The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review
Abstract
1. Introduction
2. Heavy Metal Ions in the Environment
2.1. Sources of Heavy Metal
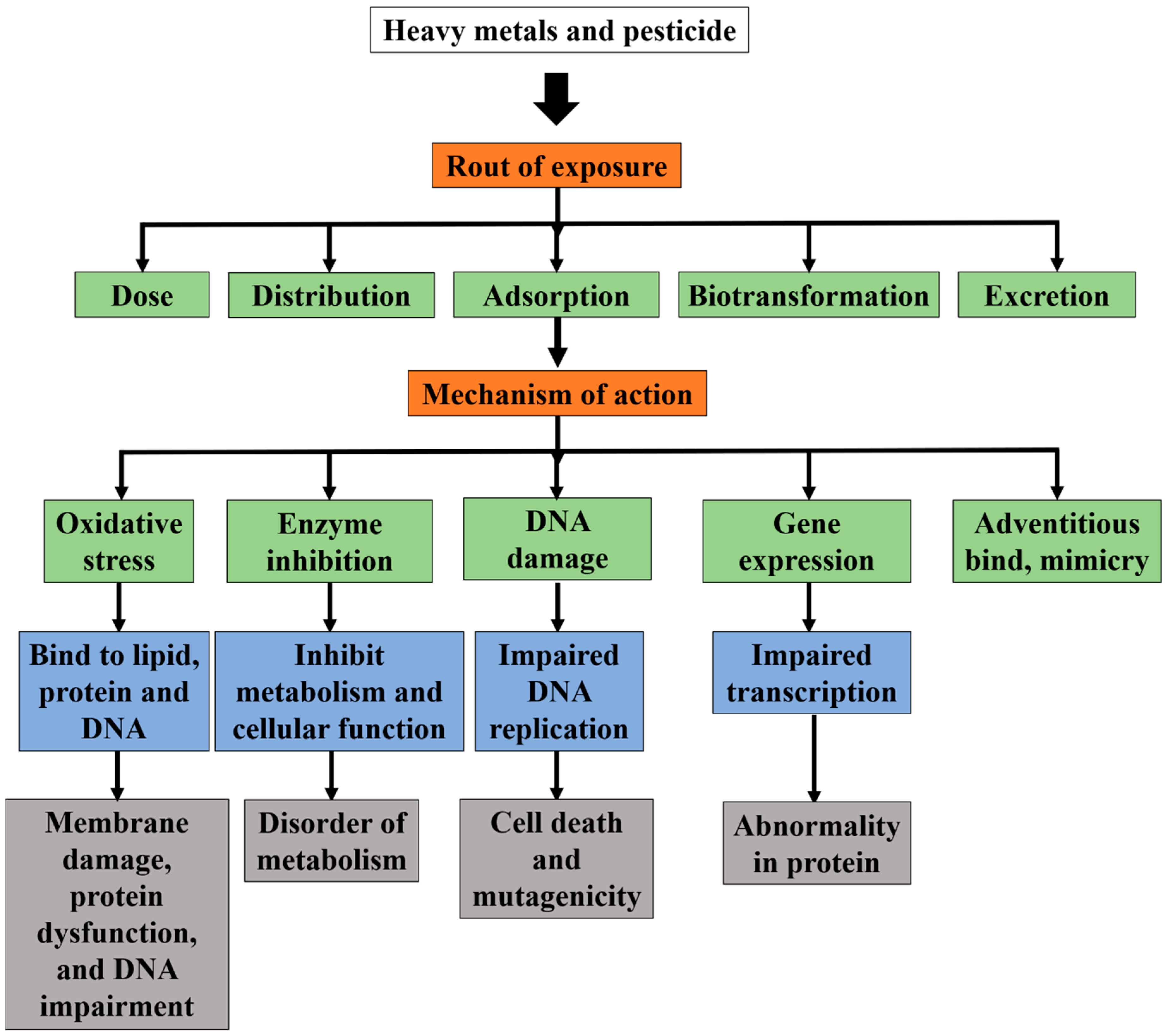
2.2. Effect of Heavy Metal
2.2.1. Effect of Heavy Metal Ions Towards the Environment
2.2.2. Effect of Heavy Metal Ions Towards Humans
2.3. The Chemistry of Heavy Metal Ions
3. Nanomaterials-Assisted Approach for Heavy Metal Removal
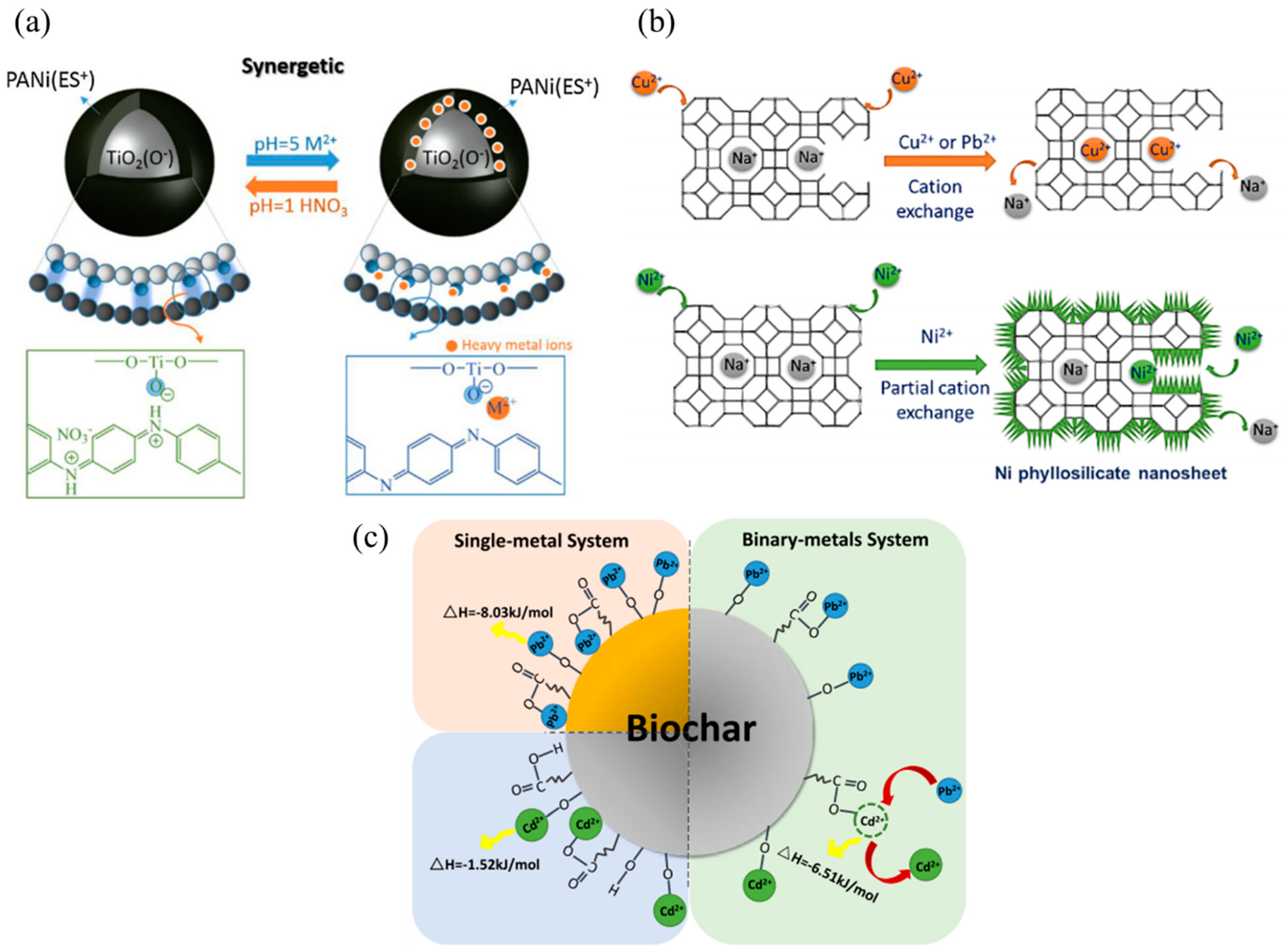
3.1. Adsorption of Heavy Metals
3.2. Photocatalytic Reduction of Heavy Metal
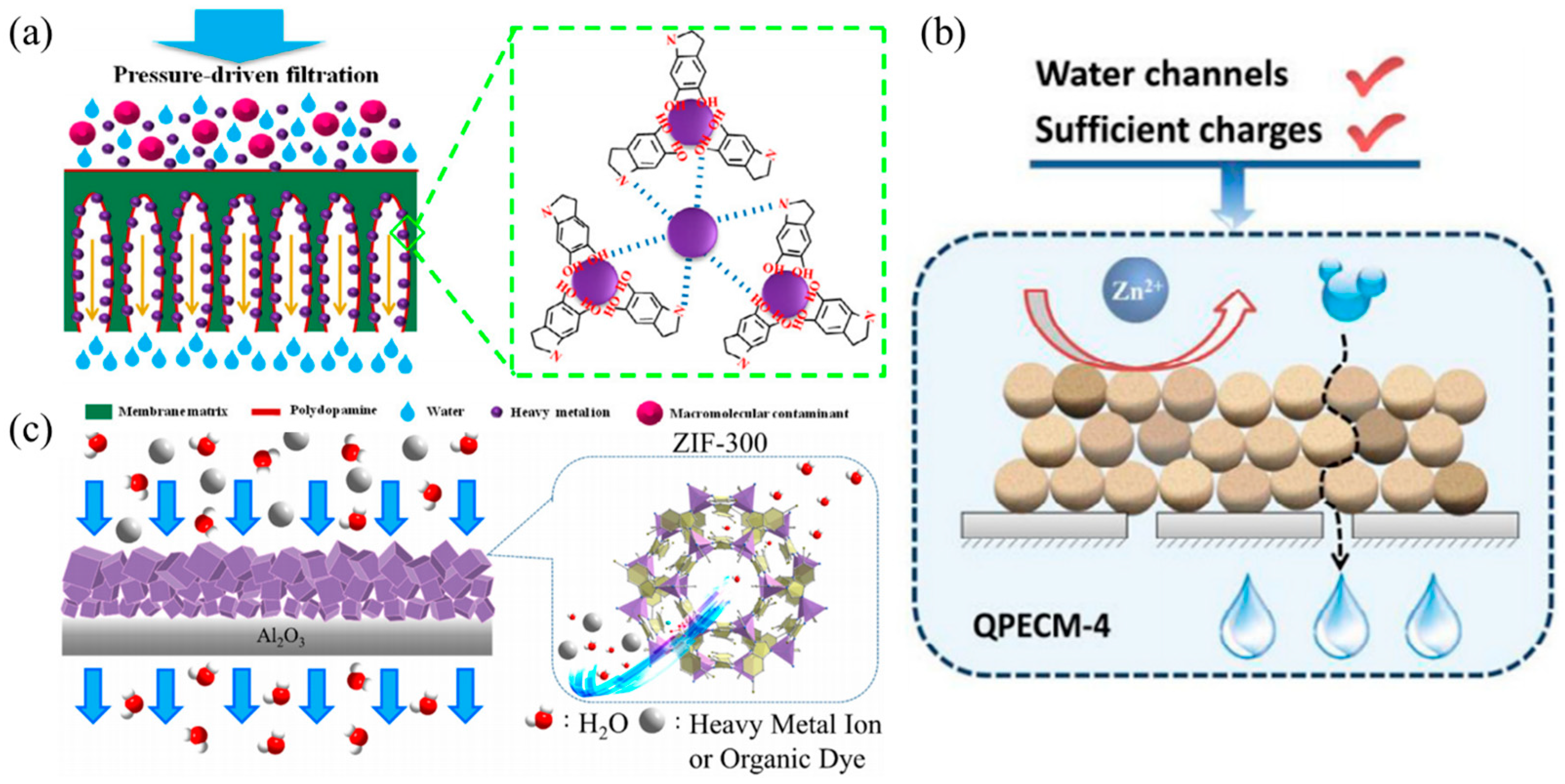
3.3. Membrane Filtration/Adsorption
4. Nanomaterials for Heavy Metal Removal
4.1. Motivation of Using Nanomaterial for Heavy Metal Removal
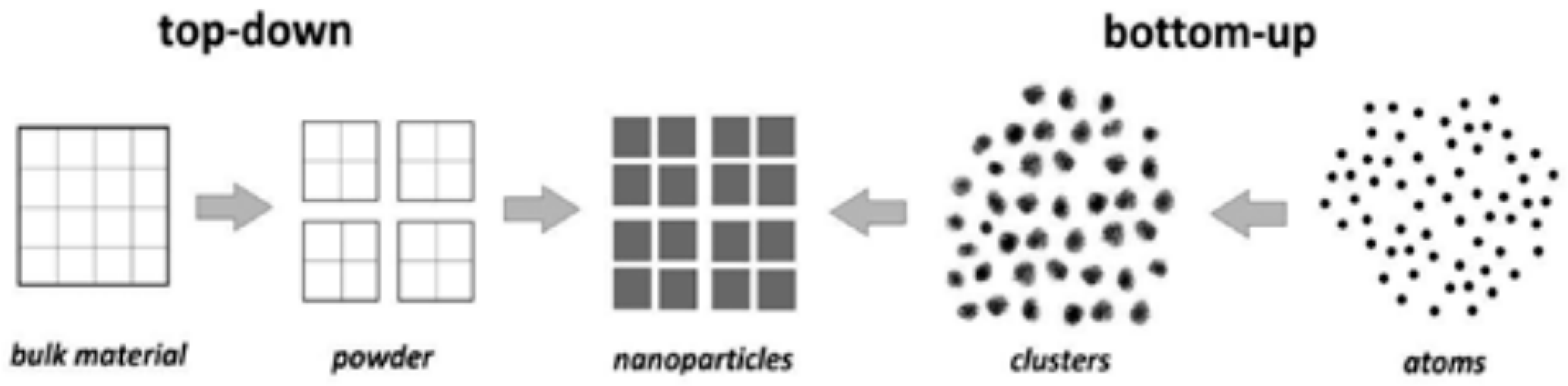
4.2. Classification of Nanomaterials
4.2.1. Metallic Nanomaterials
4.2.2. Non-Metallic Nanomaterials
4.2.3. Hybrid Nanomaterials
4.3. Synthesis and Modification of Nanomaterials
5. Recent Progress and Performance Evaluation
5.1. Adsorption of Heavy Metal
5.2. Photocatalysis of Heavy Metal
5.3. Membrane Composite for Removal of Heavy Metal
6. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Duncan, A.E.; de Vries, N.; Nyarko, K.B. Assessment of heavy metal pollution in the sediments of the River Pra and its tributaries. Water Air Soil Pollut. 2018, 229, 272. [Google Scholar] [CrossRef]
- Thakur, L.S. Heavy metal Cu, Ni and Zn: Toxicity, health hazards and their removal techniques by low cost adsorbents: A short overview. Int. J. Plant Sci. 2015, 3, 143–157. [Google Scholar]
- Akpor, O.B.; Ohiobor, G.O.; Olaolu, T.D. Heavy metal pollutants in wastewater effluents: Sources, effects and remediation. Adv. Biosci. Bioeng. 2014, 2, 37–43. [Google Scholar] [CrossRef]
- Yi, Y.; Tang, C.; Yi, T.; Yang, Z.; Zhang, S. Ecotoxicology and environmental safety health risk assessment of heavy metals in fi sh and accumulation patterns in food web in the upper Yangtze River, China. Ecotoxicol. Environ. Saf. 2017, 145, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Madzin, Z.; Kusin, F.M.; Yusof, F.M.; Muhammad, S.N. Assessment of water quality index and heavy metal contamination in active and abandoned iron ore mining sites in Pahang, Malaysia. MATEC Web Conf. 2017, 103, 05010. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Thanabalan, M.; Anjum, H.; Arunagiri, A.; Johari, K.; Gnanasundaram, N.; Regupathi, I. A review on adsorptive removal of oil pollutants (BTEX) from wastewater using carbon nanotubes. J. Mol. Liq. 2018, 277, 1005–1025. [Google Scholar]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Ou, H.H.; Lo, S.L. Review of titania nanotubes synthesized via the hydrothermal treatment: Fabrication, modification, and application. Sep. Purif. Technol. 2007, 58, 179–191. [Google Scholar] [CrossRef]
- Sheet, I.; Kabbani, A.; Holail, H. Removal of heavy metals using nanostructured graphite oxide, silica nanoparticles and silica/graphite oxide composite. Energy Procedia 2014, 50, 130–138. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Ghahremani, M. Surface functionalization of graphene oxide and graphene oxide-magnetite nanocomposite with molybdenum-bidentate Schiff base complex. J. Phys. Chem. Solids 2019, 130, 6–12. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Azzouz, A.; Kailasa, S.K.; Lee, S.S.; Rascón, A.J.; Ballesteros, E.; Zhang, M.; Kim, K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal. Chem. 2018, 108, 347–369. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Ozuni, E.; Dhaskali, L.; Abeshi, J.; Zogaj, M.; Haz Iri, I.; Beqiraj, D.; Latifi, F. Heavy metals in fish for public consumption and consumer protection. Nat. Montenegrina 2010, 9, 843–851. [Google Scholar]
- Krieger, R. Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010; Volume 3. [Google Scholar]
- Maouni, A.; Lamarti, A.; Aidoun, A.; Khaddor, M.; Badoc, A. Effect of benzimidazole fungicides and calcium chloride on Alternaria alternata and Penicillium expansum rot during storage of pears. Afr. J. Biotechnol. 2007, 6, 1289–1292. [Google Scholar]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef]
- Popova, E.; System, S.; Popova, E. Accumulation of heavy metals in the “soil-plant” system. AIP Conf. Proc. 2016, 1772, 050006. [Google Scholar]
- Nouri, J.; Khorasani, N.; Lorenstani, B.; Karami, M.; Hassani, A.H.; Yousefi, N. Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ. Earth Sci. 2009, 59, 315–323. [Google Scholar] [CrossRef]
- Yozukmaz, A.; Sel, F. Heavy metal accumulation in the leaves, stem and root of the invasive submerged macrophyte Myriophyllum spicatum L. (Haloragaceae): An Example of Kadın Creek (Mugla, Turkey). Braz. Arch. Biol. Technol. 2014, 57, 434–440. [Google Scholar]
- Vongdala, N.; Tran, H.; Xuan, T.D.; Teschke, R. Heavy metal accumulation in water soil, and plants of municipal solid waste landfill in Vientiane, Laos. Int. J. Environ. Res. Public Health 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Tangahu, B.V.; Rozaimah, S.; Abdullah, S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assesment 2015, 187, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Suvarapu, L.N.; Baek, S.O. Determination of heavy metals in the ambient atmosphere: A review. Toxicol. Ind. Health 2016, 33, 79–96. [Google Scholar] [CrossRef]
- Gimeno-García, E.; Andreu, V.; Boluda, R. Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ. Pollut. 1996, 92, 19–25. [Google Scholar] [CrossRef]
- Grinang, J.; Sim, S.F.; Rajendran, M.; Nyanti, L.; Ling, T.Y.; Liew, J.J. Assessment of trace metals in water and sediment in a tropical river potentially affected by land use activities in northern Sarawak, Malaysia. Int. J. Environ. Res. 2017, 11, 99–110. [Google Scholar]
