Tantalum Oxynitride Thin Films: Assessment of the Photocatalytic Efficiency and Antimicrobial Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structural and Morphological Characterization
2.3. Photoactivity Testing
2.4. Antibiofilm Capacity Assay
3. Results and Discussion
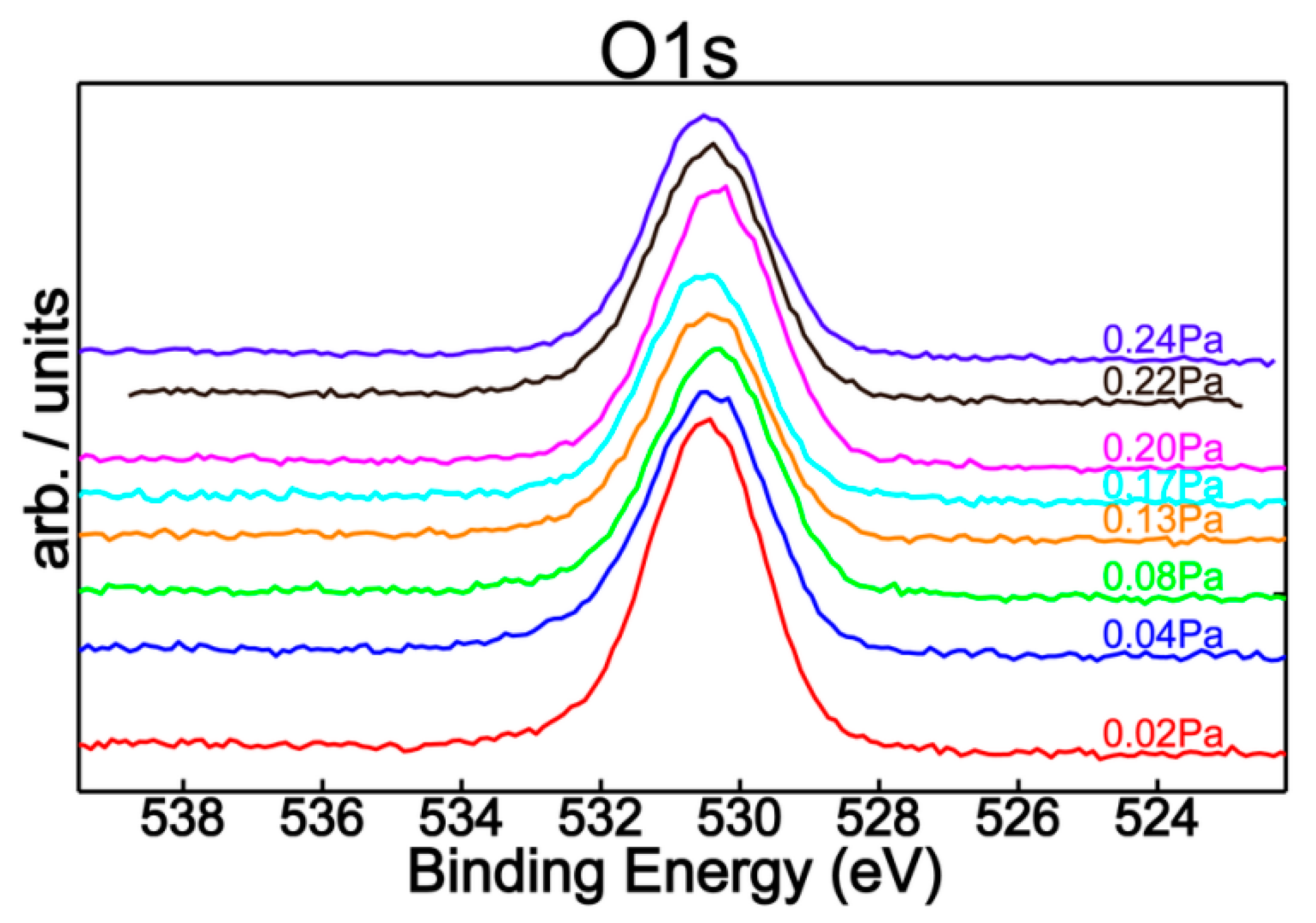
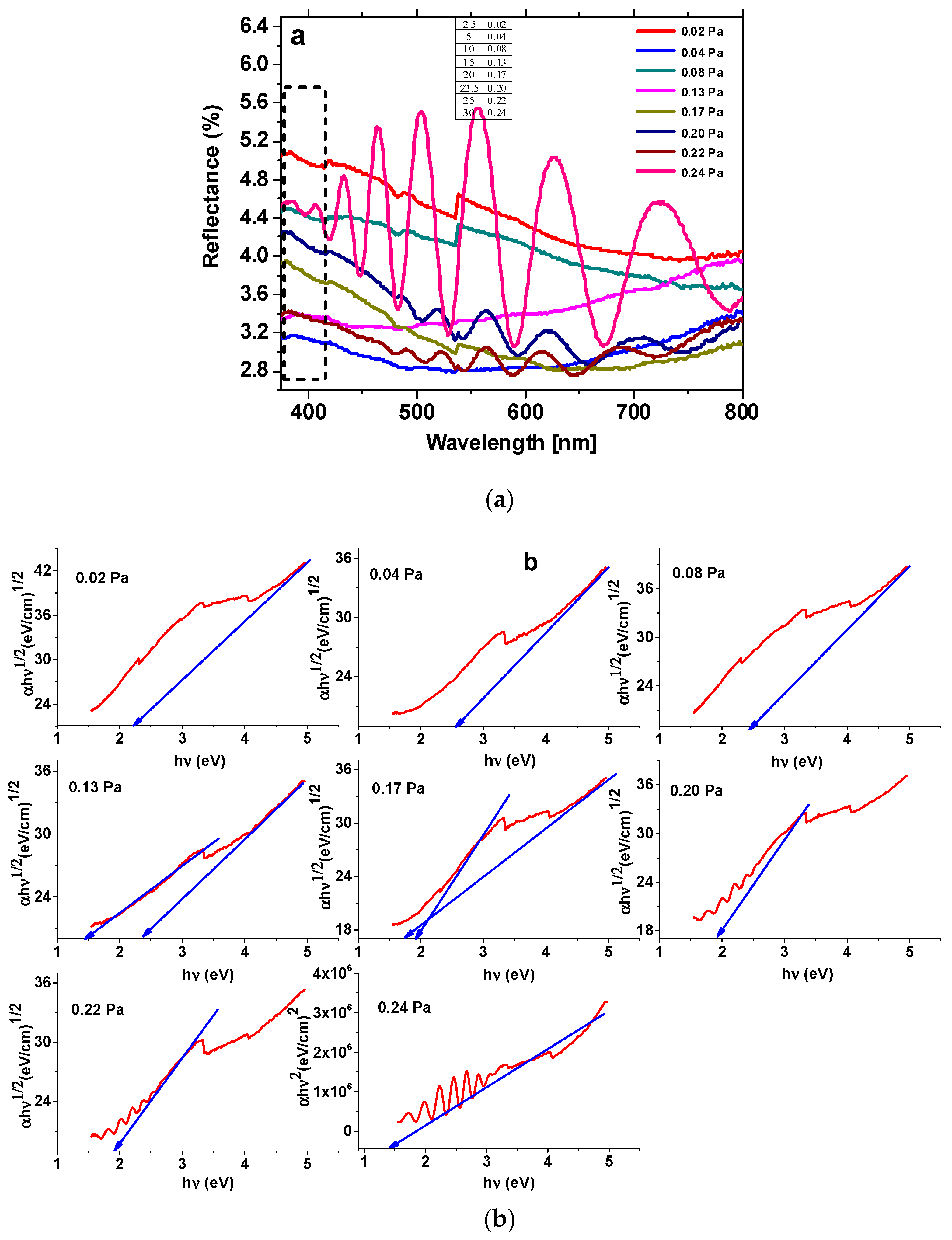
3.1. Spectral Characterization of the Thin Films
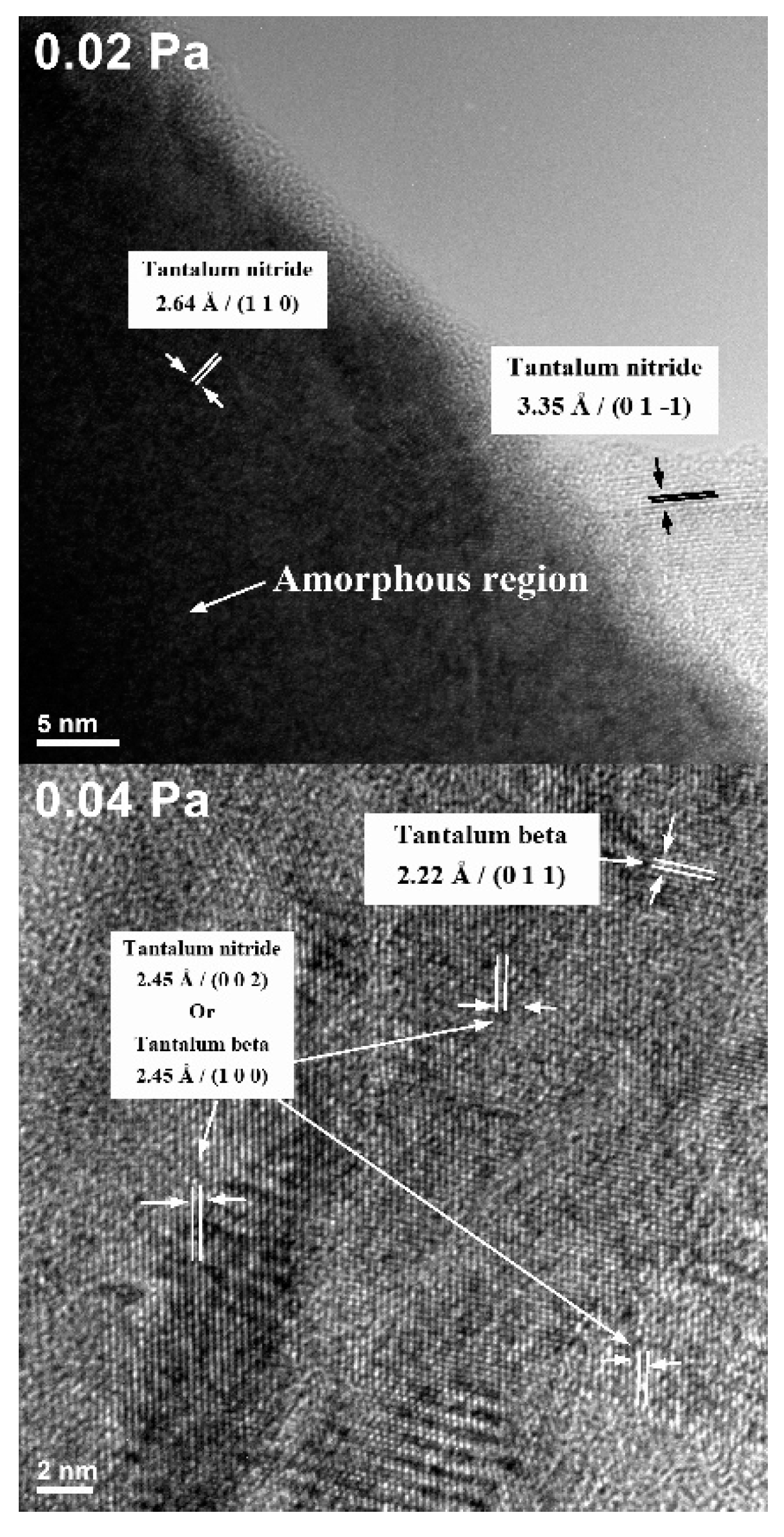
3.2. Transmission Electron Microscopy (TEM)
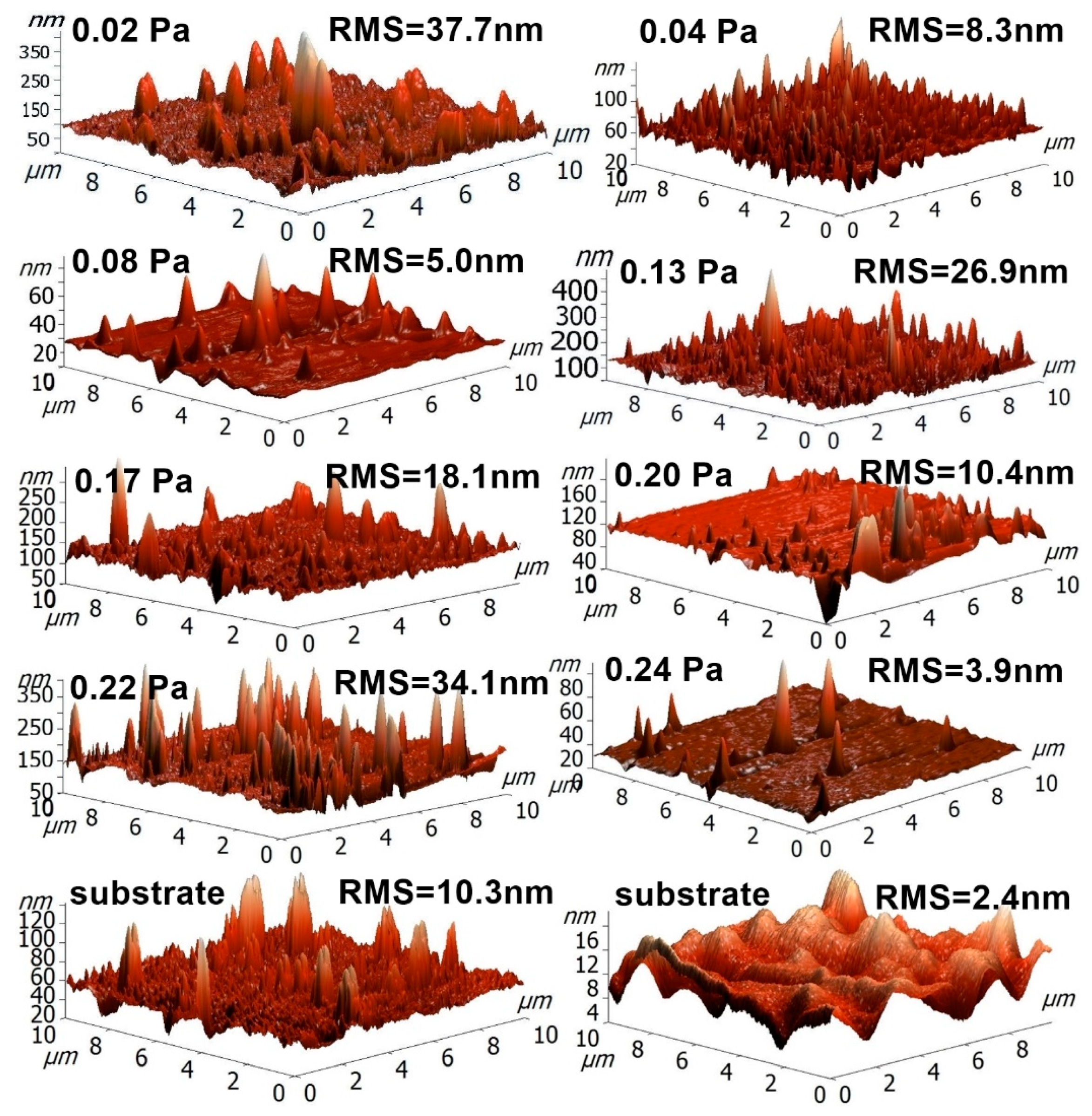
3.3. Surface Morphology (AFM)
3.4. Photodegradation Efficiency
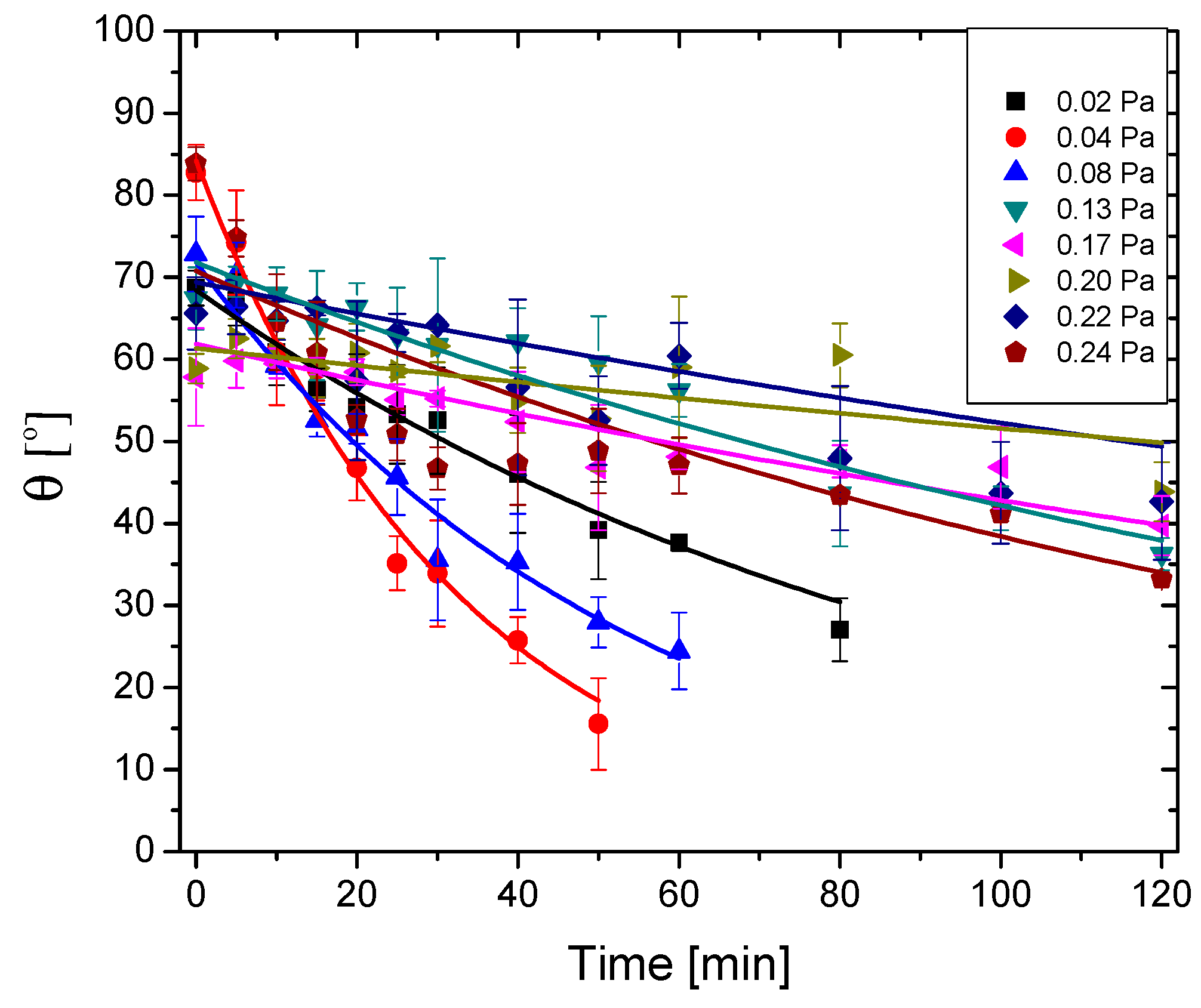
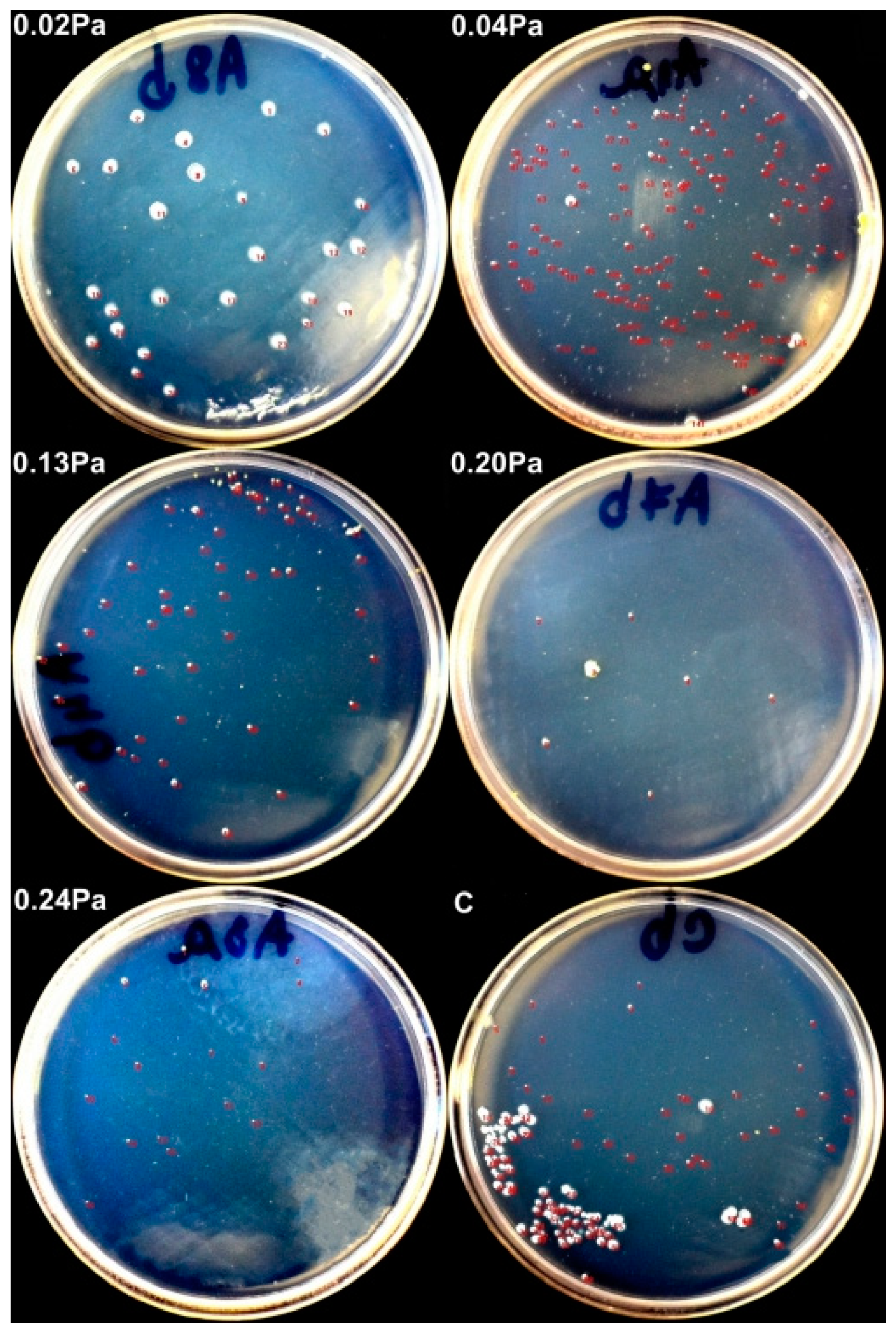
3.5. Antibacterial/Antibiofilm Capacity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banakh, O.; Csefalvay, C.; Steinmann, P.A.; Fenker, M.; Kappl, H. Evaluation of adhesion and tribological behaviour of tantalum oxynitride thin films deposited by reactive magnetron sputtering onto steel substrates. Surf. Coat. Technol. 2006, 200, 6500–6504. [Google Scholar] [CrossRef]
- Banakh, O.; Steinmann, P.A.; Dumitrescu-Buforn, L. Optical and mechanical properties of tantalum oxynitride thin films deposited by reactive magnetron sputtering. Thin Solid Films 2006, 513, 136–141. [Google Scholar] [CrossRef]
- Kato, K.; Toyota, H.; Jin, Y.; Ono, T. Characterization of tantalum oxy-nitrides deposited by ECR sputtering. Vacuum 2008, 83, 592–595. [Google Scholar] [CrossRef]
- Chung, C.K.; Chen, T.S.; Chang, N.W. Effect of reactive gases flow ratios on the microstructure and electrical resistivity of Ta-N-O thin films by reactive co-sputtering. Thin Solid Films 2011, 519, 5099–5102. [Google Scholar] [CrossRef]
- Misra, E.; Wang, Y.; Theodore, N.D.; Alford, T.L. Evaluation of diffusion barrier and electrical properties of tantalum oxynitride thin films for silver metallization. Thin Solid Films 2004, 457, 338–345. [Google Scholar] [CrossRef]
