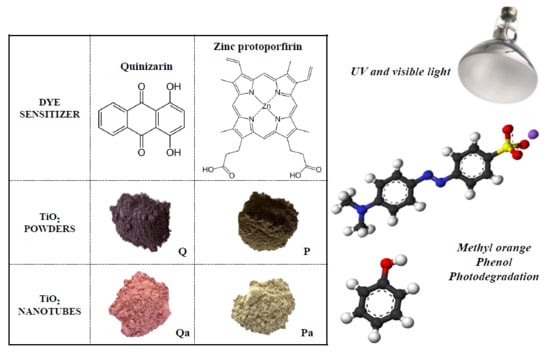
Powder and Nanotubes Titania Modified by Dye Sensitization as Photocatalysts for the Organic Pollutants Elimination
Abstract
1. Introduction
2. Materials and Methods
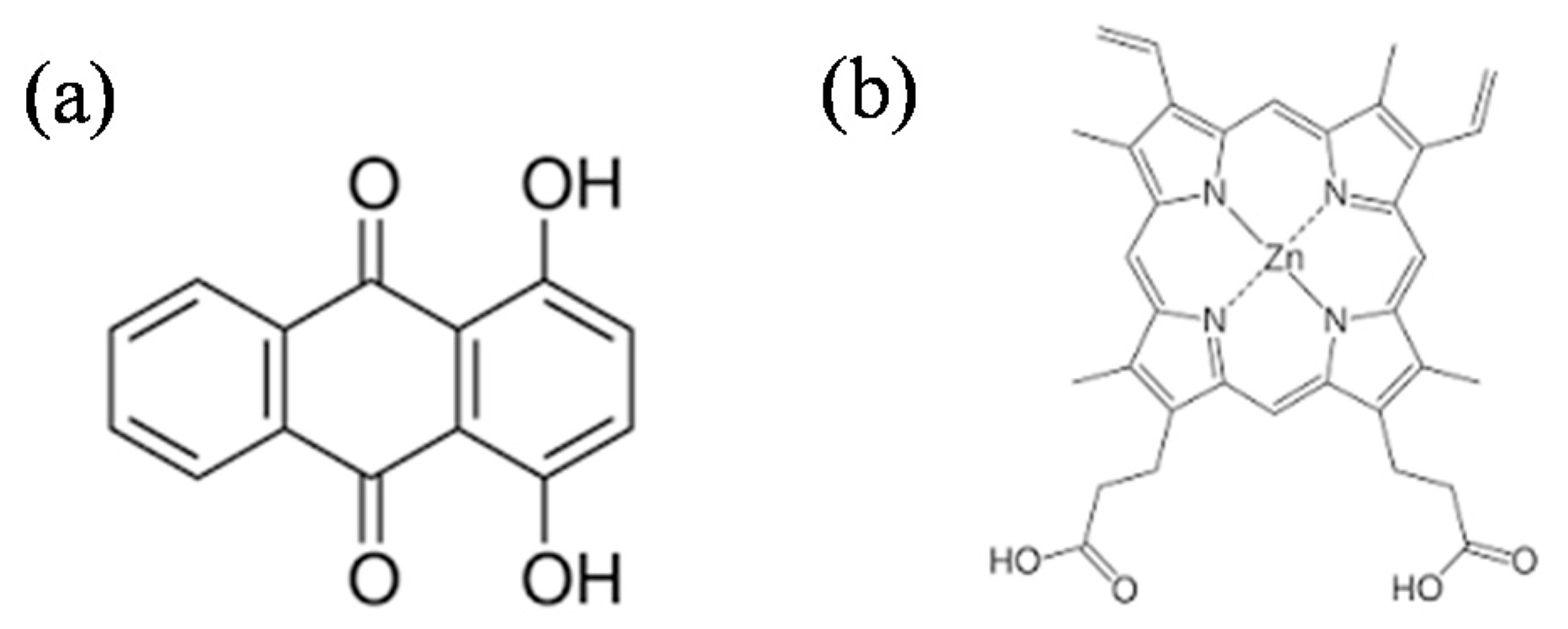
2.1. Photocatalysts Preparation
2.2. Photocatalysts Characterization
2.3. Photocatalytic Tests
3. Results and Discussion
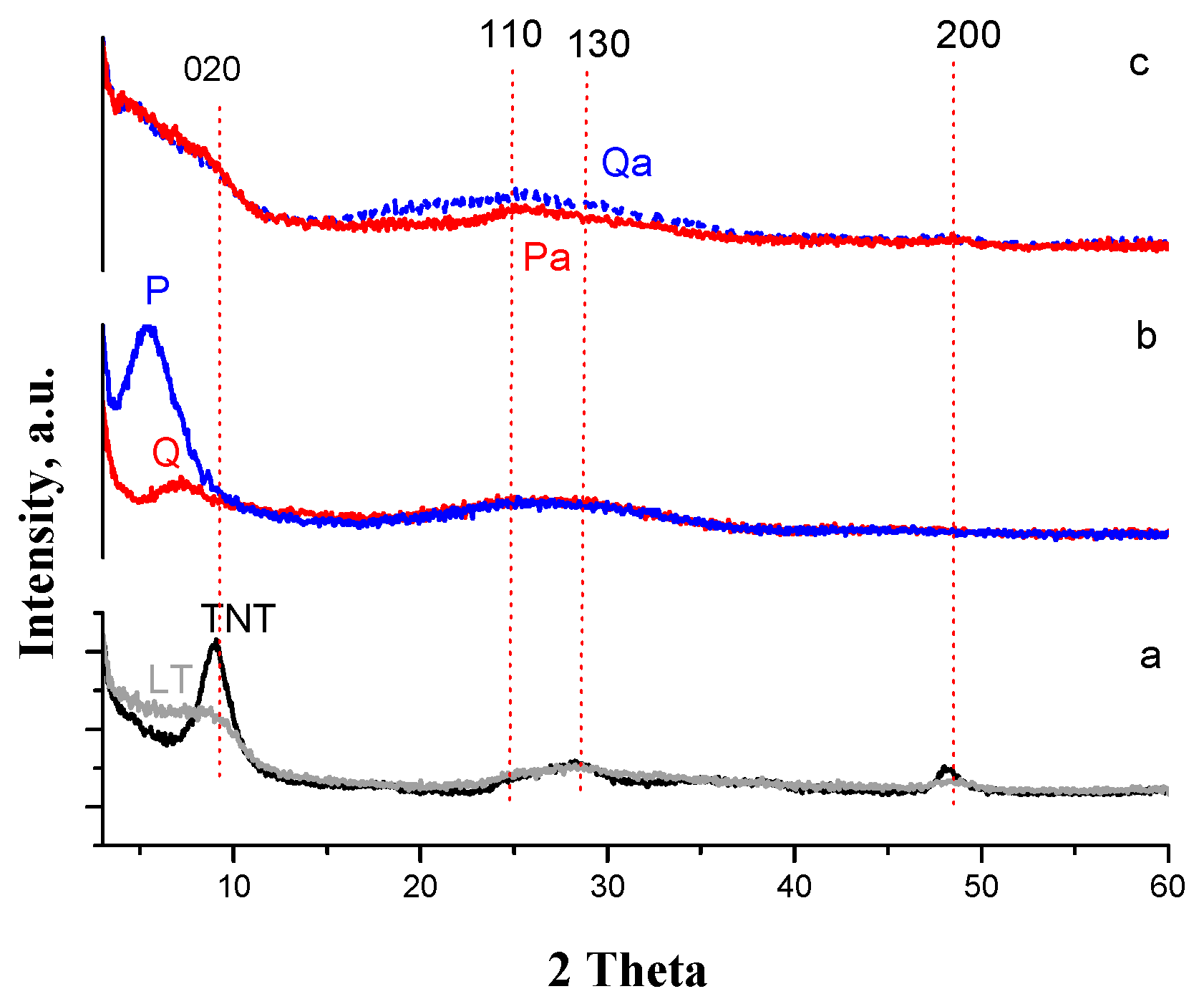
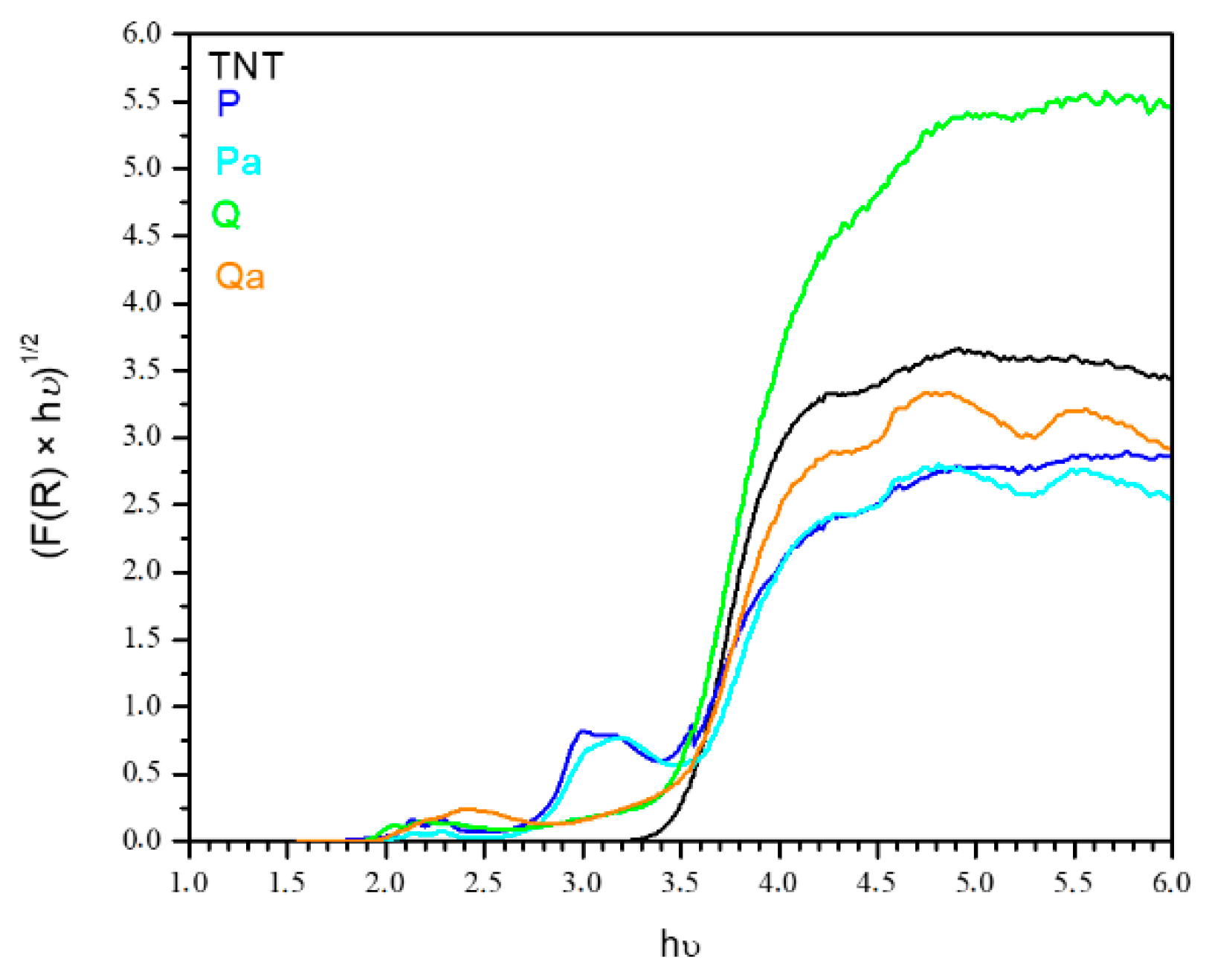
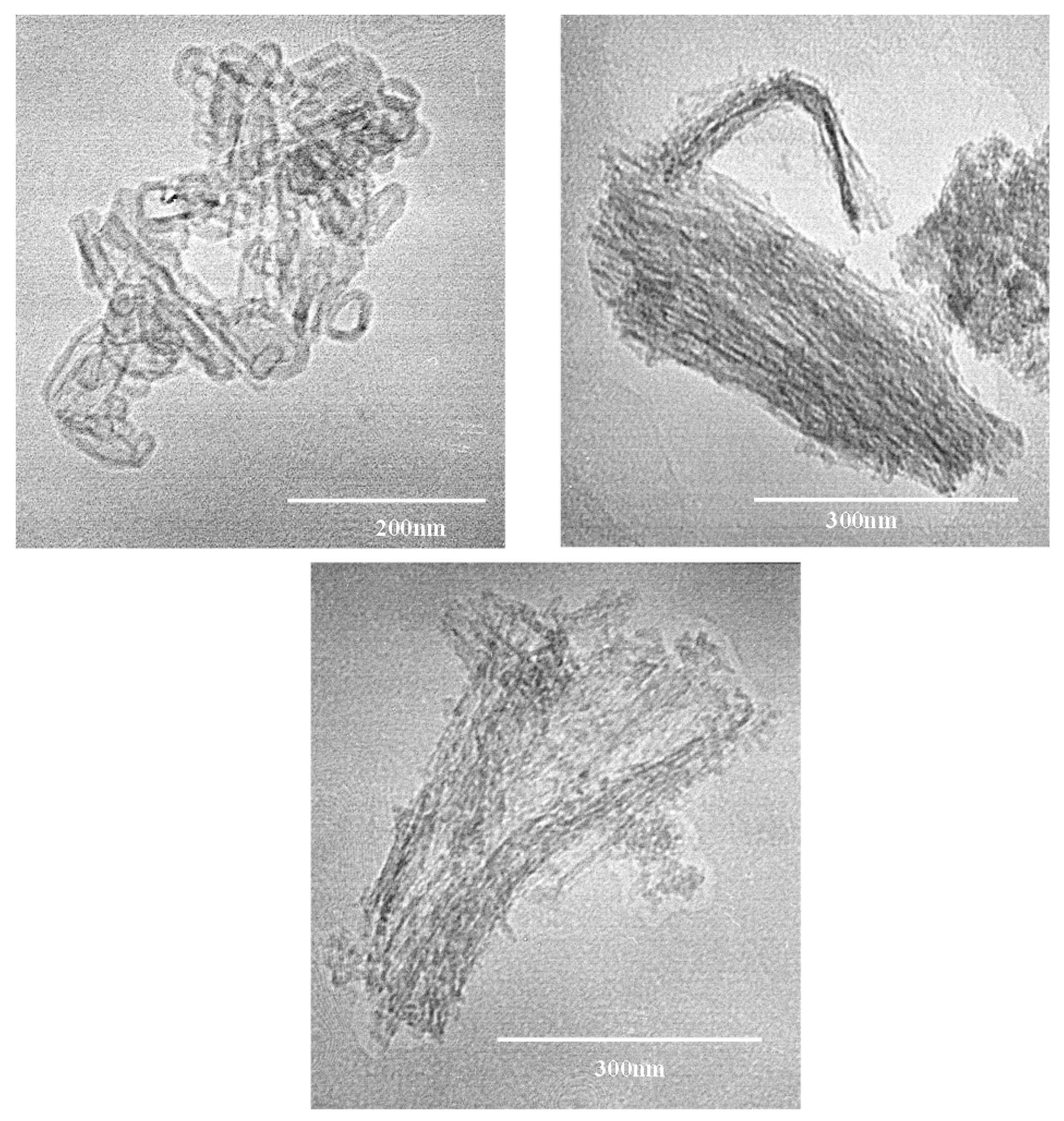
3.1. Photocatalysts Characterization
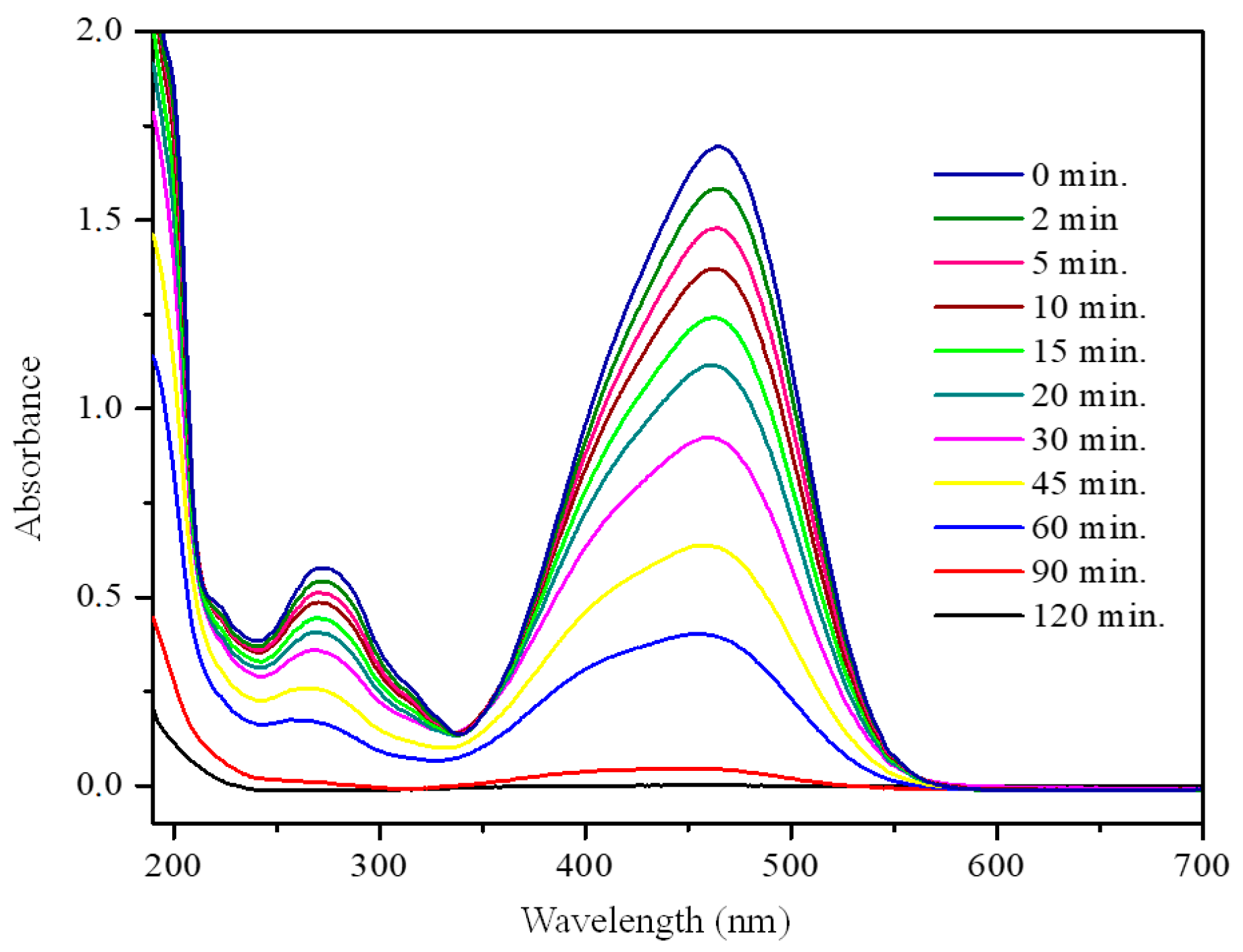
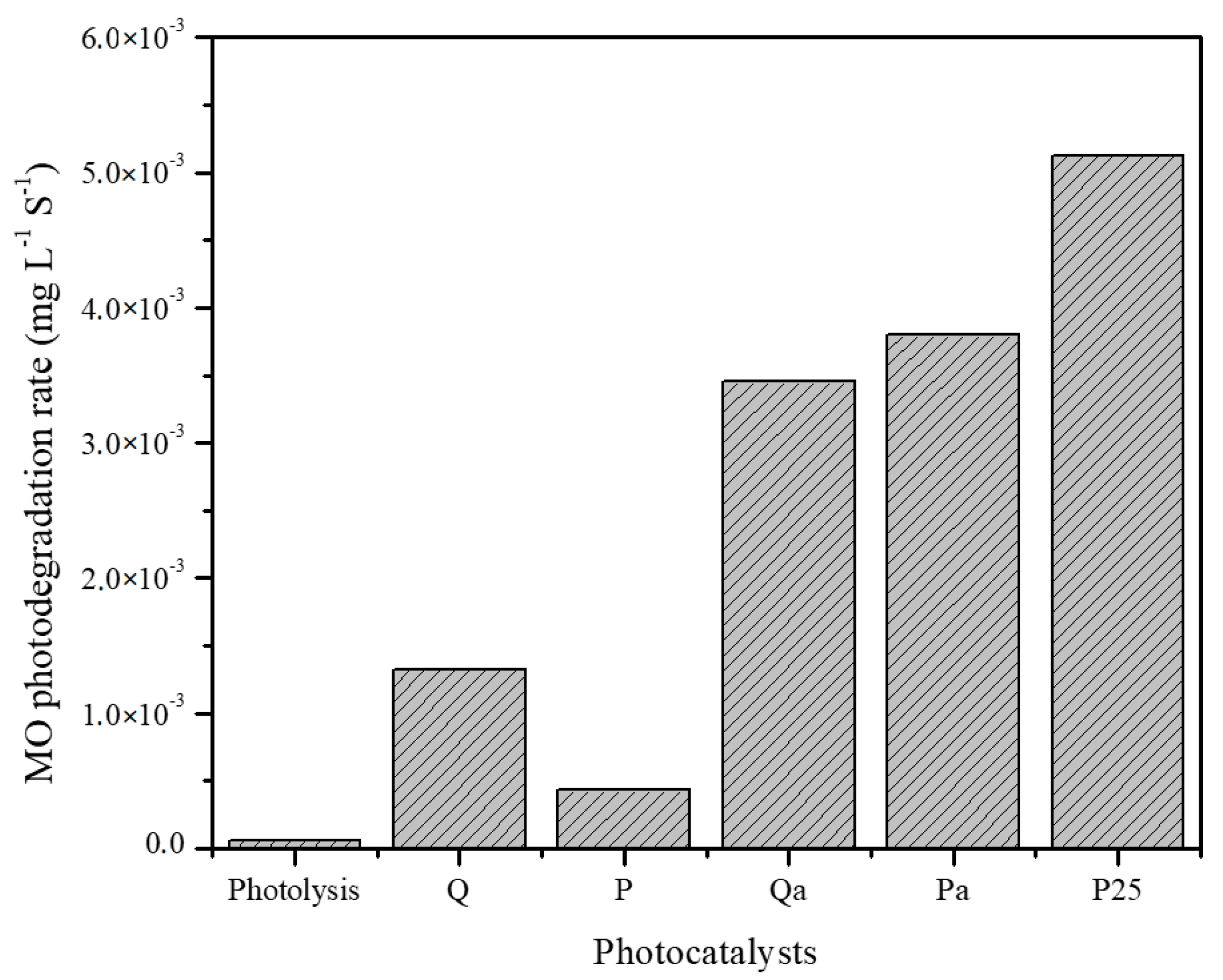
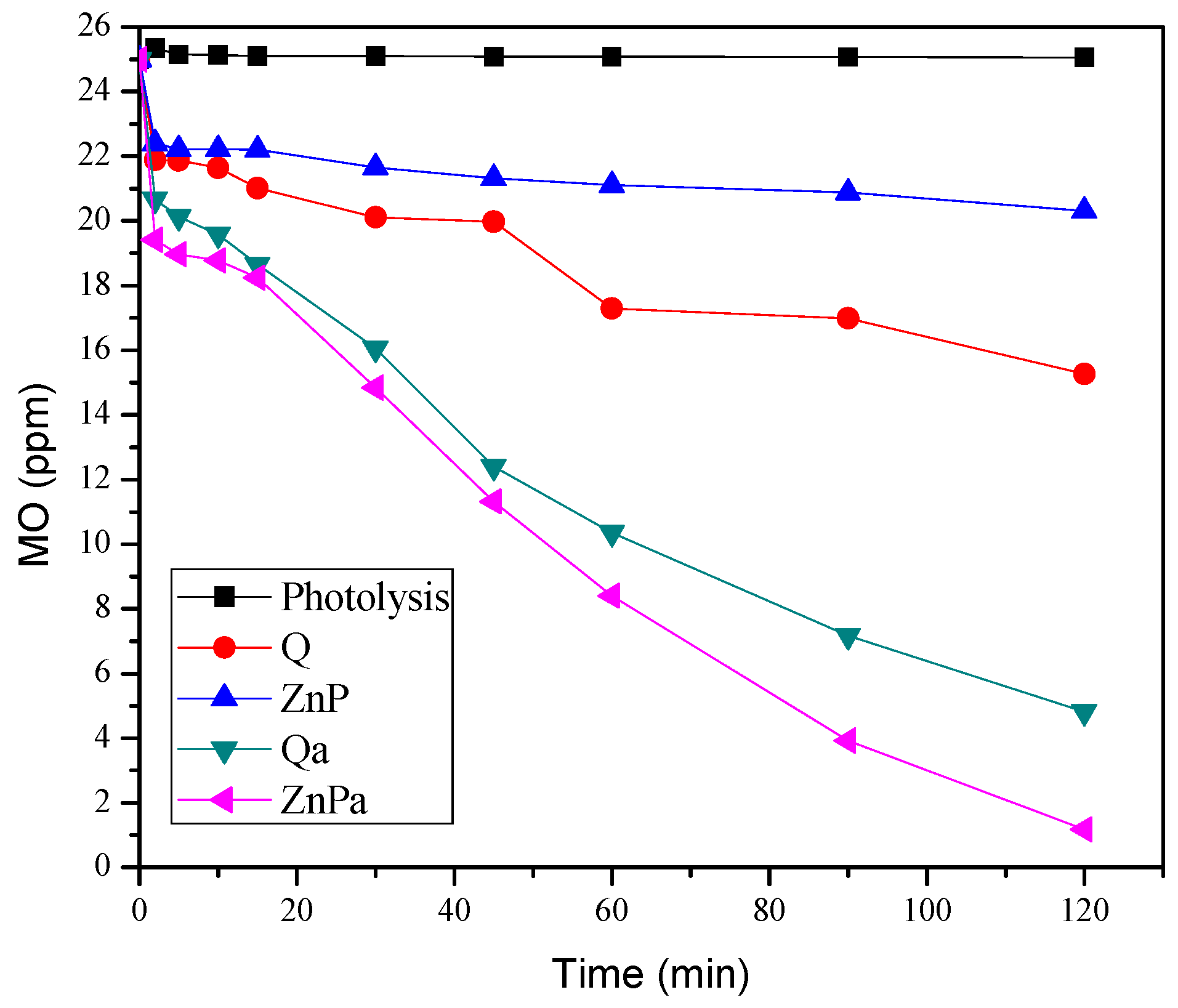
3.2. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.J.; Reckhow, D.A.; Li, X.; Humpage, A.R.; Joll, C.; Hrudey, S.E. Potential carcinogenic hazards of non-regulated disinfection by-products: Haloquinones, halo-cyclopentene and cyclohexene derivatives, N-halamines, halonitriles, and heterocyclic amines. Toxicology 2011, 286, 1–19. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Murcia, J.J.; Hidalgo, M.C.; Navío, J.A. Gas-phase Photocatalytic Partial Oxidation of Cyclohexane to Cyclohexanol and Cyclohexanone on Au/TiO2 Photocatalysts. J. Adv. Oxid. Technol. 2013, 16, 71–82. [Google Scholar] [CrossRef]
- Lee, S.K.; Mills, A. Platinum and Palladium in Semiconductor Photocatalytic Systems Factors Affecting the Purification of Water and Air. Platin. Met. Rev. 2003, 47, 61–72. [Google Scholar]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef] [PubMed]
- West, J.L.; Halas, N.J. Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Protti, S.; Fagnoni, M. The sunny side of chemistry: Green synthesis by solar light. Photochem. Photobiol. Sci. 2009, 8, 1499–1516. [Google Scholar] [CrossRef]
- Ollis, D.F.; Al-Ekabi, H. Photocatalytic Purification of Water and Air; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Murcia, J.J.; Guarín, J.R.; Cely, Á.C.; Rojas, H.; Cubillos, J.A.; Hidalgo, M.C.; Navío, J.A. Methylene blue degradation over M-TiO2 photocatalysts (M = Au or Pt). Ciencia En Desarrollo 2017, 8, 109–117. [Google Scholar] [CrossRef]