- Singh, N.; Gupta, V.K.; Kumar, A.; Sharma, B. Synergistic effects of heavy metals and pesticides in living systems. Front. Chem. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Mathew, B.B.; Tiwari, A.; Jatawa, S.K. A review on free radicals and antioxidants. J. Pharm. Res. 2011, 4, 4340–4343. [Google Scholar]
- Flora, S.J.S.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501–523. [Google Scholar]
- Ojezele, O.J.; Ojezele, M.O.; Adeosun, A.M. Cooking utensils as probable source of heavy metal toxicity. Middle-East J. Sci. Res. 2016, 24, 2216–2220. [Google Scholar]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human Health Risk Assessment for Aluminium, Aluminium Oxide, and Aluminium Hydroxide. J. Toxicol. Environ. Health B. Crit. Rev. 2008, 11, 1–269. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Rani, A. Textile dyeing and printing industry: An environmental hazard. Asian Dye 2013, 10, 51–54. [Google Scholar]
- Huang, D.; Wu, J.; Wang, L.; Liu, X.; Meng, J.; Tang, X.; Tang, C.; Xu, J. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem. Eng. J. 2019, 358, 1399–1409. [Google Scholar] [CrossRef]
- Bi, J.; Huang, X.; Xie, C.; Yang, J.; Wang, J.; Hao, H. Design and synthesis of core–shell Fe3O4@PTMT composite magnetic microspheres for adsorption of heavy metals from high salinity wastewater. Chemosphere 2018, 206, 513–521. [Google Scholar]
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption study of heavy metal ions from aqueous solution by nanoparticle of wild herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Zhang, Z.; Guo, J. Unravelling the adsorption disparity mechanism of heavy-metal ions on the biomass-derived hierarchically porous carbon. Appl. Surf. Sci. 2019, 471, 615–620. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pieroti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Procedia 2011, 4, 562–567. [Google Scholar] [CrossRef]
- Kim, W.-H.; Chung, H.-K.; Park, J.; Park, P.-K.; Cho, J.; Jeong, T.-Y. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar]
- Ghosal, P.S.; Gupta, A.K. Development of a generalized adsorption isotherm model at solid-liquid interface: A novel approach. J. Mol. Liq. 2017, 240, 21–24. [Google Scholar] [CrossRef]
- Chen, J.; Wang, N.; Liu, Y.; Zhu, J.; Feng, J.; Yan, W. Synergetic effect in a self-doping polyaniline/TiO2 composite for selective adsorption of heavy metal ions. Synth. Met. 2018, 245, 32–41. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Ni, B.; Huang, Q.; Wang, C.; Ni, T.; Sun, J.; Wei, W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lv, L.; Wang, H.; Tan, X. Enhanced photocatalytic treatment of Cr(VI) and phenol by monoclinic BiVO4 with {010}-orientation growth. Mater. Res. Bull. 2018, 107, 248–254. [Google Scholar] [CrossRef]
- Du, X.-D.; Deng, J.; Yi, X.-H.; Wang, C.-C.; Zheng, W.; Deng, J.; Wanga, C.C. Robust photocatalytic reduction of Cr(VI) on UiO-66-NH2(Zr/Hf) metal-organic framework membrane under sunlight irradiation. Chem. Eng. J. 2018, 356, 393–399. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Memb. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, X.; Li, J.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Zhao, S. Developing new adsorptive membrane by modification of support layer with iron oxide microspheres for arsenic removal. J. Colloid Interface Sci. 2018, 514, 760–768. [Google Scholar] [CrossRef]
- Mahdavi, H.R.; Arzani, M.; Isanejad, M.; Mohammadi, T. Effect of hydrophobic and hydrophilic nanoparticles loaded in D2EHPA/M2EHPA-PTFE supported liquid membrane for simultaneous cationic dyes pertraction. J. Environ. Manag. 2018, 213, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Huang, D.; Qin, L.; Zeng, G.; Lai, C.; Cheng, M.; Ye, S.; Song, B.; Ren, X.; Guo, X. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Ng, B.C.; Ismail, A.F. AT-POME colour removal through photocatalytic submerged filtration using antifouling PVDF-TNT nanocomposite membrane. Sep. Purif. Technol. 2018, 191, 266–275. [Google Scholar] [CrossRef]
- Zhang, Q.; Quan, X.; Wang, H.; Chen, S.; Su, Y.; Li, Z. Constructing a visible-light-driven photocatalytic membrane by g-C3N4 quantum dots and TiO2 nanotube array for enhanced water treatment. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Zhang, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal. Chem. Eng. J. 2017, 314, 38–49. [Google Scholar] [CrossRef]
- Ye, C.; An, Q.; Wu, J.; Zhao, F.; Zheng, P.; Wang, N. Nanofiltration membranes consisting of quaternized polyelectrolyte complex nanoparticles for heavy metal removal. Chem. Eng. J. 2019, 359, 994–1005. [Google Scholar] [CrossRef]
- Yuan, J.; Hung, W.; Zhu, H.; Guan, K.; Ji, Y.; Mao, Y.; Liu, G.; Lee, K.; Jin, W. Fabrication of ZIF-300 membrane and its application for efficient removal of heavy metal ions from wastewater. J. Memb. Sci. 2019, 572, 20–27. [Google Scholar] [CrossRef]
- Attia, H.; Johnson, D.J.; Wright, C.J.; Hilal, N. Comparison between dual-layer (superhydrophobic–hydrophobic) and single superhydrophobic layer electrospun membranes for heavy metal recovery by air-gap membrane distillation. Desalination 2018, 439, 31–45. [Google Scholar] [CrossRef]
- Mahmud, H.N.M.E.; Huq, A.K.O.; Yahya, R. Polymer-based adsorbent for heavy metals removal from aqueous solution. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012100. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 1–30. [Google Scholar] [CrossRef]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ananda, K.; Ismail, A.F. Fabrication of polydopamine functionalized halloysite nanotube/polyetherimide membranes for heavy metal removal. J. Mater. Chem. A 2016, 4, 764–774. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Dutta, M.; De, S. A novel ultrafiltration grade nickel iron oxide doped hollow fiber mixed matrix membrane: Spinning, characterization and application in heavy metal removal. Sep. Purif. Technol. 2017, 188, 155–166. [Google Scholar] [CrossRef]
