Sesbania Gum-Supported Hydrophilic Electrospun Fibers Containing Nanosilver with Superior Antibacterial Activity
Abstract
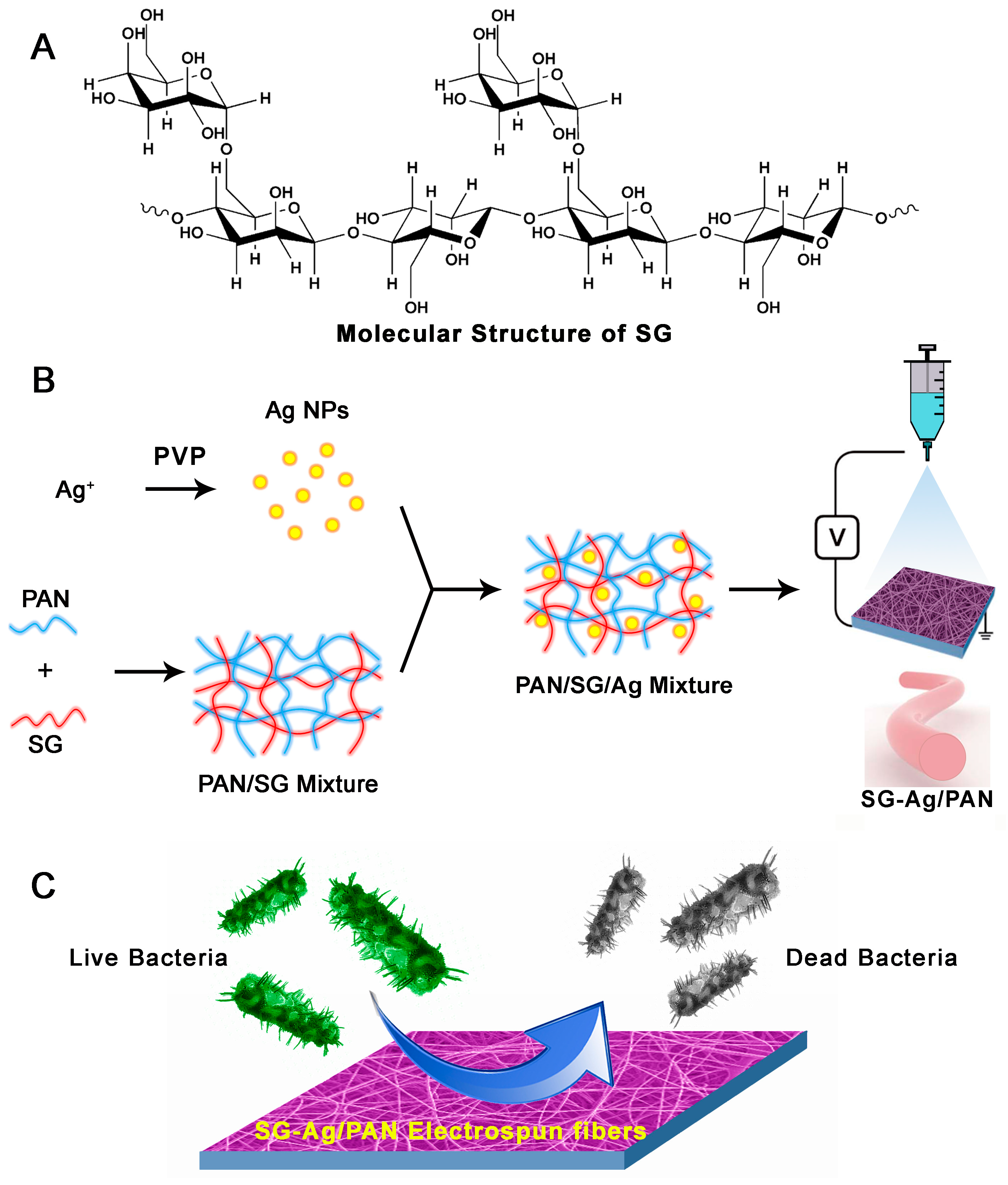
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ag NP Dispersion
2.3. Synthesis of SG-Ag/PAN Electrospun Fibers
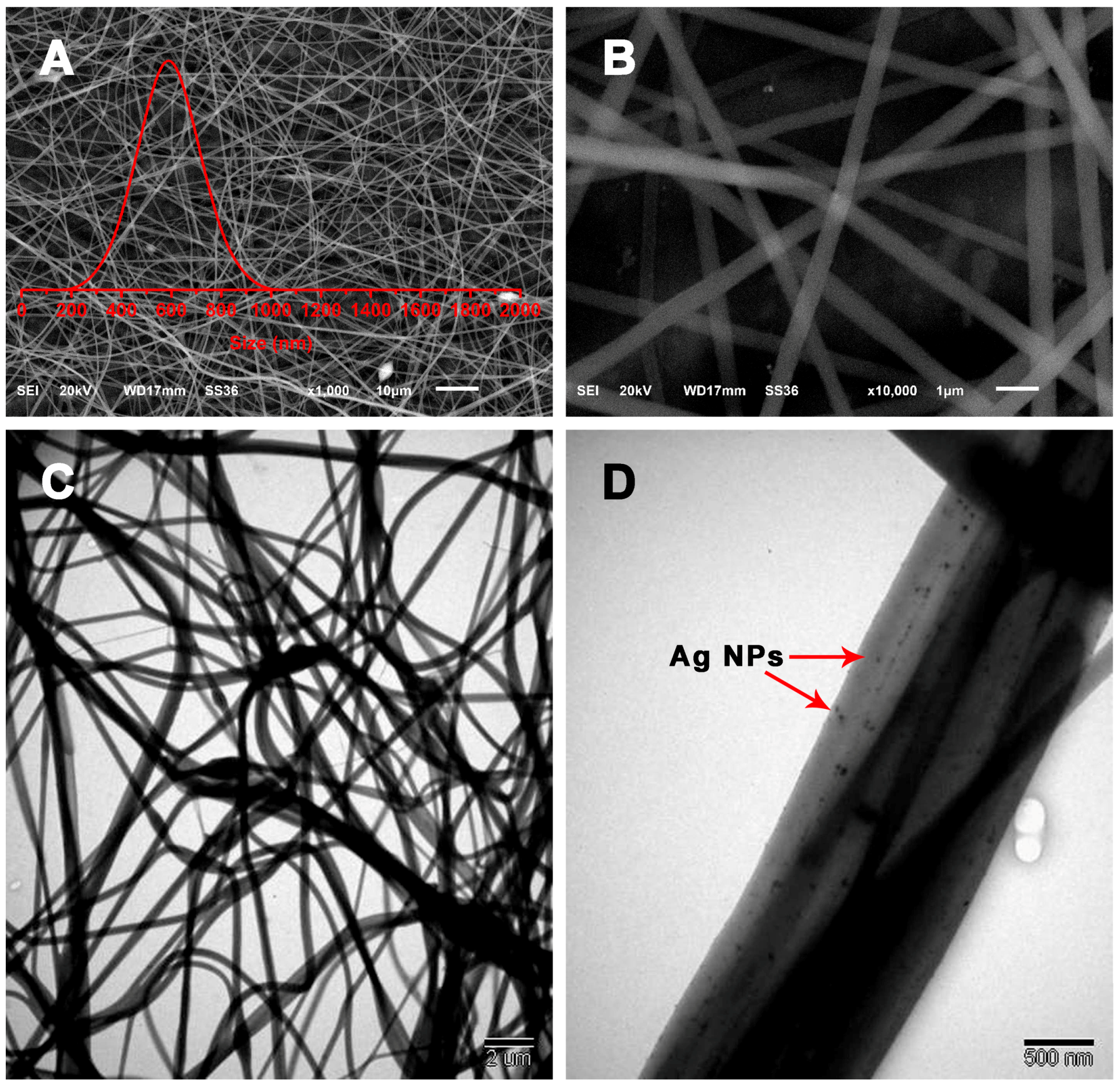
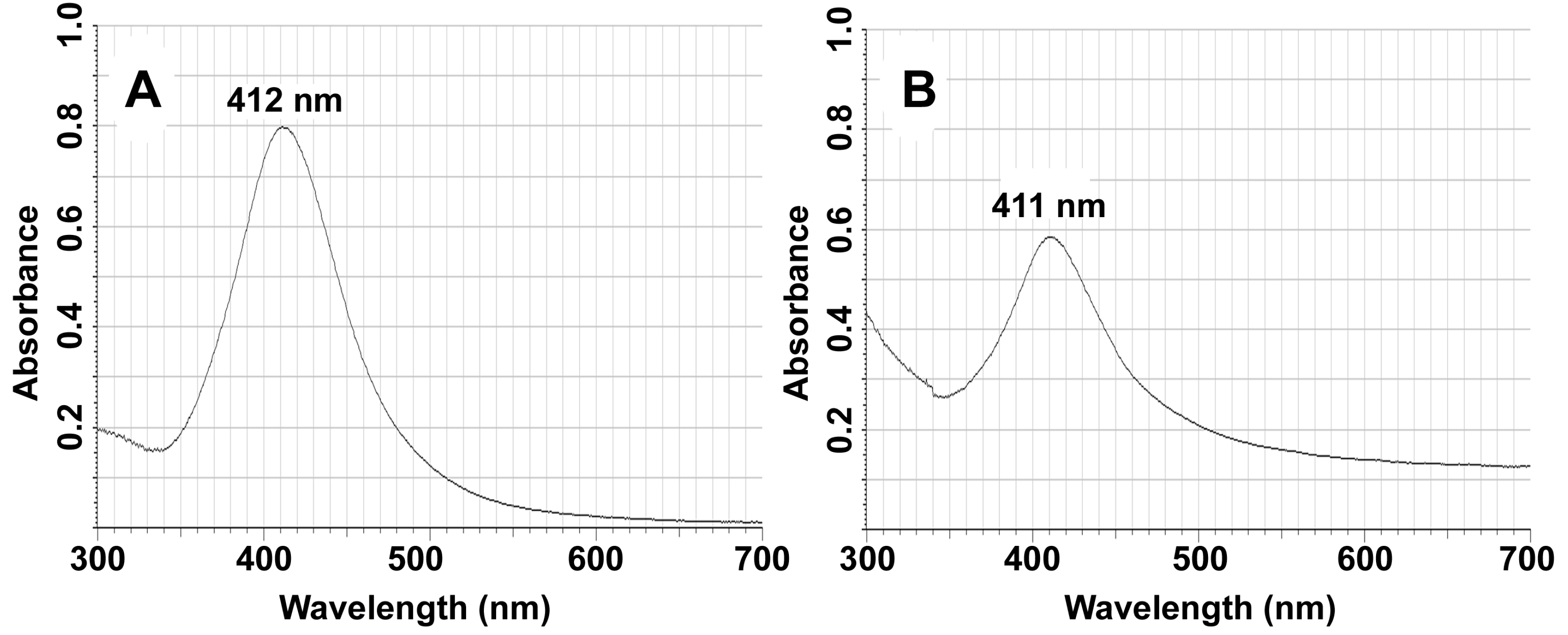
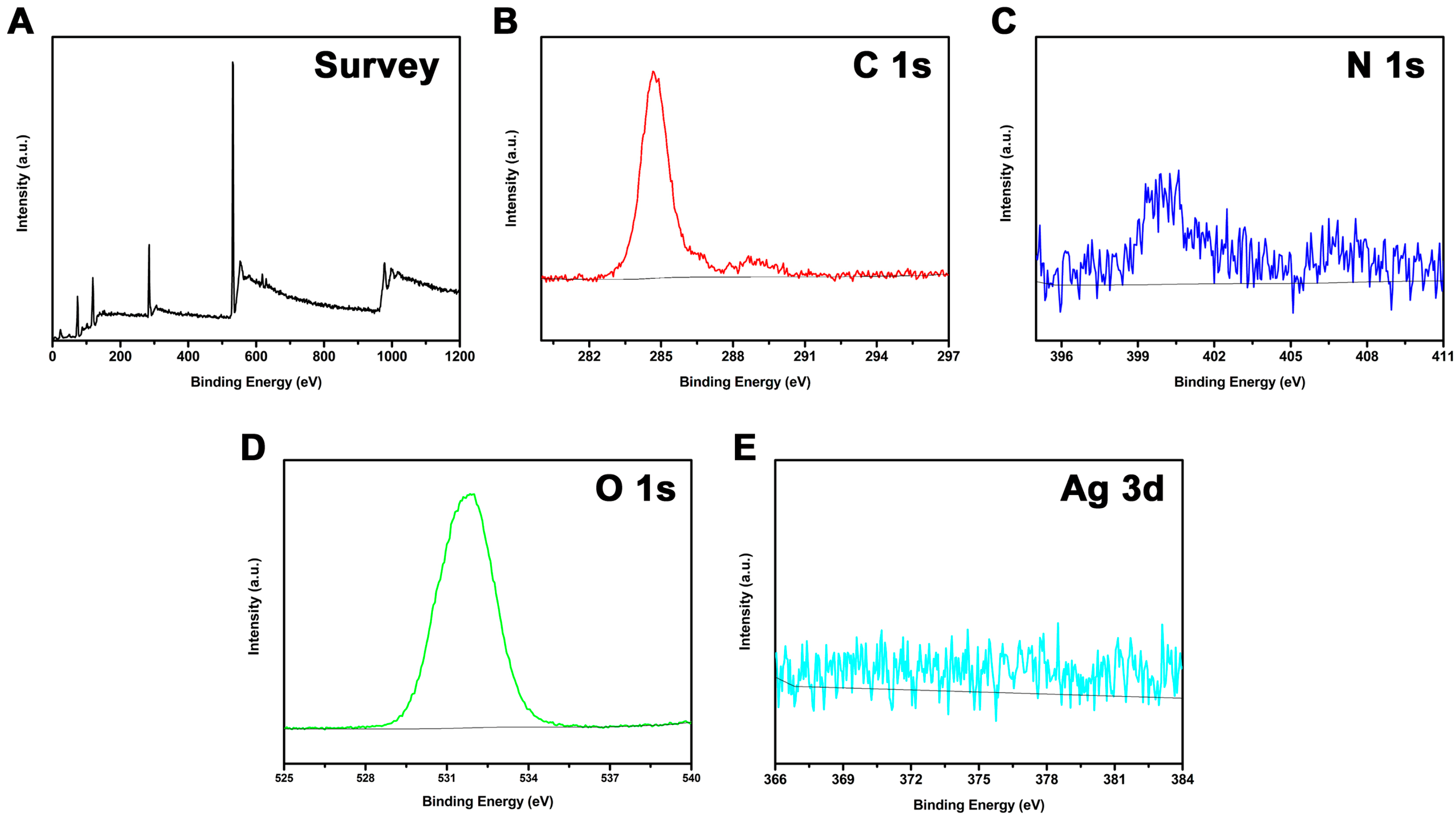
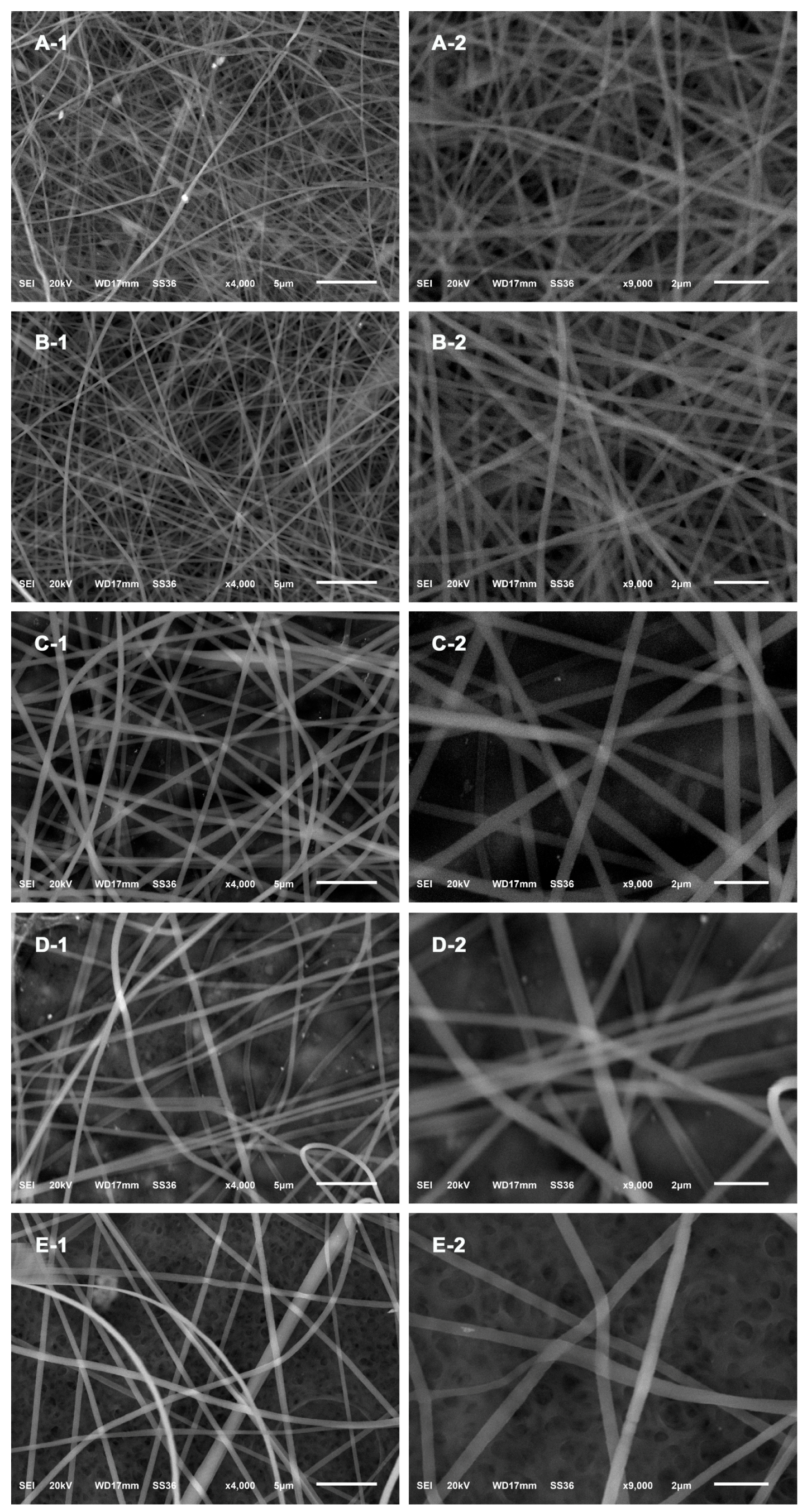
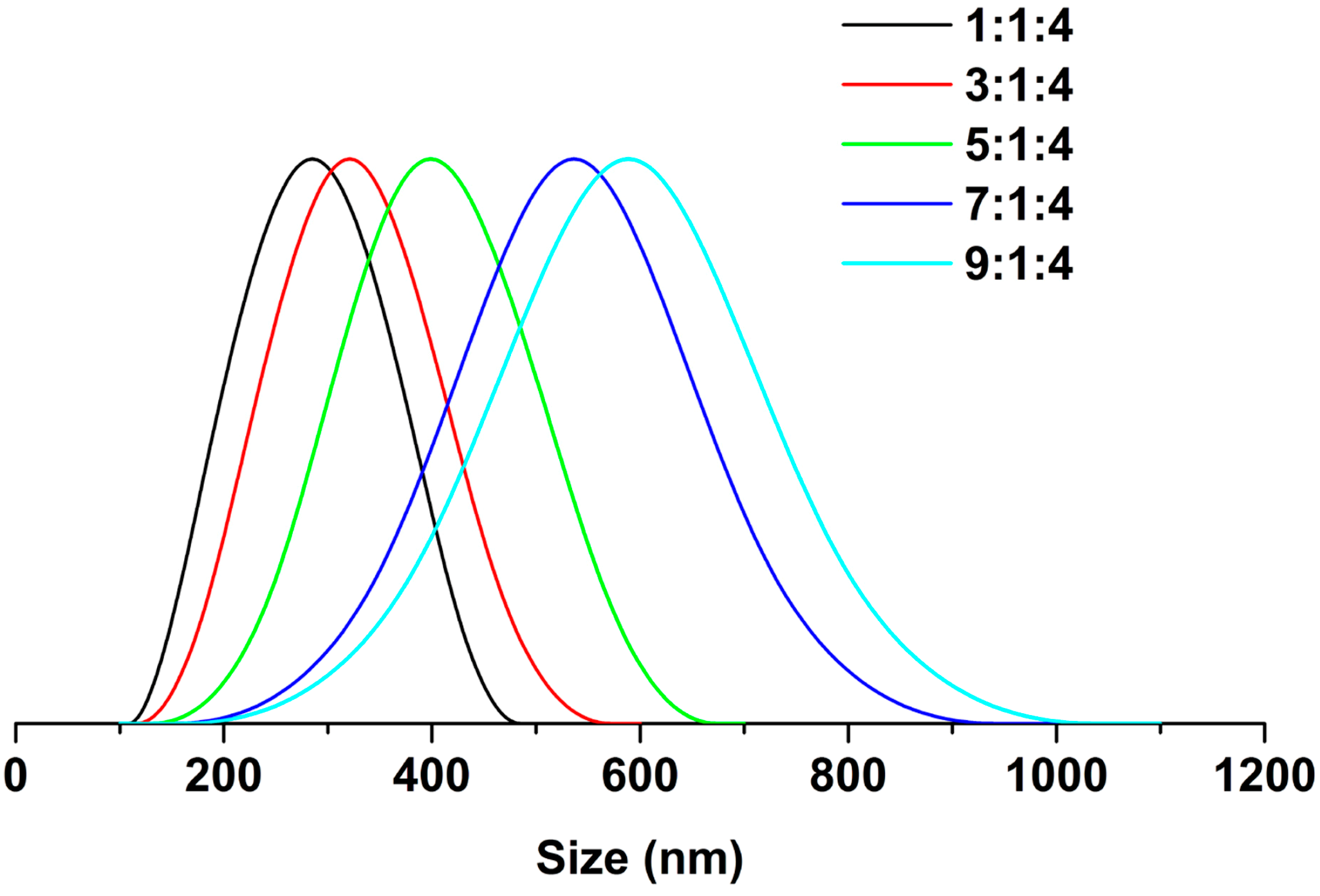
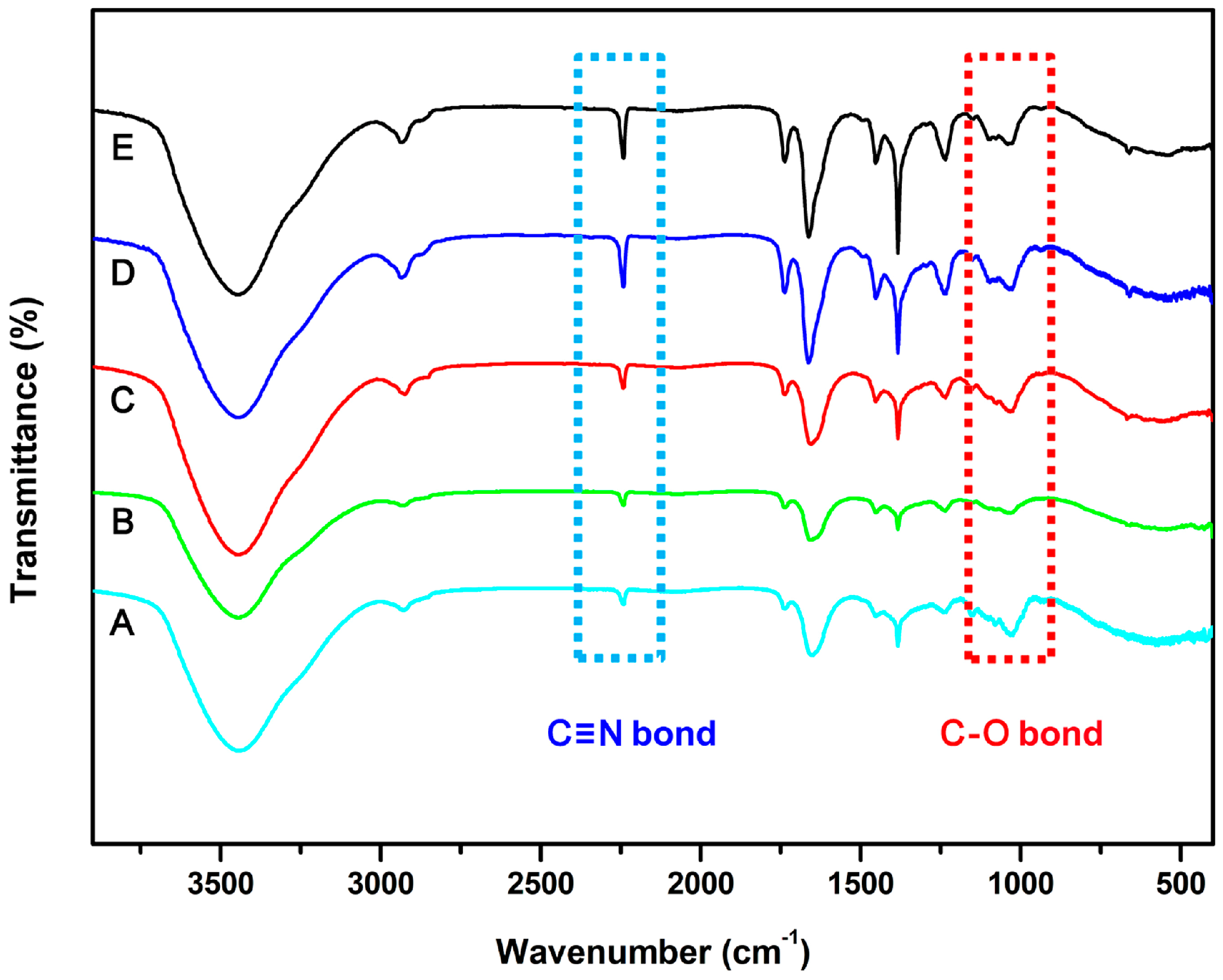
2.4. Characterization
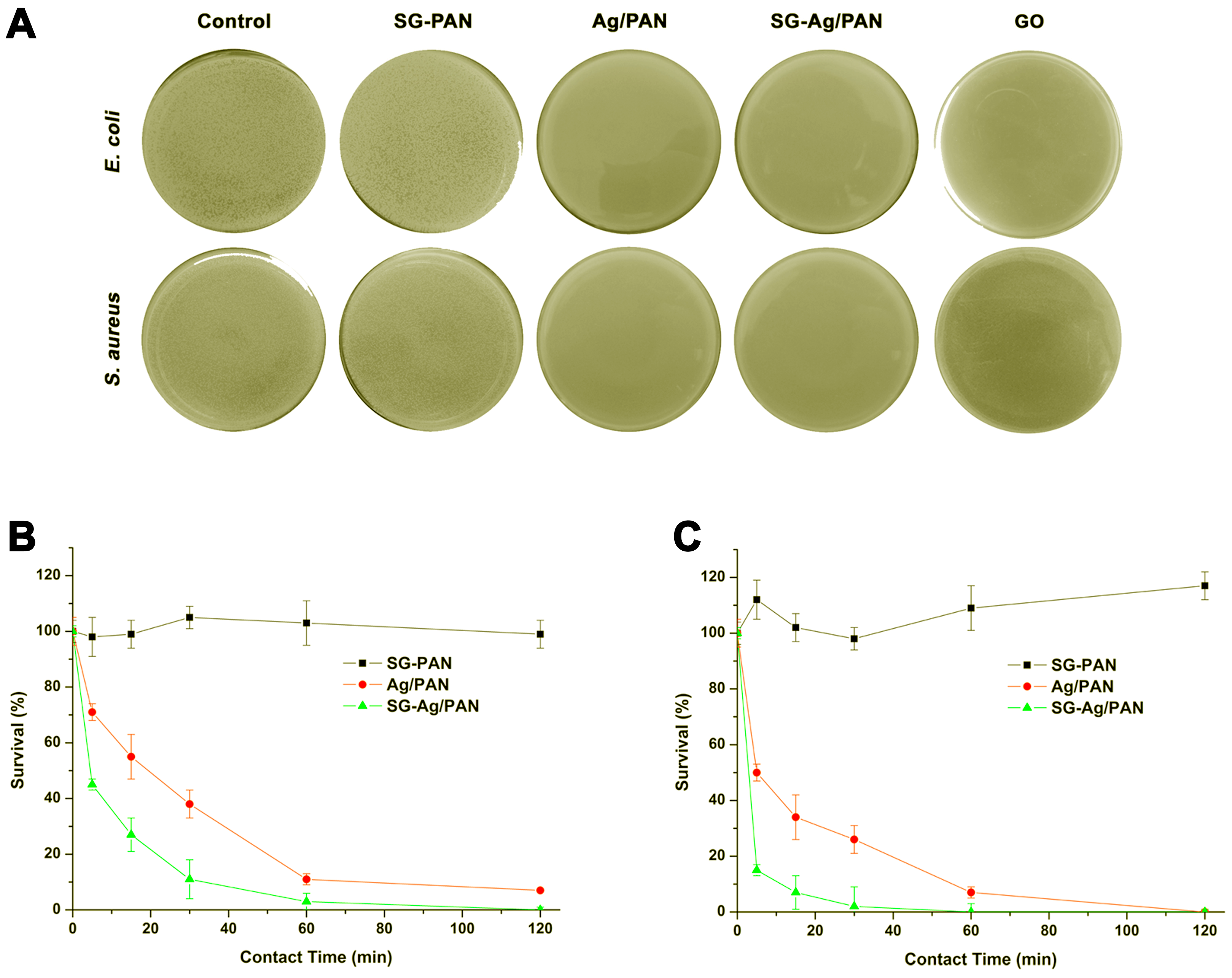
2.5. Antibacterial Testing
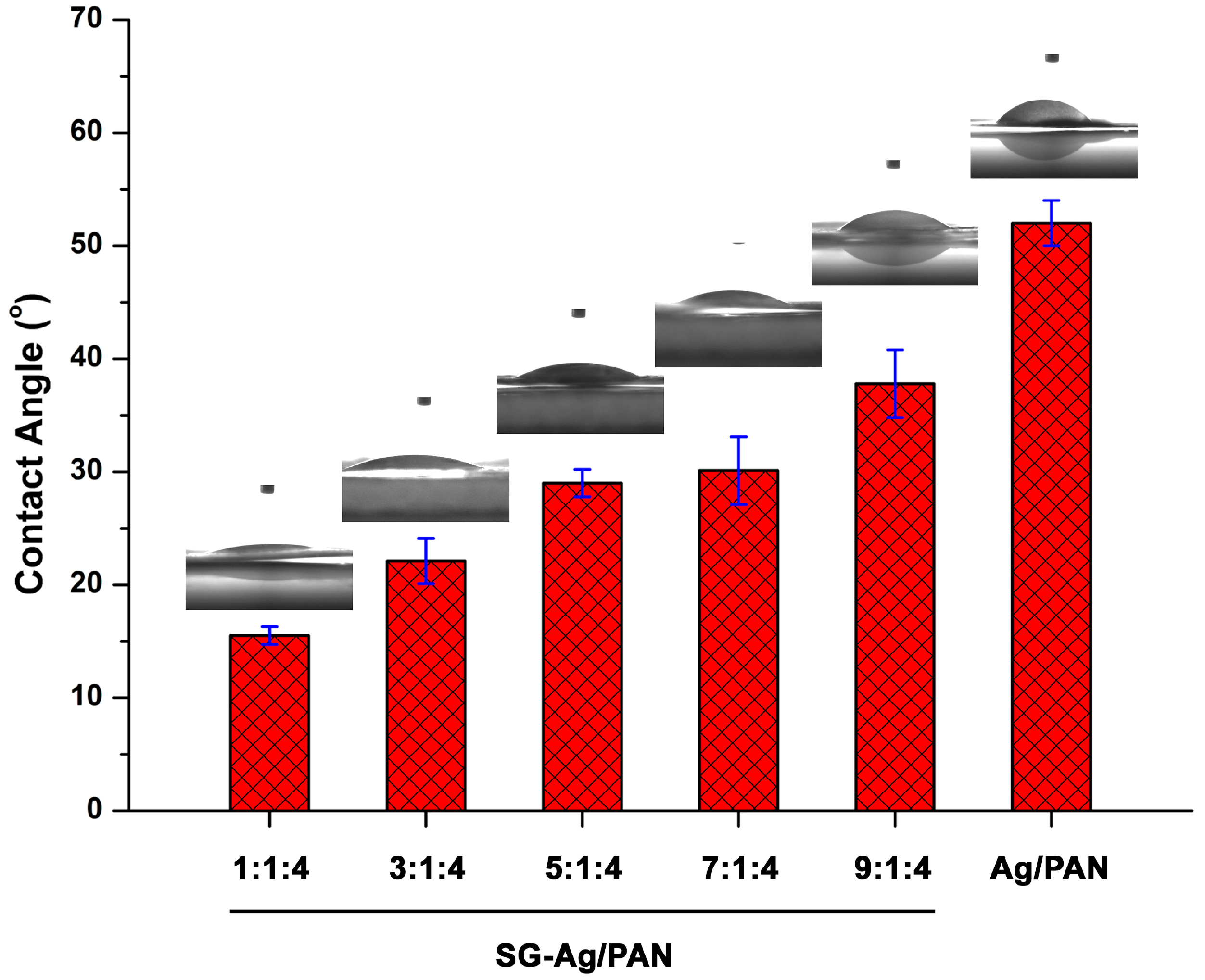
2.6. Contact Angle Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, A.; Lan, S.; Huang, J.; Wang, T.; Zhao, T.; Xiao, L.; Wang, W.; Zheng, X.; Liu, F.; Gao, G.; et al. Modifying Fe3O4-functionalized nanoparticles with N-halamine and their magnetic/antibacterial properties. ACS Appl. Mater. Interfaces 2011, 3, 4228–4235. [Google Scholar] [CrossRef]
- Dong, A.; Zhang, Q.; Wang, T.; Wang, W.; Liu, F.; Gao, G. Immobilization of cyclic N-halamine on polystyrene-functionalized silica nanoparticles: Synthesis, characterization, and biocidal activity. J. Phys. Chem. C 2010, 114, 17298–17303. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Steed, B.W.; Yufit, D.S.; Musa, O.M.; Berry, D.J.; Steed, J.W. Halogen and hydrogen bonding in povidone-iodine and related co-phases. Cryst. Growth Des. 2017, 17, 5552–5558. [Google Scholar] [CrossRef]
- Dong, A.; Huang, J.; Lan, S.; Wang, T.; Xiao, L.; Wang, W.; Zhao, T.; Zheng, X.; Liu, F.; Gao, G.; et al. Synthesis of N-halamine-functionalizedsilica-polymer core-shell nanoparticlesand their enhanced antibacterial activity. Nanotechnology 2011, 22, 295602. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.; Sun, Y.; Lan, S.; Wang, Q.; Cai, Q.; Qi, X.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. Barbituric acid-based magnetic N-halamine nanoparticles as recyclable antibacterial agents. ACS Appl. Mater. Interfaces 2013, 5, 8125–8133. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hu, C.; Yang, Y.; Wu, Y.; Yang, L.; Wang, Y.; Liu, Y.; Jiang, Z. Facile fabrication of carbonaceous nanospheres loaded with silver nanoparticles as antibacterial materials. J. Mater. Chem. 2012, 22, 8121–8126. [Google Scholar] [CrossRef]
