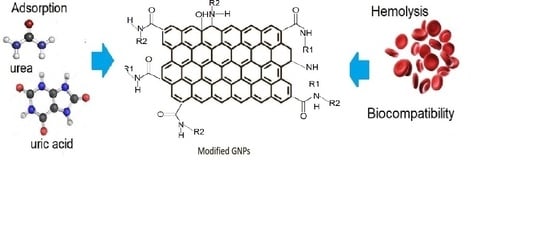
Graphene Nanoplatelets Modified with Amino-Groups by Ultrasonic Radiation of Variable Frequency for Potential Adsorption of Uremic Toxins
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Chemical Modification of Graphene by Ultrasonic Tip
2.3. Characterization
2.4. Adsorption of Uremic Toxins
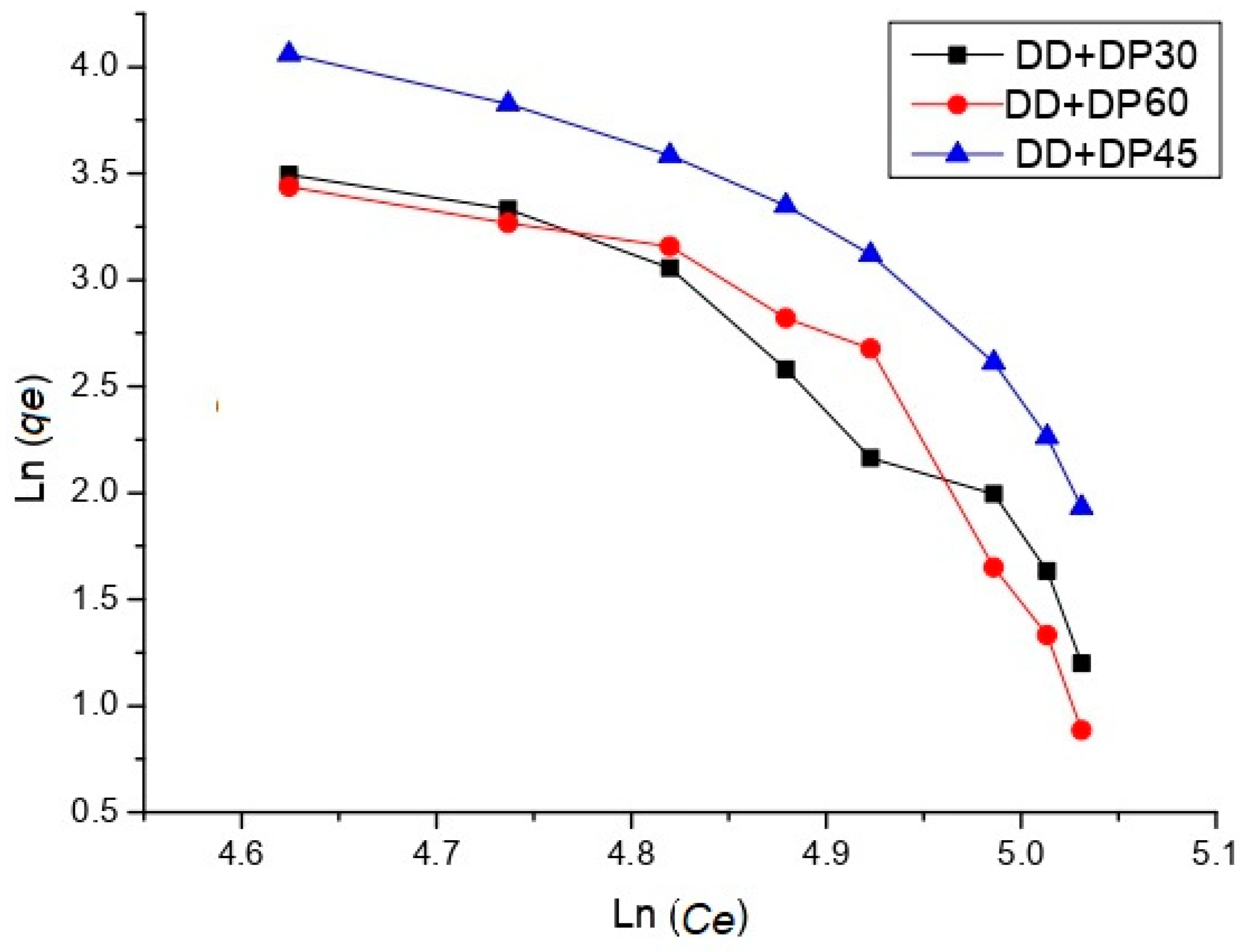
2.4.1. Adsorption Isotherm
2.4.2. In Vitro Blood Compatibility
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
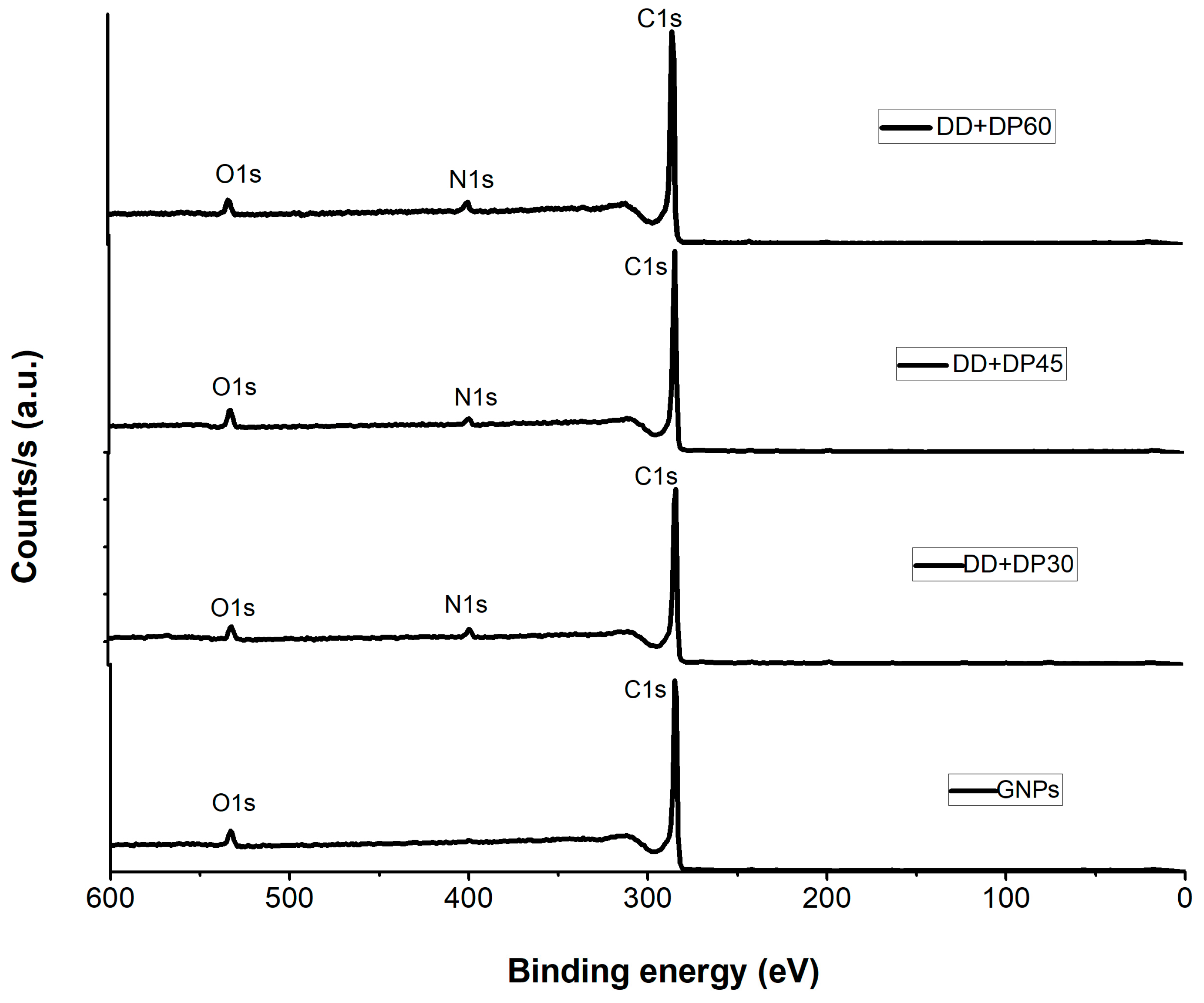
3.2. X-Ray Photoelectron Spectroscopy (XPS)
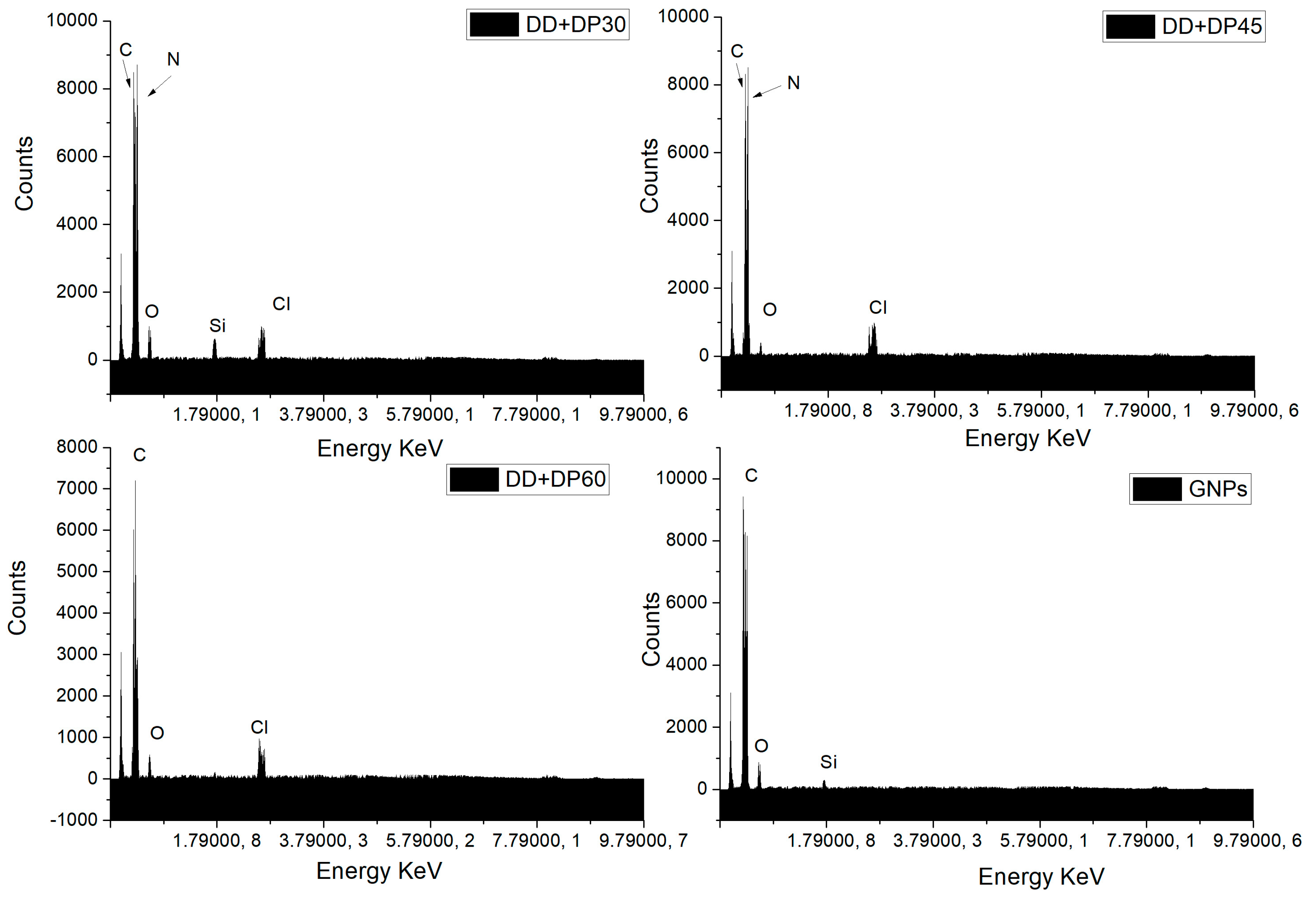
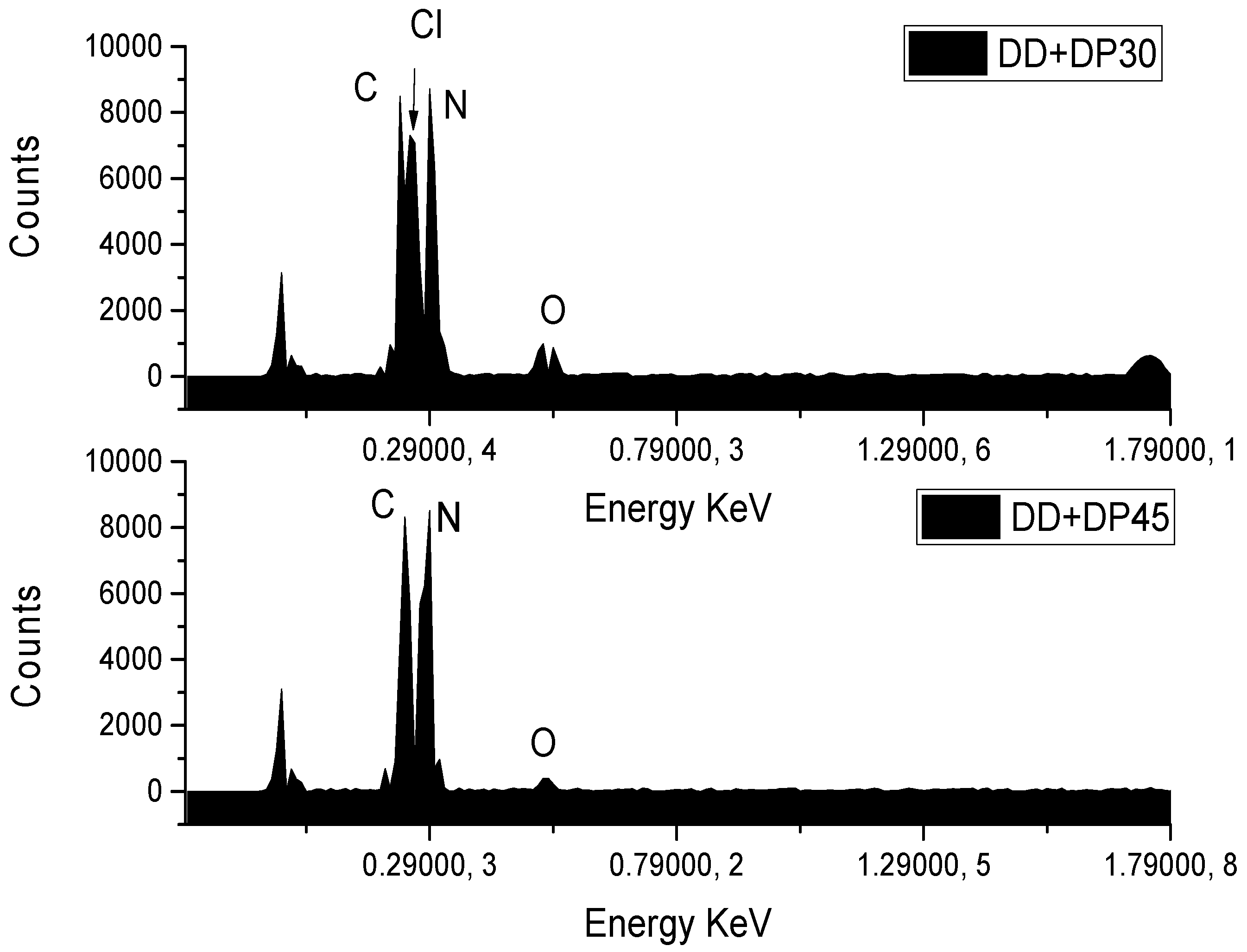
3.3. Scanning Electron Microscopy (SEM-EDS)
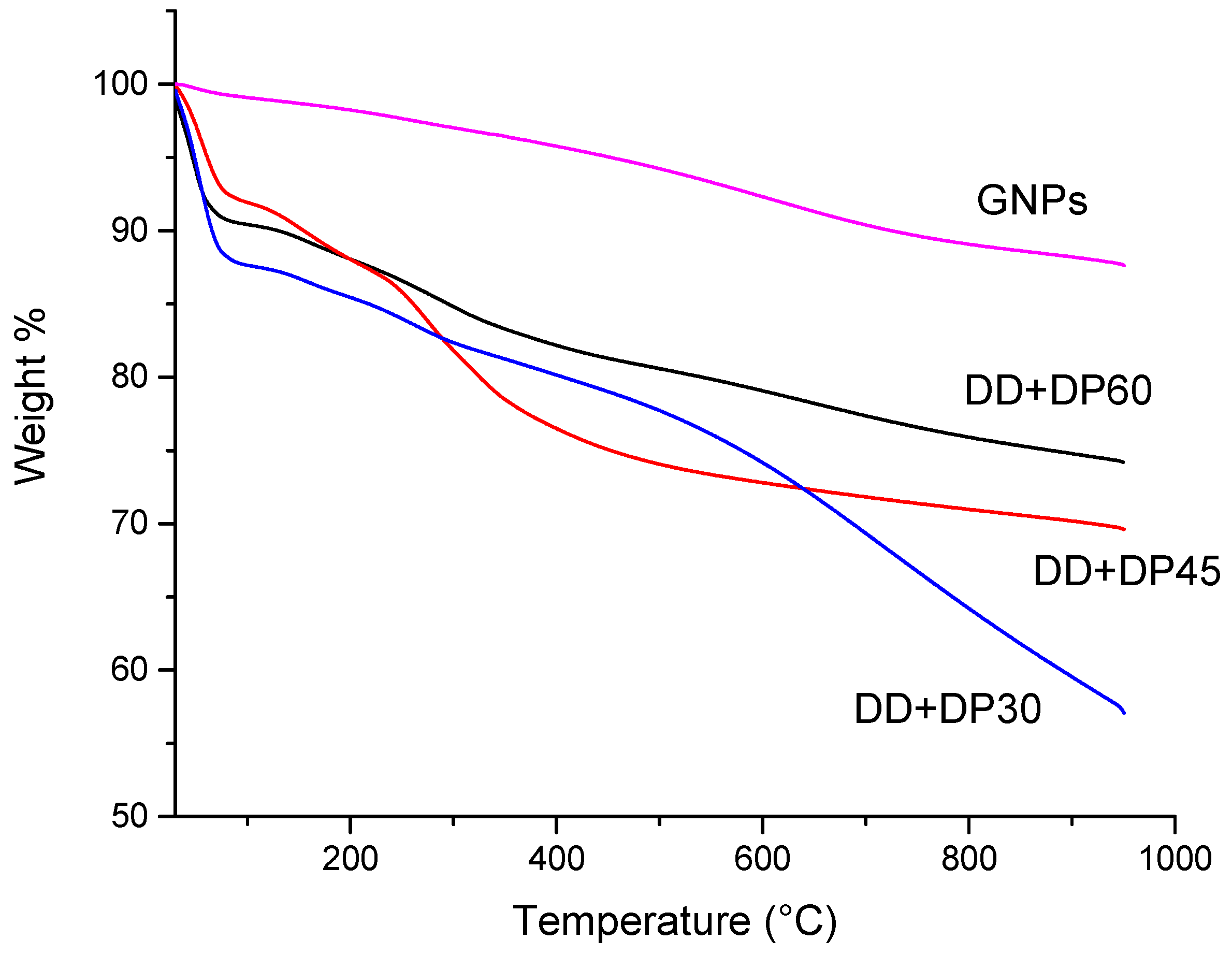
3.4. Thermogravimetric Aanalysis (TGA)
3.5. Dispersion Test
3.6. Proposal for Surface Modification in Graphene Platelets
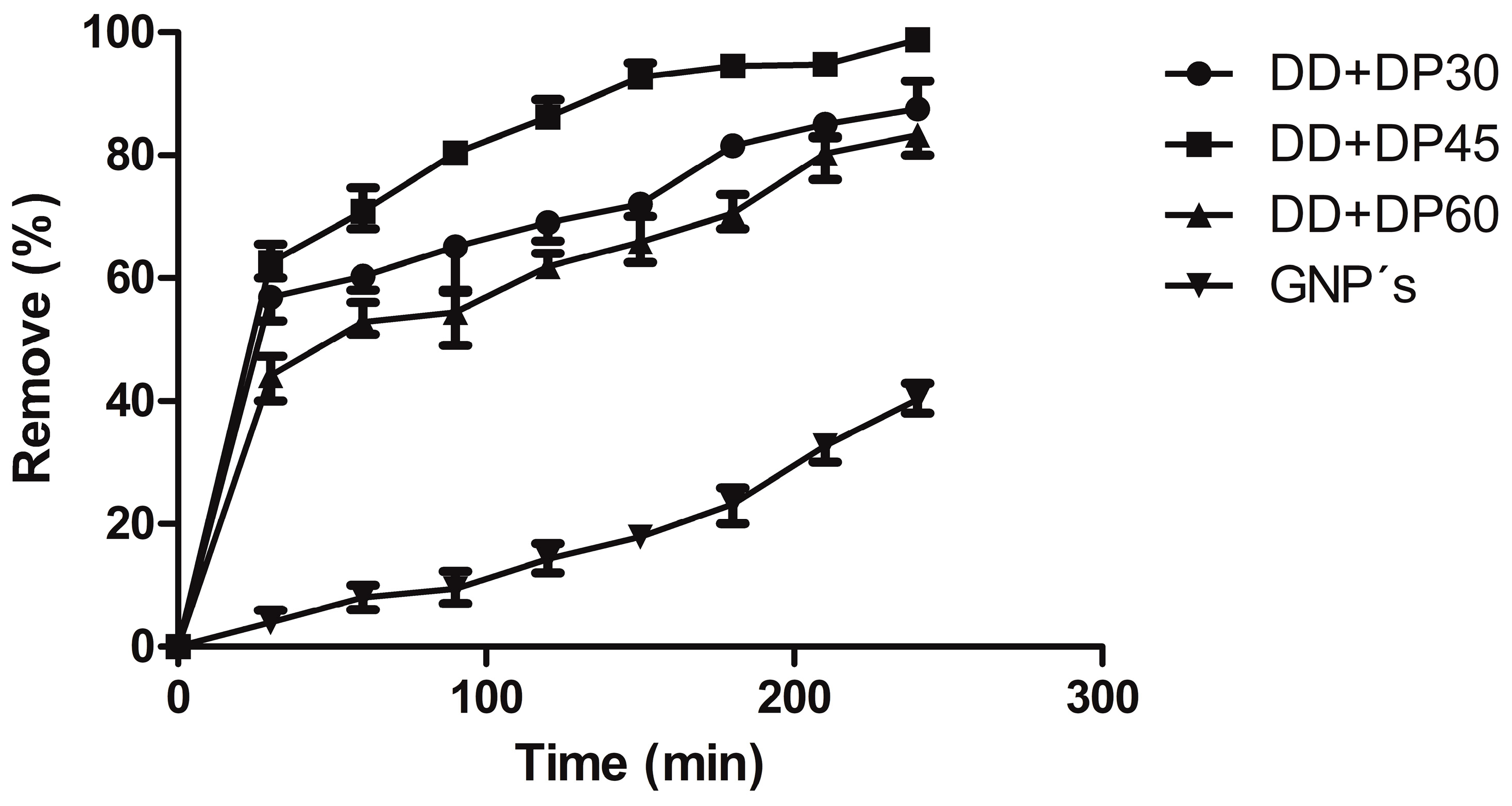
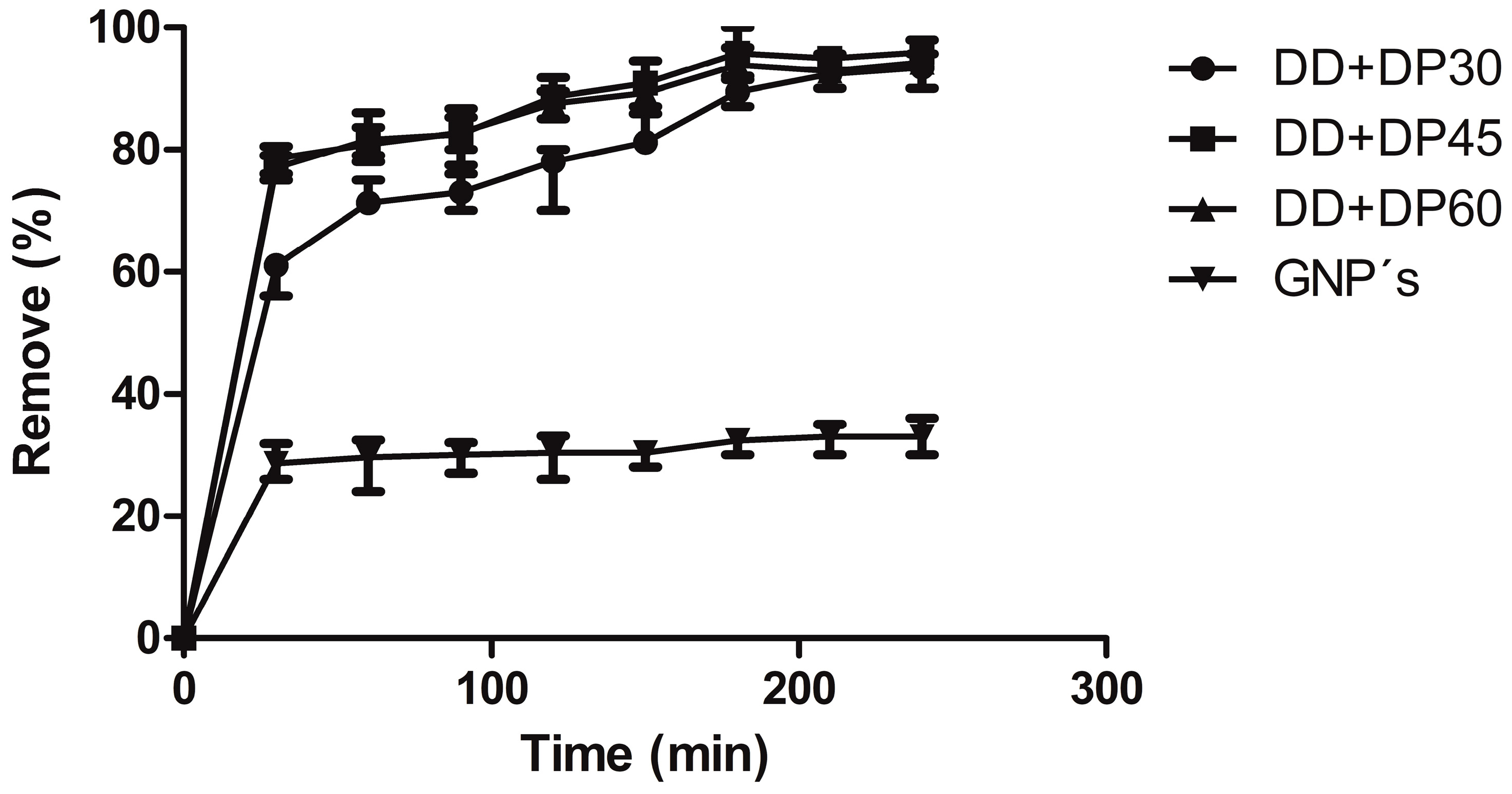
3.7. Uremic Toxin Adsorption
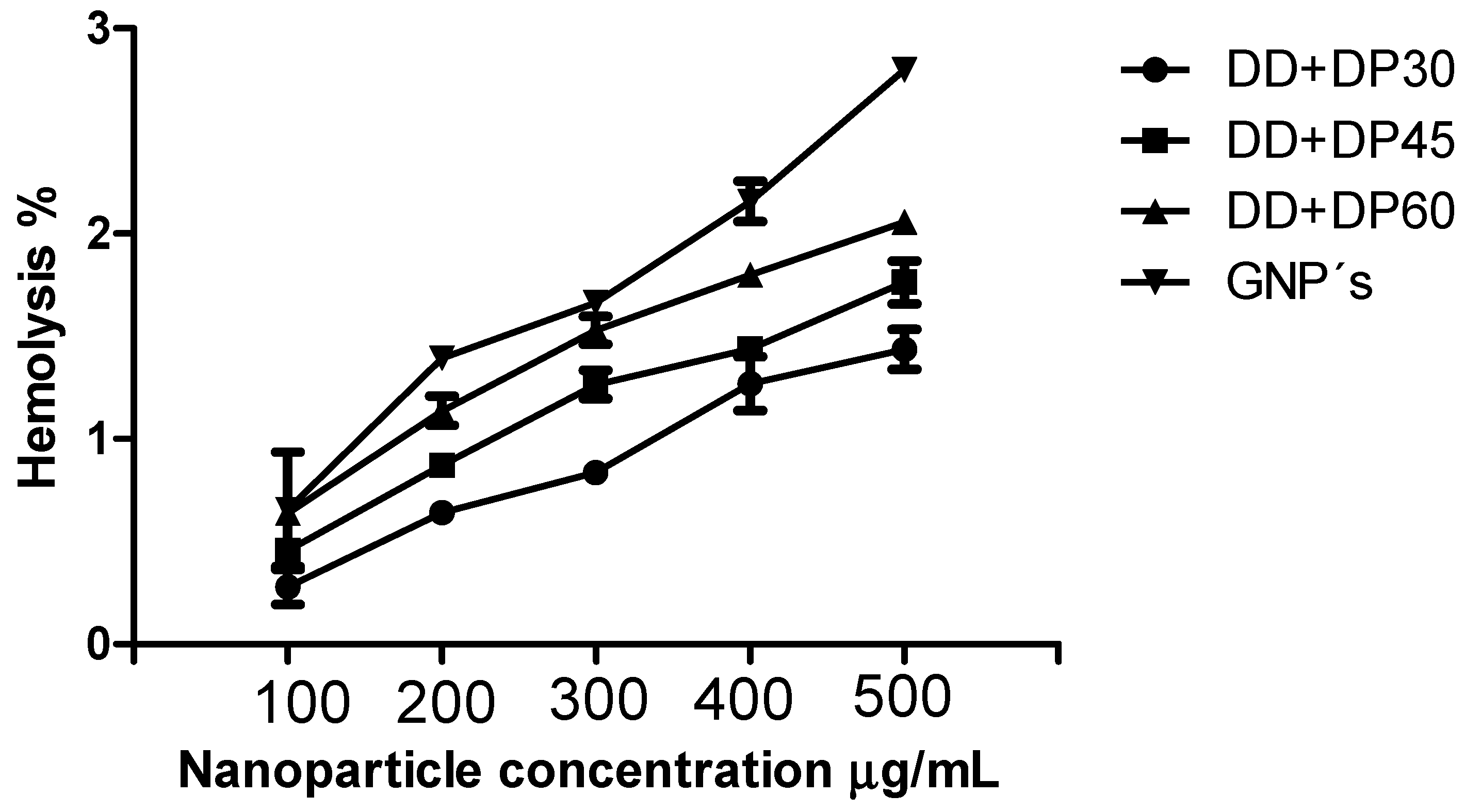
3.8. Hemolysis Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Benli, E.; Cirakoglu, A.; Ayyıldız, S.N.; Yüce, A. Comparison of serum uric acid levels between prostate cancer patients and a control group. Cent. Eur. J. Urol. 2018, 71, 242–247. [Google Scholar] [CrossRef]
- Edathil, A.A.; Zain, J.H.; Abu Haija, M.; Banat, F. Scalable synthesis of an environmentally benign graphene–sand based organic–inorganic hybrid for sulfide removal from aqueous solution: An insight into the mechanism. New J. Chem. 2019, 43, 3500–3512. [Google Scholar] [CrossRef]
