Preparation, Characterization and Properties of Porous PLA/PEG/Curcumin Composite Nanofibers for Antibacterial Application
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PLA/PEG/Cur CNFs
2.3. Characterizations and Measurements
2.4. In Vitro Release of Cur
2.5. Antimicrobial Tests
3. Results and Discussion
3.1. Properties Characterization of Spinning Solutions
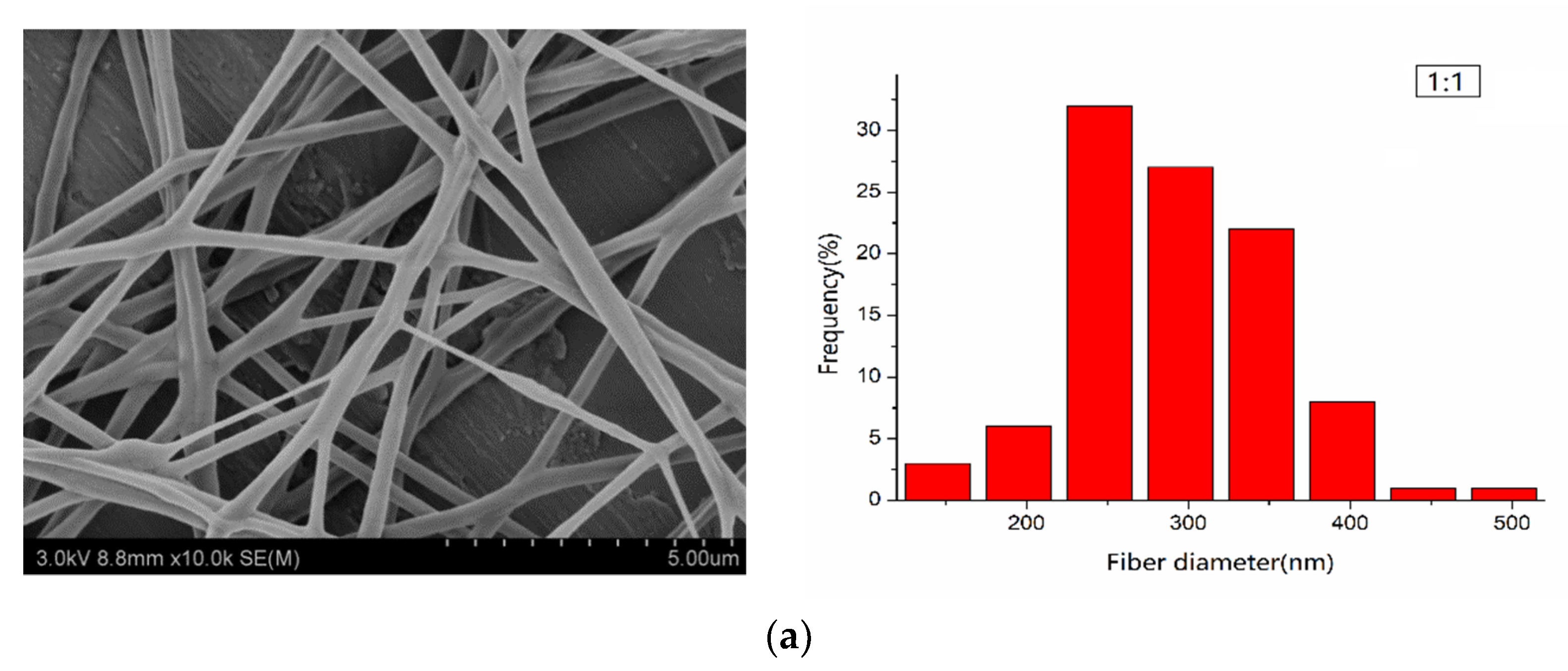
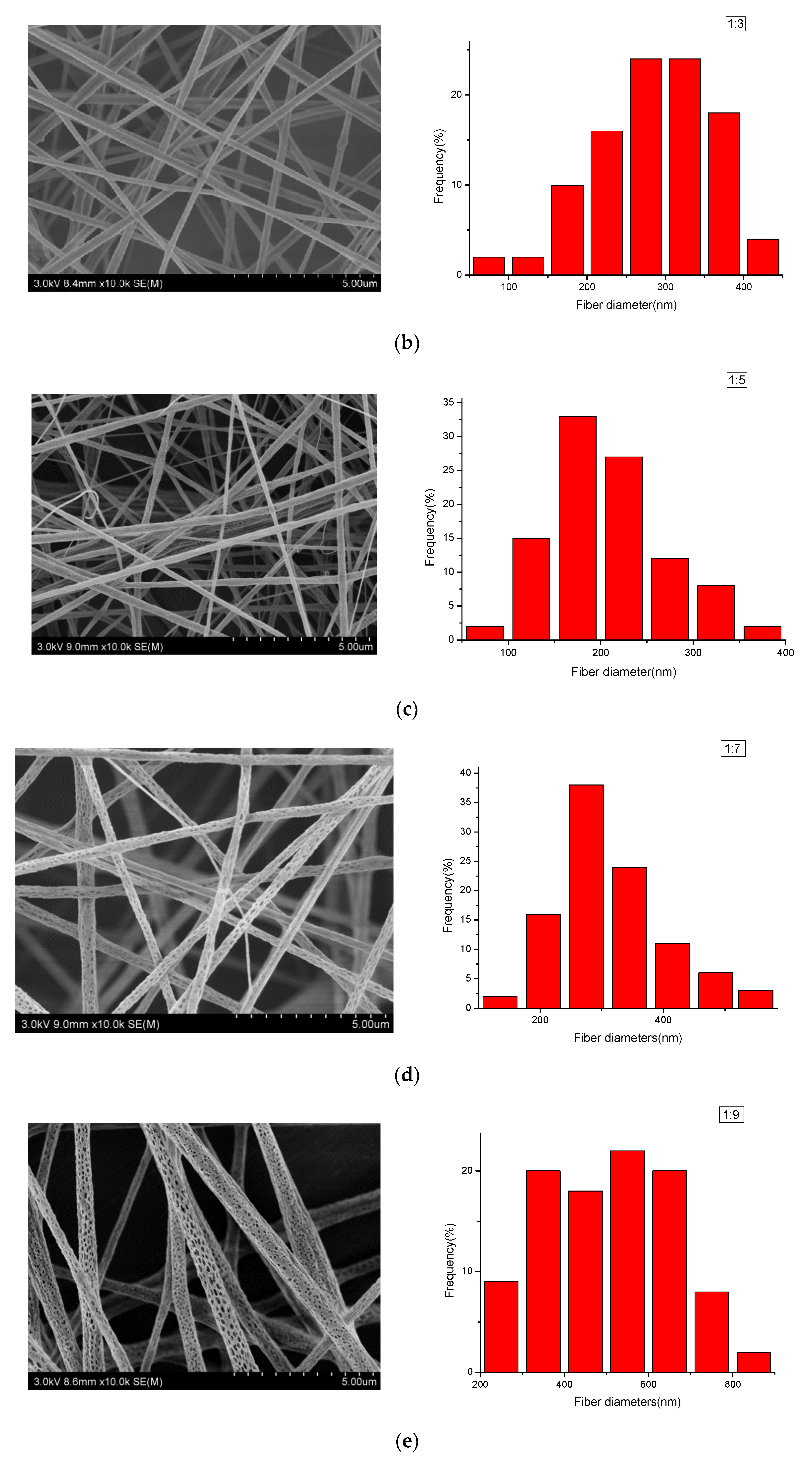
3.2. Morphological Characterization of PLA/PEG/Cur CNFs (SEM)
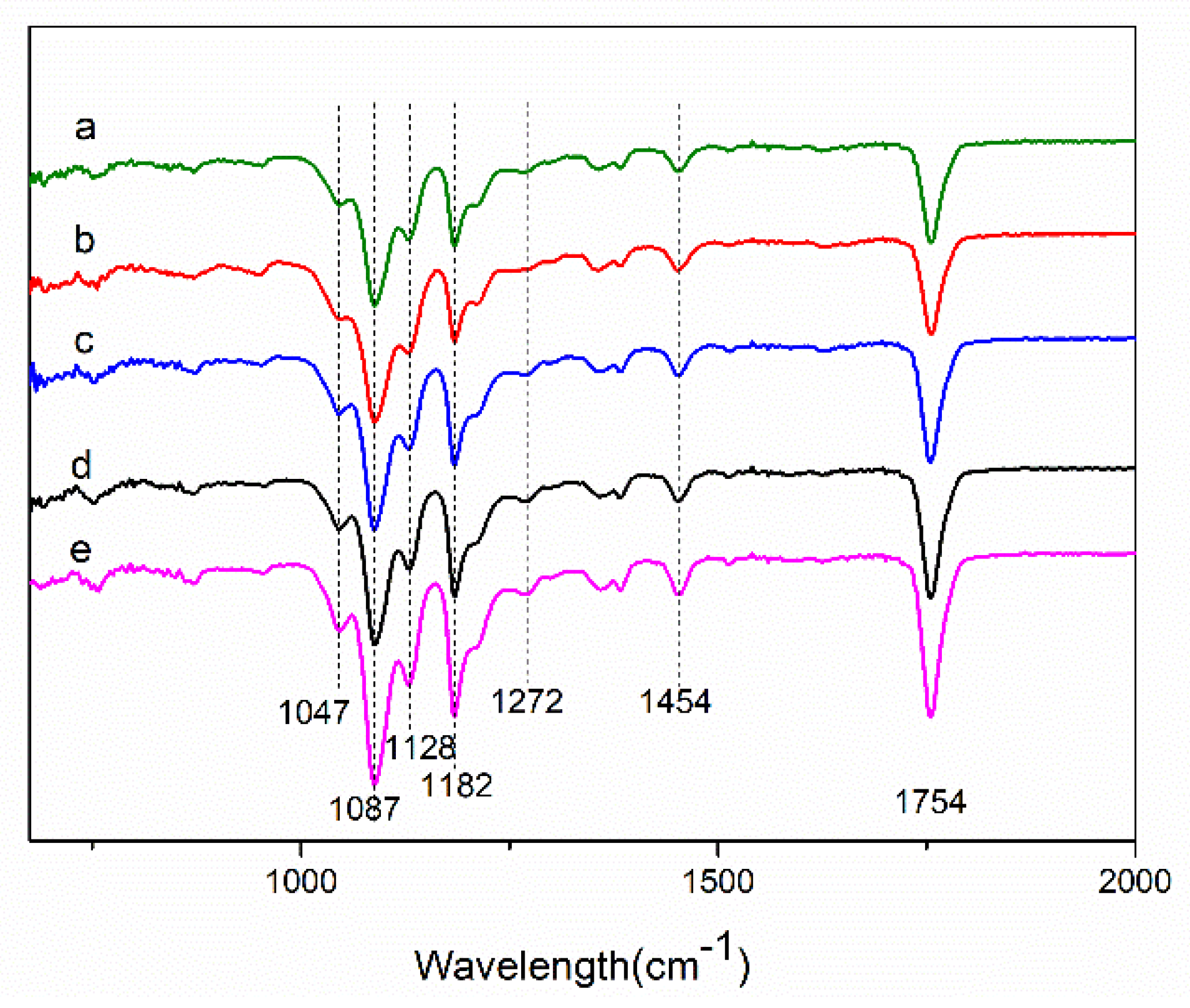
3.3. Fourier-Transform Infrared (FTIR) and Raman Spectrum Analysis
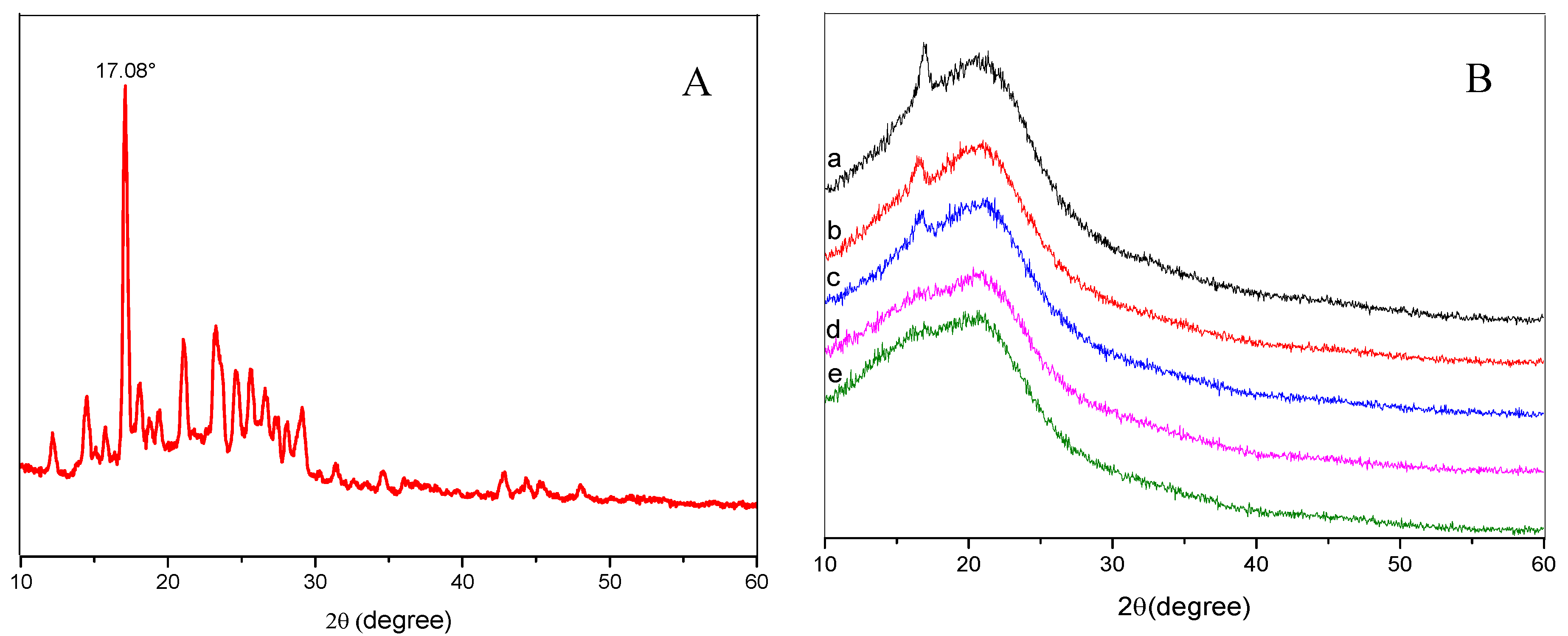
3.4. X-ray Diffraction (XRD) Spectrum Analysis
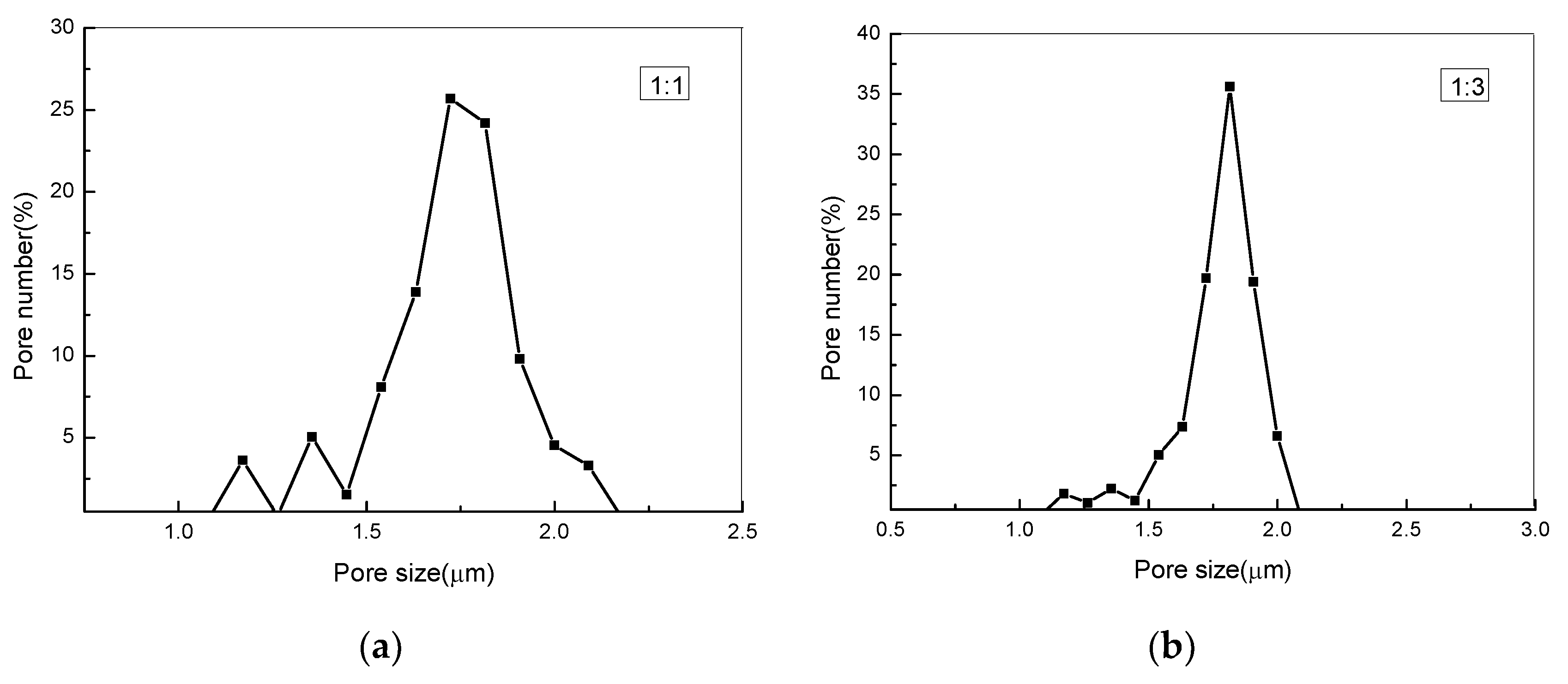
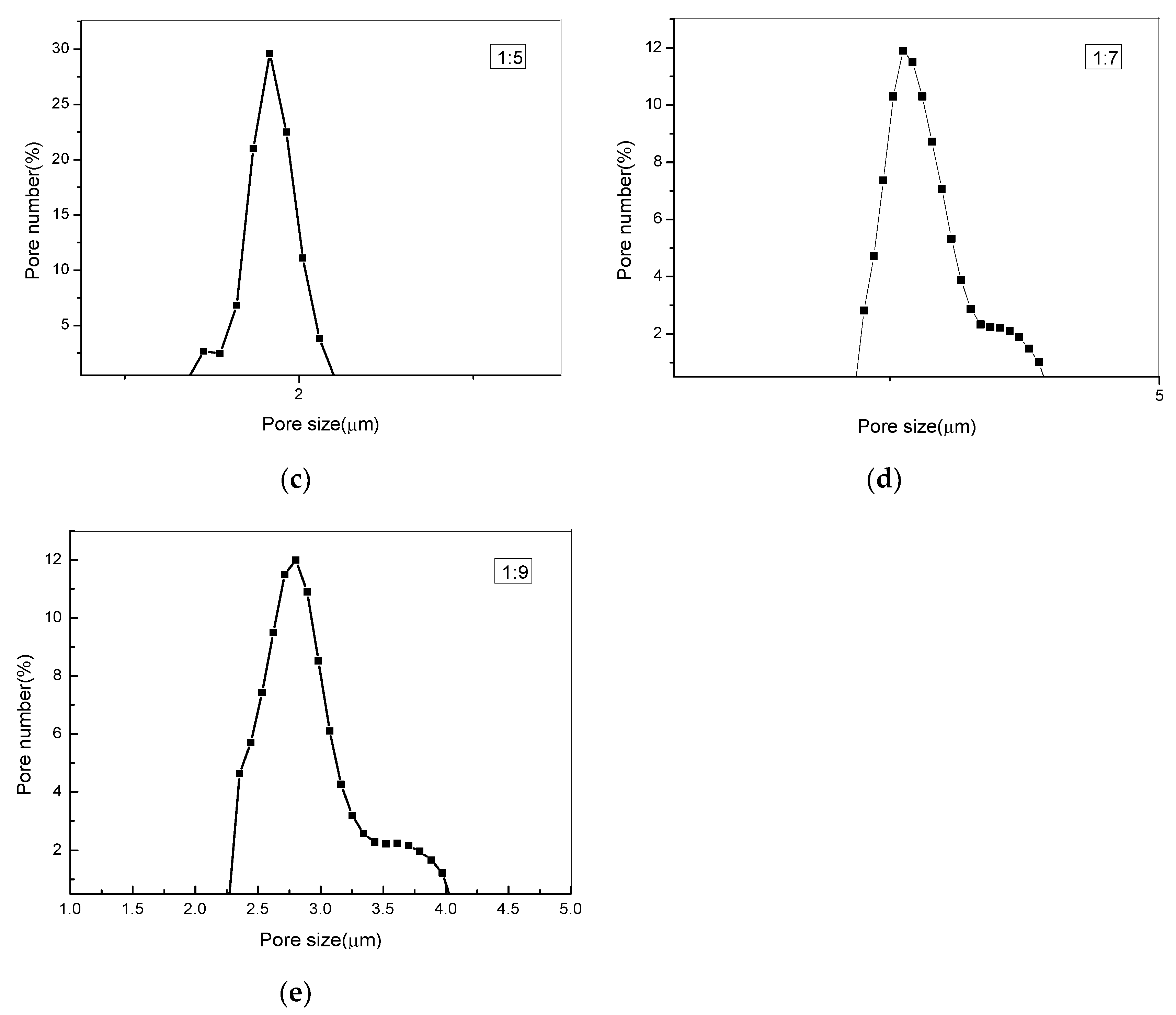
3.5. Measuring Pore Size Distribution Composite Membranes
3.6. Wetting Properties
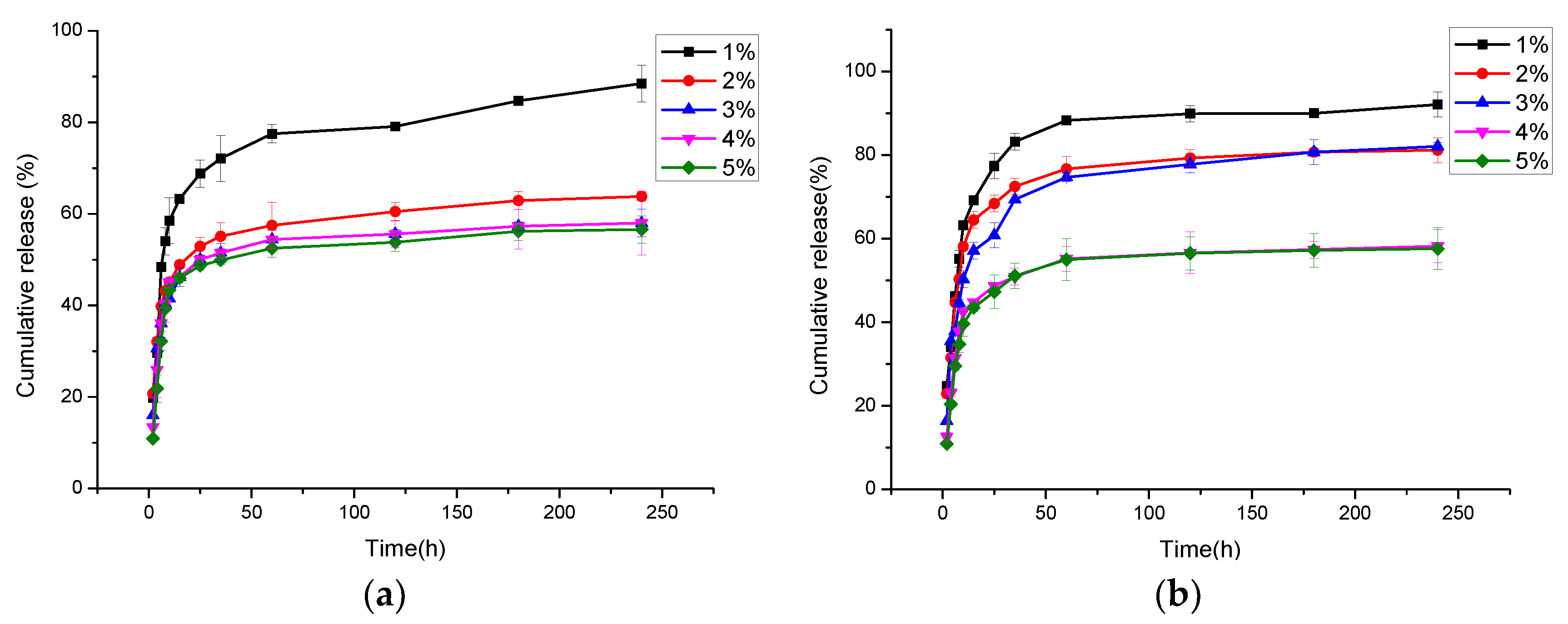
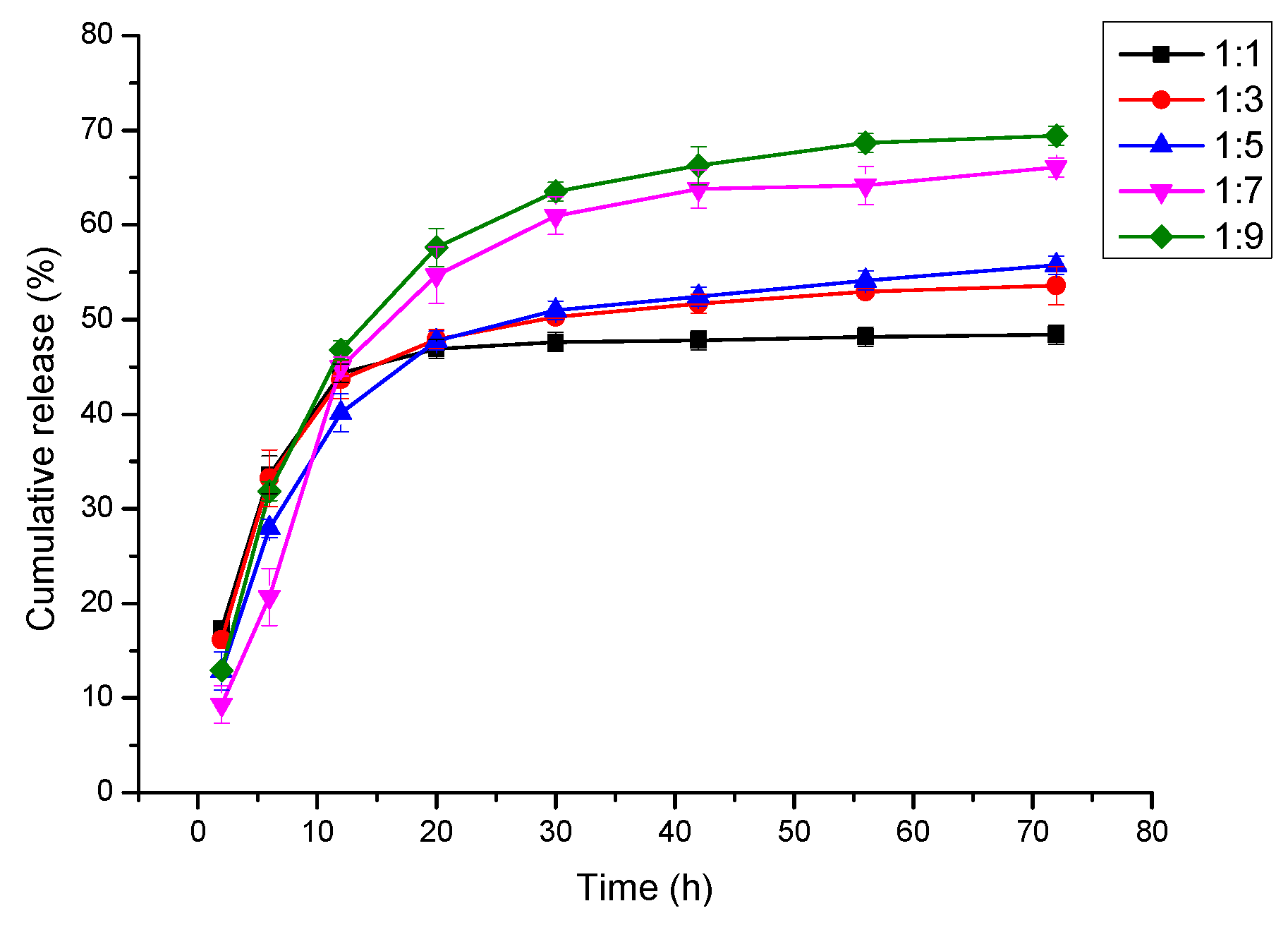
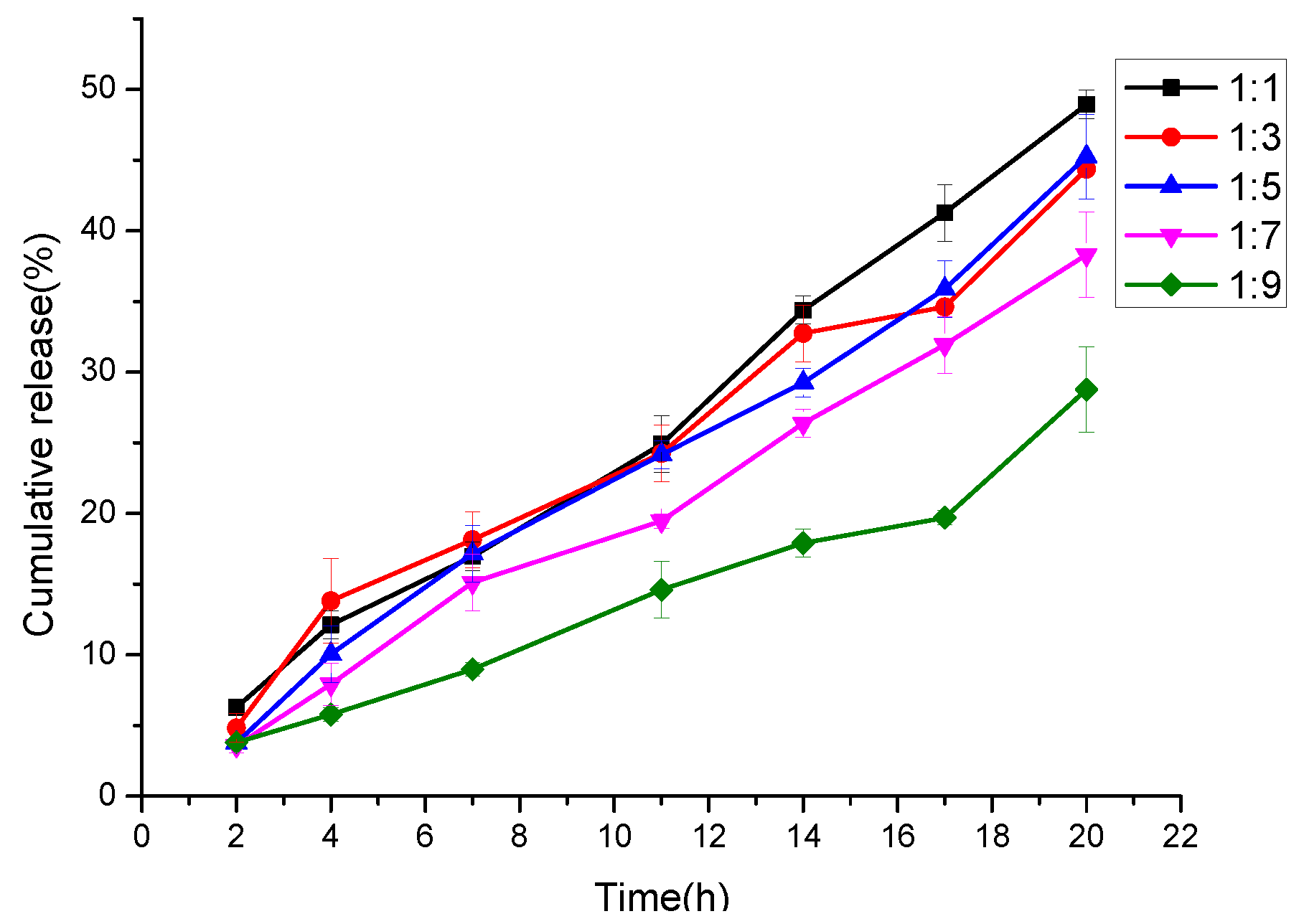
3.7. In Vitro Cur Release
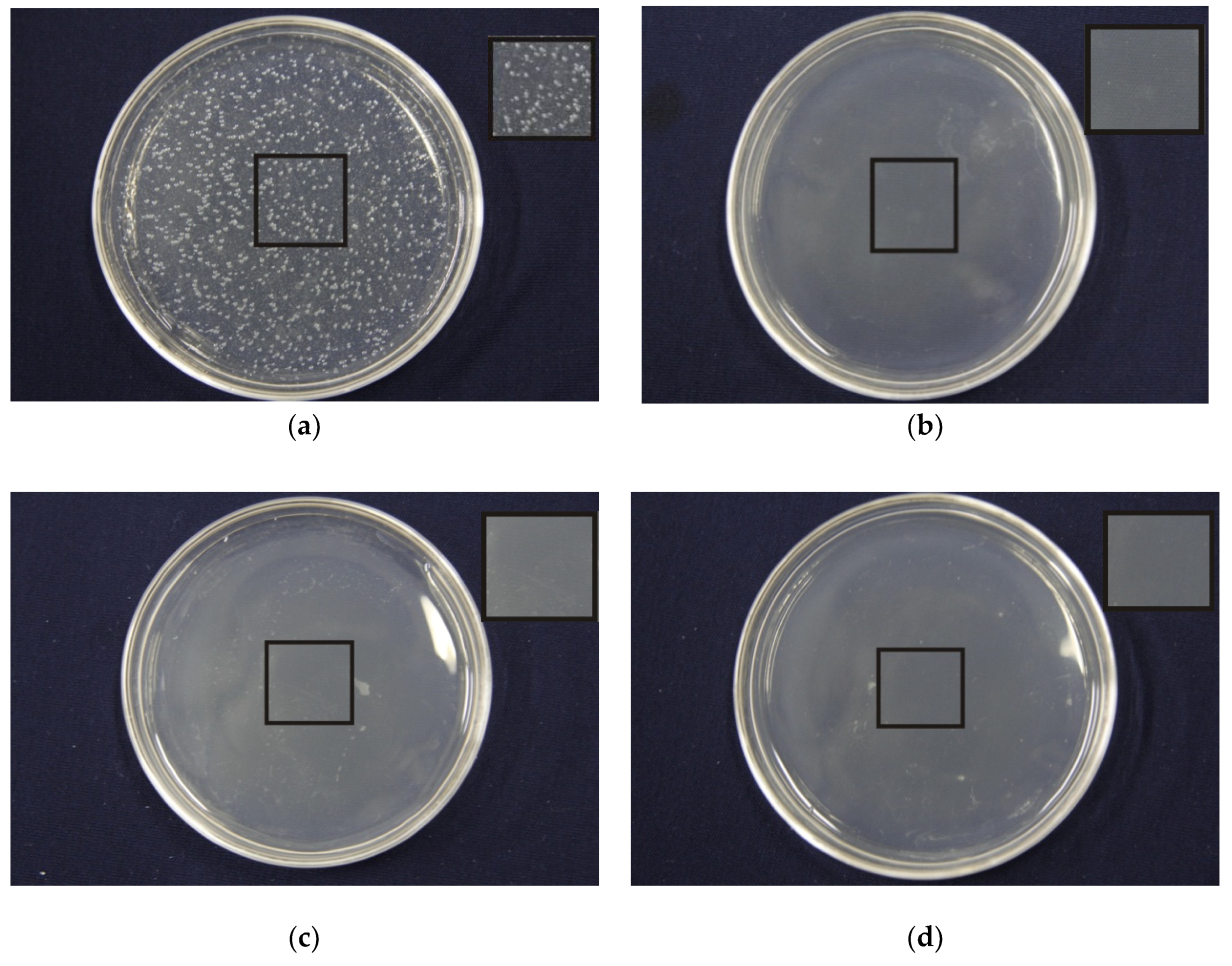
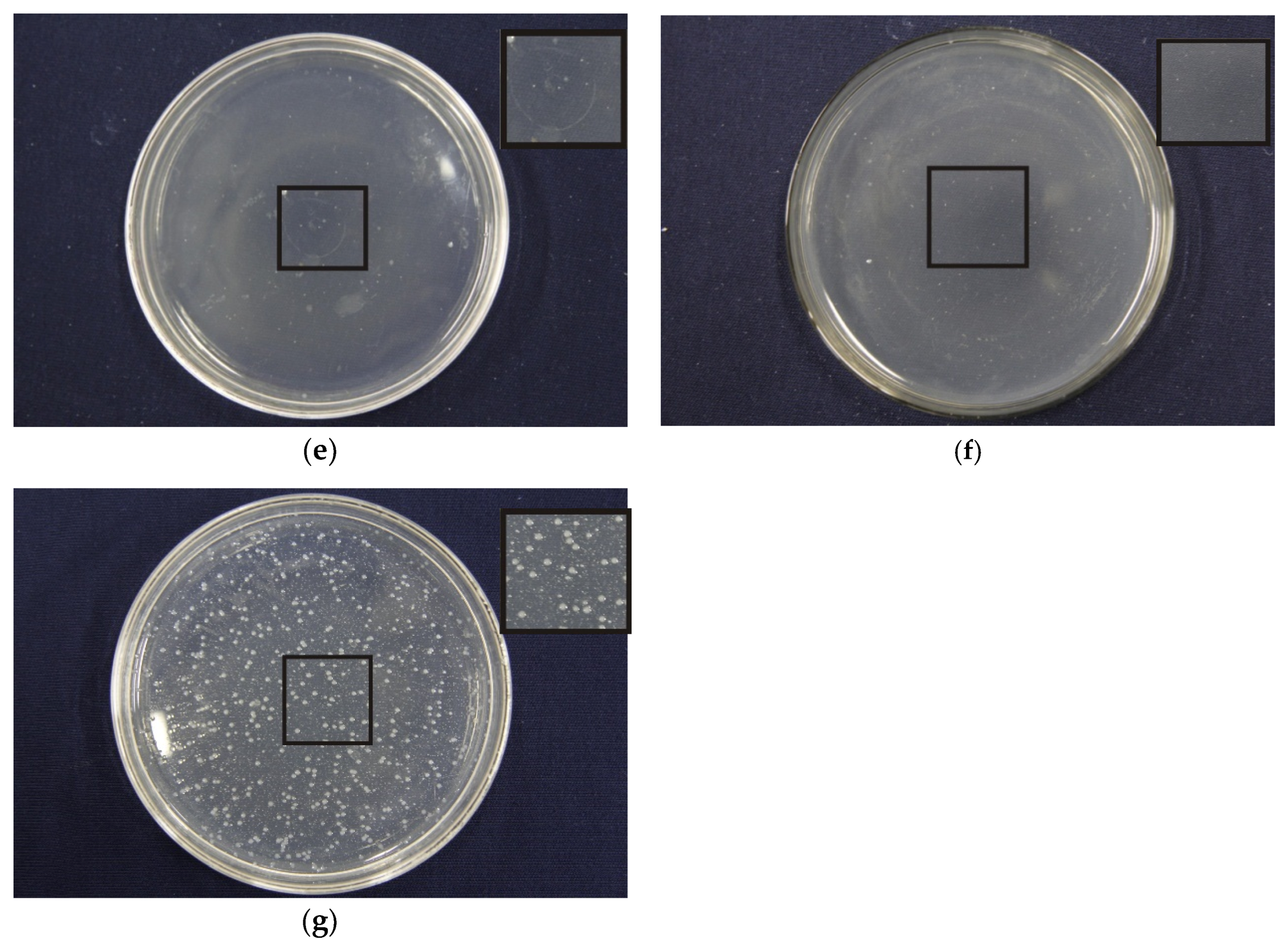