- Sun, L.; Qin, Y.; Cao, Q.; Hu, B.; Huang, Z.; Ye, L.; Tang, X. Novel photocatalytic antibacterial activity of TiO2 microspheres exposing 100% reactive {111} facets. Chem. Commun. 2011, 47, 12628–12630. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Gao, F.; Lu, Q. Morphology effect on antibacterial activity of cuprous oxide. Chem. Commun. 2009, 48, 1076–1078. [Google Scholar] [CrossRef]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef]
- Su, Y.; Tian, L.; Yu, M.; Gao, Q.; Wang, D.; Xi, Y.; Yang, P.; Lei, B.; Ma, P.X.; Li, P. Cationic peptide polysaccharides synthesized by‘click’ chemistry with enhanced broad-spectrum antimicrobial activities. Polym. Chem. 2017, 8, 3788–3800. [Google Scholar] [CrossRef]
- Gao, Q.; Li, P.; Zhao, H.; Chen, Y.; Jiang, L.; Ma, P.X. Methacrylate-ended polypeptides and polypeptoids for antimicrobial and antifouling coatings. Polym. Chem. 2017, 8, 6386–6397. [Google Scholar] [CrossRef]
- Li, P.; Sun, S.; Dong, A.; Hao, Y.; Shi, S.; Sun, Z.; Gao, G.; Chen, Y. Developing of a novel antibacterial agent by functionalization of graphene oxide with guanidine polymer with enhanced antibacterial activity. Appl. Surf. Sci. 2015, 355, 446–452. [Google Scholar] [CrossRef]
- Li, P.; Gao, Y.; Sun, Z.; Chang, D.; Gao, G.; Dong, A. Synthesis, characterization, and bactericidal evaluation of chitosan/guanidine functionalized graphene oxide composites. Molecules 2017, 22, 12. [Google Scholar] [CrossRef]
- Dong, Q.; Cai, Q.; Gao, Y.; Zhang, S.; Gao, G.; Harnoode, C.; Morigen, M.; Dong, A. Synthesis and bactericidal evaluation of imide N-halamine-loaded PMMA nanoparticles. New J. Chem. 2015, 39, 1783–1791. [Google Scholar] [CrossRef]
- Dong, A.; Lan, S.; Huang, J.; Wang, T.; Zhao, T.; Wang, W.; Xiao, L.; Zheng, X.; Liu, F.; Gao, G.; et al. Preparation of magnetically separable N-halamine nanocomposites for the improved antibacterial application. J. Colloid Interface Sci. 2011, 364, 333–340. [Google Scholar] [CrossRef]