- Yuan, K.; Li, Y.; Huang, X.; Liang, Y.; Liu, Q.; Jiang, G. Templated synthesis of a bifunctional Janus graphene for enhanced enrichment of both organic and inorganic targets. Chem. Commun. 2019, 55, 4957–4960. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, S.T.; Yilihamu, A.; Wu, D. Advances in the applications of graphene adsorbents: From water treatment to soil remediation. Rev. Inorg. Chem. 2019, 39, 47–76. [Google Scholar] [CrossRef]
- Ramírez, C.; Figueiredo, F.M.; Miranzo, P.; Poza, P.; Osendi, M.I. Graphene nanoplatelet/silicon nitride composites with high electrical conductivity. Carbon 2012, 50, 3607–3615. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A.; Bacsa, R. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Li, Z.; Li, Z.; Wu, K.; Jiao, Y.; Zhou, C. Construction of blood compatible chitin/graphene oxide composite aerogel beads for the adsorption of bilirubin. Carbohydr. Polym. 2019, 207, 704–712. [Google Scholar] [CrossRef]
- Song, X.; Cui, S.; Li, Z.; Jiao, Y.; Zhou, C. Fabrication of chitin/graphene oxide composite sponges with higher bilirubin adsorption capacity. J. Mater. Sci. Mater. Electron. 2018, 29, 108. [Google Scholar] [CrossRef]
- Radhakumary, C.; Sreenivasan, K.; Krishna, A.S. Detection and imaging of fatty plaques in blood vessels using functionalized carbon dots. Anal. Methods 2015, 7, 9482–9488. [Google Scholar]
