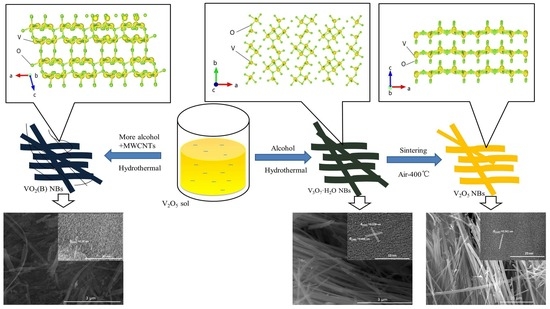
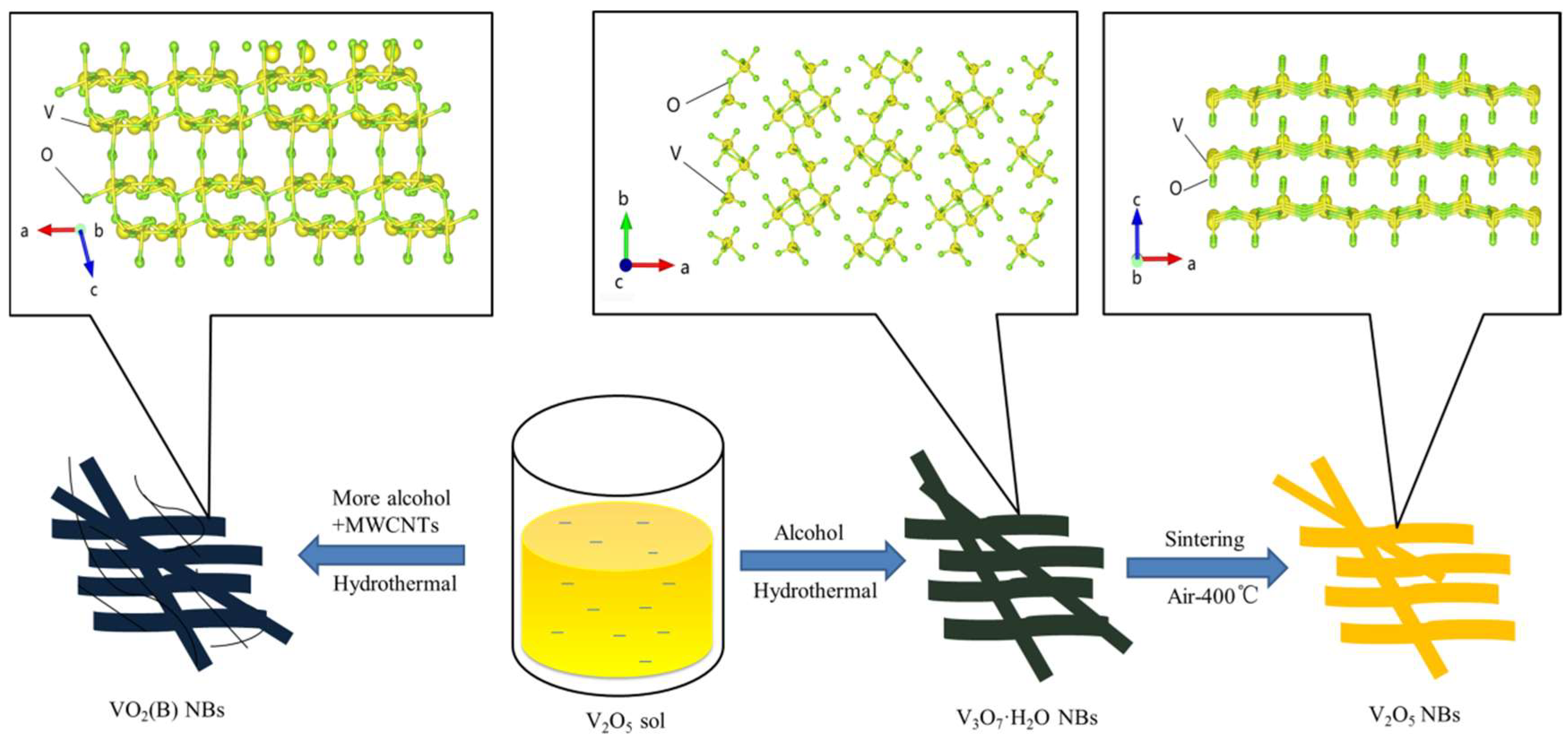
Controlled Hydrothermal Growth and Li+ Storage Performance of 1D VOx Nanobelts with Variable Vanadium Valence
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparations
2.2. Characterizations
2.3. Electrochemical Tests
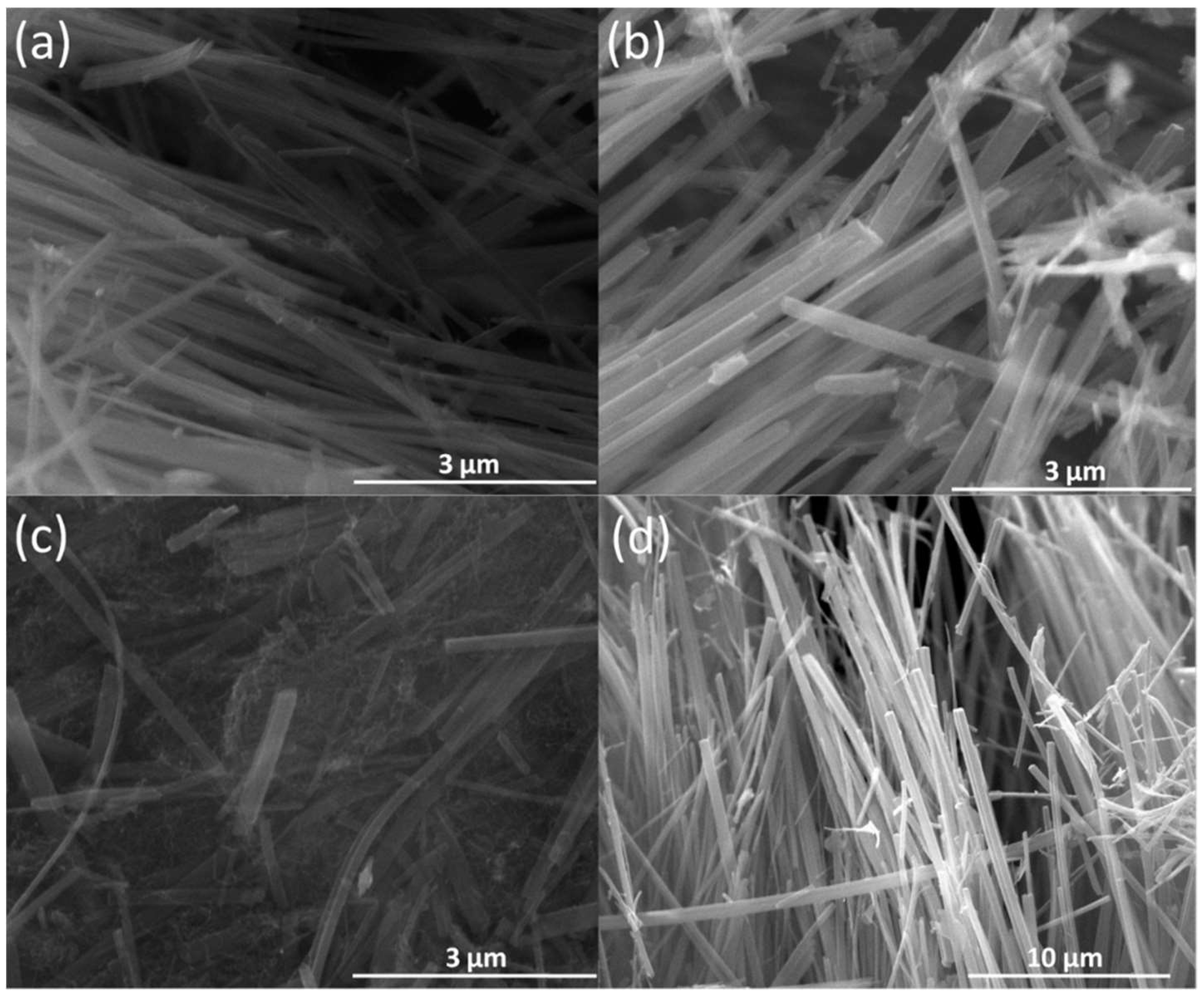
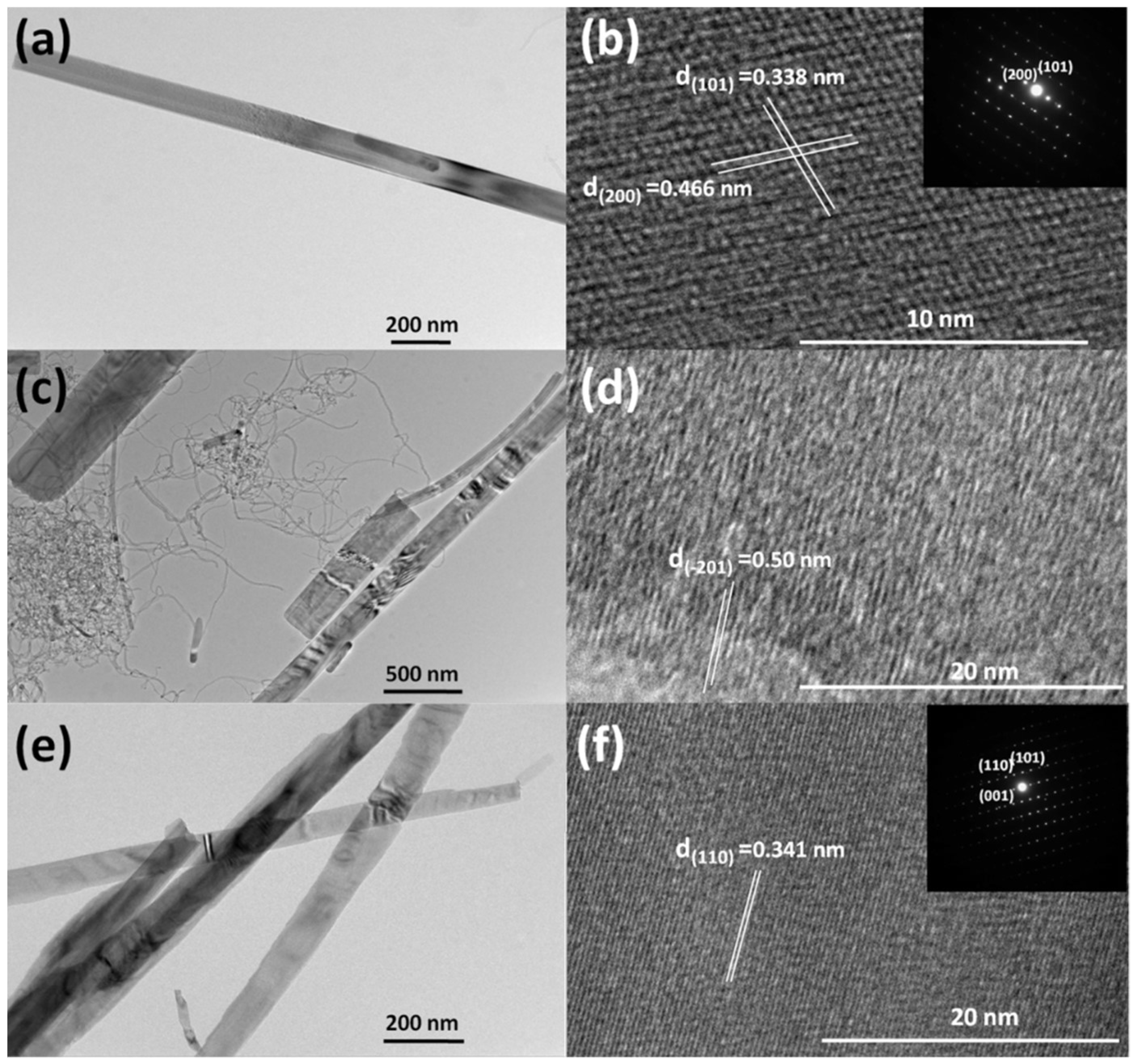
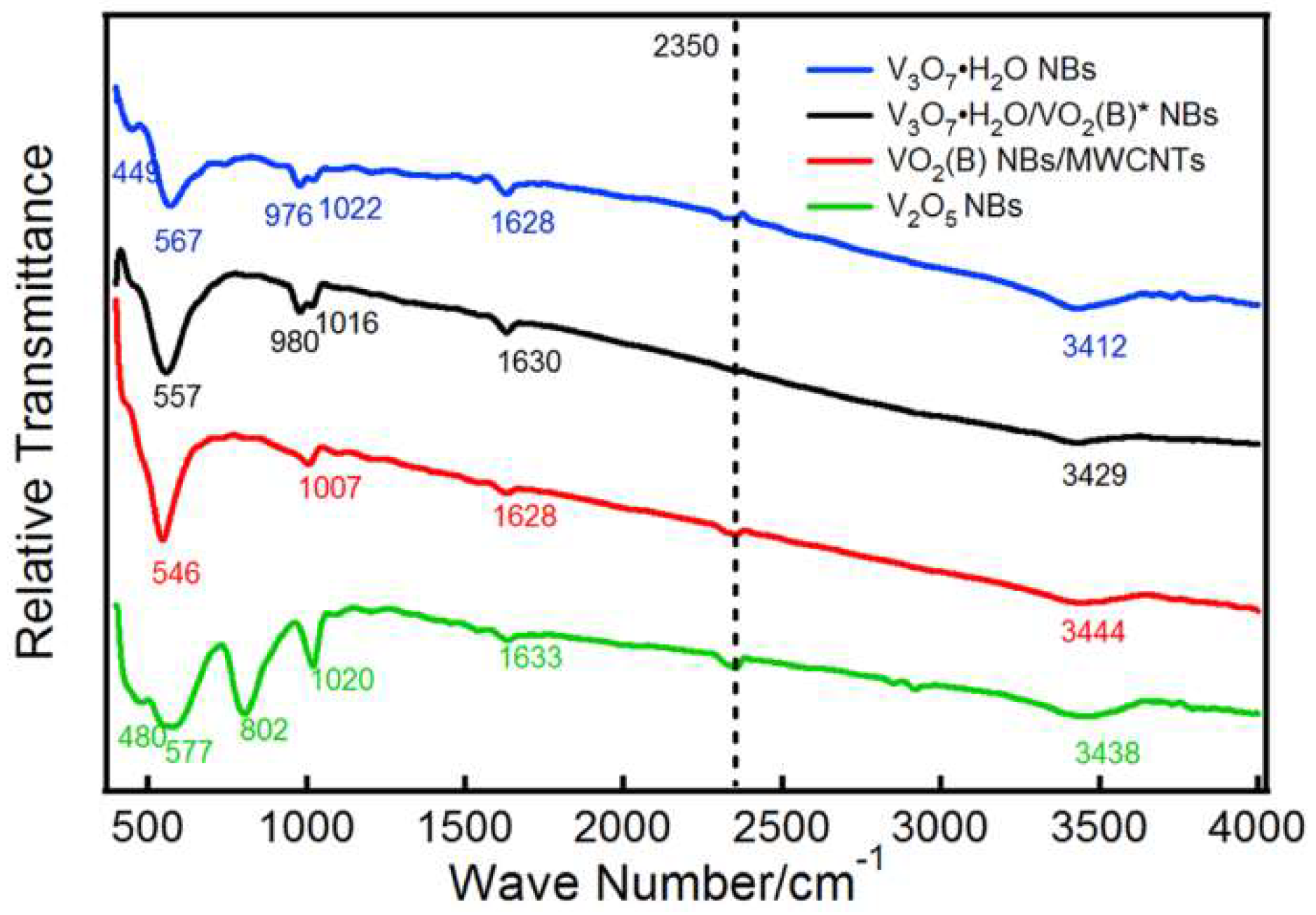
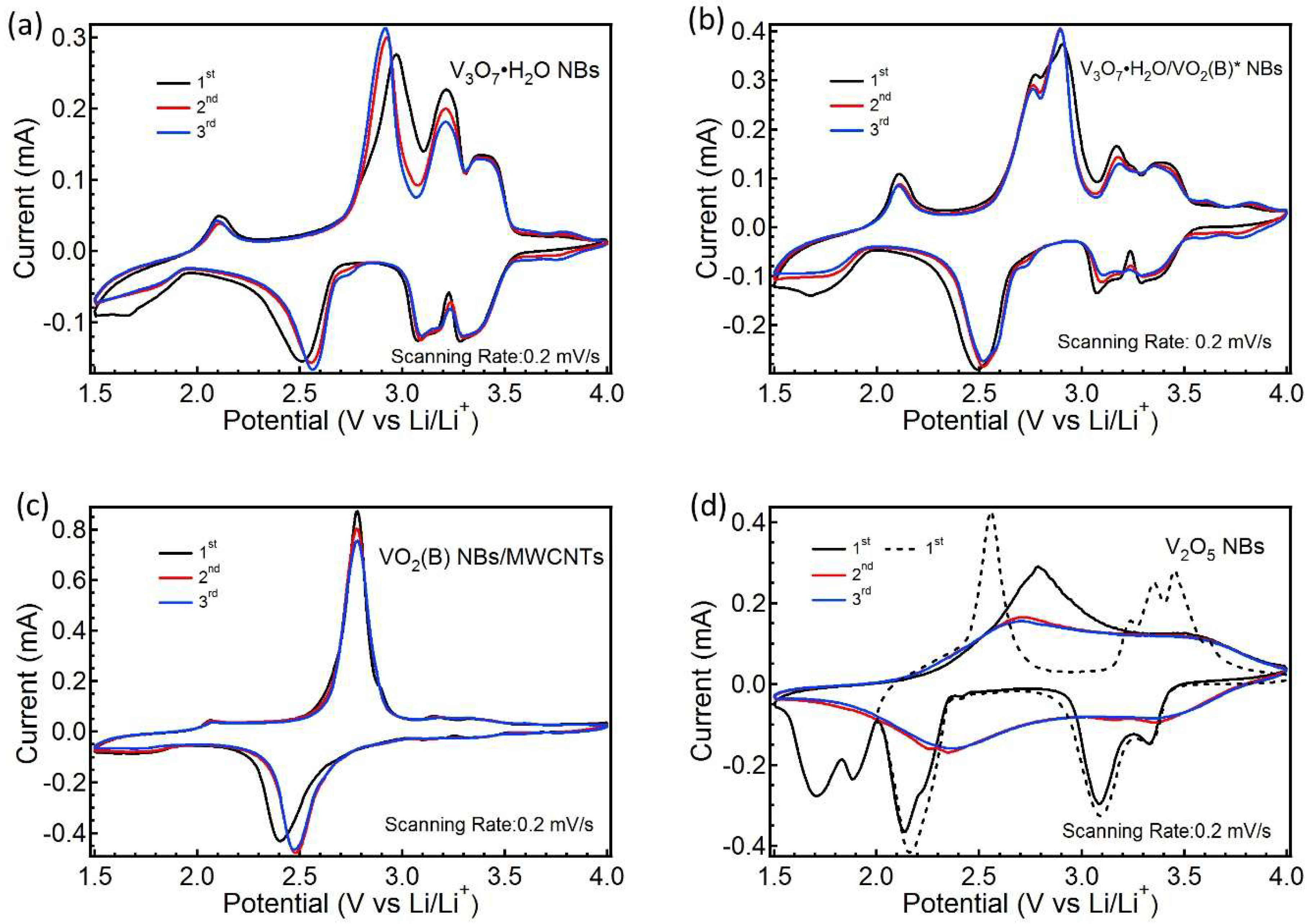
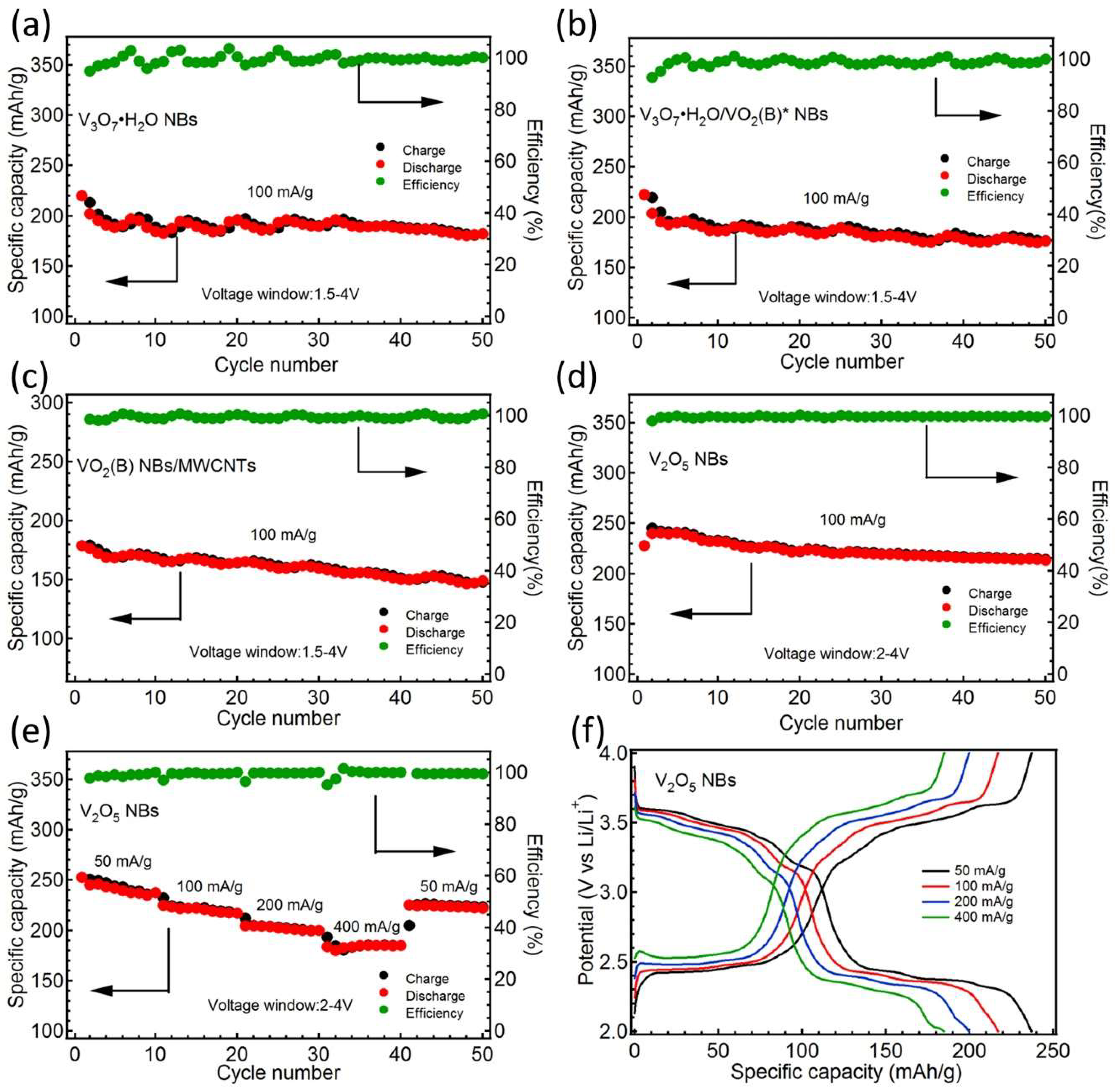
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manthiram, A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, G. Synthesis and Enhanced Intercalation Properties of Nanostructured Vanadium Oxides. Cheminform 2006, 37, 2787–2804. [Google Scholar]
- McNulty, D.; Buckley, D.N.; O’Dwyer, C. Synthesis and electrochemical properties of vanadium oxide materials and structures as Li-ion battery positive electrodes. J. Power Sources 2014, 267, 831–873. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Yang, H.; Zhou, K.; Pan, D. V2O5-Based nanomaterials: Synthesis and their applications. RSC Adv. 2018, 8, 4014–4031. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, Y.; Liang, S.; Pan, A. Controllable Preparation of V2O5/Graphene Nanocomposites as Cathode Materials for Lithium-Ion Batteries. Nanoscale Res. Lett. 2016, 11, 549. [Google Scholar] [CrossRef]