- Gao, Y.; Song, N.; Liu, W.; Dong, A.; Wang, Y.-J.; Yang, Y.-W. Construction of antibacterial N-halamine polymer nanomaterials capable of bacterial membrane disruption for efficient anti-infective wound therapy. Macromol. Biosci. 2019, 19, 1800453. [Google Scholar] [CrossRef]
- Bai, R.; Kang, J.; Simalou, O.; Liu, W.; Ren, H.; Gao, T.; Gao, Y.; Chen, W.; Dong, A.; Jia, R. Novel N-Br bond-containing N-halamine electrospun fibers with antibacterial activities. ACS Biomater. Sci. Eng. 2018, 4, 2193–2202. [Google Scholar] [CrossRef]
- Ren, H.; Du, Y.; Su, Y.; Guo, Y.; Zhu, Z.; Dong, A. A Review on recent achievements and current challenges in antibacterial electrospun N-halamines. Colloid Interface Sci. Commun. 2018, 24, 24–34. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, Q.; Li, L.; Li, P.; Wang, Y.; Simalou, O.; Zhang, Y.; Gao, G.; Dong, A. N-Halamine-containing electrospun fibers kill bacteria via a contact/release co-determined antibacterial pathway. ACS Appl. Mater. Interfaces 2016, 8, 31530–31540. [Google Scholar] [CrossRef]
- Dong, A.; Wang, Y.; Gao, Y.; Gao, T.; Gao., G. Chemical insights into antibacterial N-halamines. Chem. Rev. 2017, 117, 4806–4862. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial carbon-based nanomaterials. Adv. Mater. 2018, 30, 1804838. [Google Scholar] [CrossRef]
- Gong, H.; Liu, M.; Li, H. In situ green preparation of silver nanoparticles/chemical pulp fiber composites with excellent catalytic performance. J. Mater. Sci. 2019, 54, 6895–6907. [Google Scholar] [CrossRef]
- Yang, T.; Wang, D.; Liu, X. Assembled gold nanorods for the photothermal killing of bacteria. Colloids Surf. B 2019, 173, 833–841. [Google Scholar] [CrossRef]
- Cai, Q.; Gang, Y.; Gao, T.; Lan, S.; Simalou, O.; Zhou, X.; Zhang, Y.; Harnoode, C.; Gao, G.; Dong, A. Insight into biological effects of zinc oxide nanoflowers on bacteria: Why morphology matters. ACS Appl. Mater. Interfaces 2016, 8, 10109–10120. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; Zhou, R. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, J. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef]