3.8. Effects of Porous Structure on Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Fan, C.; Tang, X.; Zhao, J.; Song, Y.; Shao, Z.; Xu, L. Characterization and antibacterial properties of porous fibers containing silver ions. Appl. Surf. Sci. 2016, 387, 828–838. [Google Scholar] [CrossRef]
- Kanehata, M.; Ding, B.; Shiratori, S. Nanoporous ultra-high specific surface inorganic fibres. Nanotechnology 2007, 18, 1–7. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.; Ma, J.; Yang, S.; Gong, J.; Xu, J. Preparation of continuous porous alumina nanofibers with hollow structure by single capillary electrospinning. Colloids Surf. A-Physicochem. Eng. Asp. 2013, 436, 489–494. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L. Preparation and characterization of porous core-shell fibers for slow release of tea polyphenols. Polymers 2018, 10, 144. [Google Scholar] [CrossRef]
- Zhao, J.; Si, N.; Xu, L. Experimental and theoretical study on the electrospinning nanoporous fibers process. Mater. Chem. Phys. 2016, 170, 294–302. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.; Zhao, J.; Pang, X.; Xi, Y.; Zhai, J. Development of a folate-modified curcumin loaded micelle delivery system for cancer targeting. Colloids Surf. B-Biointerfaces 2014, 121, 206–213. [Google Scholar] [CrossRef]