- Verchiani, M.; Bouyssou, E.; Fiannaca, G.; Cantin, F.; Anceau, C.; Ranson, P. Reliability study of TaON capacitors: From leakage current characterization to ESD robustness prediction. Microelectron. Reliab. 2008, 48, 1412–1416. [Google Scholar] [CrossRef]
- Ishihara, A.; Doi, S.; Mitsushima, S.; Ota, K. Tantalum (oxy)nitrides prepared using reactive sputtering for new nonplatinum cathodes of polymer electrolyte fuel cell. Electrochim. Acta 2008, 53, 5442–5450. [Google Scholar] [CrossRef]
- Chen, M.C.; Chang, T.C.; Chiu, Y.C.; Chen, S.C.; Huang, S.Y.; Chang, K.C.; Tsai, T.M.; Yang, K.H.; Sze, S.M.; Tsai, M.J. The resistive switching characteristics in TaON films for nonvolatile memory applications. Thin Solid Films 2013, 528, 224–228. [Google Scholar] [CrossRef]
- Venkataraj, S.; Kittur, H.; Drese, R.; Wuttig, M. Multi-technique characterization of tantalum oxynitride films prepared by reactive direct current magnetron sputtering. Thin Solid Films 2006, 514, 1–9. [Google Scholar] [CrossRef]
- Jong, C.A.; Chin, T.S. Optical characteristics of sputtered tantalum oxynitride Ta(N,O) films. Mater. Chem. Phys. 2002, 74, 201–209. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Chang, C.C.; Cherng, J.S.; Hsu, F.Y. optical properties and hydrophilic behaviors of TaOxNy thin films with and without rapid thermal annealing. Thin Solid Films 2009, 517, 4711–4714. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Li, C.; Liang, H.C. Structures and photocatalytic behavior of tantalum-oxynitride thin films. Thin Solid Films 2011, 519, 4699–4704. [Google Scholar] [CrossRef]
- Dabirian, A.; van’t Spijker, H.; van de Krol, R. Wet ammonia synthesis of semiconducting N:Ta2O5, Ta3N5 and beta-TaON films for photoanode applications. Energy Procedia 2012, 22, 15–22. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Chang, C.C.; Chang, Y.K.; Cherng, J.S. Photocatalytic and antibacterial properties of TaON-Ag nanocomposite thin films. Thin Solid Films 2010, 518, 7263–7266. [Google Scholar] [CrossRef]