- Sun, Q.; Qiu, S.; Gu, H.; Yao, M.; Swamy, M.; Siniah, U.; Umar, A.; Akhtar, M. Biosynthesis, characterization and biological of silver nanoparticles from pogostemon cablin benth. methanolic leaf extract. J. Nanosci. Nanotechnol. 2019, 19, 4109–4115. [Google Scholar]
- Hassabo, A.; EI-Naggar, M.; Mohamed, A.; Hebelsh, A. Development of multifunctional modified cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide. Carbohydr. Polym. 2019, 210, 144–156. [Google Scholar] [CrossRef]
- Rafinska, K.; Pomastowskl, P.; Buszewskl, B. Study of Bacillus subtilis response to different froms of silver. Sci. Total Environ. 2019, 661, 120–129. [Google Scholar] [CrossRef]
- Akturk, A.; Taygun, M.; Guler, F.; Goller, G.; Kucukbayrak, S. Fabrication of antibacterial polyvinylalcohol nanocomposite mats with soluble starch coated silver nanoparticles. Colloids Surf. A 2019, 562, 255–262. [Google Scholar] [CrossRef]
- Ge, J.; Kim, J.; Yoon, S.; Chol, N. Fabrication of low-cost and high-performance coal fly ash nanofibrous membranes via electrospinning for the control of harmful substances. Fuel 2019, 273, 236–244. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Ruini, F.; Ceresa, C.; Gentile, P.; Varela, P.; Ferreira, A.; Fracchia, L.; Clardell, G. Nanostructured scaffold with biomimetic and antibacterial properties for wound healing produced by green electrospinning. Colloids Surf. B 2018, 172, 233–243. [Google Scholar] [CrossRef]
- Song, J.; Kim, H.; Jang, Y.; Jang, J. Enhanced antibacterial activity of silver/polyrhodanine- composite-decorated silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 11563–11568. [Google Scholar] [CrossRef]
- Wang, L.; Lv, H.; Li, B.; Zhao, Y.; Sun, L. Synthesis and antibacterial activity of Ag/CeO2 hybrid architectures. J. Sol-Gel Sci. Technol. 2018, 88, 654–659. [Google Scholar] [CrossRef]
- Ou, J.; Wu, B.; Xue, M.; Wang, F. Silver ions anchored fabric via coordination: Evaluation on washing durability and antibacterial activity. Mater. Lett. 2019, 237, 134–136. [Google Scholar] [CrossRef]