- Heni, R.; Yanda, Y.; Rahma, A.; Mase, N. Curcumin-Loaded PLA Nanoparticles: Formulation and Physical Evaluation. Sci. Pharm. 2016, 84, 191–202. [Google Scholar] [CrossRef]
- Lv, L.; Shen, Y.; Li, M.; Xu, X.; Li, M.; Guo, S.; Huang, S. Novel 4-Arm Poly(Ethylene Glycol)-Block-Poly(Anhydride-Esters) Amphiphilic Copolymer Micelles Loading Curcumin: Preparation, Characterization, and In Vitro Evaluation. Biomed. Res. Int. 2013, 1–11. [Google Scholar] [CrossRef]
- Bhattarai, N.; Li, Z.; Gunn, J.; Leung, M.; Cooper, A.; Edmondson, D.; Veiseh, O.; Chen, M.; Zhang, Y.; Ellenbogen, R.; et al. Natural-Synthetic Polyblend Nanofibers for Biomedical Applications. Adv. Mater. 2009, 21, 2792–2797. [Google Scholar] [CrossRef]
- Lu, H.; Qiu, Y.; Wang, Q.; Li, G.; Wei, Q. Nanocomposites prepared by electrohydrodynamics and their drug release properties. Mater. Sci. Eng. C 2018, 91, 26–35. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xiao, Y.; Liu, P.; Wang, S.; Zhao, Y.; Shen, M.; Shi, X. Electrospun PEGylated PLGA nanofibers for drug encapsulation and release. Mater. Sci. Eng. C 2018, 91, 255–262. [Google Scholar] [CrossRef]