- Armer, C.F.; Yeoh, J.S.; Li, X.; Lowe, A. Electrospun vanadium-based oxides as electrode materials. J. Power Sources 2018, 395, 414–429. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Burke, D.M.; Gabriel, T.; O’Regan, C.; O’Dwyer, C.; Petkov, N.; Holmes, J.D. Carbon nanocage supported synthesis of V2O5 nanorods and V2O5/TiO2 nanocomposites for Li-ion batteries. J. Mater. Chem. A 2013, 1, 12568. [Google Scholar] [CrossRef]
- Liu, M.; Su, B.; Tang, Y.; Jiang, X.; Yu, A. Recent Advances in Nanostructured Vanadium Oxides and Composites for Energy Conversion. Adv. Energy Mater. 2017, 7, 1700885. [Google Scholar] [CrossRef]
- Petnikota, S.; Chua, R.; Zhou, Y.; Edison, E.; Srinivasan, M. Amorphous Vanadium Oxide Thin Films as Stable Performing Cathodes of Lithium and Sodium-Ion Batteries. Nanoscale Res. Lett. 2018, 13, 363. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Liu, J.; Lu, Y.; Yang, Q.; Yang, L.Y.; Yang, H.R.; Liu, S.; Lei, M.; Han, M. Carbon fiber cloth@VO2 (B): Excellent binder-free flexible electrodes with ultrahigh mass-loading. J. Mater. Chem. A 2016, 4, 6426–6432. [Google Scholar] [CrossRef]