- Wu, H.; Shi, H.; Wang, Y.; Jia, X.; Tang, C.; Zhang, J.; Yang, S. Hyaluronic acid conjugated graphene oxide for targeted drug delivery. Carbon 2014, 69, 379–389. [Google Scholar] [CrossRef]
- Irfan, M.; Irfan, M.; Idris, A.; Baig, N.; Saleh, T.A.; Nasiri, R.; Khalid, H. Fabrication and performance evaluation of blood compatible hemodialysis membrane using carboxylic multiwall carbon nanotubes and low molecular weight polyvinylpyrrolidone based nanocomposites. J. Biomed. Mater. Res. A 2019, 107, 513–525. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Cabello-Alvarado, C.; Cruz-Delgado, V.J.; Bartolo-Perez, P.; León-Martínez, D.; Sáenz-Galindo, A.; Cadenas-Pliego, G.; Ávila-Orta, C.A. Surface Modification of Graphene Nanoplatelets by Organic Acids and Ultrasonic Radiation for Enhance Uremic Toxins Adsorption. Materials 2019, 12, 715. [Google Scholar] [CrossRef]
- Gonçalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J.; Nogueira, H.S. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 2012, 22, 21032. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef]
- Das, A.; Kotadia, H. Effect of high-intensity ultrasonic irradiation on the modification of solidification microstructure in a Si-rich hypoeutectic Al–Si alloy. Mater. Chem. Phys. 2011, 125, 853–859. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Sonication-Assisted Fabrication and Post-Synthetic Modifications of Graphene-Like Materials. Chem. Eur. J. 2010, 16, 5246–5259. [Google Scholar] [CrossRef]
