Direct Spectroscopy for Probing the Critical Role of Partial Covalency in Oxygen Reduction Reaction for Cobalt-Manganese Spinel Oxides
Abstract
1. Introduction
2. Experimental Method
2.1. Synthesis of CoxMn3-xO4 Oxides and Electrochemical Characterization
2.2. Soft X-ray Absorption Spectroscopy (sXAS)
3. Results and Discussion
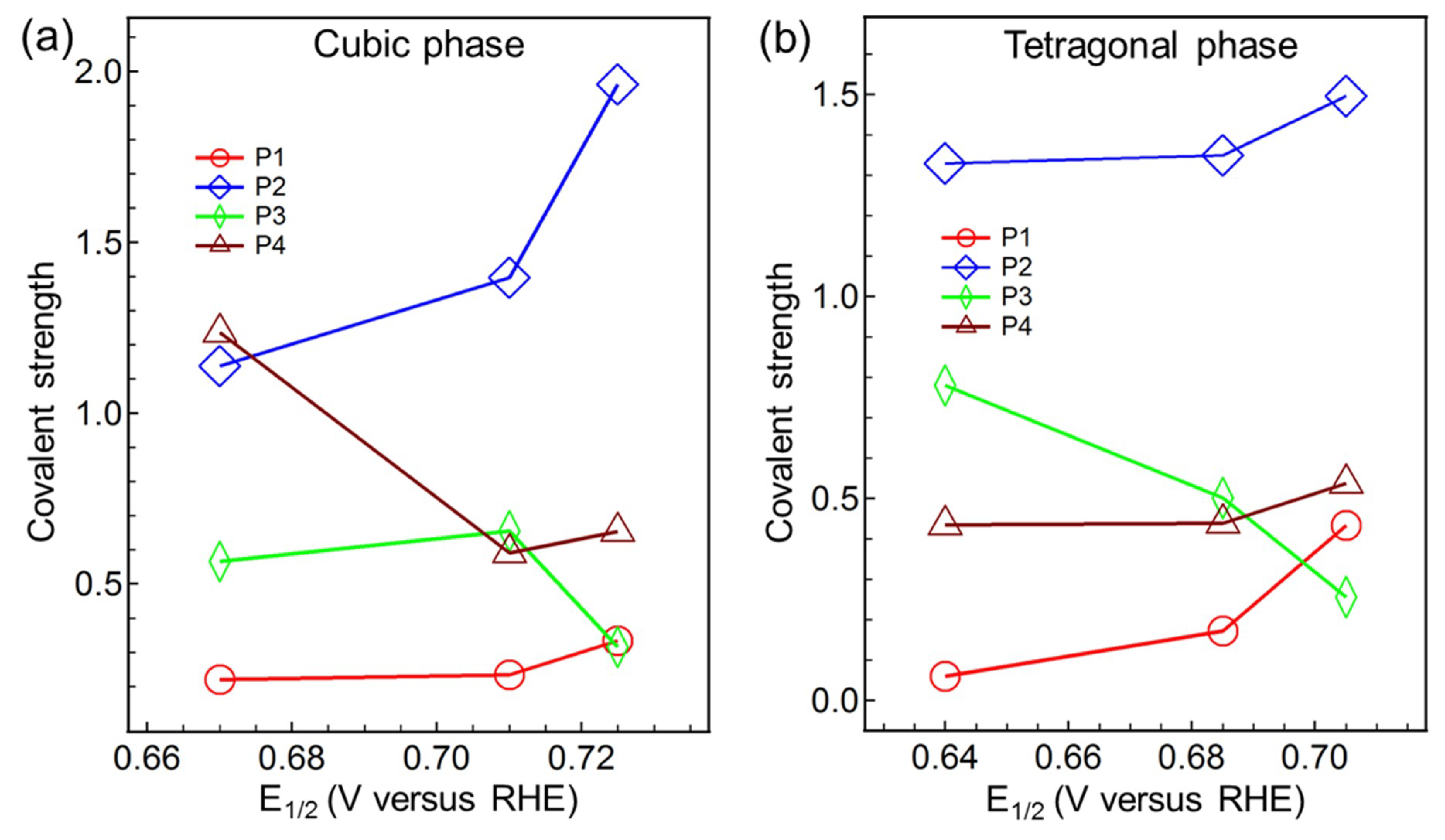
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gray, H.B. Powering the Planet with Solar Fuel. Nat. Chem. 2009, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Hydrogen Cars: Fad or the Future? Science 2009, 324, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Luo, Z.; Marti-Sanchez, S.; Nafria, R.; Joshua, G.; de la Mata, M.; Guardia, P.; Flox, C.; Martinez-Boubeta, C.; Simeonidis, K.; Llorca, J.; Morante, J.R.; Arbiol, J.; Ibanez, M.; Cabot, A. Fe3O4@NiFexOy Nanoparticles with Enhanced Electrocatalytic Properties for Oxygen Evolution in Carbonate Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 29461–29469. [Google Scholar] [CrossRef]
- Davodi, F.; Tavakkoli, M.; Lahtinen, J.; Kallio, T. Straightforward synthesis of nitrogen-doped carbon nanotubes as highly active bifunctional electrocatalysts for full water splitting. J. Catal. 2017, 353, 19–27. [Google Scholar] [CrossRef]