- Li, Z.; Sun, H.; Xu, J.; Zhu, Q.; Chen, W.; Zakharova, G.S. The synthesis, characterization and electrochemical properties of V3O7·H2O/CNT Nanocomposite. Solid State Ion. 2014, 262, 30–34. [Google Scholar] [CrossRef]
- Niu, C.; Li, J.; Jin, H.; Shi, H.; Zhu, Y.; Wang, W.; Cao, M. Self-template processed hierarchical V2O5 nanobelts as cathode for high performance lithium ion battery. Electrochim. Acta 2015, 182, 621–628. [Google Scholar] [CrossRef]
- Rui, X.; Sim, D.; Xu, C.; Liu, W.; Tan, H.; Wong, K.; Hng, H.H.; Lim, T.M.; Yan, Q. One-pot synthesis of carbon-coated VO2(B) nanobelts for high-rate lithium storage. RSC Adv. 2012, 2, 1174–1180. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Wang, X.; Chang, B.; Wang, J.; Ahmad, M.; Sun, H. Oxygen vacancies enhance lithium storage performance in ultralong vanadium pentoxide nanobelt cathodes. J. Colloid Interface Sci. 2018, 539, 118–125. [Google Scholar] [CrossRef]
- Zhang, C.; Song, H.; Zhang, C.; Liu, C.; Liu, Y.; Cao, G. Interface Reduction Synthesis of H2V3O8 Nanobelts–Graphene for High-Rate Li-Ion Batteries. J. Phys. Chem. C 2015, 119, 11391–11399. [Google Scholar] [CrossRef]