- Cabello, C.; Sáenz, A.; Pérez, C.; López, L.; Barajas, L.; Cantú, L.; Ávila, C. Modificación de nanotubos de carbono de pared múltiple (MWNTC´ s) usando ácido acético y anilina mediante radiación ultrasónica. Rev. Lat. Metal. Mater. 2015, 35, 27–33. [Google Scholar]
- Daza, C.; Guáqueta, S.; Gallego, R. Estudio morfológico y estructural de una arcilla Colombiana pilarizada en presencia de ultrasonido y microondas. Sci. Tech. 2011, 3, 292–297. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Sauter, C.; Emin, M.; Schuchmann, H.; Tavman, S. Influence of hydrostatic pressure and sound amplitude on the ultrasound induced dispersion and de-agglomeration of nanoparticles. Ultrason. Sonochem. 2008, 15, 517–523. [Google Scholar] [CrossRef]
- Cabello, C.; Rincón, S.; Bartolo, P.; Ruiz-Espinoza, J.; Zepeda, A. Incorporation of organic groups on the surface of multi-walled carbon nanotubes using an ultrasonic tip. Fuller. Nanotub. Car. Nanostruct. 2018, 26, 502–509. [Google Scholar] [CrossRef]
- Cabello, C.; Galindo, A.; Berumen, C.; Cortez, J.; Orta, C.; Toledano, C. Modificación de nanotubos de carbono de pared múltiple utilizando energía de ultrasonido a diferentes potencias. Afinidad 2016, 73, 285–290. [Google Scholar]
- Guan, J.; Sacks, M.S.; Beckman, E.J.; Wagner, W.R. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane) ureas based on poly(caprolactone) and putrescine. J. Biomed. Mater. Res. 2002, 61, 493–503. [Google Scholar] [CrossRef]