- Ramírezagudelo, R.; Scheuermann, K.; Galagarcía, A.; Monteiro, A.; Pinzóngarcía, A.D.; Cortés, M.E.; Sinisterra, R.D. Hybrid nanofibers based on poly-caprolactone/gelatin/hydroxyapatite nanoparticles-loaded Doxycycline: Effective anti-tumoral and antibacterial activity. Mater. Sci. Eng. C 2018, 83, 25–34. [Google Scholar] [CrossRef]
- Ma, B.; Huang, Y.; Zhu, C.; Chen, C.; Chen, X.; Fan, M.; Sun, D. Novel Cu@SiO2/bacterial cellulose nanofibers: Preparation and excellent performance in antibacterial activity. Mater. Sci. Eng. C 2016, 62, 656–661. [Google Scholar] [CrossRef]
- Phan, Q.; Mai, H.; Le, T.; Tran, T.; Xuan, P.; Ha, P. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano systems with various PLA: PEG ratios. Int. J. Pharm. 2016, 507, 32–40. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: Enhanced water solubility and slow release of curcumin. Int. J. Pharm. 2017, 518, 177–184. [Google Scholar] [CrossRef]
- Zhong, T.; Jiao, Y.; Guo, L.; Ding, J.; Nie, Z.; Tan, L.; Huang, R. Investigations on porous PLA composite scaffolds with amphiphilic block PLA-b-PEG to enhance the carrying property for hydrophilic drugs of excess dose. J. Appl. Polym. Sci. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Hussain, S.A.; Patil, G.R.; Reddi, S.; Yadav, V.; Pothuraju, R.; Singh, R.R.B.; Kapila, S. Aloe vera (Aloe barbadensis Miller) supplemented probiotic lassi, prevents Shigella, infiltration from epithelial barrier into systemic blood flow in mice model. Microb. Pathog. 2017, 102, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of Fish Gelatin and Tea Polyphenol Coating on the Spoilage and Degradation of Myofibril in Fish Fillet During Cold Storage. Food Bioprocess Technol. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Serrano-Aroca, A.; Iskandar, L.; Deb, S. Green synthetic routes to alginate-graphene oxide composite hydrogels with enhanced physical properties for bioengineering applications. Eur. Polym. J. 2018, 103, 198–206. [Google Scholar] [CrossRef]
- Ma, M.; Wan, R.; Gong, H.; Lv, X.; Chu, S.; Li, D.; Peng, C. Study on the In Vitro and In Vivo Antibacterial Activity and Biocompatibility of Novel TiN/Ag Multilayers Immobilized onto Biomedical Titanium. J. Nanosci. Nanotechnol. 2019, 19, 3777–3791. [Google Scholar] [CrossRef]
- Xu, L.; Si, N.; Lee, E.W.M. Effect of humidity on the surface morphology of a charged jet. Heat Transf. Res. 2013, 44, 441–445. [Google Scholar] [CrossRef]
- Achoh, A.; Zabolotsky, V.; Melnikov, S. Conversion of water-organic solution of sodium naphtenates into naphtenic acids and alkali by electrodialysis with bipolar membranes. Sep. Purif. Technol. 2019, 212, 929–940. [Google Scholar] [CrossRef]
- Yan, C.; Yu, Z.; Yang, B. Improvement of thermoregulating performance for outlast/silk fabric by the incorporation of polyurethane microcapsule containing paraffin. Fibers Polym. 2013, 14, 1290–1294. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Li, C.; Fan, N.; Wang, J.; He, Z.; Sun, J. Viewing Molecular and Interface Interactions of Curcumin Amorphous Solid Dispersions for Comprehending Dissolution Mechanisms. Mol. Pharm. 2017, 14, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Si, N.; Liu, H.Y. Fabrication and characterization of chinese drug-loaded nanoporous materials. J. Nano Res. 2014, 27, 103–109. [Google Scholar] [CrossRef]
- Frıgols, B.; Martı, M.; Salesa, B.; Herna´ndezOliver, C.; Aarstad, O.; Teialeret Ulset, A.-S.; Inger Sætrom, G.; Lillelund Aachman, F.; Serrano-Aroca, A. Graphene oxide in zinc alginate films: Antibacterial activity, cytotoxicity, zinc release, water sorption/diffusion, wettability and opacity. PLoS ONE 2019, 14, e0212819. [Google Scholar] [CrossRef] [PubMed]