- Kim, S.-H.; Ngo, H.H.; Shon, H.K.; Vigneswaran, S. Adsorption and photocatalysis kinetics of herbicide onto titanium oxide and powdered activated carbon. Sep. Purif. Technol. 2008, 58, 335–342. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Kasuga, T. Formation of titanium oxide nanotubes using chemical treatments and their characteristic properties. Thin Solid Films 2006, 496, 141–145. [Google Scholar] [CrossRef]
- Alam, U.; Fleisch, M.; Kretschmer, I.; Bahnemann, D.; Muneer, M. One-step hydrothermal synthesis of Bi-TiO2 nanotube/graphene composites: An efficient photocatalyst for spectacular degradation of organic pollutants under visible light irradiation. Appl. Catal. B 2017, 218, 758–769. [Google Scholar] [CrossRef]
- Sanabria, B.E.; Mazare, A.; Yoo, J.; Nguyen, N.T.; Hejazi, S.; Bian, H.; Diamanti, M.V.; Pedeferri, M.P.; Schmuki, P. Intrinsic AuPt-alloy particles decorated on TiO2 nanotubes provide enhanced photocatalytic degradation. Electrochim. Acta 2018, 292, 865–870. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kitano, M.; Fukumoto, S.; Iyatani, K.; Takeuchi, M.; Anpo, M. The effect of the hydrothermal treatment with aqueous NaOH solution on the photocatalytic and photoelectrochemical properties of visible light-responsive TiO2 thin films. Catal. Today 2008, 132, 159–164. [Google Scholar] [CrossRef]
- Morawski, A.W.; Kusiak-Nejmana, E.; Wanag, A.; Kapica-Kozar, J.; Wróbel, R.J.; Ohtani, B.; Aksienionek, M.; Lipinska, L. Photocatalytic degradation of acetic acid in the presence of visible light-active TiO2-reduced graphene oxide photocatalysts. Catal. Today 2017, 280, 108–113. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. A critical review on the recent progress of synthesizing techniques and fabrication of TiO2-based nanotubes photocatalysts. Appl. Catal. A 2014, 481, 127–142. [Google Scholar] [CrossRef]
- Ivanova, S.; Penkova, A.; Hidalgo, M.C.; Navío, J.A.; Romero-Sarria, F.; Centeno, M.Á.; Odriozola, J.A. Synthesis and application of layered titanates in the photocatalytic degradation of phenol. Appl. Catal. B 2015, 163, 23–29. [Google Scholar] [CrossRef]