- Leroy, C.M.; Sanjines, R.; Sivula, K.; Cornuz, M.; Xanthopoulos, N.; Laporte, V.; Gratzel, M. TaOxNy Sputtered Photoanodes For Solar Water Splitting. Energy Procedia 2012, 22, 119–126. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Chang, C.C.; Li, C.; Liu, S.J.; Chang, Y.K. Effects of Ag contents on antibacterial behaviors of TaON-Ag nanocomposite thin films. Surf. Coat. Technol. 2010, 205, S337–S340. [Google Scholar] [CrossRef]
- Du, Y.X.; Zhao, L.; Zhang, Y.Y. Roles of TaON and Ta3N5 in the visible-Fenton-like degradation of atrazine. J. Hazard. Mater. 2014, 267, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Hitoki, G.; Takata, T.; Kondo, J.N.; Kobayashi, H.; Domen, K. TaON and Ta3N5 as new visible light driven photocatalysts. Catal. Today 2003, 78, 555–560. [Google Scholar] [CrossRef]
- Mungchamnankit, A.; Limsuwan, P.; Thongcham, K.; Meejoo, S. The electron spin resonance study of Gd3+ in natural zircon. J. Magn. Magn. Mater. 2008, 320, 479–482. [Google Scholar] [CrossRef]
- Harrison, P.L.; Harrison, T.; Stockley, I.; Smith, T.J. Does tantalum exhibit any intrinsic antimicrobial or antibiofilm properties? Bone Joint J. 2017, 99B, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Schildhauer, T.A.; Robie, B.; Muhr, G.; Koller, M. Bacterial adherence to tantalum versus commonly used orthopedic metallic implant materials. J. Orthop. Trauma 2006, 20, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.Y.; Huang, H.L.; Chen, H.J.; Lai, C.H.; Wen, C.Y. Antibacterial properties and cytocompatibility of tantalum oxide coatings. Surf. Coat. Technol. 2014, 259, 193–198. [Google Scholar] [CrossRef]
- Huang, H.L.; Chang, Y.Y.; Lai, M.C.; Lin, C.R.; Lai, C.H.; Shieh, T.M. Antibacterial TaN-Ag coatings on titanium dental implants. Surf. Coat. Technol. 2010, 205, 1636–1641. [Google Scholar] [CrossRef]
- Cristea, D.; Constantin, D.; Crisan, A.; Abreu, C.S.; Gomes, J.R.; Barradas, N.P.; Alves, E.; Moura, C.; Vaz, F.; Cunha, L. Properties of tantalum oxynitride thin films produced by magnetron sputtering: The influence of processing parameters. Vacuum 2013, 98, 63–69. [Google Scholar] [CrossRef]