- Davodi, F.; Muhlhausen, E.; Tavakkoli, M.; Sainio, J.; Jiang, H.; Gokce, B.; Marzun, G.; Kallio, T. Catalyst Support Effect on the Activity and Durability of Magnetic Nanoparticles: Toward Design of Advanced Electrocatalyst for Full Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 31300–31311. [Google Scholar] [CrossRef]
- Rios, E.; Gautier, J.-L.; Poillerat, G.; Chartier, P. Mixed Valency Spinel Oxides of Transition Metals and Electrocatalysis: Case of the MnxCo3-xO4 System. Electrochim. Acta 1998, 44, 1491–1497. [Google Scholar] [CrossRef]
- Ponce, J.; Rehspringer, J.-L.; Poillerat, G.; Gautier, J.L. Electrochemical study of nickel–aluminium–manganese spinel NixAl1-xMn2O4. Electrocatalytical properties for the oxygen evolution reaction and oxygen reduction reaction in alkaline media. Electrochim. Acta 2001, 46, 3373–3380. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Li, J.-B.; Li, X.; Yang, J.; Tao, J. Core-Ring Structured NiCo2O4 Nanoplatelets: Synthesis, Characterization, and Electrocatalytic Applications. Adv. Funct. Mater. 2008, 18, 1440–1447. [Google Scholar] [CrossRef]
- Li, Y.; Hasin, P.; Wu, Y. NixCo3-xO4 nanowire arrays for electrocatalytic oxygen evolution. Adv. Mater. 2010, 22, 1926–1929. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Zheng, G.; Li, Y.; Cui, Y.; Dai, H. Rechargeable Li–O2 batteries with a covalently coupled MnCo2O4–graphene hybrid as an oxygen cathode catalyst. Energy Environ. Sci. 2012, 5, 7931. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Zhang, K.; Xu, G.; He, X.; Dong, S.; Liu, Z.; Huang, C.; Gu, L.; Cui, G. Mesoporous NiCo2O4 nanoflakes as electrocatalysts for rechargeable Li-O2 batteries. Chem. Commun. 2013, 49, 3540–3542. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Woo, M.A.; Regis, M.; Choi, K.-S. Electrochemical Synthesis of Spinel Type ZnCo2O4 Electrodes for Use as Oxygen Evolution Reaction Catalysts. J. Phys. Chem. Lett. 2014, 5, 2370–2374. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.; Chartier, P.; Gautier, J.-L. Oxygen Evolution Electrocatalysis in Alkaline Medium at thin MnxCo3-xO4 (0 < x < 1) Spinel Films on Glass/SnO2: F Prepared by Spray Pyrolysis. Solid State Sci. 1999, 1, 267–277. [Google Scholar]
- Restovic, A.; Rı’os, E.; Barbato, S.; Ortiz, J.; Gautier, J.L. Oxygen Reduction in Alkaline Medium at Thin MnxCo3-xO4(0<x<1) Spinel Films Prepared by Spray Pyrolysis. Effect of Oxide Cation Composition on the Reaction Kinetics. J. Electroanal. Chem. 2002, 522, 141–151. [Google Scholar]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Feng, Z.; Scherer, G.G.; Barber, J.; Shao-Horn, Y.; Xu, Z.J. Cations in Octahedral Sites: A Descriptor for Oxygen Electrocatalysis on Transition-Metal Spinels. Adv. Mater. 2017, 29, 1606800. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, S.; Xi, S.; Duan, Y.; Sritharan, T.; Du, Y.; Xu, Z.J. Superexchange Effects on Oxygen Reduction Activity of Edge-Sharing [CoxMn1-xO6] Octahedra in Spinel Oxide. Adv. Mater. 2018, 30, 1705407. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.N.; Machala, M.L.; Bluhm, H.; Chueh, W.C. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nat. Commun. 2015, 6, 6097. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; Hong, W.T.; Lee, Y.-L.; Rondinelli, J.M.; Yang, W.; Goodenough, J.B.; Dabrowski, B.; Freeland, J.W.; Shao-Horn, Y. Estimating Hybridization of Transition Metal and Oxygen States in Perovskites from O K-edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2014, 118, 1856–1863. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, J.; Peng, B.; Pan, Y.; Tao, Z.; Chen, J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 2011, 3, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.C.; Chen, C.I.; Tseng, P.C.; Lin, H.F.; Dann, T.E.; Song, Y.F.; Huang, L.R.; Chen, C.C.; Chuang, J.M.; Tsang, K.L.; et al. Soft X-ray spectroscopy beamline 6 m high energy spherical grating monochromator at SRRC: Optical design and first performance tests. Rev. Sci. Instrum. 1995, 66, 1655–1657. [Google Scholar] [CrossRef]
- Morales, F.; de Groot, F.M.F.; Glatzel, P.; Kleimenov, E.; Bluhm, H.; Havecker, M.; Knop-Gericke, A.; Weckhuysen, B.M. In Situ X-ray Absorption of Co/Mn/TiO2 Catalysts for Fischer-Tropsch Synthesis. J. Phys. Chem. B 2004, 108, 16201–16207. [Google Scholar] [CrossRef]