- Dvorak, F.; Zazpe, R.; Krbal, M.; Sopha, H.; Prikryl, J.; Ng, S.; Hromadko, L.; Bures, F.; Macak, J.M. One-dimensional anodic TiO2 nanotubes coated by atomic layer deposition: Towards advanced applications. Appl. Mater. Today 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Tang, C.-H.; Hsiao, P.-H.; Chen, C.-Y. Efficient Photocatalysts Made by Uniform Decoration of Cu2O Nanoparticles on Si Nanowire Arrays with Low Visible Reflectivity. Nanoscale Res. Lett. 2018, 13, 312–319. [Google Scholar] [CrossRef]
- Tang, C.-H.; Chen, K.-Y.; Chen, C.-Y. Solution-processed ZnO/Si based heterostructures with enhanced photocatalytic performance. New J. Chem. 2018, 42, 13797–13802. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Hsiao, P.-H.; Wei, T.-C.; Chen, T.-C.; Tang, C.-H. Well incorporation of carbon nanodots with silicon nanowire arrays featuring excellent photocatalytic performances. Phys. Chem. Chem. Phys. 2017, 19, 11786–11792. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.C.; Maicu, M.; Navío, J.A.; Colón, G. Effect of Sulfate Pretreatment on Gold-Modified TiO2 for Photocatalytic Applications. J. Phys. Chem. C 2009, 113, 12840–12847. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hidalgo, M.C.; Navío, J.A.; Araña, J.; Doña-Rodríguez, J.M. Correlation study between photo-degradation and surface adsorption properties of phenol and methyl orange on TiO2 Vs platinum-supported TiO2. Appl. Catal. B 2014, 150, 107–115. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Han, F.; Subba, V.; Kambala, R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Preat, J.; Laurent, A.D.; Michaux, C.; Perpète, E.A.; Jacquemin, D. Impact of tautomers on the absorption spectra of neutral and anionic alizarin and quinizarin dyes. J. Mol. Struct. TEOCHEM 2009, 901, 24–30. [Google Scholar] [CrossRef]
- Fain, Y.; Zaitsev, B.E.; Ryabov, M.A. Electronic Absorption Spectra and Tautomerism of Quinizarin and Its Substituted Derivatives. Russ. J. Gen. Chem. 2003, 73, 1595–1602. [Google Scholar] [CrossRef]