- Akbal, F. Photocatalytic degradation of organic dyes in the presence of titanium dioxide under UV and solar light: Effect of operational parameters. Environ. Prog. 2005, 24, 317–322. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, V. TiO2 mediated photocatalytic degradation studies of Reactive Red 198 by UV irradiation. J. Hazard. Mater. 2007, 141, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, J.; Radovic, M.; Bojic, D.; Andelkovic, T.; Purenovic, M.; Bojic, A. Decolorization of the textile azo dye Reactive Orange 16 by the UV/H2O2 process. J. Serb. Chem. Soc. 2012, 77, 465–481. [Google Scholar] [CrossRef]
- Liu, X.J.; Gan, K.; Liu, H.; Song, X.Q.; Chen, T.J.; Liu, C.C. Antibacterial properties of nano-silver coated PEEK prepared through magnetron sputtering. Dent. Mater. 2017, 33, E348–E360. [Google Scholar] [CrossRef]
- Yamajo, S.; Yoon, S.; Liang, J.; Sodabanlu, H.; Watanabe, K.; Sugiyama, M.; Yasui, A.; Ikenaga, E.; Shigekawa, N. Hard X-ray photoelectron spectroscopy investigation of annealing effects on buried oxide in GaAs/Si junctions by surface-activated bonding. Appl. Surf. Sci. 2019, 473, 627–632. [Google Scholar] [CrossRef]
- Rumble, J.R.; Bickham, D.M.; Powell, C.J. The Nist X-Ray Photoelectron-Spectroscopy Database. Surf. Interface Anal. 1992, 19, 241–246. [Google Scholar] [CrossRef]
- Arranz, A.; Palacio, C. Tantalum nitride formation by low-energy (0.5-5 keV) nitrogen implantation. Surf. Interface Anal. 2000, 29, 653–658. [Google Scholar] [CrossRef]
- Chun, W.J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Mei, X.X.; Yang, D.Z.; Chen, F.X.; Ma, T.C.; Wang, Y.M.; Teng, F.N. Preparation, structure and properties of TaN and TaC films obtained by ion beam assisted deposition. Nucl. Instrum. Methods Phys. Res. Sect. B 1997, 127, 664–668. [Google Scholar] [CrossRef]
- Shi, L.; Yang, Z.H.; Chen, L.Y.; Qian, Y.T. Synthesis and characterization of nanocrystalline TaN. Solid State Commun. 2005, 133, 117–120. [Google Scholar] [CrossRef]
