TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen
Abstract
1. Introduction
2. Experimental Procedure
2.1. Preparation of the Photocatalyst
2.2. Characterization of the Photocatalyst
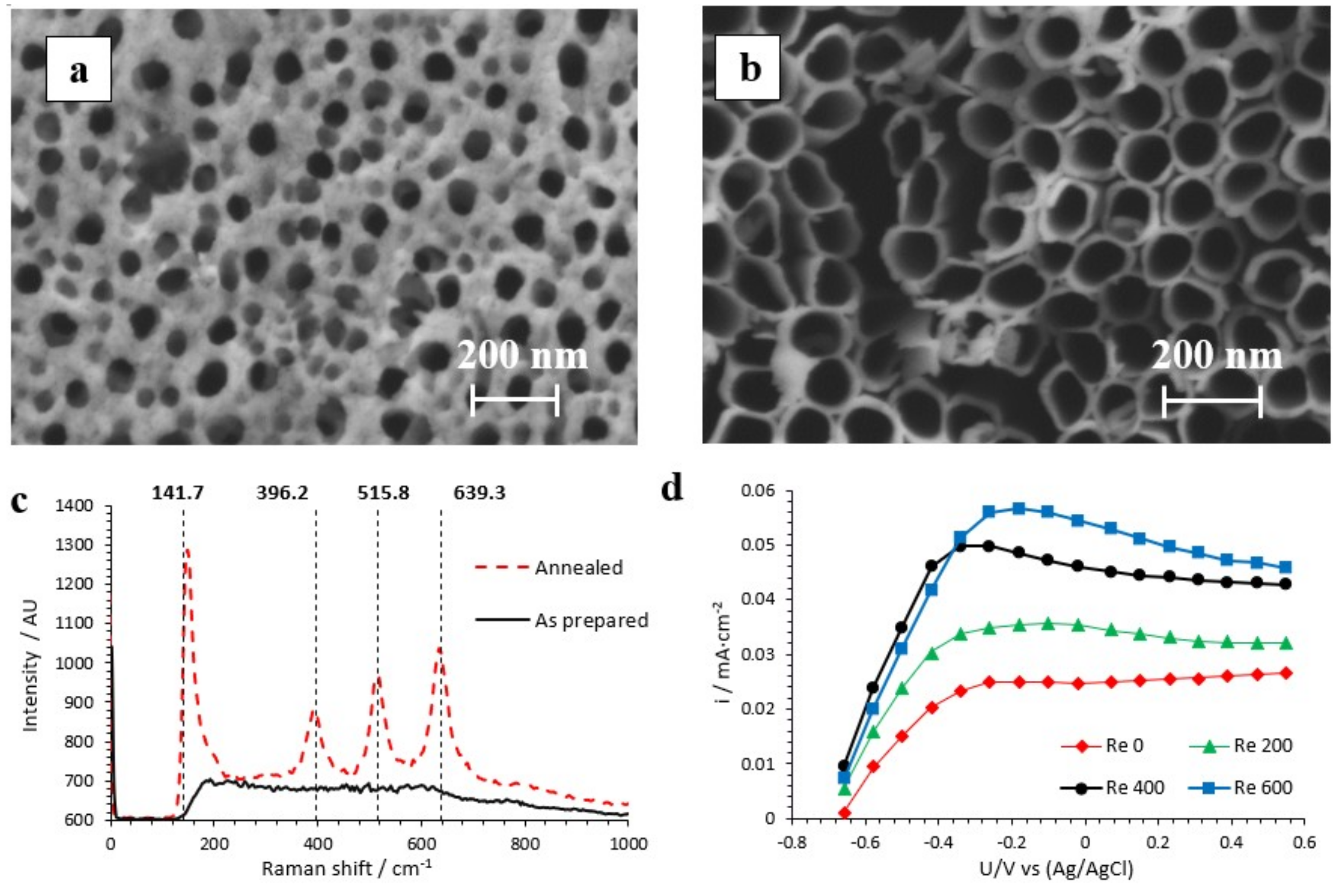
2.2.1. Morphological and Structural Characterization
2.2.2. Photoelectrochemical Characterization
2.3. Photoelectrocatalysis Experiments
3. Results and Discussion
3.1. Photocatalyst Characterization
3.2. Reaction Kinetics
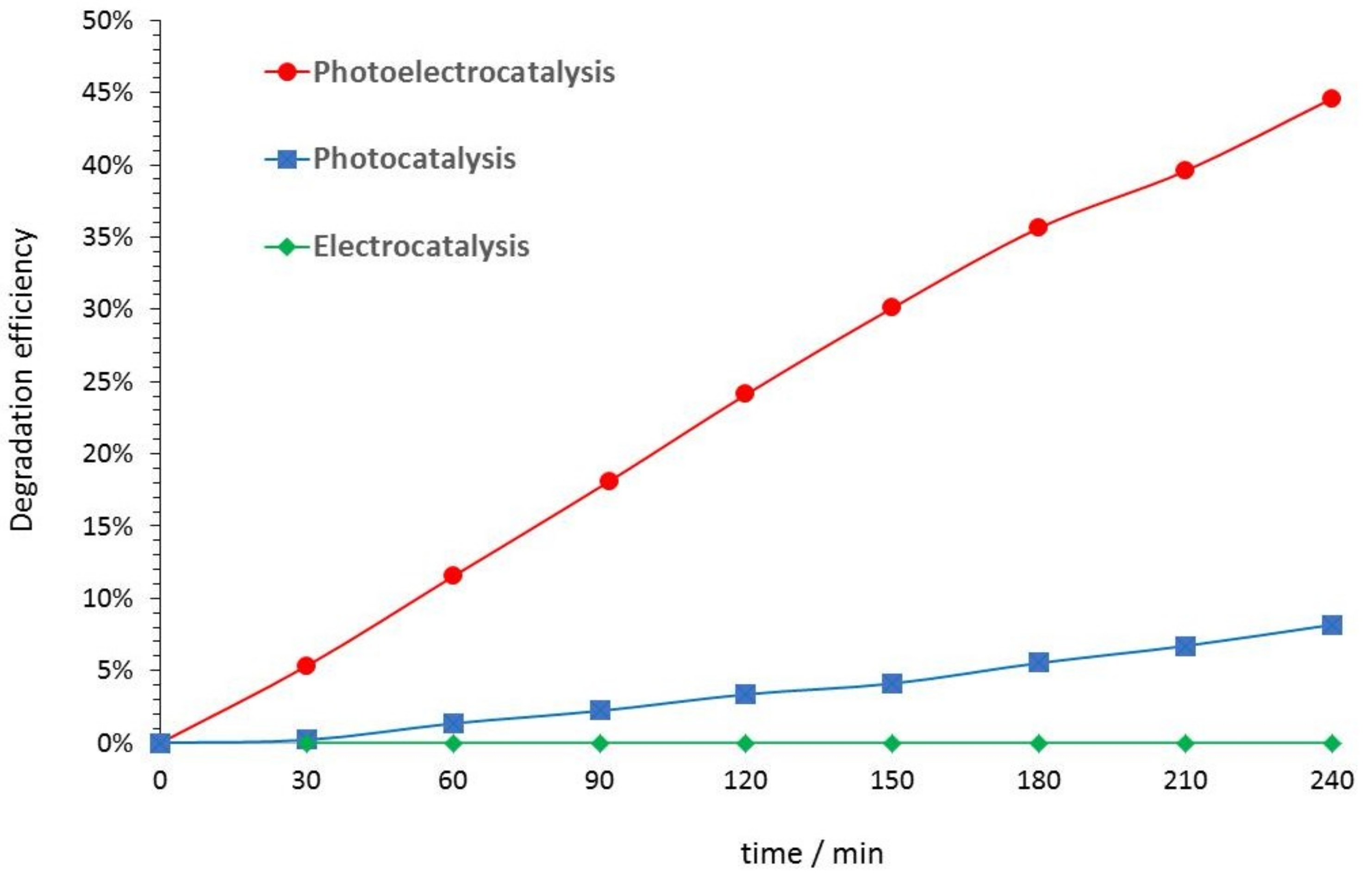
3.3. Electrocatalysis, Photocatalysis, and Photoelectrocatalysis
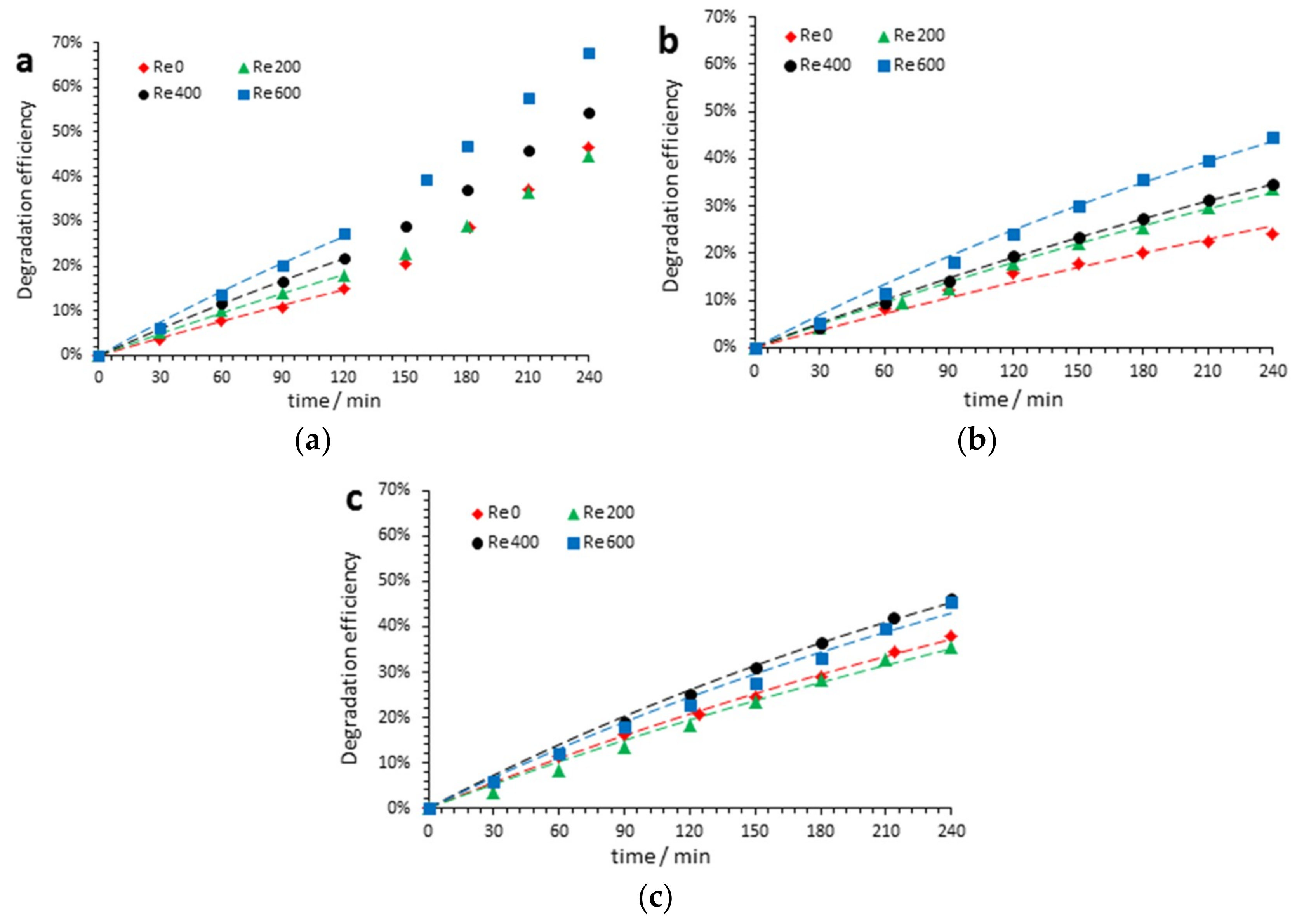
3.4. Effect of the Reynolds Number and pH on Acetaminophen Degradation
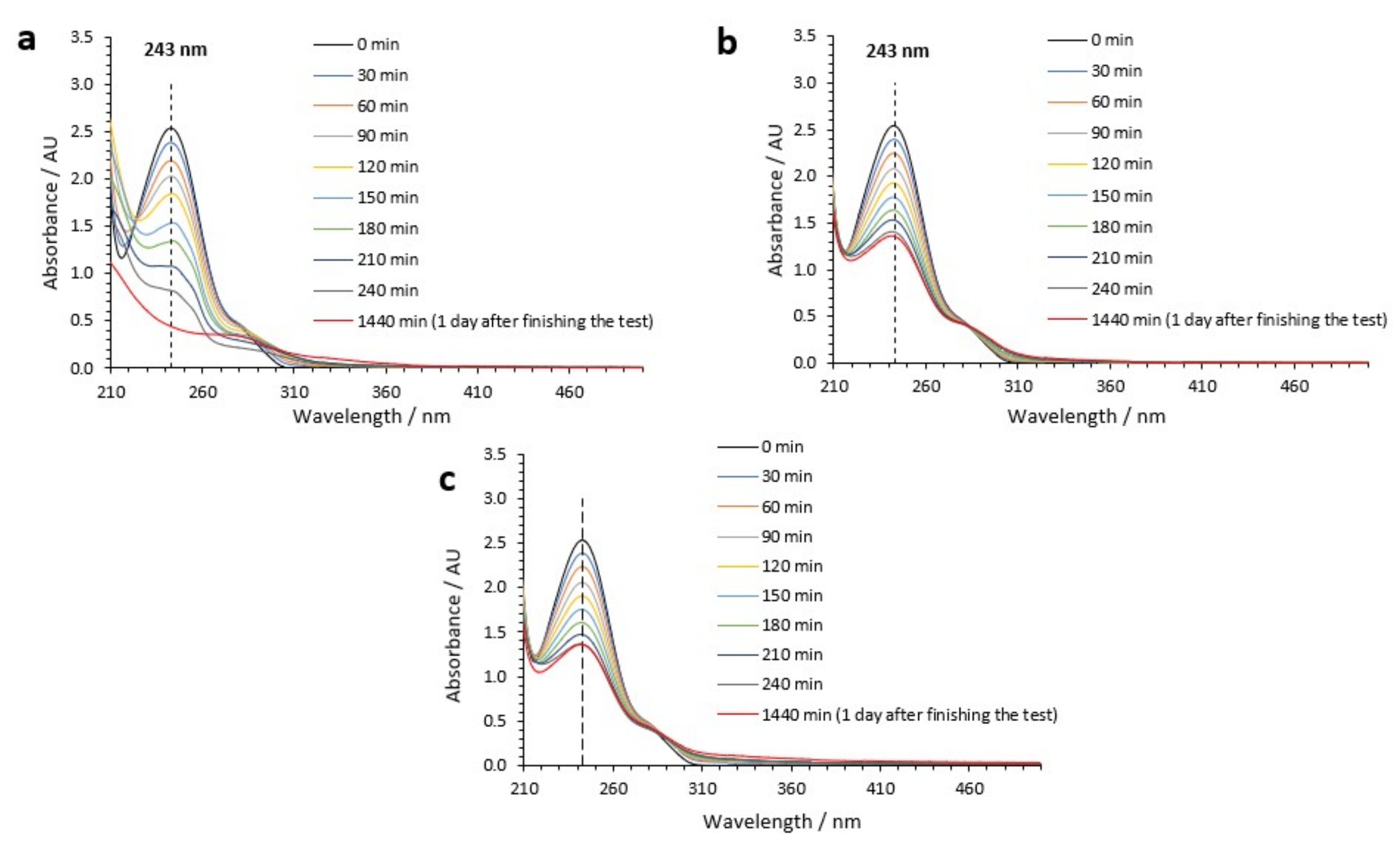
3.5. Study of the Byproducts
3.6. COD and TOC Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.A.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Solís-Casados, D.; Escobar-Alarcón, L.; Gómez-Oliván, L.; Haro-Poniatowski, E.; Klimova, T. Photodegradation of pharmaceutical drugs using Sn-modified TiO2 powders under visible light irradiation. Fuel 2017, 198, 3–10. [Google Scholar] [CrossRef]
- Xie, G.; Chang, X.; Adhikari, B.R.; Thind, S.S.; Chen, A. Photoelectrochemical degradation of acetaminophen and valacyclovir using nanoporous titanium dioxide. Chin. J. Catal. 2016, 37, 1062–1069. [Google Scholar] [CrossRef]