- Van der Bruggen, B. The Separation power of nanotubes in membranes: A review. ISRN Nanotechnol. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, S.; Huang, X.; Xiao, K.; Ma, Z.; Wang, H.; Lu, P. A facile approach to fabrication of superhydrophilic ultrafiltration membranes with surface-tailored nanoparticles. Sep. Purif. Technol. 2018, 203, 251–259. [Google Scholar]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Zou, S.W.; Nanayakkara, K.G.N.; Matsuura, T.; Chen, J.P. Adsorptive removal of arsenic from aqueous solution by a PVDF/zirconia blend flat sheet membrane. J. Memb. Sci. 2011, 374, 1–11. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Hilal, N. Treatment of highly concentrated dye solution by coagulation/flocculation-sand filtration and nanofiltration. Water Resour. Ind. 2013, 3, 23–34. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Bai, H. Photocatalytic removal of NO and NO2 using titania nanotubes synthesized by hydrothermal method. J. Environ. Sci. (China) 2014, 26, 1180–1187. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, Y.W.L.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and titania nanostructured materials for environmental and energy applications: A review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Kijima, T. Inorganic and metallic nanotubular materials: Recent technologies and applications. Top. Appl. Phys. 2010, 117, 17–32. [Google Scholar]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Suarez-Martinez, I.; Grobert, N.; Ewels, C.P. Nomenclature of sp2 carbon nanoforms. Carbon 2012, 50, 741–747. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Liu, Z.; Deng, Z.; Wang, W. Application of titanate nanoflowers for dye removal: A comparative study with titanate nanotubes and nanowires. Chem. Eng. J. 2012, 191, 38–44. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Megiel, E.; Ali, G.A.M.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Gupta, V.K.; Sillanpää, M.; Sadegh, H. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostructure Chem. 2017, 7, 1–14. [Google Scholar]
- Sadegh, H.; Shahryari-Ghoshekandi, R.; Agarwal, S.; Tyagi, I.; Asif, M.; Gupta, V.K. Microwave-assisted removal of malachite green by carboxylate functionalized multi-walled carbon nanotubes: Kinetics and equilibrium study. J. Mol. Liq. 2015, 206, 151–158. [Google Scholar] [CrossRef]
- Aprianti, T.; Miskah, S.; Selpiana; Komala, R.; Hatina, S. Heavy metal ions adsorption from pulp and paper industry wastewater using zeolite/activated carbon-ceramic composite adsorbent. AIP Conf. Proc. 2018, 2014, 020127. [Google Scholar]
- Sharma, M.; Singh, J.; Hazra, S.; Basu, S. Adsorption of heavy metal ions by mesoporous ZnO and TiO2@ZnO monoliths: Adsorption and kinetic studies. Microchem. J. 2019, 145, 105–112. [Google Scholar] [CrossRef]
- González, F.; Castro, L.; Blázquez, M.L.; Ballester, A.; Muñoz, J.A. Heavy metal adsorption using biogenic iron compounds. Hydrometallurgy 2018, 179, 44–51. [Google Scholar]
- Zhao, W.; Wei, Z.; Ma, L.; Liang, J.; Zhang, X. Ag2S quantum dots based on flower-like SnS2 as matrix and enhanced photocatalytic degradation. Materials 2019, 12, 582. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Liu, C.; Tang, C.; Wang, X.; Ding, G.; Ding, Y.; Yang, L. TiO2 nanotubes/Ag/MoS2 meshy photoelectrode with excellent photoelectrocatalytic degradation activity for tetracycline hydrochloride. Nanomaterials 2018, 8, 666. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Zheng, W.; Chen, S.; Zhao, J. Preparation of N-doped carbon nanosheets from sewage sludge for adsorption studies of Cr(VI) from aqueous solution. Nanomaterials 2019, 9, 265. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Liu, Y.-C. Microstructures and photodegradation performance toward methylene orange of sputtering-assisted decoration of ZnFe2O4 crystallites onto TiO2 nanorods. Nanomaterials 2019, 9, 205. [Google Scholar] [CrossRef]
- Jiang, X.F.; Weng, Q.; Wang, X.B.; Li, X.; Zhang, J.; Golberg, D.; Bando, Y. Recent progress on fabrications and applications of boron nitride nanomaterials: A review. J. Mater. Sci. Technol. 2015, 31, 589–598. [Google Scholar] [CrossRef]
- Bernholc, J.; Brenner, D.; Buongiorno Nardelli, M.; Meunier, V.; Roland, C. Mechanical and electrical properties of nanotubes. Annu. Rev. Mater. Res. 2002, 32, 347–375. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 nanotubes: Recent advances in synthesis and gas sensing properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Bäumer, M.; Weissmüller, J.; Biener, J.; Wittstock, A.; Hamza, A.; Baumann, T. Surface chemistry in nanoscale materials. Materials 2009, 2, 2404–2428. [Google Scholar]
- Shende, P.; Kasture, P.; Gaud, R.S. Nanoflowers: The future trend of nanotechnology for multi-applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Bhanjana, G.; Dilbaghi, N.; Kim, K.H.; Kumar, S. Low temperature synthesis of copper oxide nanoflowers for lead removal using sonochemical route. J. Mol. Liq. 2017, 244, 506–511. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Du, M.; Wang, X.; Ji, X.; He, Z. The preparation of dual-functional hybrid nanoflower and its application in the ultrasensitive detection of disease-related biomarker. Biosens. Bioelectron. 2017, 92, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R.; Du, G.; Zhang, W.; Liu, L.; Liu, Y.; Mei, L.; Li, Z. Photocatalytic degradation of methylene blue using TiO2 Impregnated diatomite. Adv. Mater. Sci. Eng. 2014, 2014, 170148. [Google Scholar] [CrossRef]