- Lamour, P.; Fioux, P.; Ponche, A.; Nardin, M.; Vallat, M.F.; Dugay, P.; Brun, J.P.; Moreaud, N.; Pinvidic, J.M. Direct measurement of the nitrogen content by XPS in self-passivated TaNx thin films. Surf. Interface Anal. 2008, 40, 1430–1437. [Google Scholar] [CrossRef]
- Maeda, K.; Terashima, H.; Kase, K.; Higashi, M.; Tabata, M.; Domen, K. Surface modification of TaON with monoclinic ZrO2 to produce a composite photocatalyst with enhanced hydrogen evolution activity under visible light. Bull. Chem. Soc. Jpn. 2008, 81, 927–937. [Google Scholar] [CrossRef]
- Hara, M.; Chiba, E.; Ishikawa, A.; Takata, T.; Kondo, J.N.; Domen, K. Ta3N5 and TaON thin films on Ta foil: Surface composition and stability. J. Phys. Chem. B 2003, 107, 13441–13445. [Google Scholar] [CrossRef]
- Khemasiri, N.; Jessadaluk, S.; Chananonnawathorn, C.; Vuttivong, S.; Lertvanithphol, T.; Horprathum, M.; Eiamchai, P.; Patthanasettakul, V.; Klamchuen, A.; Pankiew, A.; et al. Optical band engineering of metal-oxynitride based on tantalum oxide thin film fabricated via reactive gas-timing RF magnetron sputtering. Surf. Coat. Technol. 2016, 306, 346–350. [Google Scholar] [CrossRef]
- Han, Q.; Zhou, Y.; Tang, L.; Li, P.; Tu, W.; Li, L.; Li, H.; Zou, Z. Synthesis of single-crystalline, porous TaON microspheres toward visible-light photocatalytic conversion of CO2 into liquid hydrocarbon fuels. RSC Adv. 2016, 6, 90792–90796. [Google Scholar] [CrossRef]
- Renaud, A.; Wilmet, M.; Truong, T.G.; Seze, M.; Lemoine, P.; Dumait, N.; Chen, W.; Saito, N.; Ohsawa, T.; Uchikoshi, T.; et al. Transparent tantalum cluster-based UV and IR blocking electrochromic devices. J. Mater. Chem. C 2017, 5, 8160–8168. [Google Scholar] [CrossRef]
- Perez, I.; Carrejo, J.L.E.; Sosa, V.; Perera, F.G.; Mancillas, J.R.F.; Galindo, J.T.E.; Rodriguez, C.I.R. Evidence for structural transition in crystalline tantalum pentoxide films grown by RF magnetron sputtering. J. Alloys Compd. 2017, 712, 303–310. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50-xSex thin films. J. Alloys Compd. 2015, 648, 280–290. [Google Scholar] [CrossRef]