- Basha, S.; Keane, D.; Nolan, K.; Oelgemöller, M.; Lawler, J.; Tobin, J.M.; Morrissey, A. UV-induced photocatalytic degradation of aqueous acetaminophen: The role of adsorption and reaction kinetics. Environ. Sci. Pollut. Res. 2015, 22, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Elghniji, K.; Trabelsi, H.; Ksibi, M. Photocatalytic degradation of paracetamol on TiO2 nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arab. J. Chem. 2017, 10, S3640–S3645. [Google Scholar] [CrossRef]
- El-Kemary, M.; El-Shamy, H.; El-Mehasseb, I. Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles. J. Lumin. 2010, 130, 2327–2331. [Google Scholar] [CrossRef]
- Lin, C.J.; Liao, S.-J.; Kao, L.-C.; Liou, S.Y.H. Photoelectrocatalytic activity of a hydrothermally grown branched ZnO nanorod-array electrode for paracetamol degradation. J. Hazard. Mater. 2015, 291, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Babu, V.J.; Ramakrishna, S. Photoelectrode nanomaterials for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 11078–11109. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-García, D.M.; García-Antón, J. Enhancement of photoelectrochemical activity for water splitting by controlling hydrodynamic conditions on titanium anodization. J. Power Sources 2015, 286, 224–231. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Mills, A.; le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Tovar, R.S.; Paramasivam, I.; Lee, K.; Schmuki, P. Influence of hydrodynamic conditions on growth and geometry of anodic TiO2 nanotubes and their use towards optimized DSSCs. J. Mater. Chem. 2012, 22, 12792–12795. [Google Scholar] [CrossRef]