| PEG:PLA | Electrical Conductivity (μs/cm) | Viscosity (mPa·s) |
|---|---|---|
| 1:1 | 0.455 ± 0.045 | 33 ± 2 |
| 1:3 | 0.432 ± 0.038 | 77 ± 4 |
| 1:5 | 0.421 ± 0.035 | 112 ± 3 |
| 1:7 | 0.267 ± 0.052 | 137 ± 3 |
| 1:9 | 0.249 ± 0.048 | 157 ± 4 |
| PEG:PLA | Average Diameter (nm) | Standard Deviation (σ) (nm) | Confidence Interval (nm) |
|---|---|---|---|
| 1:1 | 290.60 | 64.08 | ±12.56 |
| 1:3 | 287.70 | 74.39 | ±16.91 |
| 1:5 | 205.16 | 59.43 | ±11.64 |
| 1:7 | 308.73 | 91.25 | ±17.89 |
| 1:9 | 552.81 | 195.53 | ±38.32 |
| PEG:PLA | Pore Size (μm) | Maximum Pore Number (/cm2) /The According Pore Size (μm) | Pore Number (/cm2) |
|---|---|---|---|
| 1:1 | 1.39–2.11 | 4.3 × 107/1.769 | 1.68 × 108 |
| 1:3 | 1.41–2.18 | 4.13 × 107/1.861 | 1.15 × 108 |
| 1:5 | 1.55–2.39 | 2.2 × 107/1.879 | 7.4 × 107 |
| 1:7 | 2.35–3.93 | 5.6 × 106/2.668 | 5.1 × 107 |
| 1:9 | 2.44–4.04 | 5.5 × 106/2.848 | 4.5 × 107 |
| Ratio | 1:1 | 1:3 | 1:5 | 1:7 | 1:9 |
|---|---|---|---|---|---|