- Mosquera, A.A.; Horwat, D.; Rashkovskiy, A.; Kovalev, A.; Miska, P.; Wainstein, D.; Albella, J.M.; Endrino, J.L. Exciton and core-level electron confinement effects in transparent ZnO thin films. Sci. Rep. 2013, 3, 1714. [Google Scholar] [CrossRef]
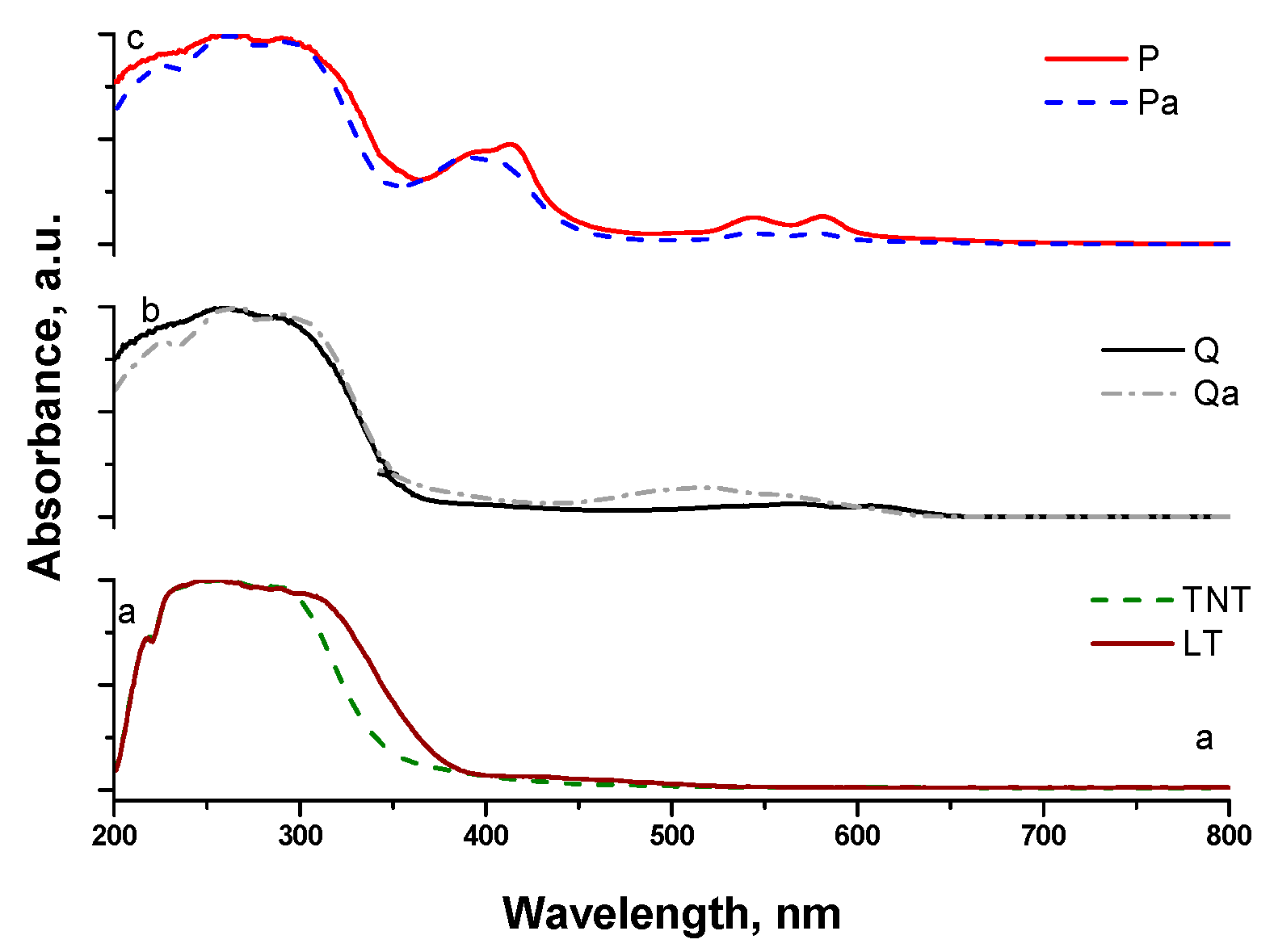
| Sample | SBET (m2/g) | Band gap, eV |
|---|---|---|
| LT | 153 | 3.4 |
| Q | 213.6 | 3.2 |
| ZnP | 172.6 | 3.2 |
| TNT | 73 | 3.5 |
| Qa | 50.3 | 3.3 |
| ZnPa | 80.6 | 3.3 |
| Photocatalysts | Irradiation | Degradation rate (mg L−1·s−1) | |
|---|---|---|---|
| MO | Phenol | ||
| Q | UV-Vis | 1.33 × 10−3 | 1.09 × 10−4 |
| Q | Vis | 1.10 × 10−3 | 1.80 × 10−4 |
| ZnP | UV-Vis | 4.31 × 10−4 | 1.05 × 10−4 |
| ZnP | Vis | 4.14 × 10−4 | — |
| Qa | Vis | 1.0 × 10−3 | — |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murcia, J.J.; Ávila-Martínez, E.G.; Rojas, H.; Cubillos, J.; Ivanova, S.; Penkova, A.; Laguna, O.H. Powder and Nanotubes Titania Modified by Dye Sensitization as Photocatalysts for the Organic Pollutants Elimination. Nanomaterials 2019, 9, 517. https://doi.org/10.3390/nano9040517
Murcia JJ, Ávila-Martínez EG, Rojas H, Cubillos J, Ivanova S, Penkova A, Laguna OH. Powder and Nanotubes Titania Modified by Dye Sensitization as Photocatalysts for the Organic Pollutants Elimination. Nanomaterials. 2019; 9(4):517. https://doi.org/10.3390/nano9040517
Chicago/Turabian StyleMurcia, Julie J., Elsa G. Ávila-Martínez, Hugo Rojas, Jairo Cubillos, Svetlana Ivanova, Anna Penkova, and Oscar H. Laguna. 2019. "Powder and Nanotubes Titania Modified by Dye Sensitization as Photocatalysts for the Organic Pollutants Elimination" Nanomaterials 9, no. 4: 517. https://doi.org/10.3390/nano9040517
APA StyleMurcia, J. J., Ávila-Martínez, E. G., Rojas, H., Cubillos, J., Ivanova, S., Penkova, A., & Laguna, O. H. (2019). Powder and Nanotubes Titania Modified by Dye Sensitization as Photocatalysts for the Organic Pollutants Elimination. Nanomaterials, 9(4), 517. https://doi.org/10.3390/nano9040517