- Renuka, L.; Anantharaju, K.S.; Vidya, Y.S.; Nagaswarupa, H.P.; Prashantha, S.C.; Sharma, S.C.; Nagabhushana, H.; Darshan, G.P. A simple combustion method for the synthesis of multi-functional ZrO2/CuO nanocomposites: Excellent performance as Sunlight photocatalysts and enhanced latent fingerprint detection. Appl. Catal. B Environ. 2017, 210, 97–115. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, S.C.; Thaweesak, S.; Luo, B.; Wang, L.Z. Tantalum (Oxy)Nitride: Narrow Bandgap Photocatalysts for Solar Hydrogen Generation. Engineering 2017, 3, 365–378. [Google Scholar] [CrossRef]
- Murphy, A.B.; Barnes, P.R.F.; Randeniya, L.K.; Plumb, I.C.; Grey, I.E.; Horne, M.D.; Glasscock, J.A. Efficiency of solar water splitting using semiconductor electrodes. Int. J. Hydrogen Energy 2006, 31, 1999–2017. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.J.; Gong, J.L. Tantalum-based semiconductors for solar water splitting. Chem. Soc. Rev. 2014, 43, 4395–4422. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.; Sautet, P.; Nurlaela, E.; Raybaud, P.; Cavallo, L.; Domen, K.; Basseta, J.M.; Takanabe, K. Tuning the properties of visible-light-responsive tantalum (oxy)nitride photocatalysts by non-stoichiometric compositions: A first-principles viewpoint. Phys. Chem. Chem. Phys. 2014, 16, 20548–20560. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Gomez, V.; Bear, J.C.; Rome, B.; Mazzali, F.; McGettrick, J.D.; Lewis, A.R.; Margadonna, S.; Al-Masry, W.A.; Dunnill, C.W. Active removal of waste dye pollutants using Ta3N5/W18O49 nanocomposite fibres. Sci. Rep. 2017, 7, 4090. [Google Scholar] [CrossRef] [PubMed]
- Granadino-Roldan, J.M.; Garzon, A.; Moral, M.; Garcia, G.; Pena-Ruiz, T.; Fernandez-Liencres, M.P.; Navarro, A.; Fernandez-Gomez, M. Theoretical estimation of the optical bandgap in a series of poly(aryl-ethynylene)s: A DFT study. J. Chem. Phys. 2014, 140, 044908. [Google Scholar] [CrossRef] [PubMed]