- Ghiasi, M.; Delgado-Jaime, M.U.; Malekzadeh, A.; Wang, R.-P.; Miedema, P.S.; Beye, M.; de Groot, F.M.F. Mn and Co Charge and Spin Evolutions in LaMn1–xCoxO3 Nanoparticles. J. Phys. Chem. C 2016, 120, 8167–8174. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Hu, Z.; Wu, H.; Burnus, T.; Hollmann, N.; Benomar, M.; Lorenz, T.; Tanaka, A.; Lin, H.J.; Hsieh, H.H.; et al. Spin Blockade, Orbital Occupation, and Charge Ordering in La1.5Sr0.5CoO4. Phys. Rev. Lett. 2009, 102, 116401. [Google Scholar] [CrossRef] [PubMed]
- Suchow, L. A Detailed, Simple Crystal Field Consideration of the Normal Spinel Structure of Co3O4. J. Chem. Educ. 1976, 53, 560. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, Y.R. Optical investigation of charge-transfer transitions in spinel Co3O4. Solid State Commun. 2003, 127, 25–28. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Wang, Y.; Olalde-Velasco, P.; Li, H.; Hu, Y.-S.; Yang, W. Direct evidence of gradient Mn(II) evolution at charged states in LiNi0.5Mn1.5O4 electrodes with capacity fading. J. Power Sources 2015, 273, 1120–1126. [Google Scholar] [CrossRef]
- Qiao, R.; Chin, T.; Harris, S.J.; Yan, S.; Yang, W. Spectroscopic fingerprints of valence and spin states in manganese oxides and fluorides. Curr. Appl. Phys. 2013, 13, 544–548. [Google Scholar] [CrossRef]