- Heseltine, P.L.; Ahmed, J.; Edirisinghe, M. Developments in pressurized gyration for the mass production of polymeric fibers. Macromol. Mater. Eng. 2018, 303, 1800218. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Mahalingam, S.; Matharu, R.K.; Edirisinghe, M. Evolution of surface nanopores in pressurized gyrospun polymeric microfibers. Polymers 2017, 9, 508. [Google Scholar] [CrossRef]
- Mahalingam, S.; Xu, Z.; Edirisinghe, M. Antibacterial activity and biosensing of PVA-lysozyme microbubbles formed by pressurized gyration. Langmuir 2015, 31, 9771–9780. [Google Scholar] [CrossRef]
- Raimi-Abraham, B.T.; Mahalingam, S.; Davies, P.J.; Edirisinghe, M. Development and characterization of amorphous nanofiber drug dispersions prepared using pressurized gyration. Mol. Pharm. 2015, 12, 3851–3861. [Google Scholar] [CrossRef]
- Pascariu, P.; Homocianu, M.; Cojocaru, C.; Samoll, P.; Alrlnel, A.; Suchea, M. Preparation of La doped ZnO ceramic nanostrctures by electrospinning-calcination method: Effect of La3+ doping on optical and photocatalytic properties. Appl. Surf. Sci. 2019, 476, 16–27. [Google Scholar] [CrossRef]
- Wang, H.; Kong, L.; Ziegier, G. Fabrication of starch-Nanocellulose composite fibers by electrospinning. Food Hydrocoll. 2019, 90, 90–98. [Google Scholar] [CrossRef]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.; Uyar, T. One-step green synthesis of antibacterial sliver nanoparticles embedded in electrospun cyclodextrin electrospun fibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fiber production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef]
- Luo, C.J.; Stride, E.; Edirisinghe. Mapping the influence of solubility and dielectric constant on electrospinning polycaprolactone solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Jatol, A.; Kim, I.; Ni, Q. Cellulose acetate electrospun fibers embedded with Ag NPs anchored TiO2 nanoparticles for long term excellent antibacterial applications. Carbohydr. Polym. 2019, 207, 640–649. [Google Scholar]