- Bremer, H.; Kohne, E.; Endres, W. The excretion of diamines in human urine. II. Cadaverine, putrescine, 1, 3-diaminopropane, 2, 2′-dithiobis (ethylamine) and spermidine in urine of patients with cystinuria and cystinlysinuria. Clin. Chim. Acta 1971, 32, 407–418. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Zhai, G. The effect of incubation conditions on the hemolytic properties of unmodified graphene oxide with various concentrations. RSC Adv. 2016, 6, 68322–68334. [Google Scholar]
- Li, H.; Lü, T.; Pan, L.; Zhang, Y.; Sun, Z. Electrosorption behavior of graphene in NaCl solutions. J. Mater. Chem. 2009, 19, 6773. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Chen, X.A.; Zhang, L.J.; He, Z.J.; Wang, W.-W.; Xu, B.; Zhong, Q.; Shuai, X.-T.; Yang, L.-Q.; Deng, Y.-B. Plasmid-encapsulated polyethylene glycol-grafted polyethylenimine nanoparticles for gene delivery into rat mesenchymal stem cells. Int. J. Nanomed. 2011, 6, 843–853. [Google Scholar]
- Mishra, P.; Bhat, B.R. Aggregative ways of graphene quantum dots with nitrogen-rich edges for direct emission spectrophotometric estimation of glucose. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117325. [Google Scholar] [CrossRef]
- Arrigo, R.; Teresi, R.; Gambarotti, C.; Parisi, F.; Lazzara, G.; Dintcheva, N.T. Sonication-Induced Modification of Carbon Nanotubes: Effect on the Rheological and Thermo-Oxidative Behaviour of Polymer-Based Nanocomposites. Materials 2018, 11, 383. [Google Scholar] [CrossRef]
