Modification of Graphene Oxide Membranes by the Incorporation of Nafion Macromolecules and Conductive Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide
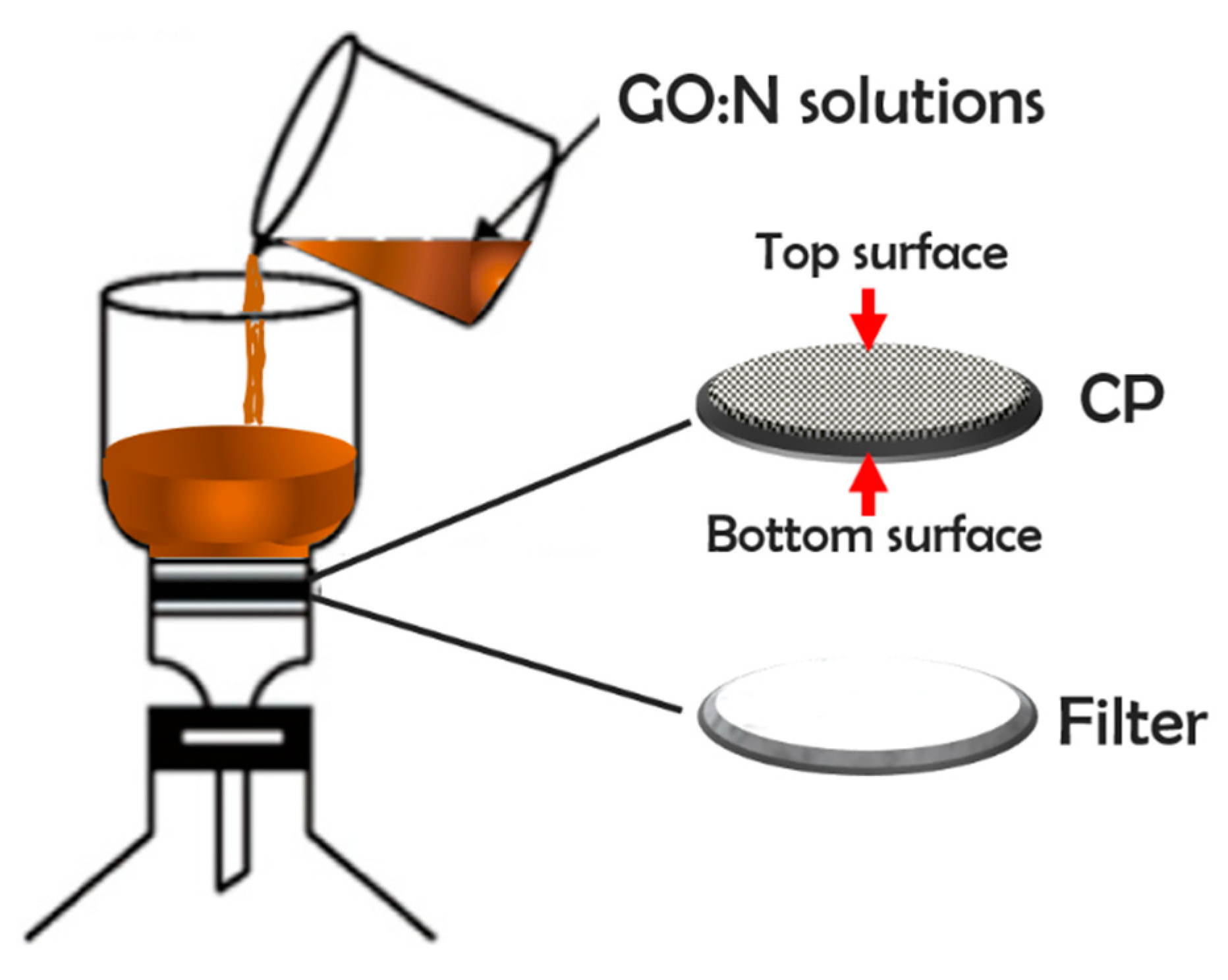
2.3. Preparation of CP Supported GO/N Membranes
2.4. Electrode Preparation for Galvanostatic Experiments
2.5. Characterization
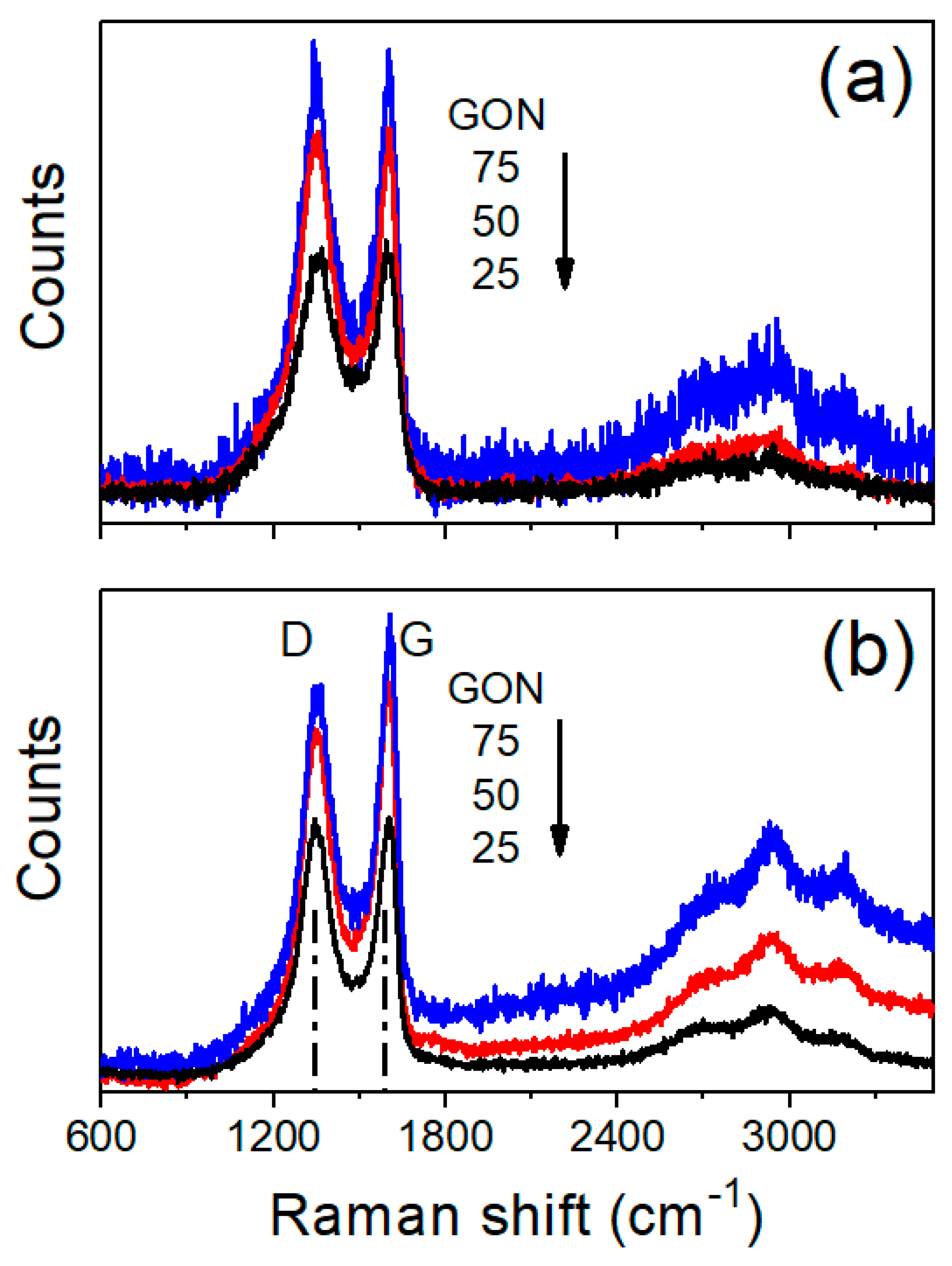
3. Results and Discussion
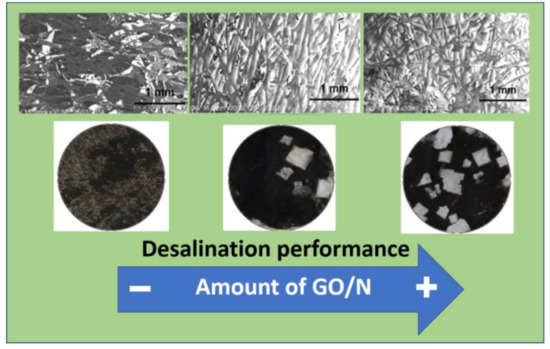
3.1. CP-Supported GO/N Membranes
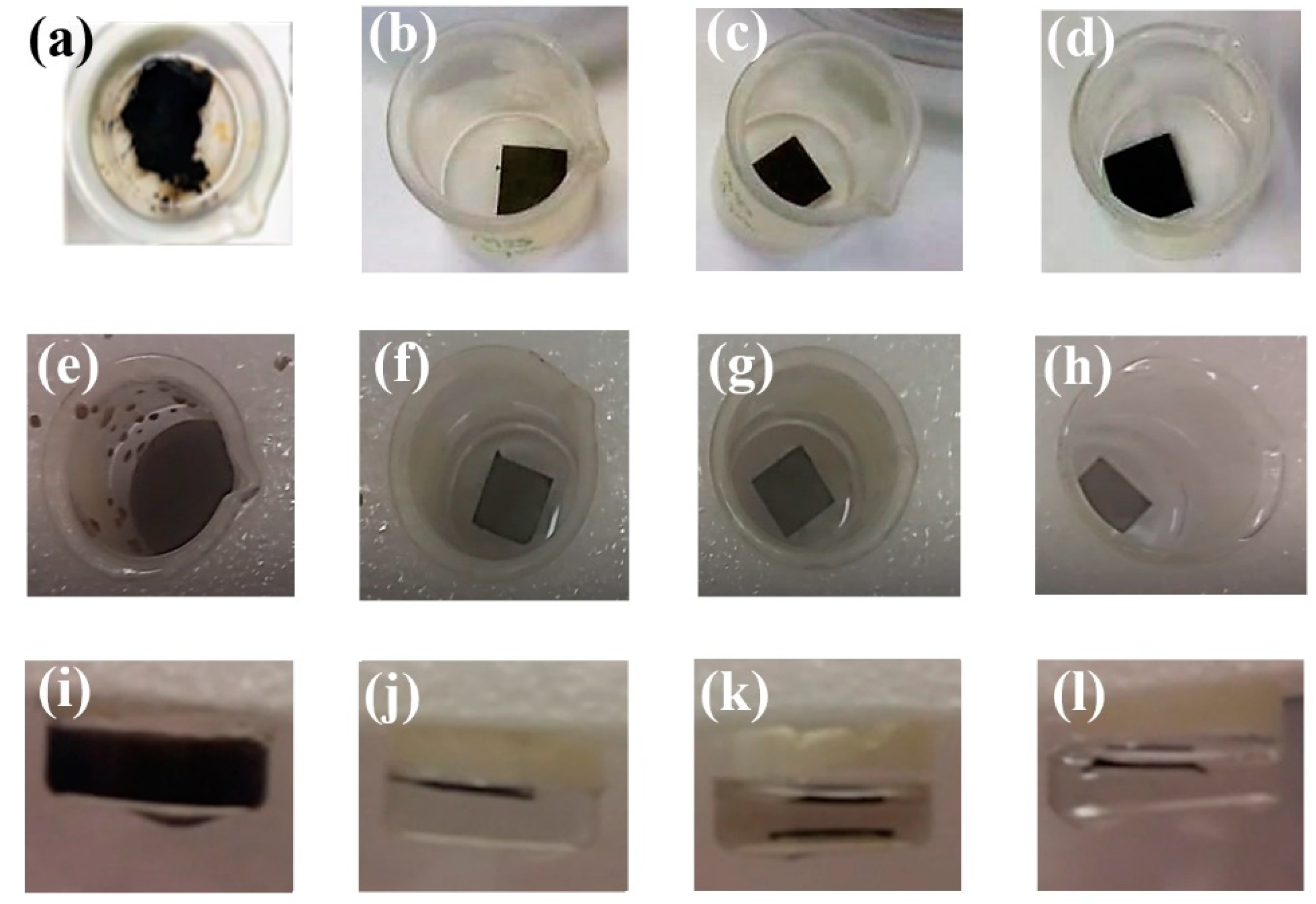
3.2. Stability of CP-GO/N Membranes
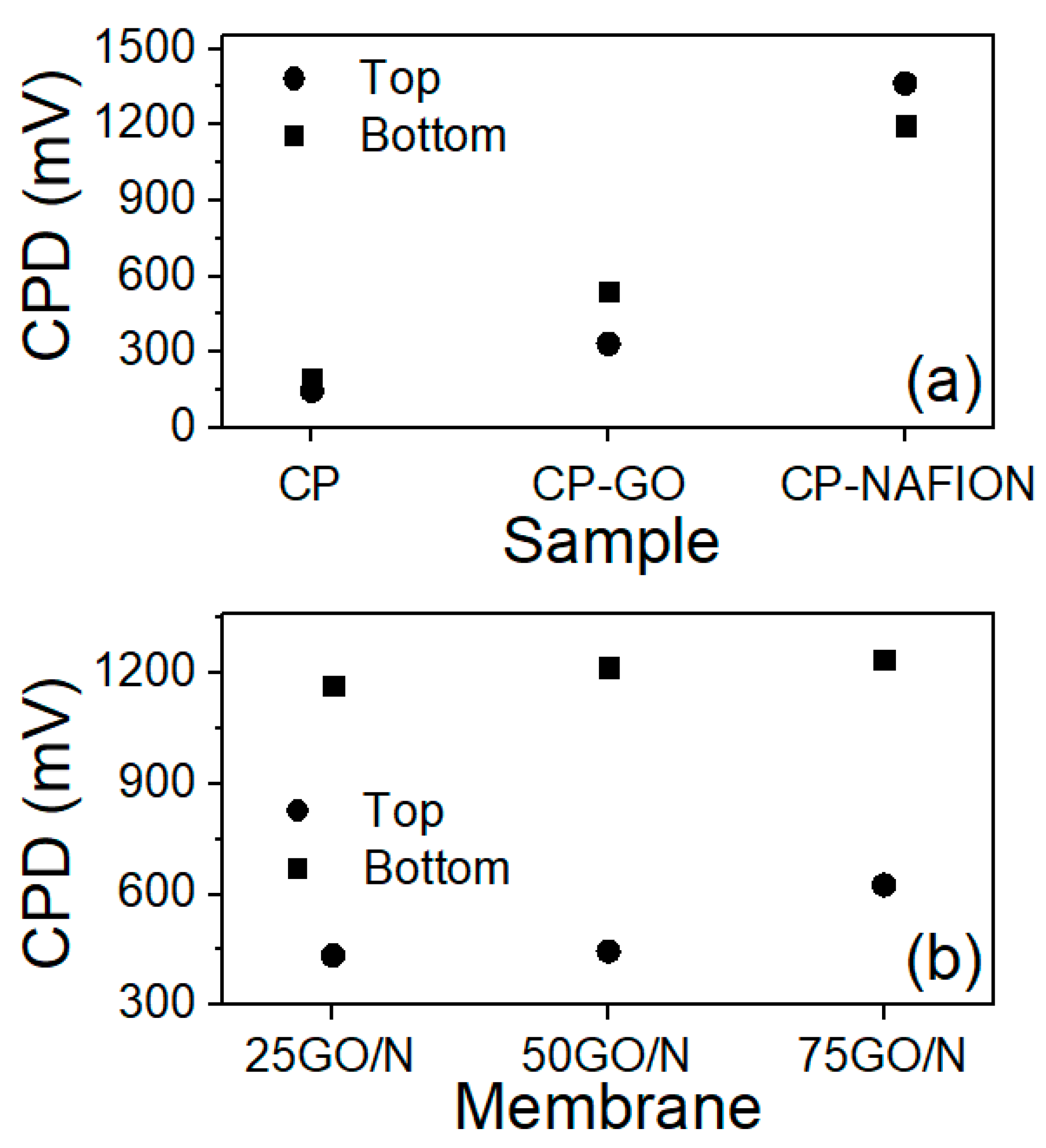
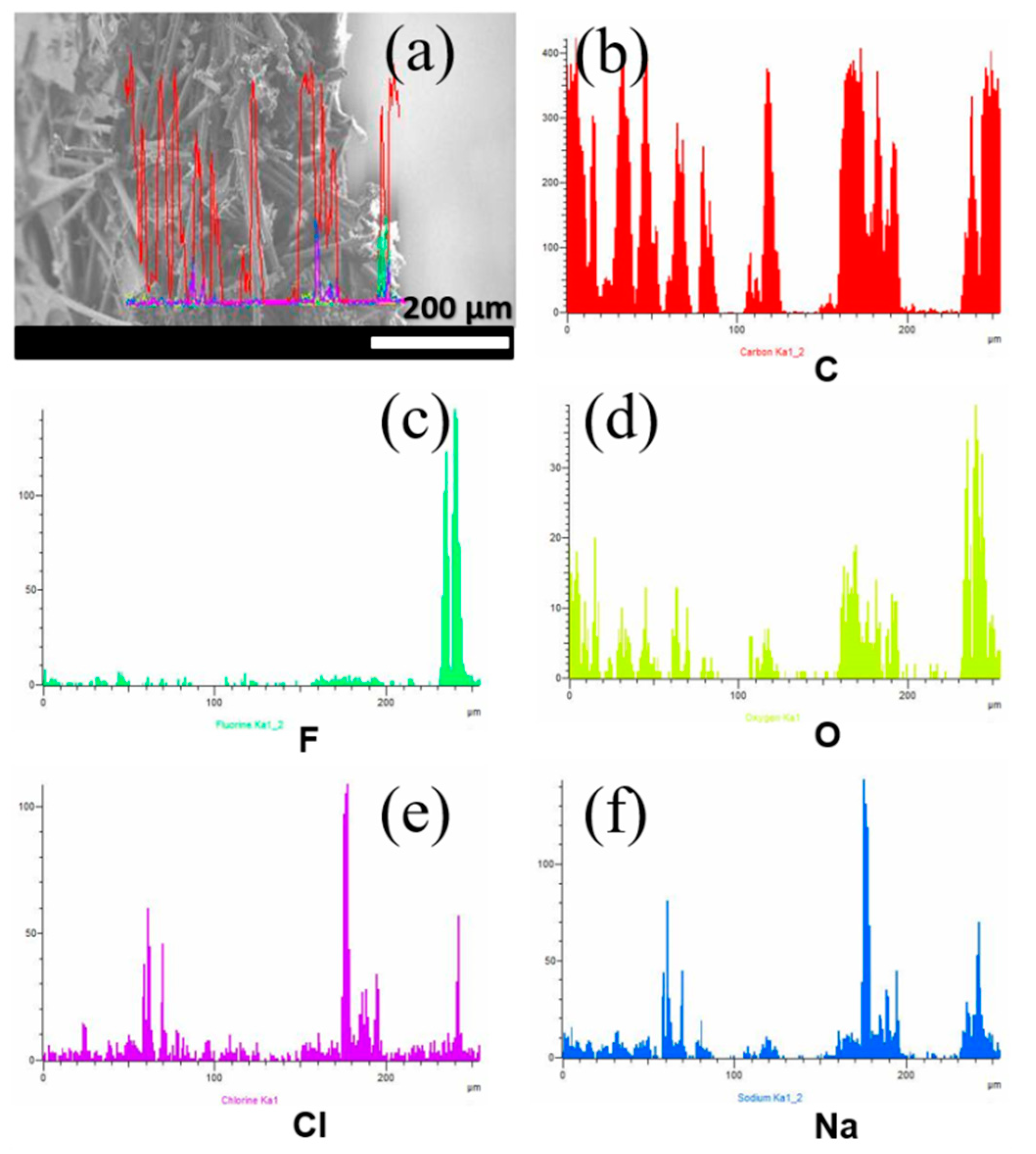
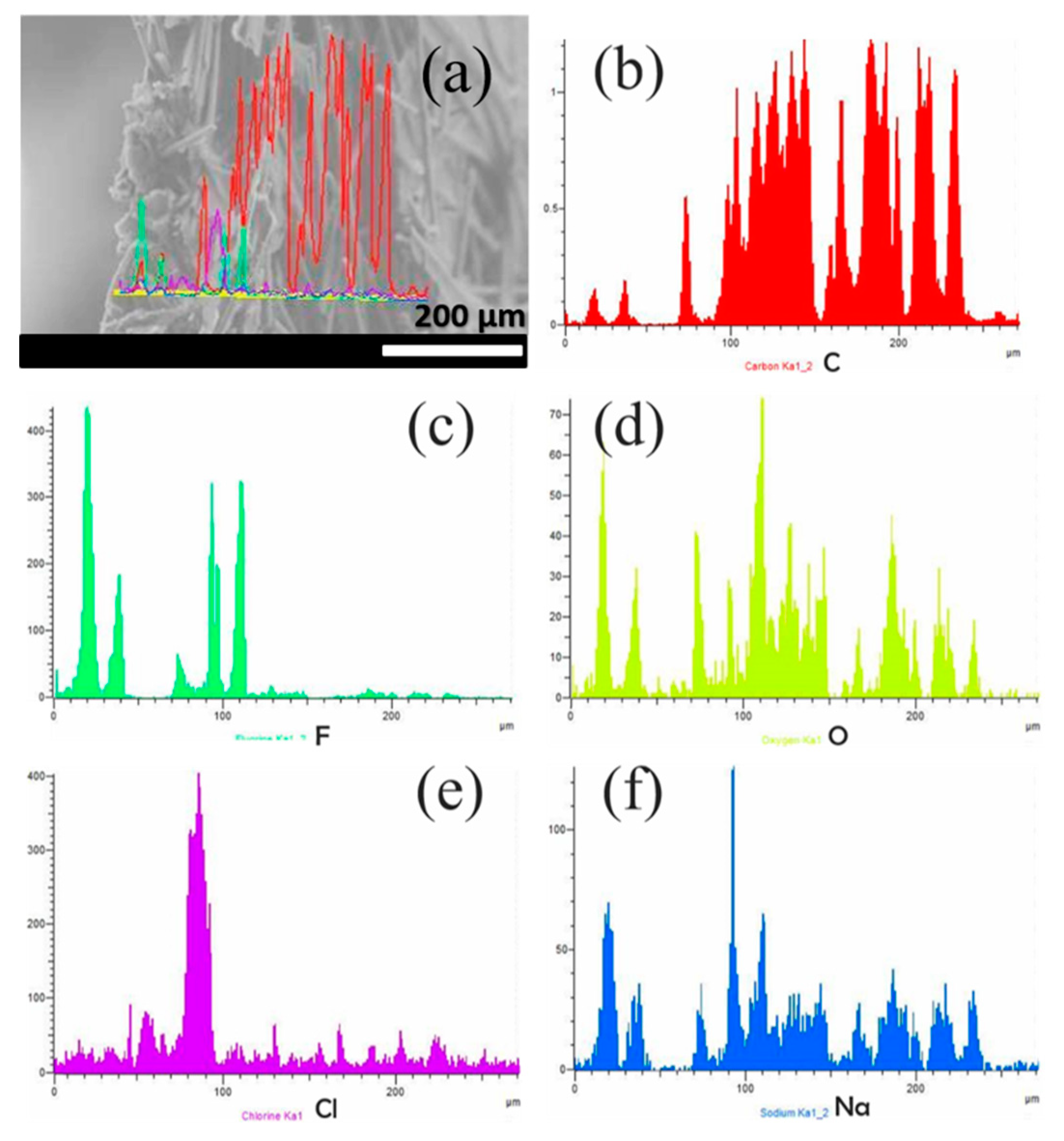
3.3. Distribution of GO and Nafion into the CP Support
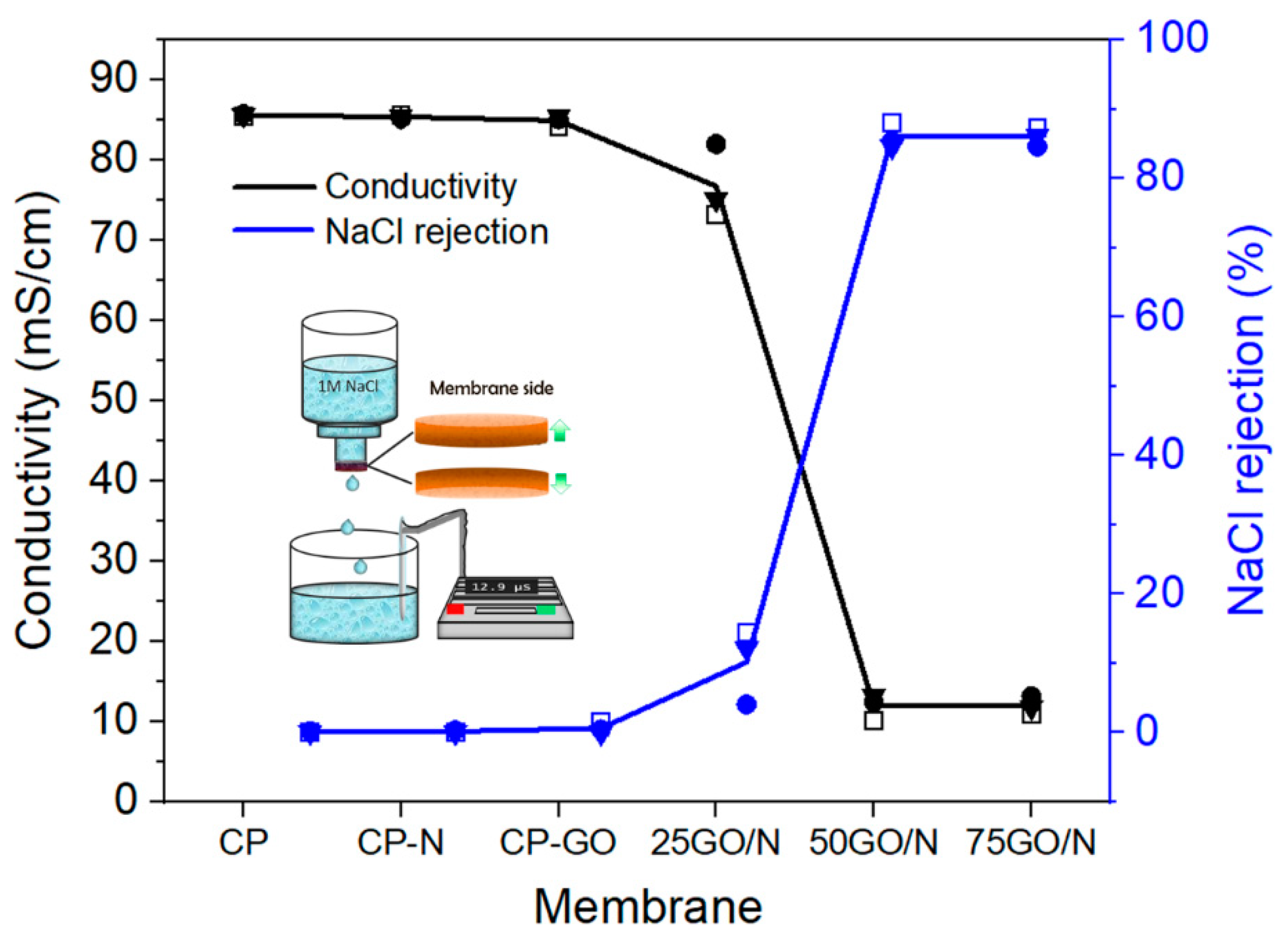
3.4. Salt Removal Experiments