- Duran-Guerrero, J.; Martinez-Rodriguz, M.; Garza-Navarro, M.; Gonzalez-Gonzalez, V.; Turres-Castro, A. Magnetic nanofibrous materials based on CMC/PVP polymeric blends. Carbohydr. Polym. 2018, 200, 289–296. [Google Scholar] [CrossRef]
- Xiao, Y.; Cao, Y.; Xin, B.; Liu, Y.; Chen, Z.; Lin, L.; Sun, Y. Fabrication and characterization of electrospun cellulose/polyacrylonitrile electrospun fibers with Cu(II) ions. Cellullose 2018, 5, 2955–2963. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Zhai, Y.; Liu, F.; Gao, G. Synthesis of sesbania gum supported dithiocarbamate chelating resin and studies on its adsorption performance for metal ions. Carbohydr. Polym. 2008, 73, 359–363. [Google Scholar] [CrossRef]
- Lan, S.; Leng, Z.; Guo, N.; Wu, X.; Gan, S. Sesbania gum-based magnetic carbonaceous nanocomposites: Facile fabrication and adsorption behavior. Colloids Surf. A 2014, 446, 163–171. [Google Scholar] [CrossRef]
- Pinki, P.; Aparna, B.; Urmi, H.; Jai, P.; Gautam, S.; Rajib, B. Conferring Antibacterial Properties on Sesbania Gum via MicrowaveAssisted Graft Copolymerization of DADMAC. J. Polym. Environ. 2018, 26, 3272–3282. [Google Scholar]
- Abdel-Halim, E.; Al-Deyab, S. Hydrogel from crosslinked polyacrylamide/guar gum graft copolymer for sorption of hexavalent chromium ion. Carbohydr. Polym. 2011, 86, 1306–1312. [Google Scholar] [CrossRef]
- Pajor-Swierzy, A.; Gawel, D.; Drzymala, E.; Socha, R.; Parlinskaw, W.; Szczepanowicz, K.; Warszynski, P. The optimization of methods of synthesis of nickel-sliver core-shell nanoparticles for conductive materials. Nanotechnology 2019, 30, 015601. [Google Scholar] [CrossRef]
- Dong, A.; Huang, Z.; Lan, S.; Wang, Q.; Bao, S.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. N-Halamine-decorated polystyrene nanoparticles based on 5-allylbarbituric acid: From controllable fabrication to bactericidal evaluation. J. Colloid Interface Sci. 2014, 413, 92–99. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, T.; Zhu, W.; Yao, W. Natural Chrysotile-based nanowires decorated with monodispersed Ag nanoparticles as a highly active and reusable hydrogenation catalyst. J. Phys. Chem. 2015, 119, 21465–21472. [Google Scholar] [CrossRef]