- Mei, X.; Ouyang, J. Ultrasonication-assisted ultrafast reduction of graphene oxide by zinc powder at room temperature. Carbon 2011, 49, 5389–5397. [Google Scholar] [CrossRef]
- Ávila Orta, C.A.; Martínez Colunga, J.G.; Bueno Baqués, D.; Raudry López, C.E.; Cruz Delgado, V.J.; González Morones, P.; Valdéz Garza, J.A.; Esparza Juárez, M.E.; Espinoza Gonzalez, C.J.; Rodríguez González, J.A. Continuous Method Assisted by Ultrasound with a Variable Amplitude and Frequency for the Preparation of Nanocompounds Based on Polymers and Nanoparticles. MX Patent 323756, 2014. [Google Scholar]
- Ong, W.; Tan, L.; Chai, S. Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like grapheme/g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 2015, 51, 858–861. [Google Scholar] [CrossRef]
- Grieser, F.; AshokKumar, M. The effect of surface active solutes on bubbles exposed to ultrasound. Adv. Colloid Interface Sci. 2001, 89, 423–438. [Google Scholar] [CrossRef]
- Awada, H.; Monplaisir, D.; Daneault, C. Growth of polyelectrolyte on lignocellulosic fibres: Study by zeta potential, FTIR, and XPS. BioRes 2012, 7, 2090–2104. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Zhang, S.; Zhang, L.; Choi, H.J.; Seo, J.M.; Xia, Z.; Dai, L.; Baek, J.-B. Edge-Selectively Sulfurized Graphene Nanoplatelets as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction: The Electron Spin Effect. Adv. Mater. 2013, 25, 6138–6145. [Google Scholar] [CrossRef]