- Baro, M.; Nayak, P.; Baby, T.T.; Ramaprabhu, S. Green approach for the large-scale synthesis of metal/metal oxidenanoparticle decorated multiwalled carbon nanotubes. J. Mater. Chem. A 2013, 1, 482–486. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Zhang, R.; Hu, H.; Shen, Y.; Liu, J. Preparation and performance of spherical FeF2.5·0.5H2O nanoparticles wrapped by MWCNTs as cathode material of lithium ion batteries. RSC Adv. 2016, 6, 97759–97769. [Google Scholar] [CrossRef]
- Li, H.; Zhai, T.; He, P.; Wang, Y.; Hosono, E.; Zhou, H. Single-crystal H2V3O8 nanowires: A competitive anode with large capacity for aqueous lithium-ion batteries. J. Mater. Chem. 2011, 21, 1780–1787. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Q.; Sheng, J.; Hu, P.; Yan, M.; Sun, R.; Xu, X.; An, Q.; Mai, L. Flexible additive free H2V3O8 nanowire membrane as cathode for sodium ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 12074–12079. [Google Scholar] [CrossRef]
- An, Q.; Zhang, P.; Wei, Q.; He, L.; Xiong, F.; Sheng, J.; Wang, Q.; Mai, L. Top-down fabrication of three-dimensional porous V2O5 hierarchical microplates with tunable porosity for improved lithium battery performance. J. Mater. Chem. A 2014, 2, 3297–3302. [Google Scholar] [CrossRef]
- Li, W.D.; Xu, C.Y.; Pan, X.L.; Huang, Y.D.; Zhen, L. High capacity and enhanced structural reversibility of β-LixV2O5 nanorods as the lithium battery cathode. J. Mater. Chem. A 2013, 1, 5361. [Google Scholar] [CrossRef]