3.5. Charged Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aani, S.A.; Wright, C.J.; Atieh, M.A.; Hilal, N. Engineering nanocomposite membranes: Addressing current challenges and future opportunities. Desalination 2017, 401, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Savage, N.; Diallo, M.S. Nanomaterials and water purification: Opportunities and challenges. J. Nanopart. Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Xue, X.; Cheng, R.; Shi, L.; Ma, Z.; Zheng, X. Nanomaterials for water pollution monitoring and remediation. Environ. Chem. Lett. 2017, 15, 23–27. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Sarkar, S.; Chakraborty, S.; Bhattacharjee, C. Photocatalytic degradation of pharmaceutical wastes by alginate supported TiO2 nanoparticles in packed bed photo reactor (PBPR). Ecotoxicol. Environ. Saf. 2015, 121, 263–270. [Google Scholar] [CrossRef]
- Johns, J.; Rao, V. Adsorption of methylene blue onto natural rubber/chitosan blends. Int. J. Polym. Mater. 2011, 60, 766–775. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-Ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef]
- Lu, Y.; Suzuki, T.; Zhang, W.; Moore, J.S.; Mariñas, B.J. Nanofiltration membranes based on rigid star amphiphiles. Chem. Mater. 2007, 19, 3194–3204. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Safajj, N.; Persin, M.; Younsi, S.A.; Albizane, A.; Cretin, M.; Larbot, A. Elaboration and characterization of microfiltration and ultrafiltration membranes deposited on raw support prepared from natural Moroccan clay: Application to filtration of solution containing dyes and salts. Appl. Clay Sci. 2006, 31, 110–119. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124, 030901. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liang, B.; Liu, Y.; Xu, T.; Wang, L.; Cao, B.; Pan, K. Graphene oxide as an effective barrier on a porous nanofibrous membrane for water treatment. ACS Appl. Mater. Interfaces 2016, 8, 6211–6218. [Google Scholar] [CrossRef]