- Chen, W.Q.; Li, Q.T.; Li, P.H.; Zhang, Q.Y.; Xu, Z.S.; Chu, P.K.; Wang, X.-B.; Yi, C.-F. In situ random co-polycondensation for preparation of reduced graphene oxide/polyimide nanocomposites with amino-modified and chemically reduced graphene oxide. J. Mater. Sci. 2015, 50, 3860–3874. [Google Scholar] [CrossRef]
- Perez-Marin, L.; Lopez-Valdivia, H.; Avila-Perez, P.; Otazo-Sánchez, E.; Macedo-Miranda, G.; Gutierrez-Lozano, O.; Carapia-Morales, L. Response mechanism of a neutral carrier Hg (II) polymeric membrane ion-selective electrode. SEM and EDAX study. Analyst 2001, 126, 501–504. [Google Scholar] [CrossRef]
- Huang, Y.; Terentjev, E.M. Dispersion of carbon nanotubes: Mixing, sonication, stabilization, and composite properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Wang, J.; Chen, B. Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem. Eng. J. 2015, 281, 379–388. [Google Scholar] [CrossRef]
- Cao, Y.; Gu, Y.; Wang, K.; Wang, X.; Gu, Z.; Ambrico, T.; Castro, M.A.; Lee, J.; Gibbons, W.; Rice, J.A. Adsorption of creatinine on active carbons with nitric acid hydrothermal modification. J. Taiwan Inst. Chem. Eng. 2016, 66, 347–356. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Fu, C.C.; Hsiao, Y.S.; Chien, C.C.; Juang, R.S. Clearance of low molecular-weight uremic toxins p-cresol, creatinine, and urea from simulated serum by adsorption. J. Mol. Liq. 2018, 252, 203–210. [Google Scholar] [CrossRef]
- Standard Test Method for Analysis of Hemolytic Properties of Nanoparticles; American Society for Testing Material: West Conshohocken, PA, USA, 2013; Volume E2524-08, pp. 1–5.