- Borràs-Ferrís, J.; Sánchez-Tovar, R.; Blasco-Tamarit, E.; Fernández-Domene, R.M.; García-Antón, J. Effect of Reynolds number and lithium cation insertion on titanium anodization. Electrochim. Acta 2016, 196, 24–32. [Google Scholar] [CrossRef]
- Zavala, M.A.L.; Estrada, E.E. Degradation of Acetaminophen and Its Transformation Products in Aqueous Solutions by Using an Electrochemical Oxidation Cell with Stainless Steel Electrodes. Water 2016, 8, 383. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association (APHA): Washington, DC, USA, 2012.
- Costa, L.L.; Prado, A.G. TiO2 nanotubes as recyclable catalyst for efficient photocatalytic degradation of indigo carmine dye. J. Photochem. Photobiol. A Chem. 2009, 201, 45–49. [Google Scholar] [CrossRef]
- Hsiao, P.-T.; Wang, K.-P.; Cheng, C.-W.; Teng, H. Nanocrystalline anatase TiO2 derived from a titanate-directed route for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2007, 188, 19–24. [Google Scholar] [CrossRef]
- Qian, L.; Du, Z.-L.; Yang, S.-Y.; Jin, Z.-S. Raman study of titania nanotube by soft chemical process. J. Mol. Struct. 2005, 749, 103–107. [Google Scholar] [CrossRef]
- Hoque, M.A.; Guzman, M.I. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Schwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating Materials Development for Photoelectrochemical Hydrogen Production: Standards for Methods, Definitions, and Reporting Protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Aguilar, C.A.; Montalvo, C.; Ceron, J.G.; Moctezuma, E. Photocatalytic Degradation of Acetaminophen. Int. J. Environ. Res. 2011, 5, 1071–1078. [Google Scholar]