- Gangwar, A.; Varghese, S.S.; Meena, S.S.; Prajapat, C.L.; Gupta, N.; Prasad, N.K. Fe3C nanoparticles for magnetic hyperthermia application. J. Magn. Magn. Mater. 2019, 481, 251–256. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Cai, T.; Xia, X. Simultaneous removal of heavy metal ions and organic pollutant by BiOBr/Ti3C2 nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 375, 201–208. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, L.; Xuan, Y.; Zhao, Z.; Kong, D.; Du, L.; Fang, Z. Research about top electrode improvement of ZnO nanowires array nanogenerator. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; pp. 1084–1087. [Google Scholar]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Bobinger, M.; Dergianlis, V.; Becherer, M.; Lugli, P. Comprehensive synthesis study of well-dispersed and solution-processed metal nanowires for transparent heaters. J. Nanomater. 2018, 2018, 7304807. [Google Scholar] [CrossRef]
- Shaban, M.; AbdAllah, H.; Said, L.; Hamdy, H.S.; Abdel Khalek, A. Titanium dioxide nanotubes embedded mixed matrix PES membranes characterization and membrane performance. Chem. Eng. Res. Des. 2015, 95, 307–316. [Google Scholar] [CrossRef]
- Malhotra, A.; Maldovan, M. Thermal transport in semiconductor nanotubes. Int. J. Heat Mass Transf. 2019, 130, 368–374. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Kamalian, S.; Shayeghi, M.; Yousefi, M.; Heidarinejad, Z.; Agarwal, S.; Gupta, V.K. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem. J. 2019, 145, 486–491. [Google Scholar] [CrossRef]
- Fu, M.; Xing, J.; Ge, Z. Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci. Total Environ. 2019, 651, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Nguyen, T.P.; Kim, S.Y.; Jung Park, T.; Hasani, A.; Choi, K.S.; Van Le, Q.; Lee, T.H.; Tekalgne, M. The role of metal dopants in WS2 nanoflowers in enhancing the hydrogen evolution reaction. Appl. Catal. A Gen. 2018, 567, 73–79. [Google Scholar]
- Runowski, M. Nanotechnology–nanomaterials, nanoparticles and multifunctional core/shell type nanostructures. Chemik 2014, 68, 766–775. [Google Scholar]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Gusatti, M.; Souza, D.A.R.; Durazzo, M.; Riella, H.G. Chemical processes for the synthesis of nanostructured materials. In Manufacturing Nanostructures; One Central Press: Chesire, UK, 2014; pp. 50–78. ISBN 9781910086070. [Google Scholar]
- Pareek, V.; Jain, N.; Panwar, J.; Bhargava, A.; Gupta, R. Synthesis and applications of noble metal nanoparticles: A review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile method to synthesize magnetic iron oxides/TiO2 hybrid nanoparticles and their photodegradation application of methylene blue. Nanoscale Res. Lett. 2011, 6, 533. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, X.; Liu, Z.; Gao, J.; Sun, H.; Fu, Y. Hydrothermal synthesis of Ag nanoparticles on the nanocellulose and their antibacterial study. Inorg. Chem. Commun. 2018, 100, 44–50. [Google Scholar]
- Zhang, N.; Chen, D.; Niu, F.; Wang, S.; Qin, L.; Huang, Y. Enhanced visible light photocatalytic activity of Gd-doped BiFeO3 nanoparticles and mechanism insight. Sci. Rep. 2016, 6, 26467. [Google Scholar] [CrossRef]
- Wu, L.; Yang, X.; Li, J.; Huang, Y.; Li, X. Fabrication of titanium dioxide nanotubes with good morphology at high calcination temperature and their photocatalytic activity. Mater. Chem. Phys. 2017, 202, 136–142. [Google Scholar] [CrossRef]
- Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. State-of-the-art strategies for the colorimetric detection of heavy metals using gold nanorods based on aspect ratio reduction. Anal. Methods 2016, 8, 2131–2137. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Teleb, N.H.; Yahia, I.S.; Zahran, H.Y.; Elhaes, H.; Ibrahim, M.A. First principles study of the adsorption of hydrated heavy metals on graphene quantum dots. J. Phys. Chem. Solids 2019, 130, 32–40. [Google Scholar] [CrossRef]
- Wei, S.; Guo, Z.; Bafana, A.; Patel, M.; Guo, J.; Qiu, B.; Lu, Y.; Wujcik, E.K.; Jeffryes, C.; Wang, X.; et al. Carbon nanotubes, graphene, and their derivatives for heavy metal removal. Adv. Compos. Hybrid Mater. 2017, 1, 56–78. [Google Scholar]
- Fu, Y.; Liu, X.; Chen, G. Adsorption of heavy metal sewage on nano-materials such as titanate/TiO2 added lignin. Results Phys. 2019, 12, 405–411. [Google Scholar] [CrossRef]
- Yarandpour, M.R.; Rashidi, A.; khajavi, R.; Eslahi, N.; Yazdanshenas, M.E. Mesoporous PAA/dextran-polyaniline core-shell nanofibers: Optimization of producing conditions, characterization and heavy metal adsorptions. J. Taiwan Inst. Chem. Eng. 2018, 93, 566–581. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, K.; Cai, J.; Hou, S.; Wang, Y.; Jiang, P.; Liang, M. Silica oxide encapsulated natural zeolite for high efficiency removal of low concentration heavy metals in water. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 388–394. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Han, T.; Cheng, M.; Zhang, W.; Long, J.; Fu, X. A biomimetic SiO2@chitosan composite as highly-efficient adsorbent for removing heavy metal ions in drinking water. Chemosphere 2019, 214, 738–742. [Google Scholar] [CrossRef]
- Luu, C.L.; Nguyen, Q.T.; Ho, S.T. Synthesis and characterization of Fe-doped TiO2 photocatalyst by the sol–gel method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 015008. [Google Scholar] [CrossRef]
- Ahmed, M.A.; El-Katori, E.E.; Gharni, Z.H. Photocatalytic degradation of methylene blue dye using Fe2O3/TiO2 nanoparticles prepared by sol–gel method. J. Alloys Compd. 2013, 553, 19–29. [Google Scholar] [CrossRef]
- Aware, D.V.; Jadhav, S.S. Synthesis, characterization and photocatalytic applications of Zn-doped TiO2 nanoparticles by sol–gel method. Appl. Nanosci. 2016, 6, 965–972. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Abdullah, N.; Lau, W.J.; Ng, B.C.; Ismail, A.F. Adsorption and photocatalytic degradation of methylene blue using high surface area titanate nanotubes (TNT) synthesized via hydrothermal method. J. Nanoparticle Res. 2017, 19, 220. [Google Scholar] [CrossRef]