| Sample | Reaction Time (min) |
|---|---|
| GNPs | 0 |
| DD+DP30 | 30 |
| DD+DP45 | 45 |
| DD+DP60 | 60 |
| Sample | C1s Peak (eV) | O1s Peak (eV) | N1s Peak (eV) | C1s At % | O1s At % | N1s At % |
|---|---|---|---|---|---|---|
| GNPs | 284.92 | 532.89 | ND | 95.85 | 4.15 | ND |
| DD+DP 30 | 284.45 | 532.42 | 399.43 | 91.62 | 4.15 | 4.23 |
| DD+DP 45 | 284.29 | 532.36 | 399.38 | 90.46 | 6.23 | 3.31 |
| DD+DP 60 | 284.97 | 532.81 | 399.9 | 92.34 | 4.26 | 3.4 |
| Sample | Weight Loss at 100 °C (%) | Weight Loss at 250 °C (%) | Weight Loss at 500 °C (%) | Weight Loss at 750 °C (%) | Residue at 950 °C (%) |
|---|---|---|---|---|---|
| GNPs | 0.9 | 2.35 | 5.7 | 10.2 | 12.5 |
| DD+DP30 | 12.6 | 16.0 | 25.9 | 33.2 | 42.9 |
| DD+DP45 | 8.1 | 14.1 | 22.2 | 28.6 | 30.4 |
| DD+DP60 | 9.6 | 13.4 | 19.6 | 23.6 | 25.8 |
| Solvent | GNPs | DD+DP30 | DD+DP45 | DD+DP60 |
|---|---|---|---|---|
| Distilled water |  |  |  |  |
| Ethanol |  |  |  |  |
| Hexane |  |  |  |  |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | Kf | R2 | |
| GNPs | 0.010 | 0.056 | 0.973 | 1.050 | 1.189 | 0.6769 |
| DD+DP30 | 0.118 | 10.20 | 0.8875 | 2.90 | 17.47 | 0.9461 |
| DD+DP45 | 0.556 | 63.13 | 0.6977 | 6.23 | 33.49 | 0.8768 |
| DD+DP60 | 0.075 | 5.46 | 0.9002 | 2.11 | 13.71 | 0.9401 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | Kf | R2 | |
| GNPs | 1.17 | 0.001 | 0.9996 | 0.009 | 0.007 | 0.9993 |
| DD+DP30 | 0.343 | 37.08 | 0.7335 | 5.02 | 27.58 | 0.8902 |
| DD+DP45 | 1.11 | 144.17 | 0.6877 | 10.04 | 52.37 | 0.91 |
| DD+DP60 | 1.58 | 210.05 | 0.6459 | 11.57 | 59.98 | 0.8911 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello-Alvarado, C.; Andrade-Guel, M.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Cortés-Hernández, D.A.; Bartolo-Pérez, P.; Ávila-Orta, C.A.; Cruz-Delgado, V.J.; Zepeda-Pedreguera, A. Graphene Nanoplatelets Modified with Amino-Groups by Ultrasonic Radiation of Variable Frequency for Potential Adsorption of Uremic Toxins. Nanomaterials 2019, 9, 1261. https://doi.org/10.3390/nano9091261
Cabello-Alvarado C, Andrade-Guel M, Pérez-Alvarez M, Cadenas-Pliego G, Cortés-Hernández DA, Bartolo-Pérez P, Ávila-Orta CA, Cruz-Delgado VJ, Zepeda-Pedreguera A. Graphene Nanoplatelets Modified with Amino-Groups by Ultrasonic Radiation of Variable Frequency for Potential Adsorption of Uremic Toxins. Nanomaterials. 2019; 9(9):1261. https://doi.org/10.3390/nano9091261
Chicago/Turabian StyleCabello-Alvarado, C., M. Andrade-Guel, M. Pérez-Alvarez, G. Cadenas-Pliego, Dora A. Cortés-Hernández, P. Bartolo-Pérez, C.A. Ávila-Orta, V.J. Cruz-Delgado, and A. Zepeda-Pedreguera. 2019. "Graphene Nanoplatelets Modified with Amino-Groups by Ultrasonic Radiation of Variable Frequency for Potential Adsorption of Uremic Toxins" Nanomaterials 9, no. 9: 1261. https://doi.org/10.3390/nano9091261
APA StyleCabello-Alvarado, C., Andrade-Guel, M., Pérez-Alvarez, M., Cadenas-Pliego, G., Cortés-Hernández, D. A., Bartolo-Pérez, P., Ávila-Orta, C. A., Cruz-Delgado, V. J., & Zepeda-Pedreguera, A. (2019). Graphene Nanoplatelets Modified with Amino-Groups by Ultrasonic Radiation of Variable Frequency for Potential Adsorption of Uremic Toxins. Nanomaterials, 9(9), 1261. https://doi.org/10.3390/nano9091261