- Chang, S.L.; Fekete, M.; Hocking, R.K.; Izgorodina, A.; Singh, A.; Zhou, F.; Macfarlane, D.R.; Spiccia, L. Role of Advanced Analytical Techniques in the Design and Characterization of Improved Catalysts for Water Oxidation. In New and Future Developments in Catalysis: Solar Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 305–339. [Google Scholar]
- Lin, J.C.-T.; De Luna, M.D.G.; Aranzamendez, G.L.; Lu, M.-C. Degradations of acetaminophen via a K2S2O8-doped TiO2 photocatalyst under visible light irradiation. Chemosphere 2016, 155, 388–394. [Google Scholar] [CrossRef]
- Malakootian, M.; Pourshaban-Mazandarani, M.; Hossaini, H.; Ehrampoush, M.H. Preparation and characterization of TiO2 incorporated 13X molecular sieves for photocatalytic removal of acetaminophen from aqueous solutions. Process Saf. Environ. Prot. 2016, 104, 334–345. [Google Scholar] [CrossRef]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A Review of Photocatalysis using Self-organized TiO2 Nanotubes and Other Ordered Oxide Nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef]
- Boxall, C.; Kelsall, G.H. Hypochlorite Electrogeneration. II. Thermodynamics and Kinetic Model of the Anode Reaction Layer; Institute of Chemical Engineers: Rugby, UK, 1992; pp. 59–70. [Google Scholar]
- Yang, L.; Yu, L.E.; Ray, M.B. Photocatalytic Oxidation of Paracetamol: Dominant Reactants, Intermediates, and Reaction Mechanisms. Environ. Sci. Technol. 2009, 43, 460–465. [Google Scholar] [CrossRef]
