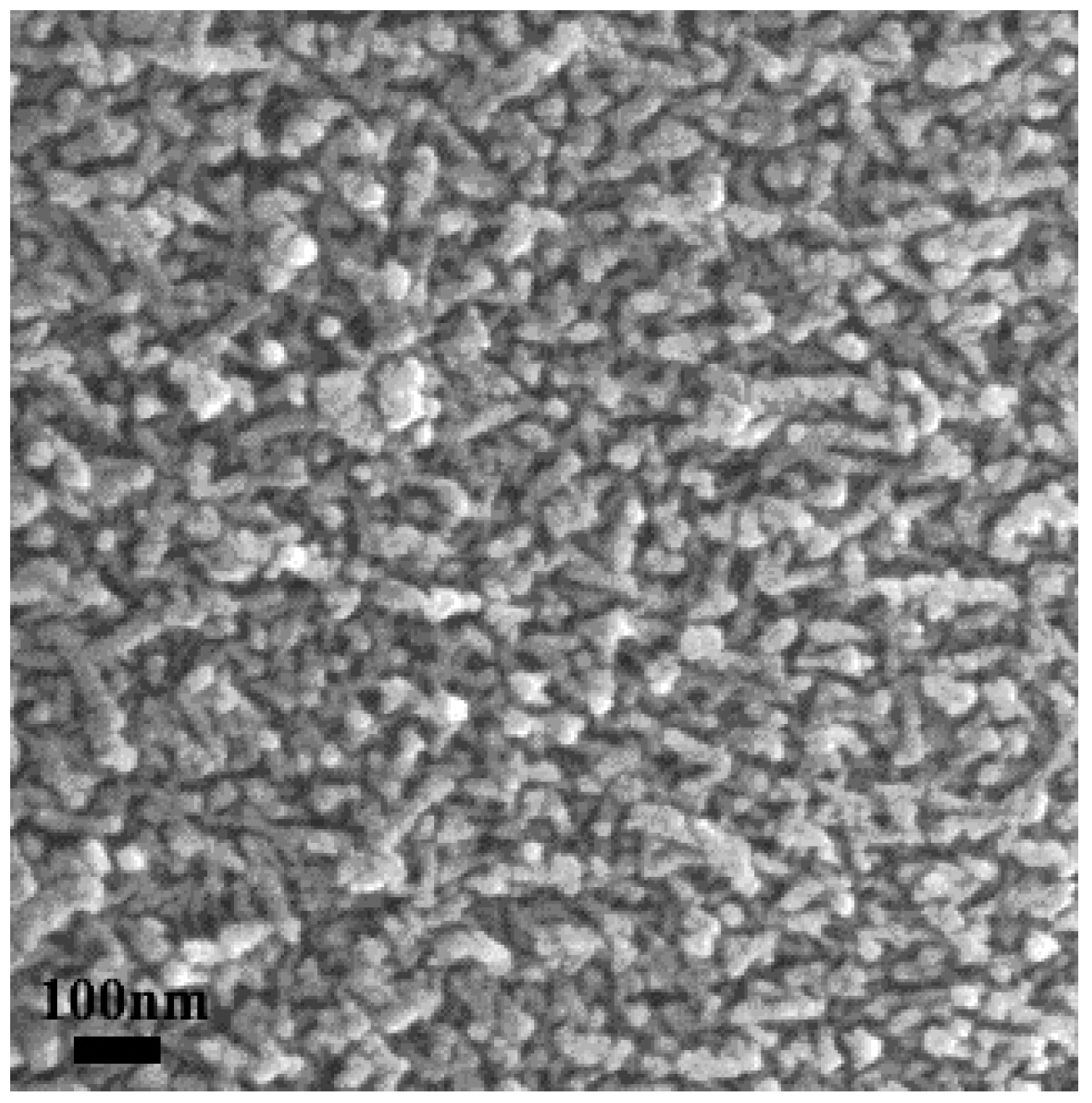
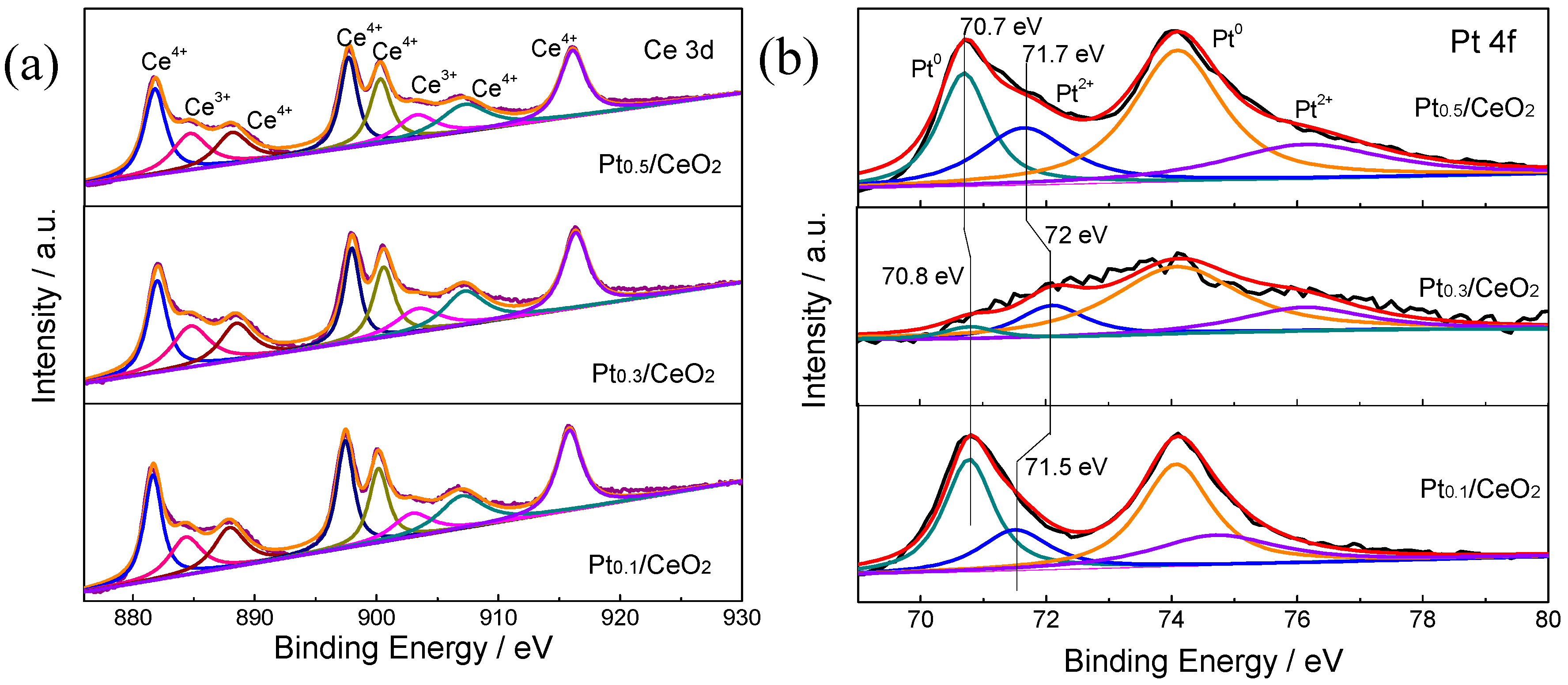
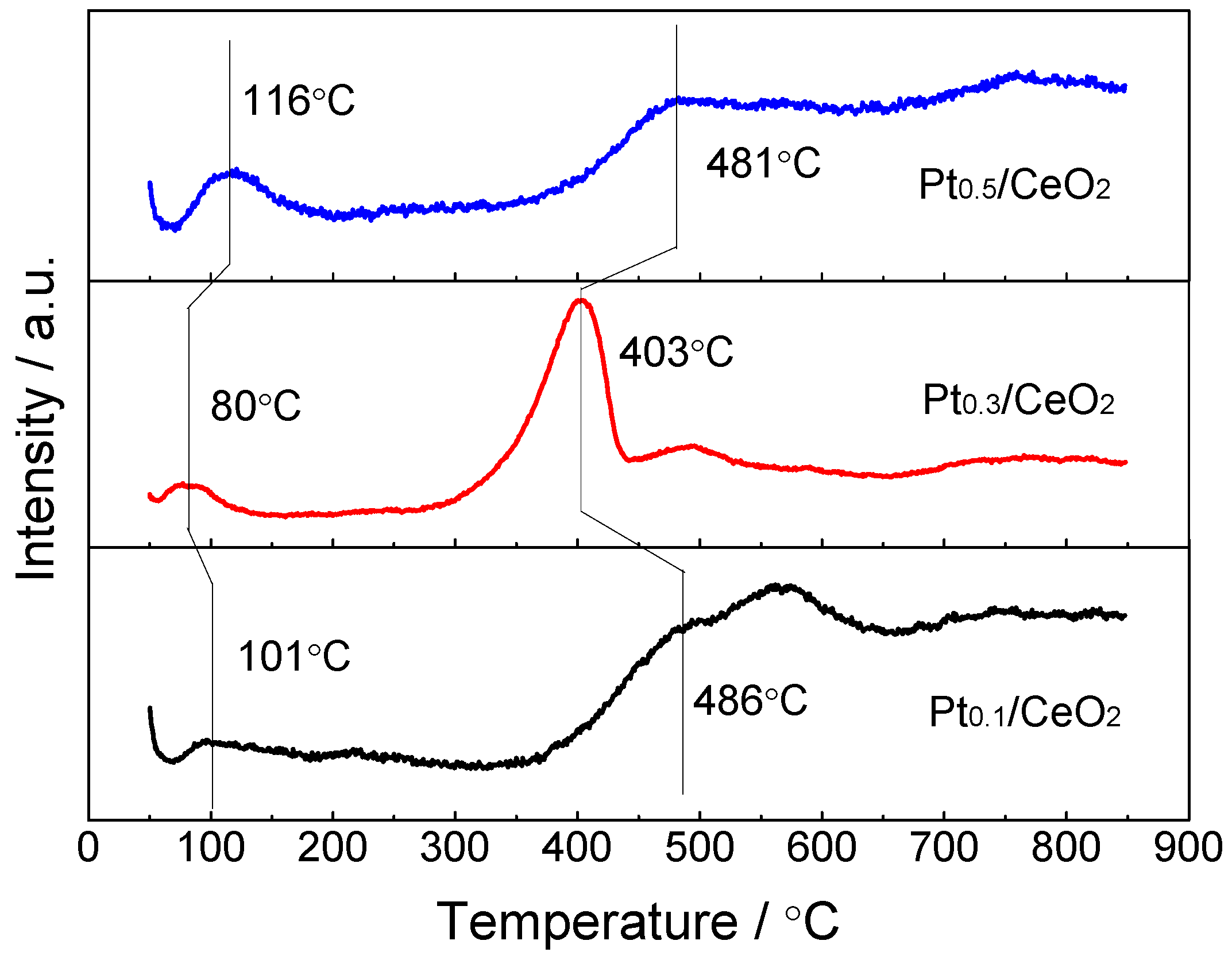
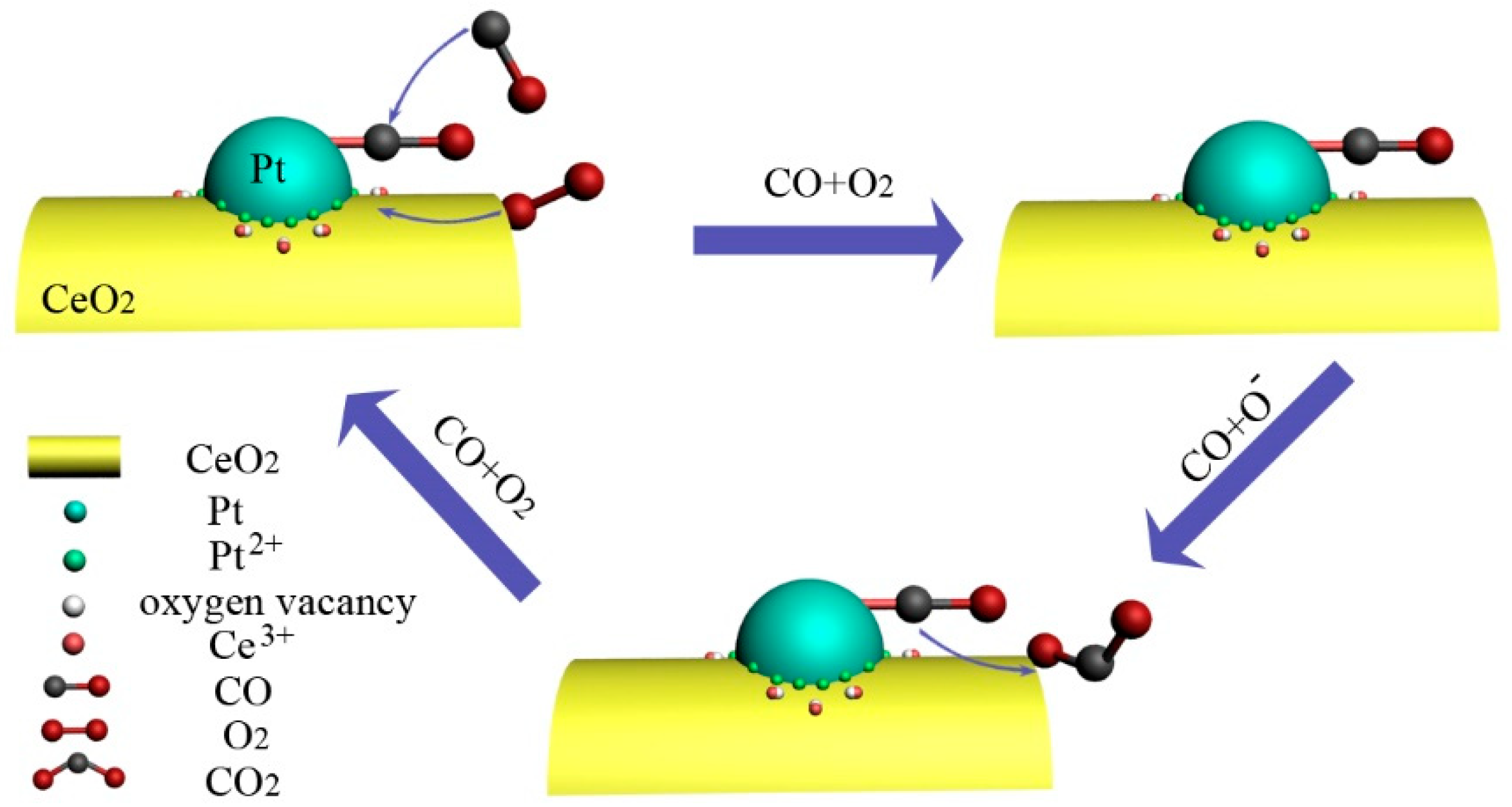
| Catalyst | Reaction Temperature (T99) (°C) | Durability Test (h) | Space Velocity (h−1) | Reference |
|---|---|---|---|---|
| Porous/hollow structured Pt/CeO2@SiO2 | 162 | Not stated | 60,000 | [44] |
| Nanorods CeO2-IMP-Pt | 90 | 10 | 30,000 | [45] |
| Pt/CeO2 hollow sphere | 155 | 12 | 80,000 | [25] |