- Ahirrao, D.J.; Mohanapriya, K.; Jha, N. V2O5 nanowires-graphene composite as an outstanding electrode material for high electrochemical performance and long-cycle-life supercapacitor. Mater. Res. Bull. 2018, 108, 73–82. [Google Scholar] [CrossRef]
- Laorodphan, N.; Pooddee, P.; Kidkhunthod, P.; Kunthadee, P.; Tapala, W.; Puntharod, R. Boron and pentavalent vanadium local environments in binary vanadium borate glasses. J. Non-Cryst. Solids 2016, 453, 118–124. [Google Scholar] [CrossRef]
- Hassan, N.; Riaz, J.; Qureshi, M.T.; Razaq, A.; Rahim, M.; Toufiq, A.M.; Shakoor, A. Vanadium oxide (V2O3) for energy storage applications through hydrothermal route. J. Mater. Sci. Mater. Electron. 2018, 29, 16021–16026. [Google Scholar] [CrossRef]
- Roppolo, M.; Jacobs, C.B.; Upreti, S.; Chernova, N.A.; Whittingham, M.S. Synthesis and characterization of layered and scrolled amine-templated vanadium oxides. J. Mater. Sci. 2008, 43, 4742–4748. [Google Scholar] [CrossRef]
- Bordelet, G.; Yamaguchi, I.; Manabe, T.; Tsuchiya, T. Low temperature vanadium oxide thin film sintering by thermal and excimer-laser-assisted Metal-Organic Deposition (MOD). Ceram. Int. 2018, 44, S26–S29. [Google Scholar] [CrossRef]