- Tao, H.; Liang, X.; Zhang, Q.; Chang, C.-T. Enhanced photoactivity of graphene/titanium dioxide nanotubes for removal of Acetaminophen. Appl. Surf. Sci. 2015, 324, 258–264. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Wu, X.; Chen, P.; Deng, N. Photodegradation of acetaminophen in TiO2 suspended solution. J. Hazard. Mater. 2008, 157, 300–307. [Google Scholar] [CrossRef]
- Çifçi, D.I.; Tunçal, T.; Pala, A.; Uslu, O. Determination of optimum extinction wavelength for paracetamol removal through energy efficient thin film reactor. J. Photochem. Photobiol. A Chem. 2016, 322–323, 102–109. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; Aguilar, C.A.; Luna, R.A.; Montalvo, C. Photocatalytic degradation of paracetamol: Intermediates and total reaction mechanism. J. Hazard. Mater. 2012, 243, 130–138. [Google Scholar] [CrossRef]
| Re | kapp (h−1) | R2 | (%) | |
| pH 3 | 0 | 0.1234 | 0.8622 | 46.47 |
| 200 | 0.1250 | 0.9272 | 44.58 | |
| 400 | 0.1655 | 0.9179 | 38.66 | |
| 600 | 0.2299 | 0.8906 | 67.73 | |
| pH 7 | 0 | 0.0747 | 0.9685 | 24.18 |
| 200 | 0.0998 | 0.9945 | 33.61 | |
| 400 | 0.1064 | 0.9984 | 34.79 | |
| 600 | 0.1438 | 0.9934 | 44.58 | |
| pH 9 | 0 | 0.1166 | 0.9969 | 37.83 |
| 200 | 0.1084 | 0.9888 | 35.54 | |
| 400 | 0.1512 | 0.9938 | 46.19 | |
| 600 | 0.1407 | 0.9824 | 45.40 | |
| Re | kapp (h−1) | R2 | ||
| pH 3 | 0 | 0.0797 | 0.9948 | |
| 200 | 0.0999 | 0.9961 | ||
| 400 | 0.1223 | 0.9993 | ||
| 600 | 0.1547 | 0.9872 |
| Estimated β | Sum of Squares | Gl | Mean Square | Fratio | p-Value | |
|---|---|---|---|---|---|---|
| Re (β1) | 15.36 | 393.22 | 1 | 393.22 | 8.18 | 0.0212 |
| pH (β2) | −8.12 | 131.87 | 1 | 131.87 | 2.74 | 0.1363 |