- Ristova, M.; Ristov, M. Silver-doped CdS films for PV application. Sol. Energy Mater. Sol. C 1998, 53, 95–102. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Influence of thermal and compositional variations on conduction mechanisms and localized state density of amorphous Cd50S50-xSex thin films. J. Non-Cryst. Solids 2018, 487, 28–36. [Google Scholar] [CrossRef]
- Balaz, S.; Porter, S.H.; Woodward, P.M.; Brinson, L.J. Electronic Structure of Tantalum Oxynitride Perovskite Photocatalysts. Chem. Mater. 2013, 25, 3337–3343. [Google Scholar] [CrossRef]
- Noguchi, D.; Kawamata, Y.; Nagatomo, T. Photocatalytic characteristics of TiO2 thin films prepared by Dc reactive magnetron sputtering with added H2O. Jpn. J. Appl. Phys. 2003, 42, 5255–5258. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.L.; Li, T. Rapid photocatalytic decolorization of methylene blue using high photon flux UV/TiO2/H2O2 process. Chem. Eng J. 2013, 217, 407–413. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laine, J.M. Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl. Catal. B Environ. 2004, 50, 259–269. [Google Scholar] [CrossRef]
- Lin, W.H.; Cheng, C.; Hu, C.C.; Teng, H.S. NaTaO3 photocatalysts of different crystalline structures for water splitting into H2 and O2. Appl. Phys. Lett 2006, 89, 211904. [Google Scholar] [CrossRef]
- Valente, J.P.S.; Padilha, P.M.; Florentino, A.O. Studies on the adsorption and kinetics of photodegradation of a model compound for heterogeneous photocatalysis onto TiO2. Chemosphere 2006, 64, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Tahir, A.A.; Bibi, S.; Mallick, T.K.; Karazhanov, S.Z. Electronic properties of beta-TaON and its surfaces for solar water splitting. Appl. Catal. B Environ. 2018, 229, 24–31. [Google Scholar] [CrossRef]
- Sakai, N.; Wang, R.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Effect of ultrasonic treatment on highly hydrophilic TiO2 surfaces. Langmuir 1998, 14, 5918–5920. [Google Scholar] [CrossRef]
- He, Y.M.; Thorne, J.E.; Wu, C.H.; Ma, P.Y.; Du, C.; Dong, Q.; Guo, J.H.; Wang, D.W. What Limits the Performance of Ta3N5 for Solar Water Splitting? Chem 2016, 1, 640–655. [Google Scholar] [CrossRef]