- Li, H.B.; Zhang, J.; Huang, G.Y.; Fu, S.H.; Ma, C.; Wang, B.Y.; Huang, Q.R.; Liao, H.W. Hydrothermal synthesis and enhanced photocatalytic activity of hierarchical flower-like Fe-doped BiVO4. Trans. Nonferrous Met. Soc. China 2017, 27, 868–875. [Google Scholar] [CrossRef]
- Huang, M.; Tang, Z.; Yang, J. A new insight for the self-assembly of graphene oxide by hydrothermal method. Diam. Relat. Mater. 2019, 94, 73–80. [Google Scholar] [CrossRef]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Kwon, S.; Zhang, F.; Stephen, B.; Kim, K.K.; Jung, R.; Kwon, S.; Chung, K.B.; Yang, W. Photocatalytic improvement of Mn-adsorbed g-C3N4. Appl. Catal. B Environ. 2017, 206, 271–281. [Google Scholar] [CrossRef]
- Miller, C.J.; Yu, H.; Waite, T.D. Degradation of rhodamine B during visible light photocatalysis employing Ag@AgCl embedded on reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 147–153. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, H.; Xu, Q.; He, J.; Lu, J.; Chen, D.; Dong, R.; Li, N. Morphology-controlled fabrication of CNT@MoS2/SnS2 nanotubes for promoting photocatalytic reduction of aqueous Cr(VI) under visible light. J. Alloys Compd. 2019, 784, 282–292. [Google Scholar]
- Chandra, R. Synthesis of mixed metal oxide nanoparticles of SnO2 with SrO via sol–gel technology: Their structural, optical, and electrical properties. J. Sol-Gel Sci. Technol. 2018, 87, 41–49. [Google Scholar]
- Lu, D.; Kumar Kondamareddy, K.; Fan, H.; Gao, B.; Wang, J.; Wang, J.; Hao, H. Highly improved visible-light-driven photocatalytic removal of Cr(VI) over yttrium doped H-Titanate nanosheets and its synergy with organic pollutant oxidation. Sep. Purif. Technol. 2019, 210, 775–785. [Google Scholar] [CrossRef]
- Bayal, N.; Jeevanandam, P. Synthesis of TiO2-MgO mixed metal oxide nanoparticles via a sol-gel method and studies on their optical properties. Ceram. Int. 2014, 40, 15463–15477. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Parvini, M.; Ghorbani, M. Adsorption of heavy metals (Cu2+ and Zn2+) on novel bifunctional ordered mesoporous silica: Optimization by response surface methodology. J. Taiwan Inst. Chem. Eng. 2018, 84, 123–141. [Google Scholar] [CrossRef]
- Deng, J.; You, Y.; Sahajwalla, V.; Joshi, R.K. Transforming waste into carbon-based nanomaterials. Carbon 2016, 96, 105–115. [Google Scholar] [CrossRef]
- Kumar, P.S.; Venkatesh, K.; Gui, E.L.; Jayaraman, S.; Singh, G.; Arthanareeswaran, G. Electrospun carbon nanofibers/TiO2-PAN hybrid membranes for effective removal of metal ions and cationic dye. Environ. Nanotechnol. Monit. Manag. 2018, 10, 366–376. [Google Scholar] [CrossRef]
- Overah, L.C.; Iwegbue, C.M.; Babalola, J.O.; Martincigh, B.S. Fabrication and characterisation of a Fe3O4/Raphia farinifera nanocomposite for application in heavy metal adsorption. Environ. Technol. Innov. 2019, 13, 11–29. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Wang, N.; Feng, J.; Yan, W. Hydrophilic polythiophene/SiO2 composite for adsorption engineering: Green synthesis in aqueous medium and its synergistic and specific adsorption for heavy metals from wastewater. Chem. Eng. J. 2018, 360, 1486–1497. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Marciniak, M.; Goscianska, J.; Frankowski, M.; Pietrzak, R. Optimal synthesis of oxidized mesoporous carbons for the adsorption of heavy metal ions. J. Mol. Liq. 2019, 276, 630–637. [Google Scholar] [CrossRef]
- Li, L.Y.; Gong, X.D.; Abida, O. Waste-to-resources: Exploratory surface modification of sludge-based activated carbon by nitric acid for heavy metal adsorption. Waste Manag. 2019, 87, 375–386. [Google Scholar] [CrossRef]
- Luo, S.; Cai, T.; Liu, C.; Zhang, Y.; Liu, Y.; Ma, J.; Wei, Y.; Ali, O.; Zhang, S. Fast adsorption of heavy metal ions by waste cotton fabrics based double network hydrogel and influencing factors insight. J. Hazard. Mater. 2017, 344, 1034–1042. [Google Scholar]
- Wang, S.; Ning, H.; Hu, N.; Huang, K.; Weng, S.; Wu, X.; Wu, L.; Liu, J. Alamusi preparation and characterization of graphene oxide/silk fibroin hybrid aerogel for dye and heavy metal adsorption. Compos. Part B Eng. 2019, 163, 716–722. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manage. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Ghiloufi, I.; El Ghoul, J.; Modwi, A.; Mir, L. El Ga-doped ZnO for adsorption of heavy metals from aqueous solution. Mater. Sci. Semicond. Process. 2016, 42, 102–106. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; Shao, X.; Yuan, S.; Yang, C.; Hu, X. Adsorption of heavy metal ions by hierarchically structured magnetite-carbonaceous spheres. Talanta 2012, 101, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Jain, P.; Kumar, V.; Gupta, R.K. Interfacial engineering of Fe2O3@BOC heterojunction for efficient detoxification of toxic metal and dye under visible light illumination. J. Environ. Chem. Eng. 2019, 7, 102843. [Google Scholar] [CrossRef]
- Wang, P.; Yi, X.; Du, X.; Wang, C.; Deng, J. Enhanced photocatalytic Cr(VI) reduction and diclofenac sodium degradation under simulated sunlight irradiation over MIL-100(Fe)/g-C3N4 heterojunctions. Chinese J. Catal. 2018, 40, 70–79. [Google Scholar] [CrossRef]
- Kumar, K.V.A.; Chandana, L.; Ghosal, P.; Subrahmanyam, C. Simultaneous photocatalytic degradation of p-cresol and Cr(VI) by metal oxides supported reduced graphene oxide. Mol. Catal. 2018, 451, 87–95. [Google Scholar] [CrossRef]
- Koh, P.W.; Hatta, M.H.M.; Ong, S.T.; Yuliati, L.; Lee, S.L. Photocatalytic degradation of photosensitizing and non-photosensitizing dyes over chromium doped titania photocatalysts under visible light. J. Photochem. Photobiol. A Chem. 2017, 332, 215–223. [Google Scholar] [CrossRef]
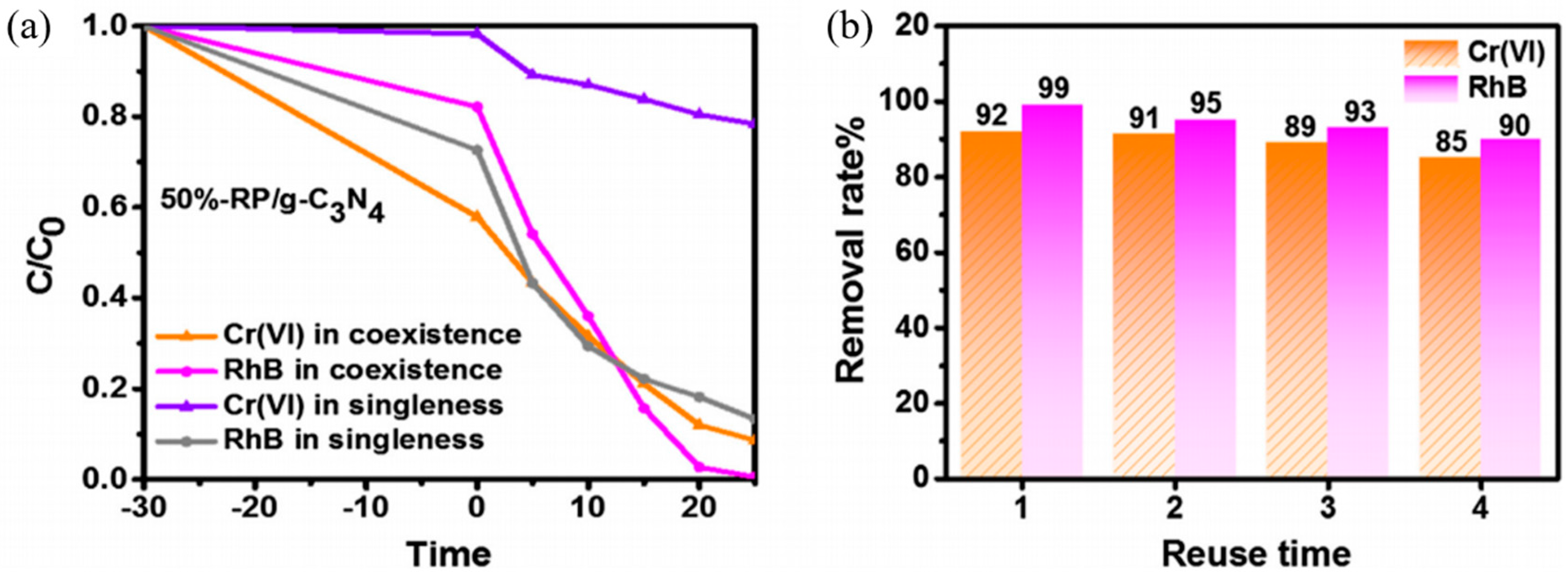