- Cramer, S.P.; DeGroot, F.M.F.; Ma, Y.; Chen, C.T.; Sette, F.; Kipke, C.A.; Eichhorn, D.M.; Chan, M.K.; Armstrong, W.H. Ligand Field Strengths and Oxidation States From Manganese L-Edge Spectroscopy. J. Am. Chem. Soc. 1991, 113, 7937–7940. [Google Scholar] [CrossRef]
- Thole, B.T.; Vanderlaan, G. Branching Ratio in X-ray Absorption Spectroscopy. Phys. Rev. B 1988, 38, 3158–3171. [Google Scholar] [CrossRef]
- Ralston, C.Y.; Wang, H.; Ragsdale, S.W.; Kumar, M.; Spangler, N.J.; Ludden, P.W.; Gu, W.; Jones, R.M.; Patil, D.S.; Cramer, S.P. Characterization of Heterogeneous Nickel Sites in CO Dehydrogenases from Clostridium thermoaceticum and Rhodospirillum rubrum by Nickel L-Edge X-ray Spectroscopy. J. Am. Chem. Soc. 2000, 122, 10553–10560. [Google Scholar] [CrossRef]
- Kurata, H.; Colliex, C. Electron-energy-loss core-edge structures in manganese oxides. Phys. Rev. B 1993, 48, 2102–2108. [Google Scholar] [CrossRef]
- De Groot, F.M.F.; Grioni, M.; Fuggle, J.C.; Ghijsen, J.; Sawatzky, G.A.; Petersen, H. Oxygen 1s X-ray-Absorption Edges of Transition-Metal Oxides. Phys. Rev. B 1989, 40, 5715–5723. [Google Scholar] [CrossRef]
- Minasian, S.G.; Keith, J.M.; Batista, E.R.; Boland, K.S.; Bradley, J.A.; Daly, S.R.; Kozimor, S.A.; Lukens, W.W.; Martin, R.L.; Nordlund, D.; et al. Covalency in metal-oxygen multiple bonds evaluated using oxygen K-edge spectroscopy and