- Xu, K.; Feng, B.; Zhou, C.; Huang, A. Synthesis of highly stable graphene oxide membranes on polydopamine functionalized supports for seawater desalination. Chem. Eng. Sci. 2016, 146, 159–165. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Christie, T.C.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wu, G.; Janicke, M.T.; Cullen, D.A.; Mukundan, R.; Baldwin, J.K.; Brosha, E.L.; Galande, C.; Ajayan, P.M.; More, K.L.; et al. Ozonated graphene oxide film as a proton-exchange membrane. Angew. Chem. Int. Ed. 2014, 53, 3588–3593. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Oweida, T.J.; Yingling, Y.G. Interfacial stability of graphene-based surfaces in water and organic solvents. J. Mater. Sci. 2018, 53, 5766–5776. [Google Scholar] [CrossRef]
- Wang, W.; Eftekhari, E.; Zhu, G.; Zhang, X.; Yan, Z.; Li, Q. Graphene oxide membranes with tunable permeability due to embedded carbon dots. Chem. Commun. 2014, 50, 13089–13092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safarpour, M.; Khatee, A.; Vatanpour, V. Preparation of a novel polyvinylidene fluoride (PVDF) ultrafiltration membrane modified with reduced graphene oxide/titanium dioxide (TiO2) Nanocomposite with enhanced hydrophilicity and antifouling properties. Ind. Eng. Chem. Res. 2014, 53, 13370–13382. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.J.; Lee, K.H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Xu, L.; Wen, L.F.; Du, Y.; Zhang, X.; Wu, J.; Xu, Z.K. Graphene oxide nanofiltration membranes stabilized by cationic porphyrin for high salt rejection. ACS Appl. Mater. Interfaces 2016, 8, 12588–12593. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.J.; Lai, J.Y.; Liu, Y.L. Nanohybrids of graphene oxide chemically-bonded with Nafion: Preparation and application for proton exchange membrane fuel cells. J. Membr. Sci. 2016, 514, 86–94. [Google Scholar] [CrossRef]
- Sengupta, S.; Lyulin, A.V. Molecular dynamics simulations of substrate hydrophilicity and confinement effects in capped Nafion films. J. Phys. Chem. B 2018, 122, 6107–6119. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Mishra, M.; Joshi, R.K.; Ojha, S.; Kanjilal, D.; Mohanty, T. Role of oxygen in the work function modification at various stages of chemically synthesized graphene. J. Phys. Chem. C 2013, 117, 19746–19750. [Google Scholar] [CrossRef]

| Membrane | Filtration volumes | GO (mg) | Nafion (mg) |
|---|---|---|---|
| 25GO/N | 25 mL GO + 12.5 mL Nafion (0.05%) | 1.2 | 0.06 |
| 50GO/N | 50 mL GO + 25 mL Nafion (0.05%) | 2.4 | 0.12 |
| 75GO/N | 75 mL GO + 37.5 mL Nafion (0.05%) | 3.6 | 0.18 |
| Sample | Diffraction Peak 2θ (°) | Interplanar Distance d002 (nm) | Size Crystal Lc (nm) | Number of Layers Lc/d002 |
|---|---|---|---|---|
| GO 1 | 11 | 0.79 ± 0.1 | - | - |
| GO/N | 11.1 | 0.81 | 9.7 | ~12 |
| CP | 26.3 | 0.33 | 9.8 | ~30 |
| Nafion | 17 | 0.51 | 7.7 | ~15 |
| Membrane | Top | Bottom | ||||
|---|---|---|---|---|---|---|
| ID | IG | ID/IG | ID | IG | ID:IG | |
| CP | 1.08 | 1.11 | 0.93 | - | - | - |
| GO | 1.38 | 1.46 | 0.95 | - | - | - |
| 25GO/N | 0.18 | 0.18 | 1.00 | 0.69 | 0.69 | 1.00 |
| 50GO/N | 0.25 | 0.25 | 1.00 | 0.89 | 1.01 | 0.88 |
| 75GO/N | 0.29 | 0.30 | 0.96 | 0.99 | 1.15 | 0.86 |
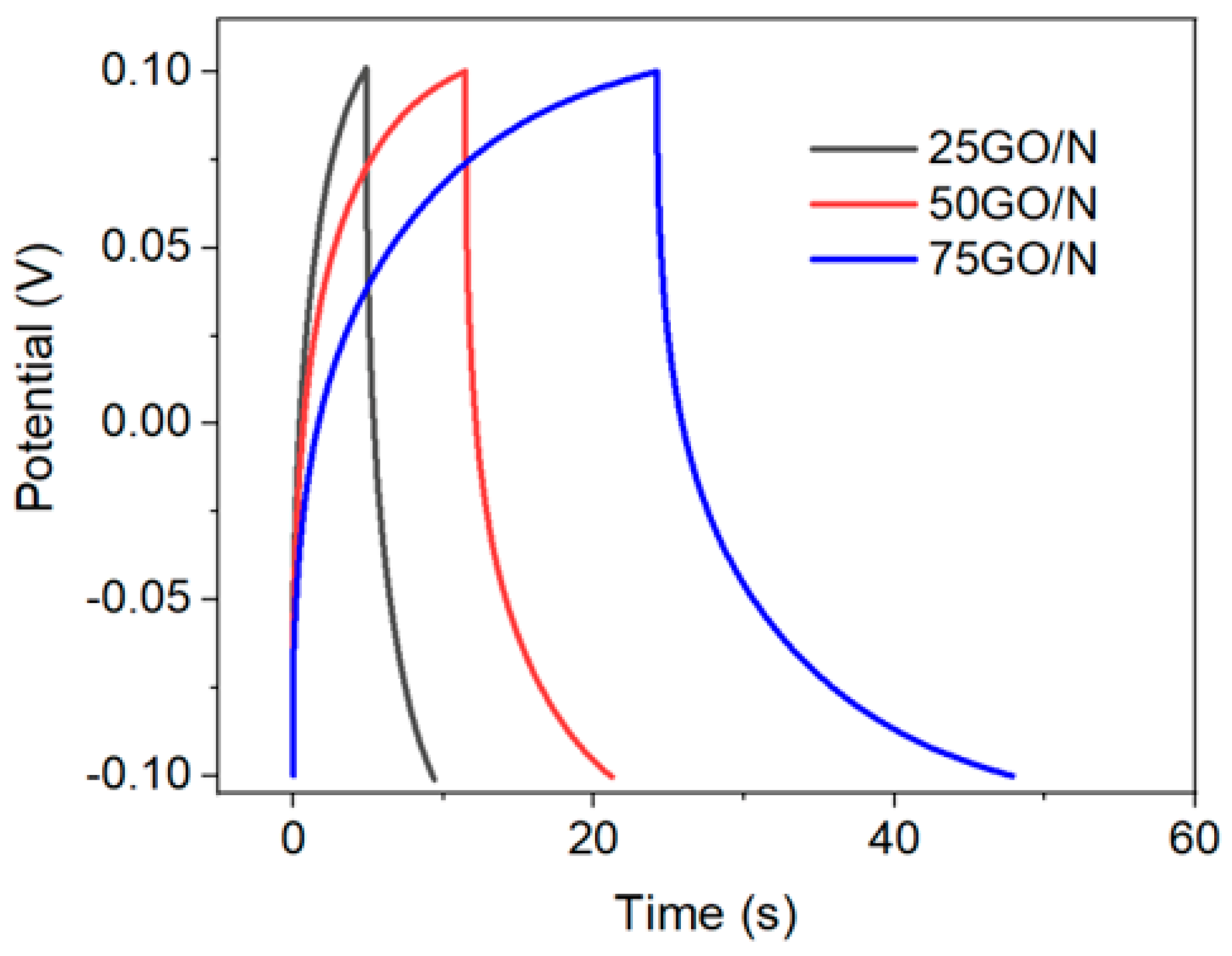
| Sample | mT (mg) | J (A g−1) | AreaGCC (V s) | C1 (F/g) | Q2 (C/g) | CNaCl3 (mM/g) |
|---|---|---|---|---|---|---|
| 25GO/N | 0.2 | 5 | 0.779 | 0.195 | 39 | 0.4 |
| 50GO/N | 0.4 | 25 | 1.912 | 2.39 | 478 | 4.95 |
| 75GO/N | 0.6 | 25 | 3.977 | 4.64 | 928 | 9.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concha-Guzmán, M.O.; Jaramillo-Quintero, O.A.; Rincón, M.E. Modification of Graphene Oxide Membranes by the Incorporation of Nafion Macromolecules and Conductive Scaffolds. Nanomaterials 2019, 9, 556. https://doi.org/10.3390/nano9040556
Concha-Guzmán MO, Jaramillo-Quintero OA, Rincón ME. Modification of Graphene Oxide Membranes by the Incorporation of Nafion Macromolecules and Conductive Scaffolds. Nanomaterials. 2019; 9(4):556. https://doi.org/10.3390/nano9040556
Chicago/Turabian StyleConcha-Guzmán, Maria O., Oscar A. Jaramillo-Quintero, and Marina E. Rincón. 2019. "Modification of Graphene Oxide Membranes by the Incorporation of Nafion Macromolecules and Conductive Scaffolds" Nanomaterials 9, no. 4: 556. https://doi.org/10.3390/nano9040556