- Gao, T.; Fan, H.; Wang, X.; Gao, Y.; Liu, W.; Chen, W.; Dong, A.; Wang, Y. Povidone-iodine-based polymeric nanoparticles for antibacterial applications. ACS Appl. Mater. Interfaces 2017, 9, 25738–25746. [Google Scholar] [CrossRef]
- Dong, A.; Xue, M.; Lan, S.; Wang, Q.; Zhao, Y.; Wang, Y.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. Bactericidal evaluation of N-halamine-functionalized silica nanoparticles based on barbituric acid. Colloids Surf. B 2014, 113, 450–457. [Google Scholar] [CrossRef]
- Pal, P.; Pandey, J.; Sen, G. Grafted sesbania gum: A novel derivative for sugarcane juice clarification. Int. J. Biol. Macromol. 2018, 114, 349–356. [Google Scholar] [CrossRef]
- Street, R.M.; Minagawa, M.; Vengrenyuk, A.; Schauer, C.L. Piezoelectric electrospun polyacrylonitrile with various tacticities. J. Appl. Polym. Sci. 2019, 136, 47530. [Google Scholar] [CrossRef]
- Nadaraia, N.S.; Amiranashvili, L.S.; Merlani, M.; Kakhabrishvili, M.L.; Barbakadze, N.N.; Geronikaki, A.; Petrou, A.; Poroikov, V.; Ciric, A.; Glamoclija, J.; et al. Novel antimicrobial agents’ discovery among the steroid derivatives. Steroids 2019, 144, 52–65. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Ciric, A.; Sokovic, M.; Morales, P.; Ferreira, I.C.F.R. Enhancing the antimicrobial and antifungal activities of a coloring extract agent rich in betacyanins obtained from Gomphrena globosa L. flowers. Food Funct. 2018, 9, 6205–6217. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, A.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Edirisinghe, M. Nanocomposites: Suitable alternatives as antimicrobial agents. Nanotechnology 2018, 29, 282001. [Google Scholar] [CrossRef]
- Matharu, R.K.; Charani, Z.; Ciric, L.; Illangakoon, U.E.; Edirisinghe, M. Antimicrobial activity of tellurium-loaded polymeric fiber meshes. J. Appl. Polym. Sci. 2018, 135, 46368. [Google Scholar] [CrossRef]
- Matharu, R.K.; Porwal, H.; Ciric, L.; Edirisinghe, M. The effect of graphene-poly(methyl methacrylate) fibres on microbial growth. Interface Focus 2018, 8, 20170058. [Google Scholar] [CrossRef]