- Liu, E.; Du, Y.; Bai, X.; Fan, J.; Hu, X. Synergistic improvement of Cr(VI) reduction and RhB degradation using RP/g-C3N4 photocatalyst under visible light irradiation. Arab. J. Chem. 2019. [Google Scholar] [CrossRef]
- Ruan, X.; Hu, H.; Che, H.; Jiang, E.; Zhang, X.; Liu, C.; Che, G. A visible-light-driven Z-scheme CdS/Bi12GeO20 heterostructure with enhanced photocatalytic degradation of various organics and the reduction of aqueous Cr(VI). J. Colloid Interface Sci. 2019, 543, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Cui, L.; Huang, Z.; Huang, Z.; Wu, P.; Dai, X.; Wang, T. Simultaneous and efficient photocatalytic reduction of Cr(VI) and oxidation of trace sulfamethoxazole under LED light by rGO@Cu2O/BiVO4 p-n heterojunction composite. Chemosphere 2019, 221, 824–833. [Google Scholar]
- Xu, S.; Dai, J.; Pang, X.; Yang, J.; Wang, Y.; Hao, J. Visible-light-driven photocatalytic degradation of 4-CP and the synergistic reduction of Cr(VI) on one-pot synthesized amorphous Nb2O5 nanorods/graphene heterostructured composites. Chem. Eng. J. 2018, 353, 100–114. [Google Scholar]
- Kaur, K.; Jindal, R. Synergistic effect of organic-inorganic hybrid nanocomposite ion exchanger on photocatalytic degradation of Rhodamine-B dye and heavy metal ion removal from industrial effluents. J. Environ. Chem. Eng. 2018, 6, 7091–7101. [Google Scholar] [CrossRef]
- Ghafoor, S.; Hussain, S.Z.; Waseem, S.; Arshad, S.N. Photo-reduction of heavy metal ions and photo-disinfection of pathogenic bacteria under simulated solar light using photosensitized TiO2 nanofibers. RSC Adv. 2018, 8, 20354–20362. [Google Scholar] [CrossRef]
- Bai, X.; Du, Y.; Hu, X.; He, Y.; He, C.; Liu, E.; Fan, J. Synergy removal of Cr(VI) and organic pollutants over RP-MoS2/rGO photocatalyst. Appl. Catal. B Environ. 2018, 239, 204–213. [Google Scholar] [CrossRef]
- Ye, M.; Wei, W.; Zheng, L.; Liu, Y.; Wu, D.; Gu, X.; Wei, A. Enhanced visible light photoreduction of aqueous Cr(VI) by Ag/Bi4O7/g-C3N4 nanosheets ternary metal/non-metal Z-scheme heterojunction. J. Hazard. Mater. 2019, 365, 674–683. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, P.; Wang, S.; Luo, Z.; Huang, C.; Wang, X. Structure-mediated charge separation in boron carbon nitride for enhanced photocatalytic oxidation of alcohol. ChemSusChem 2018, 11, 3949–3955. [Google Scholar] [CrossRef]
- Shinde, S.S.; Kher, J.A. A review on polyaniline and its noble metal composites. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 16570–16576. [Google Scholar] [CrossRef]
- Xie, M.; Huang, L.; Liu, J.; Xu, Y.; Deng, J.; Li, H.; Xu, H.; Jing, L. Three dimensional polyaniline/MgIn2S4 nanoflower photocatalysts accelerated interfacial charge transfer for the photoreduction of Cr(VI), photodegradation of organic pollution and photocatalytic H2 production. Chem. Eng. J. 2018, 360, 1601–1612. [Google Scholar]
- Wang, G.; Fan, W.; Li, Q.; Deng, N. Enhanced photocatalytic new coccine degradation and Pb(II) reduction over graphene oxide-TiO2 composite in the presence of aspartic acid-B-cyclodextrin. Chemosphere 2019, 216, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Dasari, A.; Belay, H.H.; Guttena, V. Fabrication of novel 1D/2D V2O5/g-C3N4 composites as Z-scheme photocatalysts for CR degradation and Cr(VI) reduction under sunlight irradiation. J. Environ. Chem. Eng. 2019, 7, 102822. [Google Scholar] [CrossRef]
- Barati, R.; Gilan, N.; Yousefi, N.; Ghasemi, S.; Ahmadian, M.; Moussavi, S.; Rahimi, S.; Fatehizadeh, A.; Rahimi, K.; Reshadat, S. Photocatalytic removal of cadmium (II) and lead (II) from simulated wastewater at continuous and batch system. Int. J. Environ. Health Eng. 2014, 3, 31. [Google Scholar]
- Wang, F.X.; Yi, X.H.; Wang, C.C.; Deng, J.G. Photocatalytic Cr(VI) reduction and organic-pollutant degradation in a sTable 2D coordination polymer. Cuihua Xuebao/Chinese J. Catal. 2017, 38, 2141–2149. [Google Scholar] [CrossRef]
- Deng, F.; Lu, X.; Luo, Y.; Wang, J.; Che, W.; Yang, R.; Luo, X.; Luo, S.; Dionysiou, D.D. Novel visible-light-driven direct Z-scheme CdS/CuInS2 nanoplates for excellent photocatalytic degradation performance and highly-efficient Cr(VI) reduction. Chem. Eng. J. 2019, 361, 1451–1461. [Google Scholar] [CrossRef]
- Kumordzi, G.; Malekshoar, G.; Yanful, E.K.; Ray, A.K. Solar photocatalytic degradation of Zn2+ using graphene based TiO2. Sep. Purif. Technol. 2016, 168, 294–301. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Vasanthakumar, V.; Karthikeyan, S.; Raj, V.; Shanavas, S.; Anbarasan, P.M. Multi-functional properties of ternary CeO2/SnO2/rGO nanocomposites: Visible light driven photocatalyst and heavy metal removal. J. Photochem. Photobiol. A Chem. 2017, 346, 32–45. [Google Scholar] [CrossRef]
- Sunil, K.; Karunakaran, G.; Yadav, S.; Padaki, M. Al-Ti2O6 a mixed metal oxide based composite membrane: A unique membrane for removal of heavy metals. Chem. Eng. J. 2018, 348, 678–684. [Google Scholar] [CrossRef]
- Ghaemi, N.; Daraei, P. Enhancement in copper ion removal by PPy@Al2O3 polymeric nanocomposite membrane. J. Ind. Eng. Chem. 2016, 40, 26–33. [Google Scholar] [CrossRef]