electronic structure theory. J. Am. Chem. Soc. 2013, 135, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Thorne, J.E.; Wu, C.H.; Liu, Y.S.; Du, C.; Jang, J.W.; Liu, E.; Wang, D.; Guo, J. Strong O 2p-Fe 3d Hybridization Observed in Solution-Grown Hematite Films by Soft X-ray Spectroscopies. J. Phys. Chem. B 2018, 122, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Yoneyama, H.; Tamura, H. Influence of the Nature of the Conduction Band of Transition Metal Oxides on Catalytic Activity for Oxygen Reduction. J. Electroanal. Chem. 1977, 83, 237–243. [Google Scholar] [CrossRef]
- Yeager, E. Dioxygen Electrocatalysis: Mechanisms in Relation to Catalyst Structure. J. Mol. Catal. 1986, 38, 5–25. [Google Scholar] [CrossRef]
- Ahmad, E.A.; Tileli, V.; Kramer, D.; Mallia, G.; Stoerzinger, K.A.; Shao-Horn, Y.; Kucernak, A.R.; Harrison, N.M. Optimizing Oxygen Reduction Catalyst Morphologies from First Principles. J. Phys. Chem. C 2015, 119, 16804–16810. [Google Scholar] [CrossRef]
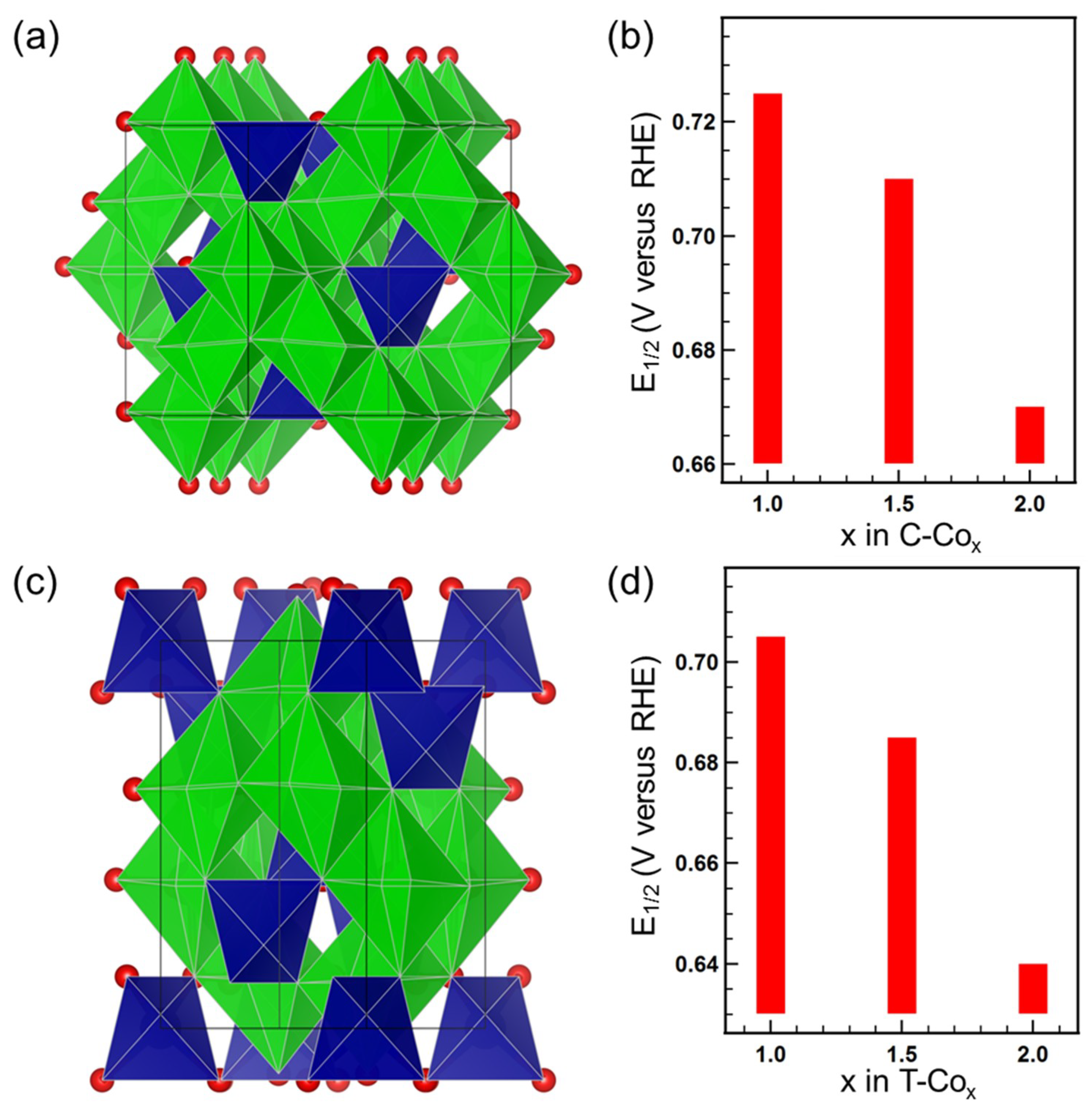
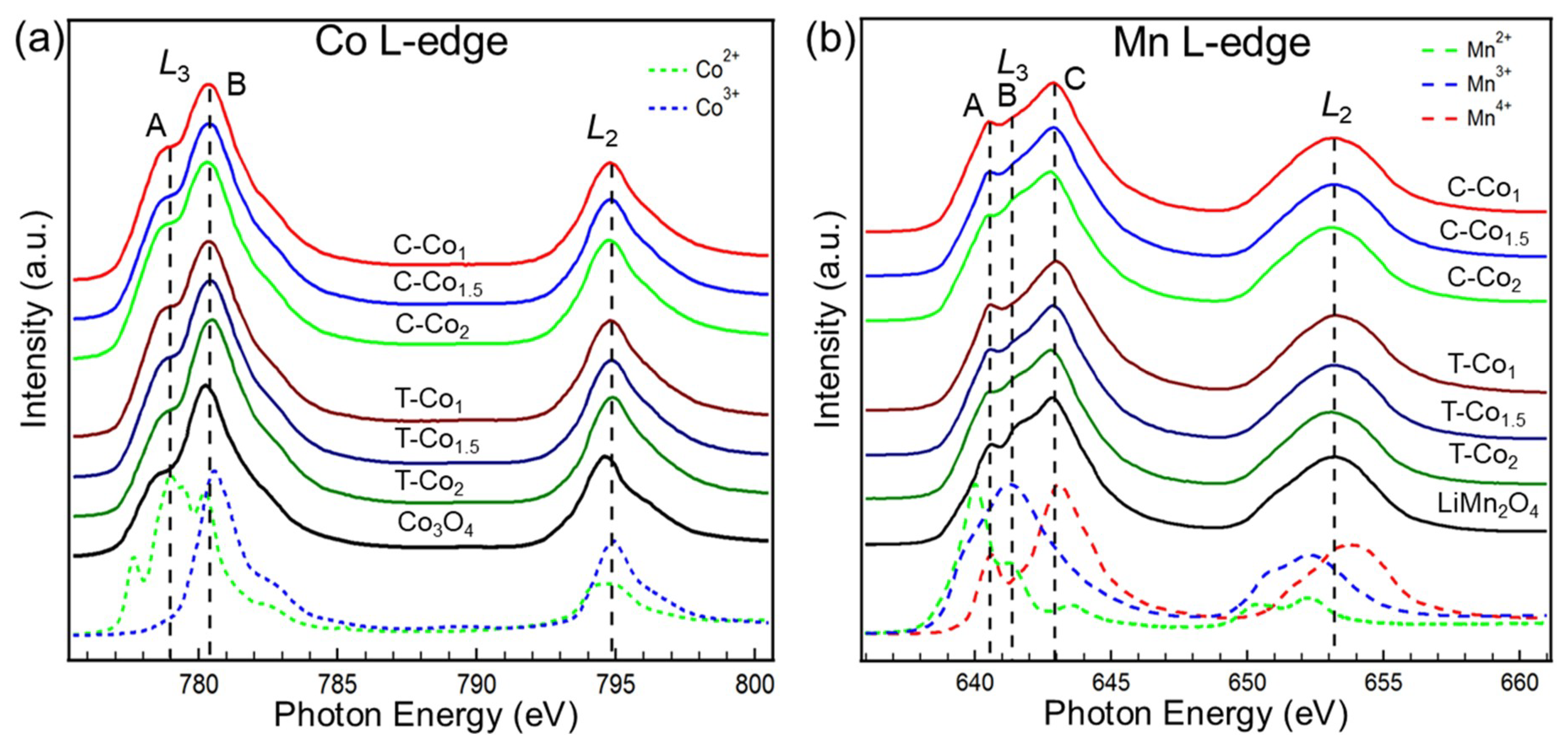
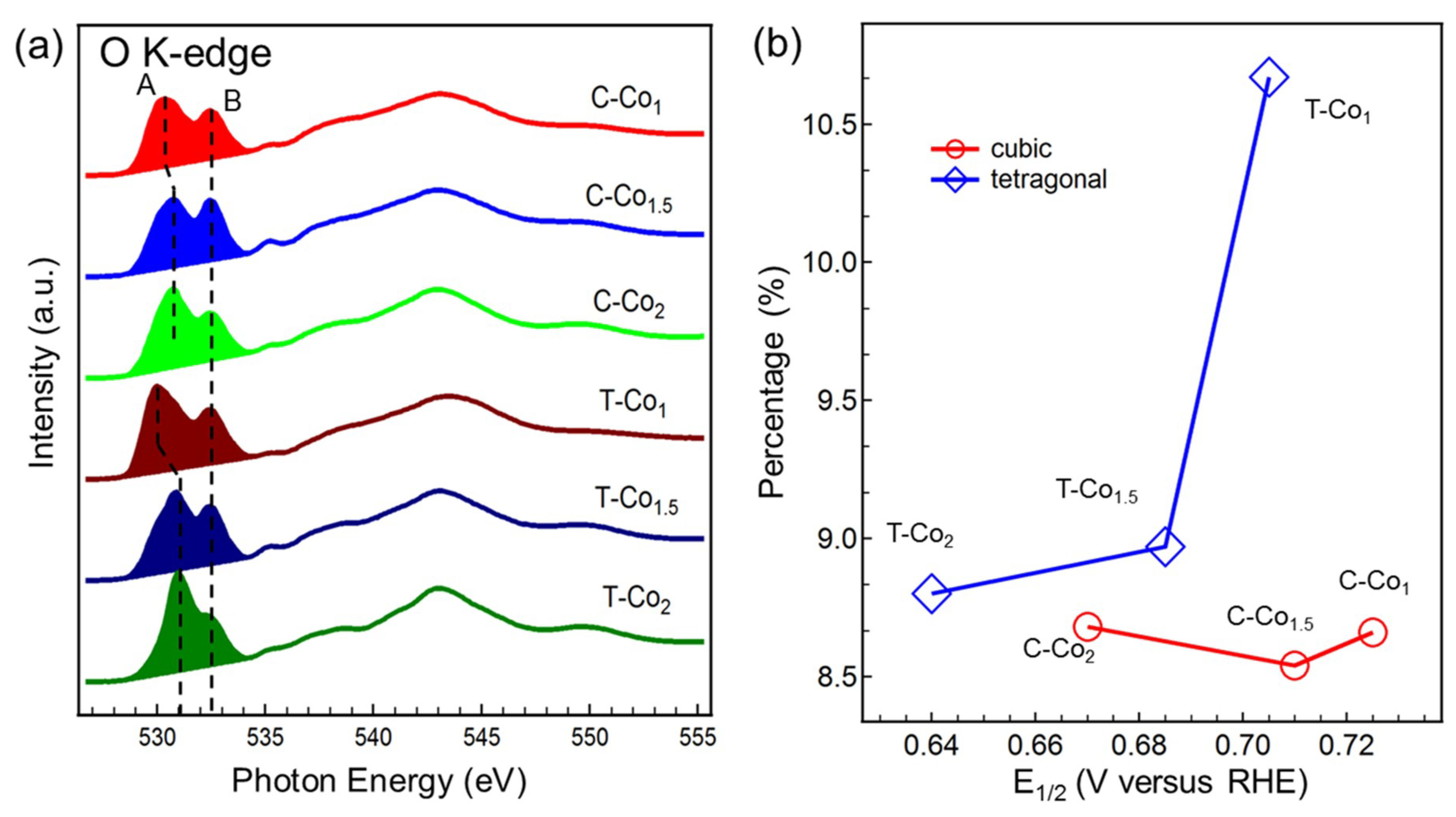
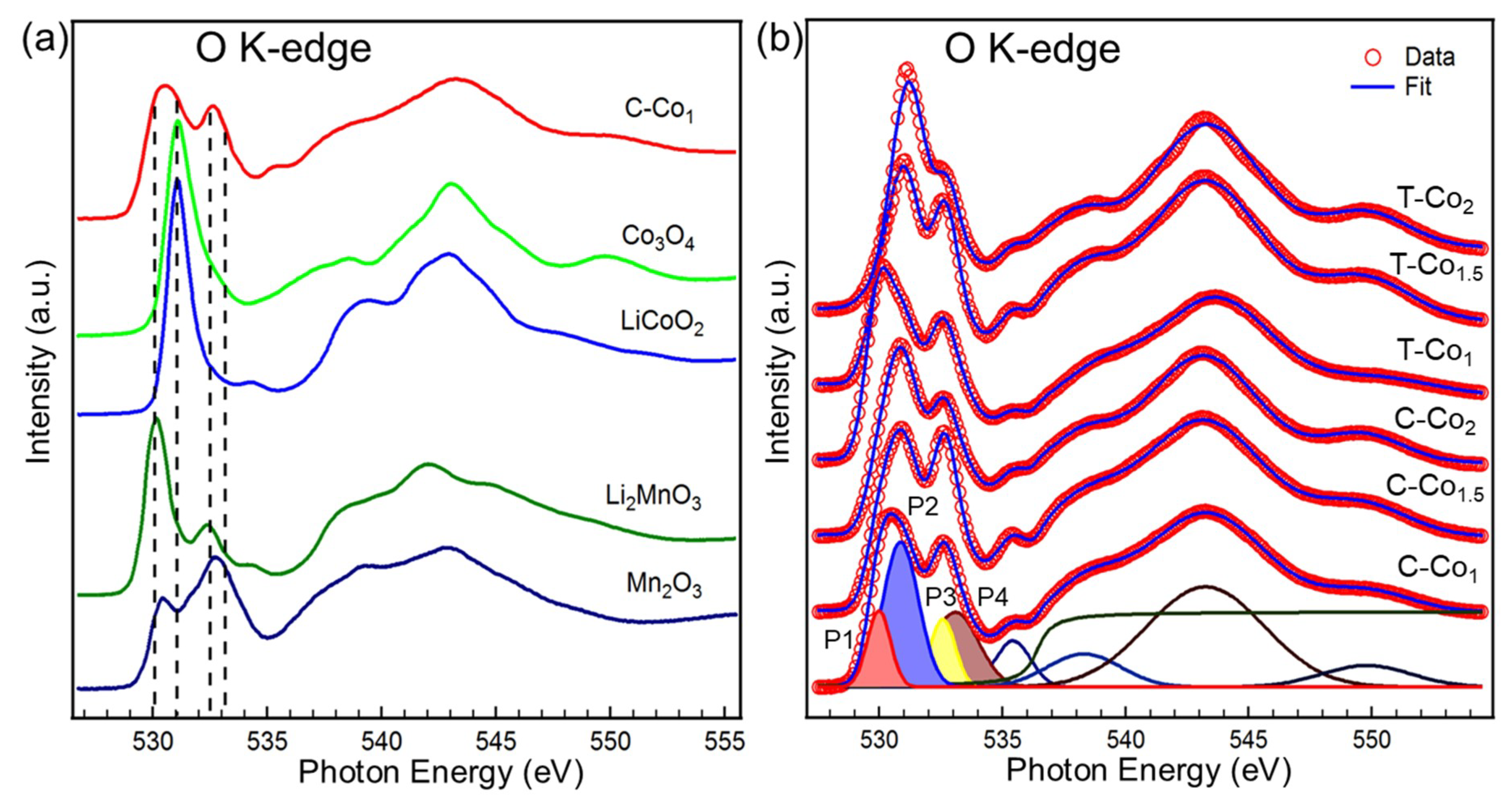
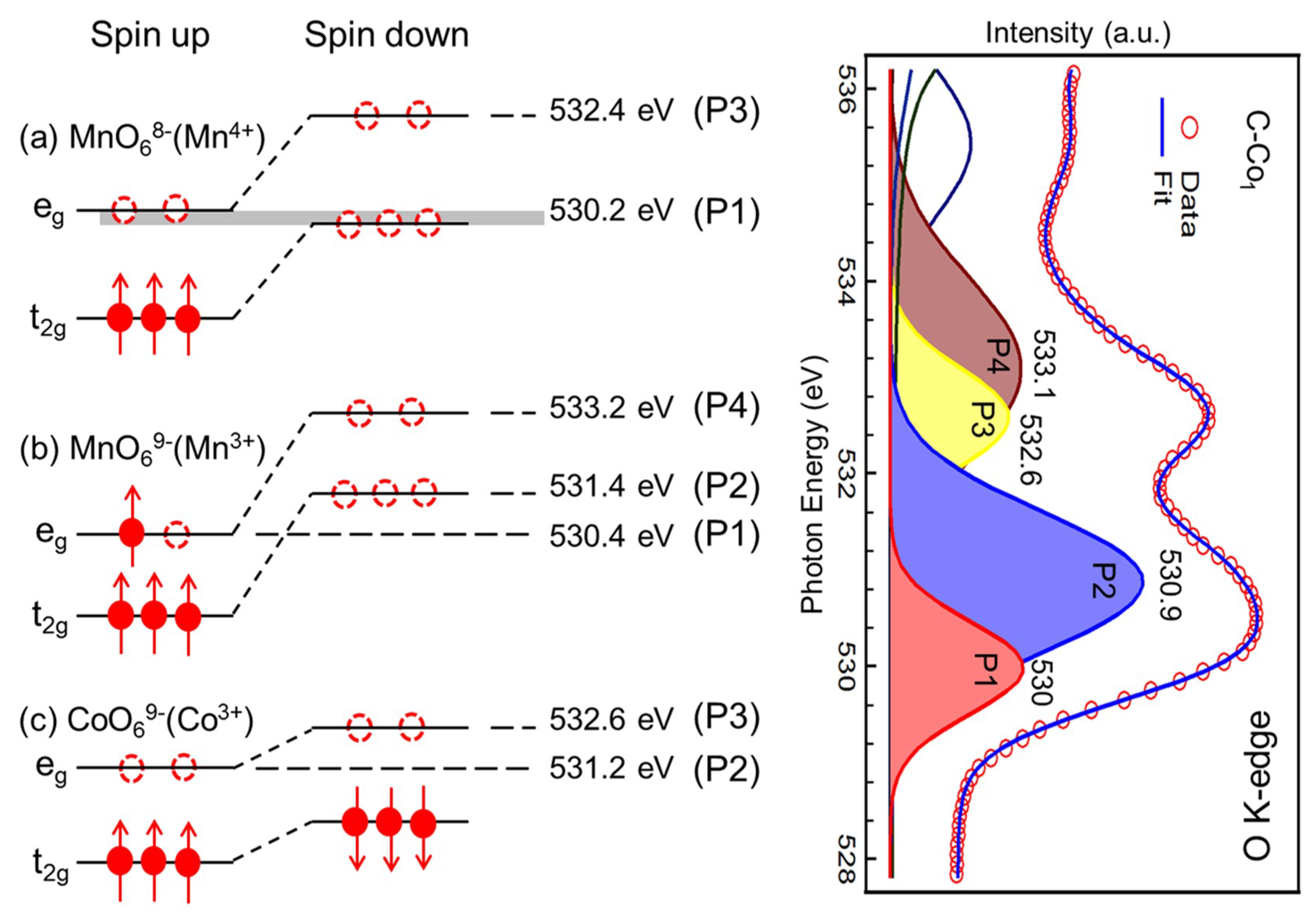
| Gaussian Peak (Energy Position) | Main Partial Covalency |
|---|---|
| P1 (~530.0 eV) | Mn4+ 3d t2g-down and eg-up orbital, Mn3+ 3d eg-up orbital |
| P2 (~530.9 eV) | Co3+ 3d eg-up orbital |