- An, Q.; Sheng, J.; Xu, X.; Wei, Q.; Zhu, Y.; Han, C.; Niu, C.; Mai, L. Ultralong H2V3O8 nanowire bundles as a promising cathode for lithium batteries. New J. Chem. 2014, 38, 2075–2080. [Google Scholar] [CrossRef]
- Liu, P.; Bian, K.; Zhu, K.; Xu, Y.; Gao, Y.; Luo, H.; Lu, L.; Wang, J.; Liu, J.; Tai, G. Ultrathin Nanoribbons of in Situ Carbon-Coated V3O7·H2O for High-Energy and Long-Life Li-Ion Batteries: Synthesis, Electrochemical Performance, and Charge-Discharge Behavior. ACS Appl. Mater Interfaces 2017, 9, 17002–17012. [Google Scholar] [CrossRef]
- Mai, L.; Wei, Q.; An, Q.; Tian, X.; Zhao, Y.; Xu, X.; Xu, L.; Chang, L.; Zhang, Q. Nanoscroll buffered hybrid nanostructural VO2 (B) cathodes for high-rate and long-life lithium storage. Adv. Mater. 2013, 25, 2969–2973. [Google Scholar] [CrossRef]
- Shao, L.; Wu, K.; Lin, X.; Shui, M.; Ma, R.; Wang, D.; Long, N.; Ren, Y.; Shu, J. Sol–gel preparation of V2O5 sheets and their lithium storage behaviors studied by electrochemical and in-situ X-ray diffraction techniques. Ceram. Int. 2014, 40, 6115–6125. [Google Scholar] [CrossRef]
- Sakunthala, A.; Reddy, M.V.; Selvasekarapandian, S.; Chowdari, B.V.R.; Selvin, P.C. Energy storage studies of bare and doped vanadium pentoxide, (V1.95M0.05)O5, M = Nb, Ta, for lithium ion batteries. Energy Environ. Sci. 2011, 4, 1712. [Google Scholar] [CrossRef]