| Contact angles | 47.1 ± 1.1° | 125.4 ± 2.6° | 130.2 ± 1.1° | 134.6 ± 2.0° | 139.0 ± 1.7° |
| PEG/PLA | Inhibition Rates (%) |
|---|---|
| 1:1 | 99.97 |
| 1:3 | 99.96 |
| 1:5 | 99.93 |
| 1:7 | 98.77 |
| 1:9 | 97.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Sun, Z.; Yin, J.; Xu, L. Preparation, Characterization and Properties of Porous PLA/PEG/Curcumin Composite Nanofibers for Antibacterial Application. Nanomaterials 2019, 9, 508. https://doi.org/10.3390/nano9040508
Wang F, Sun Z, Yin J, Xu L. Preparation, Characterization and Properties of Porous PLA/PEG/Curcumin Composite Nanofibers for Antibacterial Application. Nanomaterials. 2019; 9(4):508. https://doi.org/10.3390/nano9040508
Chicago/Turabian StyleWang, Feifei, Zhaoyang Sun, Jing Yin, and Lan Xu. 2019. "Preparation, Characterization and Properties of Porous PLA/PEG/Curcumin Composite Nanofibers for Antibacterial Application" Nanomaterials 9, no. 4: 508. https://doi.org/10.3390/nano9040508
APA StyleWang, F., Sun, Z., Yin, J., & Xu, L. (2019). Preparation, Characterization and Properties of Porous PLA/PEG/Curcumin Composite Nanofibers for Antibacterial Application. Nanomaterials, 9(4), 508. https://doi.org/10.3390/nano9040508