- Ghaemi, N.; Zereshki, S.; Heidari, S. Removal of lead ions from water using PES-based nanocomposite membrane incorporated with polyaniline modified GO nanoparticles: Performance optimization by central composite design. Process Saf. Environ. Prot. 2017, 111, 475–490. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- He, J.; Matsuura, T.; Chen, J.P. A novel Zr-based nanoparticle-embedded PSF blend hollow fiber membrane for treatment of arsenate contaminated water: Material development, adsorption and filtration studies, and characterization. J. Memb. Sci. 2014, 452, 433–445. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Chen, J.P. Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res. 2016, 101, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Delavar, M.; Bakeri, G.; Hosseini, M. Fabrication of polycarbonate mixed matrix membranes containing hydrous manganese oxide and alumina nanoparticles for heavy metal decontamination: Characterization and comparative study. Chem. Eng. Res. Des. 2017, 120, 240–253. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Lau, W.J.; Matsuura, T.; Halakoo, E.; Ismail, A.F. Adsorptive removal of Pb(II) from aqueous solution by novel PES/HMO ultrafiltration mixed matrix membrane. Sep. Purif. Technol. 2013, 120, 59–68. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Lv, Y.; Liu, J.; Zhang, D.; Shao, Z. Halloysite nanotubes and Fe3O4 nanoparticles enhanced adsorption removal of heavy metal using electrospun membranes. Appl. Clay Sci. 2018, 161, 225–234. [Google Scholar] [CrossRef]
- Khadijah, S.; Ha, M.; Othman, D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A novel green ceramic hollow fiber membrane (CHFM) derived from rice husk ash as combined adsorbent-separator for efficient heavy metals removal. Ceram. Int. 2017, 43, 4716–4720. [Google Scholar]
- Ali, S.; Aziz, S.; Rehman, U.; Ali, I.; Usman, M. Efficient removal of zinc from water and wastewater effluents by hydroxylated and carboxylated carbon nanotube membranes: Behaviors and mechanisms of dynamic filtration. J. Hazard. Mater. 2019, 365, 64–73. [Google Scholar] [CrossRef]
- Attia, H.; Alexander, S.; Wright, C.J.; Hilal, N. Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 2017, 420, 318–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Gao, J.; Chung, T. Layer-by-layer construction of graphene oxide (GO) framework composite membranes for highly efficient heavy metal removal. J. Memb. Sci. 2016, 515, 230–237. [Google Scholar] [CrossRef]
- Lee, C.; Chiang, C.; Liu, S. Electrospun nanofibrous rhodanine/polymethylmethacrylate membranes for the removal of heavy metal ions. Sep. Purif. Technol. 2013, 118, 737–743. [Google Scholar] [CrossRef]
- Soghra, S.; Zahed, H.; Khanlari, S.; Mohammadi, T. Hydrous metal oxide incorporated polyacrylonitrile-based nanocomposite membranes for Cu(II) ions removal. Sep. Purif. Technol. 2019, 213, 151–161. [Google Scholar]
- Sevinc, P.C.; Dhital, B.; Govind Rao, V.; Wang, Y.; Lu, H.P. Probing electric field effect on covalent interactions at a molecule-semiconductor interface. J. Am. Chem. Soc. 2016, 138, 1536–1542. [Google Scholar] [CrossRef]
- Chavez, S.; Rao, V.G.; Linic, S. Unearthing the factors governing site specific rates of electronic excitations in multicomponent plasmonic systems and catalysts. Faraday Discuss. 2019. [Google Scholar] [CrossRef]


| Agency | Permissible Level of HM (mg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd (II) | Cr (III) | Co (II) | Cu (II) | Pb (II) | Fe (II) | Mn (II) | Hg (II) | Ni (II) | |
| National Agency for Food and Drug Administration and Control (NAFDAC) | 0.0 | NM | NM | NM | 0.0 | NM | NM | 0.0 | NM |
| United States Environmental Protection Agency (USEPA) | 0.005 | 0.1 | 0.1 | 1.3 | 0.015 | 0.3 | 0.05 | 0.002 | 0.1 |
| World Health Organization (WHO) | 0.003 | NM | NM | 0.01 | 0.01 | 0.3 | 0.4 | 0.001 | 0.07 |
| Department of Environment (DOE), Malaysia | 0.005 | 0.05 | NM | 1.0 | 0.1 | 1.0 | 0.2 | NM | NM |
| Material | Classification | Unique Feature | Synthesis Technique | Application | Reference |
|---|---|---|---|---|---|
| Streptavidin (SA)-horseradish peroxidase (HRP) | Nanoflowers | Improved biocompatibility and attachment of the protein | Wet chemical synthesis | Biomarker detection | [95] |
| TiO2 and diatomite | Nanoparticle | High surface area, improved absorbability | Wet chemical precipitation | Photocatalyst | [96] |
| Fe3C | Nanoparticle | Good heating ability in magnetic fields | Hydrothermal and sonication | Magnetic hyperthermia | [97] |
| BiOBr/Ti3C2 | Nanoparticle | Surface functionalisation | Self-assembly | HM photoreduction | [98] |
| ZnO | Nanorods | Improved electrode performance | Hydrothermal and sputtering | Energy nanogenerators | [99] |
| Au | Nanorods | Huge electric field enhancements | Direct growth and fabrication | Plasmonic spectroscopies | [100] |
| Ag | Nanorods | Increased dispersion and stability | Wet chemical synthesis | Transparent heaters | [101] |
| TiO2 | Nanotubes | High hydrophilicity, surface area | Hydrothermal | Membrane filler | [102] |
| SiO2-Ge | Nanotubes | Excellent thermal transport, large surface-to-volume ratio. | - | Phonon transport | [103] |
| Carbon | Nanotubes | High surface area and adsorption capacity | - | Adsorption of diazinon | [104] |
| Cu3(PO4)2. | Nanoflowers | Spherical, porous and hierarchical structure | Wet chemical synthesis | Photodegradation of phenol | [105] |
| WS2 | Nanoflowers | Increased reaction sites | Hydrothermal and reduction | Hydrogen generation | [106] |
| Product | Materials | Method | Parameters | Nanomaterial Characteristics | Reference |
|---|---|---|---|---|---|
| Mn-g-C3N4 | Mn and g-C3N4 | Combustion | Heated at a rate of 520 °C with a rate of 4 °C/min (2 h) | Improved ROS creation, lower band gap (1.25 eV) | [132] |
| Ag-GO | Ag and reduced GO | Modified Tour’s method | Oxidized under 15 °C, heated to 50 °C, washed and freeze-dried | Visible light absorption improved oxidant generation capacity | [133] |
| CNT@MoS2/SnS2 nanotubes | CNT, MoS2, and SnS2 | Hydrothermal | Autoclave for 180 °C for 20 h washed with water | Faster reduction of Cr (VI), the narrow bandgap | [134] |
| SnO2-SrO | SnO2 and SrO | Sol-gel | Gel formed, digested and dried in an oven at 100 °C, washed with ammoniated water | Lower band gap (2.23 eV, impart gas sensing | [135] |
| BiOBr/Ti3C2 | BiOBr Ti3C2 | Self-assembly method | BiOBr and Ti3C2 co-precipitated under magnetic stirring | Visible light photodegradation, surface reactivity | [98] |