- Xu, Y.; Dunwell, M.; Fei, L.; Fu, E.; Lin, Q.; Patterson, B.; Yuan, B.; Deng, S.; Andersen, P.; Luo, H.; et al. Two-dimensional V2O5 sheet network as electrode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 20408–20413. [Google Scholar] [CrossRef]
- Tang, K.; Yu, X.; Sun, J.; Li, H.; Huang, X. Kinetic analysis on LiFePO4 thin films by CV, GITT, and EIS. Electrochim. Acta 2011, 56, 4869–4875. [Google Scholar] [CrossRef]
- Jiang, H.; Wei, Z.; Cai, X.; Lai, L.; Ma, J.; Huang, W. A cathode for Li-ion batteries made of vanadium oxide on vertically aligned carbon nanotube arrays/graphene foam. Chem. Eng. J. 2019, 359, 1668–1676. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Garcia, B.B.; Zhang, Q.; Pan, A.; Jeong, Y.H.; Cao, G. V2O5 xerogel electrodes with much enhanced lithium-ion intercalation properties with N2 annealing. J. Mater. Chem. 2009, 19, 8789. [Google Scholar] [CrossRef]
- Song, H.; Luo, M.; Wang, A. High Rate and Stable Li-Ion Insertion in Oxygen-Deficient LiV3O8 Nanosheets as a Cathode Material for Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 2875–2882. [Google Scholar] [CrossRef]


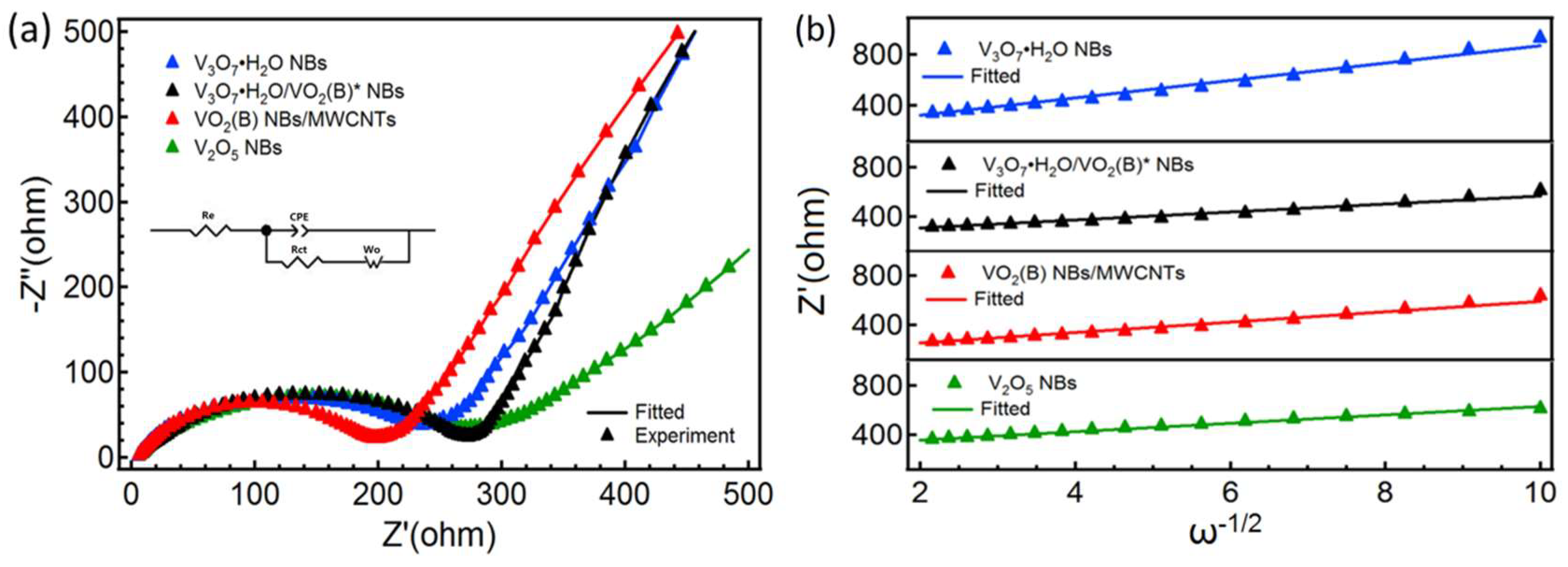
| Samples | Re (Ω) | Rct (Ω) | Aw (Ω·s−1/2) |