| pH2 (β4) | 22.02 | 323.25 | 1 | 323.25 | 6.72 | 0.0320 |
| Residual error | 384.67 | 8 | 48.08 | |||
| Total | 1233.00 | 11 |
| Re | (COD0 − COD)/COD0 | |
| pH 3 | 0 | 0.30 |
| 200 | 0.45 | |
| 400 | 0.66 | |
| 600 | 0.60 | |
| pH 7 | 0 | 0.27 |
| 200 | 0.37 | |
| 400 | 0.32 | |
| 600 | 0.35 | |
| pH 9 | 0 | 0.21 |
| 200 | 0.13 | |
| 400 | 0.32 | |
| 600 | 0.49 | |
| Re | (TOC0 − TOC)/TOC0 | |
| pH 3 | 600 | 0.45 |
| pH 7 | 0.21 | |
| pH 9 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borràs-Ferrís, J.; Sánchez-Tovar, R.; Blasco-Tamarit, E.; Muñoz-Portero, M.J.; Fernández-Domene, R.M.; García-Antón, J. TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen. Nanomaterials 2019, 9, 583. https://doi.org/10.3390/nano9040583
Borràs-Ferrís J, Sánchez-Tovar R, Blasco-Tamarit E, Muñoz-Portero MJ, Fernández-Domene RM, García-Antón J. TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen. Nanomaterials. 2019; 9(4):583. https://doi.org/10.3390/nano9040583
Chicago/Turabian StyleBorràs-Ferrís, Joan, Rita Sánchez-Tovar, Encarnación Blasco-Tamarit, Maria José Muñoz-Portero, Ramón M. Fernández-Domene, and José García-Antón. 2019. "TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen" Nanomaterials 9, no. 4: 583. https://doi.org/10.3390/nano9040583
APA StyleBorràs-Ferrís, J., Sánchez-Tovar, R., Blasco-Tamarit, E., Muñoz-Portero, M. J., Fernández-Domene, R. M., & García-Antón, J. (2019). TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen. Nanomaterials, 9(4), 583. https://doi.org/10.3390/nano9040583