| Yttrium/H-titanate | Yttrium and TiO2 | Hydrothermal | Ti(SO4)2 and hydrazine hydrate (N2H4·H2O) reacted in an autoclave for 130 °C | Reduction in the efficiency of charge separation | [136] |
| TiO2-MgO | (titanium isopropoxide and magnesium methoxide | Sol-gel | Sol-gel formed, dried at 100 °C and calcined at 900 °C | Visible light sensitivity, uniform hybrid material | [137] |
| Nanomaterial | Synthesis Technique | Features | Metal Species | Adsorption Capacity | Optimum pH | Reference |
|---|---|---|---|---|---|---|
| Mesoporous carbon | Hard template technique | High uniformity of porous structure, surface functionalized | Ni (II) | 140.9 mg/g | 5 | [144] |
| Co (II) | 129.9 mg/g | |||||
| Hierarchically porous carbon | Pyrolysis and chemical activation | KOH activated | Cd (II) | 180.0 mg/g | 6 | [40] |
| Pb (II) | 220.0 mg/g | |||||
| Cu (II) | 215.0 mg/g | |||||
| Zn (II) | 95.0 mg/g | |||||
| Cr(III) | 140.0 mg/g | |||||
| Geopolymers | Alkali activation of aluminosilicate | Alkali activated, inorganic polymers | Ni (II) | 85.3 mg/g | 10 | [148] |
| Pb (II) | 111.0 mg/g | |||||
| Cd (II) | 130.5 mg/g | |||||
| Polyaniline/TiO2 | Chemical oxidative polymerisation | Self-doping, highly selective adsorption | Zn (II) | 51.6 mg/g | 5 | [45] |
| Pb (II) | 96.2 mg/g | |||||
| Cu (II) | 18.2 mg/g | |||||
| Ga-doped ZnO | Sol-gel | Improved electrical conductivity | Cr (II) | 52.2 mg/g | 3–5 | [149] |
| Cd (II) | 28.3 mg/g | |||||
| Polythiophene/SiO2 | Sol-gel | Stable and highly selective | Pb (II) | 70.9 mg/g | 5 | [142] |
| Cu (II) | 35.3 mg/g | |||||
| Zn (II) | 34.6 mg/g | |||||
| Zeolite | Amino acid as mesoporogens | Partial cation exchange | Cu (II) | 171 mg/g | 11 | [46] |
| Ni (II) | 99.1 mg/g | |||||
| Pb (II) | 514.0 mg/g | |||||
| Wild herb nanoparticle | Ball milling | Environmentally friendly | Cd (II) | 52.9 mg/g | 12 | [39] |
| Co (II) | 40.8 mg/g | |||||
| Li (II) | 181.8 mg/g | |||||
| Fe3O4 | Carbon microsphere | High BET surface area, hierarchical as well as mesoporous structures | Pb (II) | 95.2% | 6 | [150] |
| Cd (II) | 96.2% | |||||
| Cr (III) | 98.2% |
| Photocatalyst | Dopant | Method | Metal Species | Removal Performance | Reference |
|---|---|---|---|---|---|
| Ag/Bi4O7/ | g-C3N4 | Thermal polymerization, hydrothermal and calcination | Cr (VI) | 90% | [162] |
| WO3 | Reduced graphene oxide (rGO) | In-situ hydrothermal | Cr (VI) | 90% | [153] |
| Fe2O3 | Bismuth carbonate (BOC) | Two-step chemical modification | Cr (VI) | >90% | [151] |
| TiO2 | Graphene | Hydrothermal | Pb (II) | 60% | [166] |
| V2O5 nanorod | g-C3N4 nanosheets | Facile impregnation | Cr (VI) | 71% | [167] |
| Zirconium | Selenophosphate | Two-step ion exchanger | Pb (II) | 100% | [159] |
| Mg (II) | 95% | ||||
| Red phosphorus | g-C3N4 nanosheets | Thermal polymerization and hydrothermal | Cr (VI) | 92% | [155] |
| TiO2 | - | - | Cd (II) | 98% | [168] |
| Pb (II) | 99% | ||||
| Metal organic framework 100 | g-C3N4 nanosheets | Calcination and hydrothermal | Cr (VI) | 98% | [152] |
| Zn | Coordination polymers (H2L and by) | Hydrothermal | Cr (VI) | 100% | [169] |
| CdS | CuInS | Hydrothermal | Cr (VI) | 100% | [170] |
| Titanate nanosheets | Yttrium | Hydrothermal | Cr (VI) | >75% | [136] |
| TiO2 | Graphene | Hydrothermal | Zn (II) | 100% | [171] |
| TiO2 | Graphene | Hydrothermal | Pb (II) | >70% | [166] |
| CeO2/SnO2/ | rGO | Hydrothermal | Pb (II) Cd (II) | 80% 80% | [172] |
| Polymer | Nanomaterial | Removal Method | Metal Species | Control Membrane Performance | Composite Membrane Performance | Reference |
|---|---|---|---|---|---|---|
| Polyethylene oxide (PEO) | Halloysite nanotubes and | Adsorption | Cr (VI) | 80 mg/g | 85 mg/g | [181] |
| Cd (II) | 105 mg/g | 115 mg/g | ||||
| Cu (II) | 120 mg/g | 135 mg/g | ||||
| Pb (II) | 145 mg/g | 155 mg/g | ||||
| Ceramic | Rice husk ash | Adsorption and Filtration | Ni (II) | - | 99.99% | [182] |
| Zn (II) | 99.97% | |||||
| Pb (II) | 99/99% | |||||
| Polyvinyl chloride (PVC) | Carboxylated CNT | Filtration | Zn (II) | 48% | 93% | [183] |
| PVDF | Superhydrophilic alumina | Filtration | Pb (II) | 84% | 92.5% | [184] |
| Alumina Substrate | Zeolite imidazolate framework-30 | Filtration | Cu (II) | - | 99.87% | [185] |
| Polyethylenimine (PEI) | GO | Filtration | Zn (II) | 0% | 96.6% | [186] |
| Poly(ethyl methacrylate) PEMA | Rhodanine | Adsorption | Ag (II) | - | 65% | [65] |
| Pb (II) | - | 58% | ||||
| PSF | Nickel/Iron oxide | Filtration | Pb (II) | - | 95% | [65] |
| Cu (II) | - | 95% | ||||
| PAN | HMO | Filtration | Cu (II) | 37% | 70% | [187] |
| PES | Polydopamine | Adsorption | Pb (II) | >1 mg/g | 20.3 mg/g | [56] |
| Cu (II) | >1 mg/g | 10.4 mg/g | ||||
| Cd (II) | >1 mg/g | 17 mg/g | ||||
| PSF | Al-Ti2O6 | Filtration | As (II) | - | 96% | [173] |
| Cd (II) | - | 98% | ||||
| Pb (II) | - | 99% | ||||
| PSF | Quaternized polyelectrolyte complex | Filtration | Mg (II) | 86.4% | 95.7% | [57] |
| Zn (II) | 87.1% | 98.3% | ||||
| Cu (II) | 80.3% | 97.9% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Ismail, A.F. The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review. Nanomaterials 2019, 9, 625. https://doi.org/10.3390/nano9040625
Subramaniam MN, Goh PS, Lau WJ, Ismail AF. The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review. Nanomaterials. 2019; 9(4):625. https://doi.org/10.3390/nano9040625
Chicago/Turabian StyleSubramaniam, Mahesan Naidu, Pei Sean Goh, Woei Jye Lau, and Ahmad Fauzi Ismail. 2019. "The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review" Nanomaterials 9, no. 4: 625. https://doi.org/10.3390/nano9040625
APA StyleSubramaniam, M. N., Goh, P. S., Lau, W. J., & Ismail, A. F. (2019). The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review. Nanomaterials, 9(4), 625. https://doi.org/10.3390/nano9040625