|---|---|---|---|
| V3O7·H2O NBs | 5.1 | 210.5 | 69.0 |
| V3O7·H2O/VO2(B)* NBs | 4.6 | 276.9 | 33.1 |
| VO2(B) NBs/MWCNTs | 5.1 | 199.8 | 42.9 |
| V2O5 NBs | 5.7 | 230.1 | 34.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhou, X.; Chen, X.; Wen, J.; Guan, L.; Shi, M.; Ren, Y.; Liu, Z. Controlled Hydrothermal Growth and Li+ Storage Performance of 1D VOx Nanobelts with Variable Vanadium Valence. Nanomaterials 2019, 9, 624. https://doi.org/10.3390/nano9040624
Jiang Y, Zhou X, Chen X, Wen J, Guan L, Shi M, Ren Y, Liu Z. Controlled Hydrothermal Growth and Li+ Storage Performance of 1D VOx Nanobelts with Variable Vanadium Valence. Nanomaterials. 2019; 9(4):624. https://doi.org/10.3390/nano9040624
Chicago/Turabian StyleJiang, Yuhan, Xiaowei Zhou, Xu Chen, Jia Wen, Linlin Guan, Mingxia Shi, Yang Ren, and Zhu Liu. 2019. "Controlled Hydrothermal Growth and Li+ Storage Performance of 1D VOx Nanobelts with Variable Vanadium Valence" Nanomaterials 9, no. 4: 624. https://doi.org/10.3390/nano9040624
APA StyleJiang, Y., Zhou, X., Chen, X., Wen, J., Guan, L., Shi, M., Ren, Y., & Liu, Z. (2019). Controlled Hydrothermal Growth and Li+ Storage Performance of 1D VOx Nanobelts with Variable Vanadium Valence. Nanomaterials, 9(4), 624. https://doi.org/10.3390/nano9040624