| P3 (~532.6 eV) | Mn4+ 3d eg-down orbital |
| P4 (~533.1e V) | Mn3+ 3d eg-down orbital |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Yu, P.; Zhang, N.; Li, C.; Feng, X.; Ren, G.; Zheng, S.; Fu, J.; Cheng, F.; Liu, X. Direct Spectroscopy for Probing the Critical Role of Partial Covalency in Oxygen Reduction Reaction for Cobalt-Manganese Spinel Oxides. Nanomaterials 2019, 9, 577. https://doi.org/10.3390/nano9040577
Long X, Yu P, Zhang N, Li C, Feng X, Ren G, Zheng S, Fu J, Cheng F, Liu X. Direct Spectroscopy for Probing the Critical Role of Partial Covalency in Oxygen Reduction Reaction for Cobalt-Manganese Spinel Oxides. Nanomaterials. 2019; 9(4):577. https://doi.org/10.3390/nano9040577
Chicago/Turabian StyleLong, Xinghui, Pengfei Yu, Nian Zhang, Chun Li, Xuefei Feng, Guoxi Ren, Shun Zheng, Jiamin Fu, Fangyi Cheng, and Xiaosong Liu. 2019. "Direct Spectroscopy for Probing the Critical Role of Partial Covalency in Oxygen Reduction Reaction for Cobalt-Manganese Spinel Oxides" Nanomaterials 9, no. 4: 577. https://doi.org/10.3390/nano9040577
APA StyleLong, X., Yu, P., Zhang, N., Li, C., Feng, X., Ren, G., Zheng, S., Fu, J., Cheng, F., & Liu, X. (2019). Direct Spectroscopy for Probing the Critical Role of Partial Covalency in Oxygen Reduction Reaction for Cobalt-Manganese Spinel Oxides. Nanomaterials, 9(4), 577. https://doi.org/10.3390/nano9040577