| Pt/CeO2 mesoporous sphere | 95 | 12 | 80,000 | [14] |
| Nanorod framework-structured Au/CeO2 | 90 | Not stated | 60,000 | [32] |
| Nanorod framework-structured Pt0.3/CeO2 | 113 | 130 | 60,000 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Duan, D.; Ma, C.; Shi, W.; Liang, M.; Wang, L.; Song, X.; Gao, L.; Sun, Z. The Preparation and Catalytic Properties of Nanoporous Pt/CeO2 Composites with Nanorod Framework Structures. Nanomaterials 2019, 9, 683. https://doi.org/10.3390/nano9050683
Wang H, Duan D, Ma C, Shi W, Liang M, Wang L, Song X, Gao L, Sun Z. The Preparation and Catalytic Properties of Nanoporous Pt/CeO2 Composites with Nanorod Framework Structures. Nanomaterials. 2019; 9(5):683. https://doi.org/10.3390/nano9050683
Chicago/Turabian StyleWang, Haiyang, Dong Duan, Chen Ma, Wenyu Shi, Miaomiao Liang, Liqun Wang, Xiaoping Song, Lumei Gao, and Zhanbo Sun. 2019. "The Preparation and Catalytic Properties of Nanoporous Pt/CeO2 Composites with Nanorod Framework Structures" Nanomaterials 9, no. 5: 683. https://doi.org/10.3390/nano9050683
APA StyleWang, H., Duan, D., Ma, C., Shi, W., Liang, M., Wang, L., Song, X., Gao, L., & Sun, Z. (2019). The Preparation and Catalytic Properties of Nanoporous Pt/CeO2 Composites with Nanorod Framework Structures. Nanomaterials, 9(5), 683. https://doi.org/10.3390/nano9050683