- Xu, Z.; Mathalingam, S.; Basnett, P.; Raimi-Abraham, B.; Roy, I.; Cragig, D.; Eirisinghe, M. Making nonwoven fibrous Poly(ε-caprolactone) constructs for antimicrobial and tissue engineering applications by pressurized melt gyration. Macromol. Mater. Eng. 2016, 301, 922–934. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Change 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Cuo, Y.; Baulin, V.A.; Kobaisi, M.A.; Crawford, R.J.; Ivanova, E.P. Grapahene induced formation of pores that kill spherical and rod-shaped bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Zhang, Y.; Fei, X.; Zhou, C.; Zhang, Y.; Li, Y.; Yang, Q.; Song, Y. Fabrication of large-scale superhydrophobic composite films with enhanced tensile properties by multinozzle convey belt electrospinning. J. Appl. Polym. Sci. 2014, 131, 39735. [Google Scholar]
- Wang, S.; Wang, J. Anti-reflective and superhydrophobic films prepared from a sol at different withdrawal speeds. Appl. Surf. Sci. 2019, 476, 1035–1048. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, S.; Lu, Y.; Li, C.; Zhao, S.; Liu, N.; Sheng, X. Sesbania Gum-Supported Hydrophilic Electrospun Fibers Containing Nanosilver with Superior Antibacterial Activity. Nanomaterials 2019, 9, 592. https://doi.org/10.3390/nano9040592
Lan S, Lu Y, Li C, Zhao S, Liu N, Sheng X. Sesbania Gum-Supported Hydrophilic Electrospun Fibers Containing Nanosilver with Superior Antibacterial Activity. Nanomaterials. 2019; 9(4):592. https://doi.org/10.3390/nano9040592
Chicago/Turabian StyleLan, Shi, Yaning Lu, Chun Li, Shuang Zhao, Naren Liu, and Xianliang Sheng. 2019. "Sesbania Gum-Supported Hydrophilic Electrospun Fibers Containing Nanosilver with Superior Antibacterial Activity" Nanomaterials 9, no. 4: 592. https://doi.org/10.3390/nano9040592
APA StyleLan, S., Lu, Y., Li, C., Zhao, S., Liu, N., & Sheng, X. (2019). Sesbania Gum-Supported Hydrophilic Electrospun Fibers Containing Nanosilver with Superior Antibacterial Activity. Nanomaterials, 9(4), 592. https://doi.org/10.3390/nano9040592