- Cong, Y.Q.; Park, H.S.; Dang, H.X.; Fan, F.R.F.; Bard, A.J.; Mullins, C.B. Tantalum Cobalt Nitride Photocatalysts for Water Oxidation under Visible Light. Chem. Mater. 2012, 24, 579–586. [Google Scholar] [CrossRef]
- Tezza, V.B.; Scarpato, M.; Bernardin, A.M. Wettability of anatase ceramic glazes. Chim. Oggi 2012, 30, 55–57. [Google Scholar]
- Ahmed, M.; Guo, X.X. A review of metal oxynitrides for photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590. [Google Scholar] [CrossRef]
- Evans, P.; Sheel, D.W. Photoactive and antibacterial TiO2 thin films on stainless steel. Surf. Coat. Technol. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Aiken, Z.A.; Hyett, G.; Dunnill, C.W.; Wilson, M.; Pratten, J.; Parkin, I.P. Antimicrobial Activity in Thin Films of Pseudobrookite-Structured Titanium Oxynitride under UV Irradiation Observed for Escherichia coli. Chem. Vapor Depos. 2010, 16, 19. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferrer, M.; Cerrada, M.L.; Serrano, C.; Sanchez-Chaves, M.; Fernandez-Garcia, M.; de Andres, A.; Rioboo, R.J.J.; Fernandez-Martin, F.; Fernandez-Garcia, M. Boosting TiO2-anatase antimicrobial activity: Polymer-oxide thin films. Appl. Catal. B Environ. 2009, 89, 441–447. [Google Scholar] [CrossRef]
- AL-Jawad, S.M.H.; Taha, A.A.; Salim, M.M. Synthesis and characterization of pure and Fe doped TiO2 thin films for antimicrobial activity. Optik 2017, 142, 42–53. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.F.; Li, Y.; Xu, H.; Qin, L.; Tay, J.H. The influence of cell and substratum surface hydrophobicities on microbial attachment. J. Biotechnol. 2004, 110, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wang, L.; Levanen, E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv. 2013, 3, 12003–12020. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinanen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed]
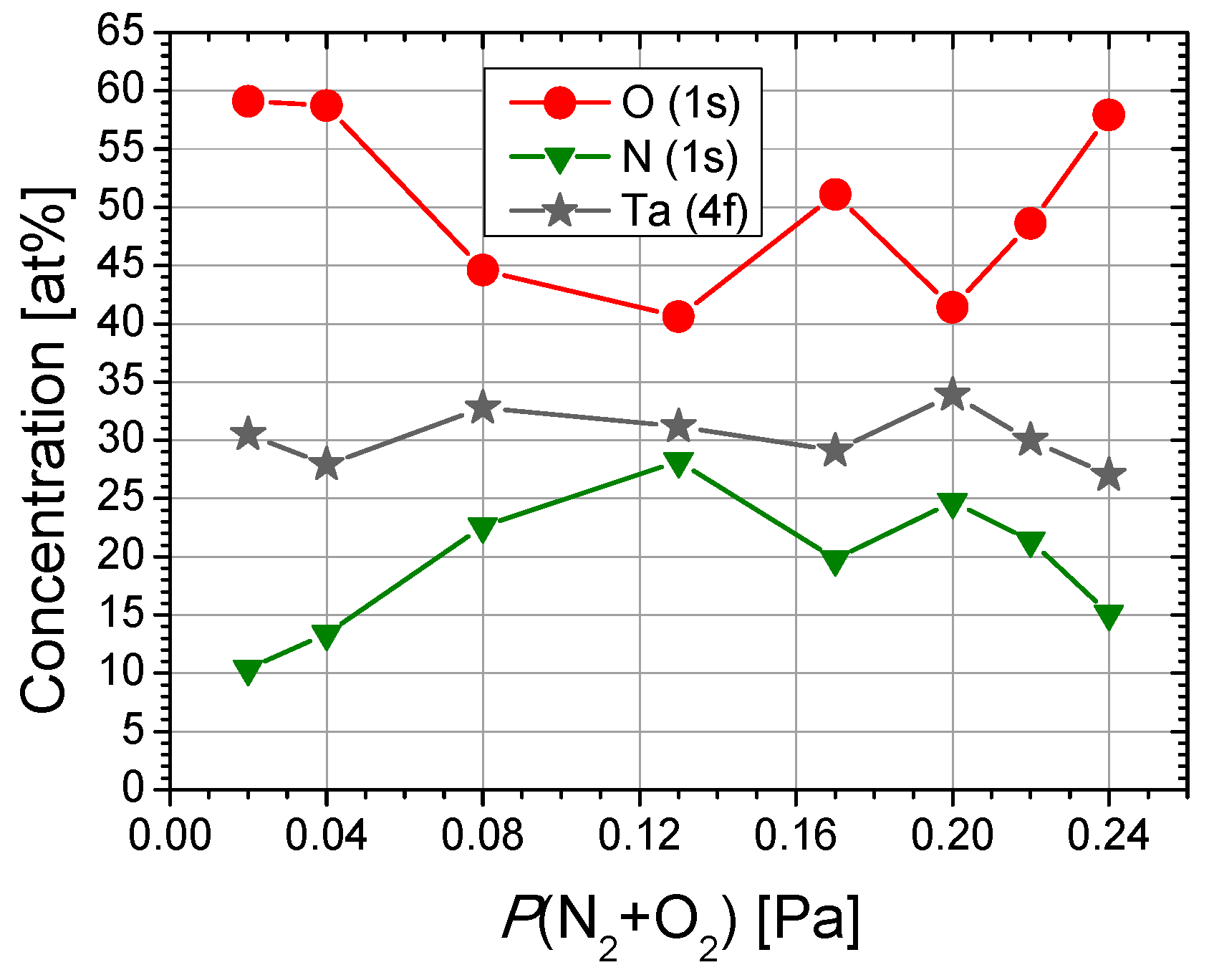

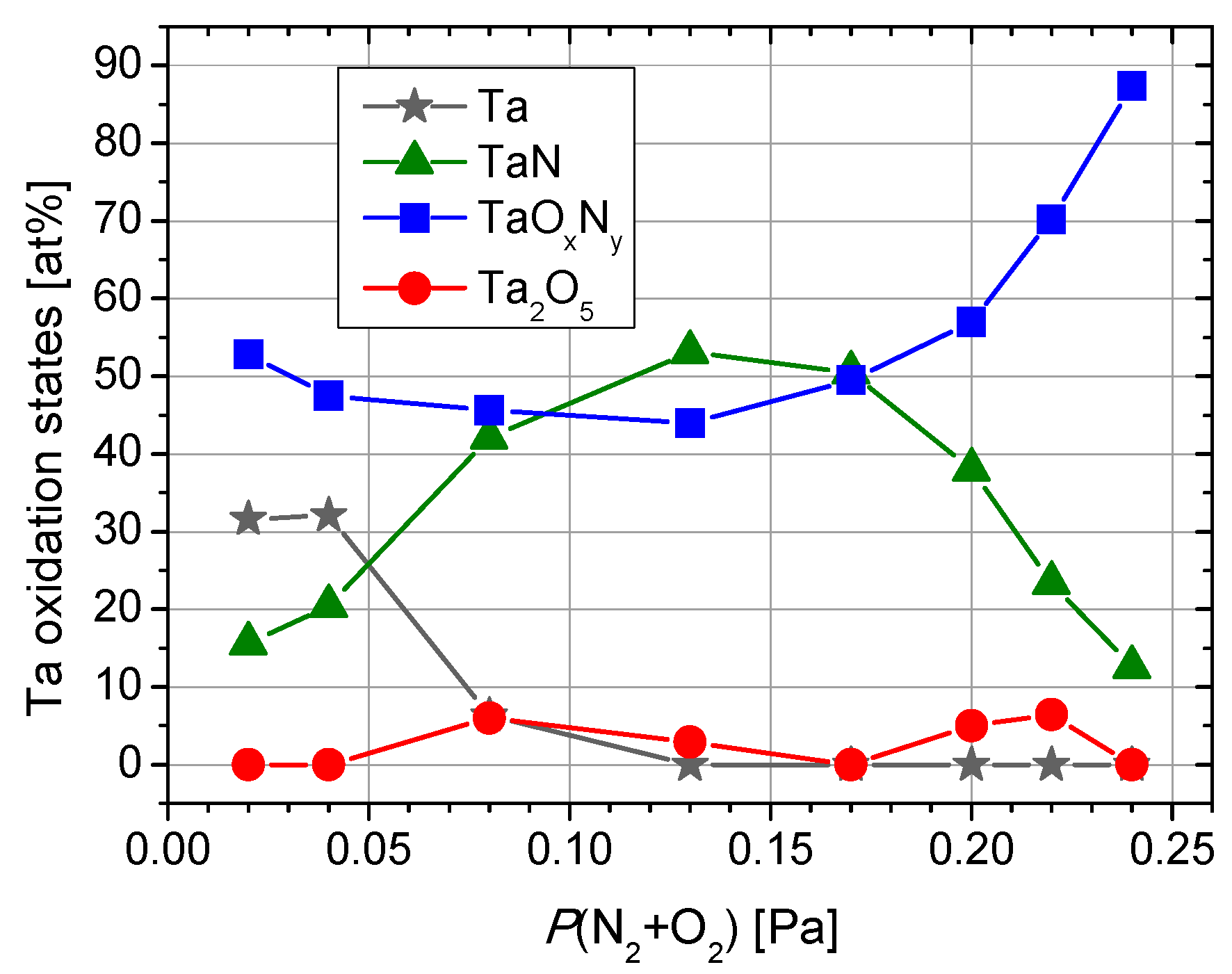
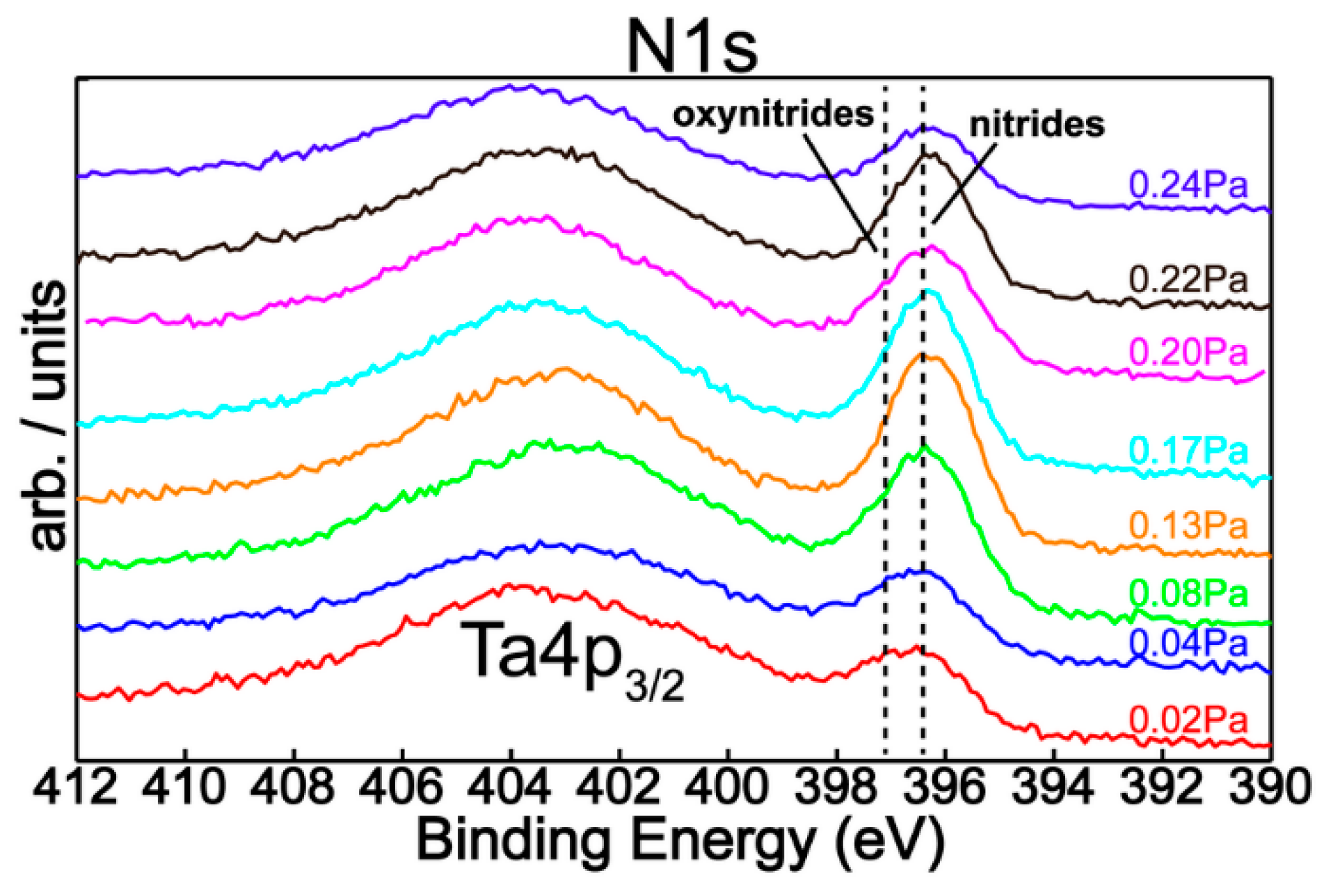
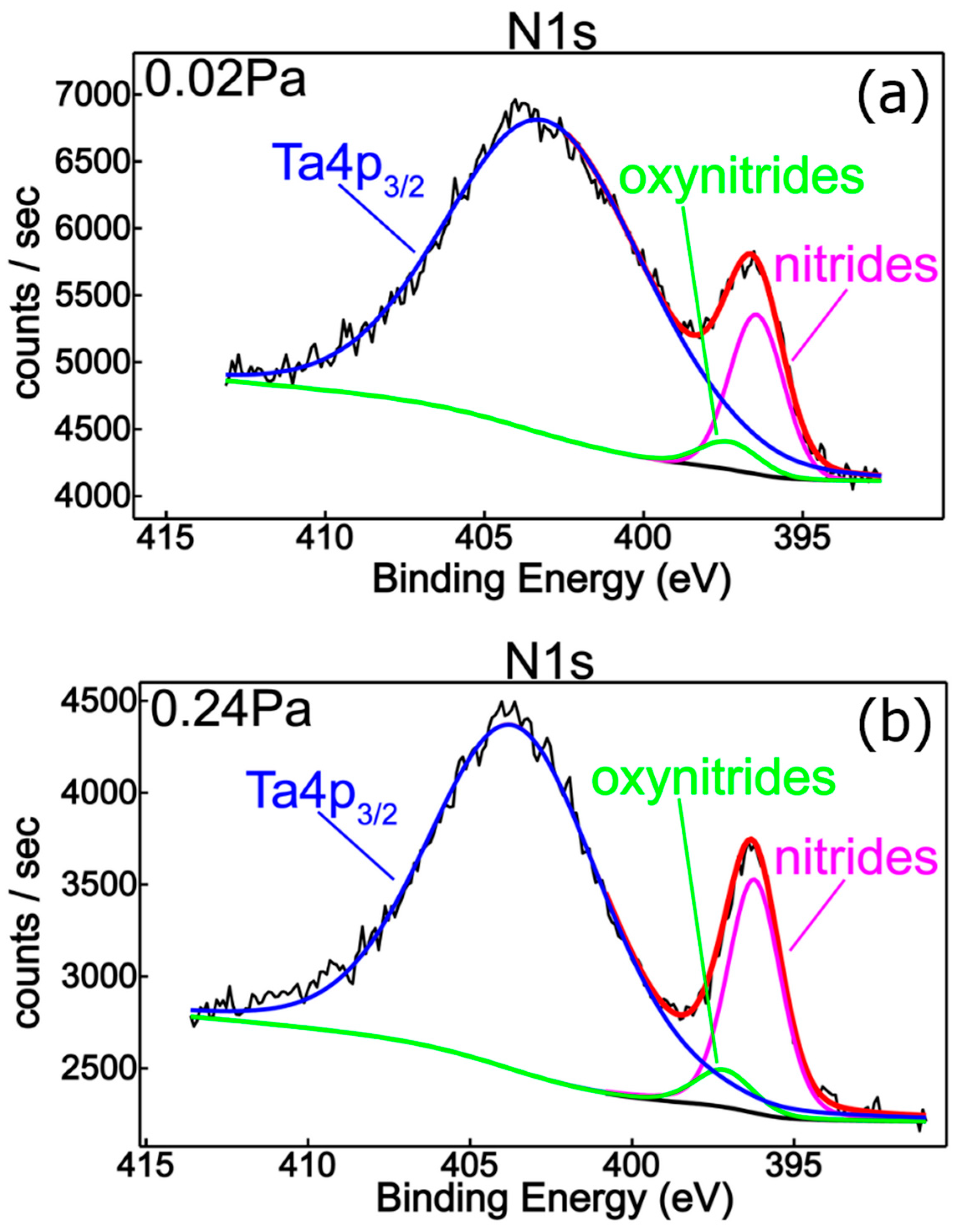
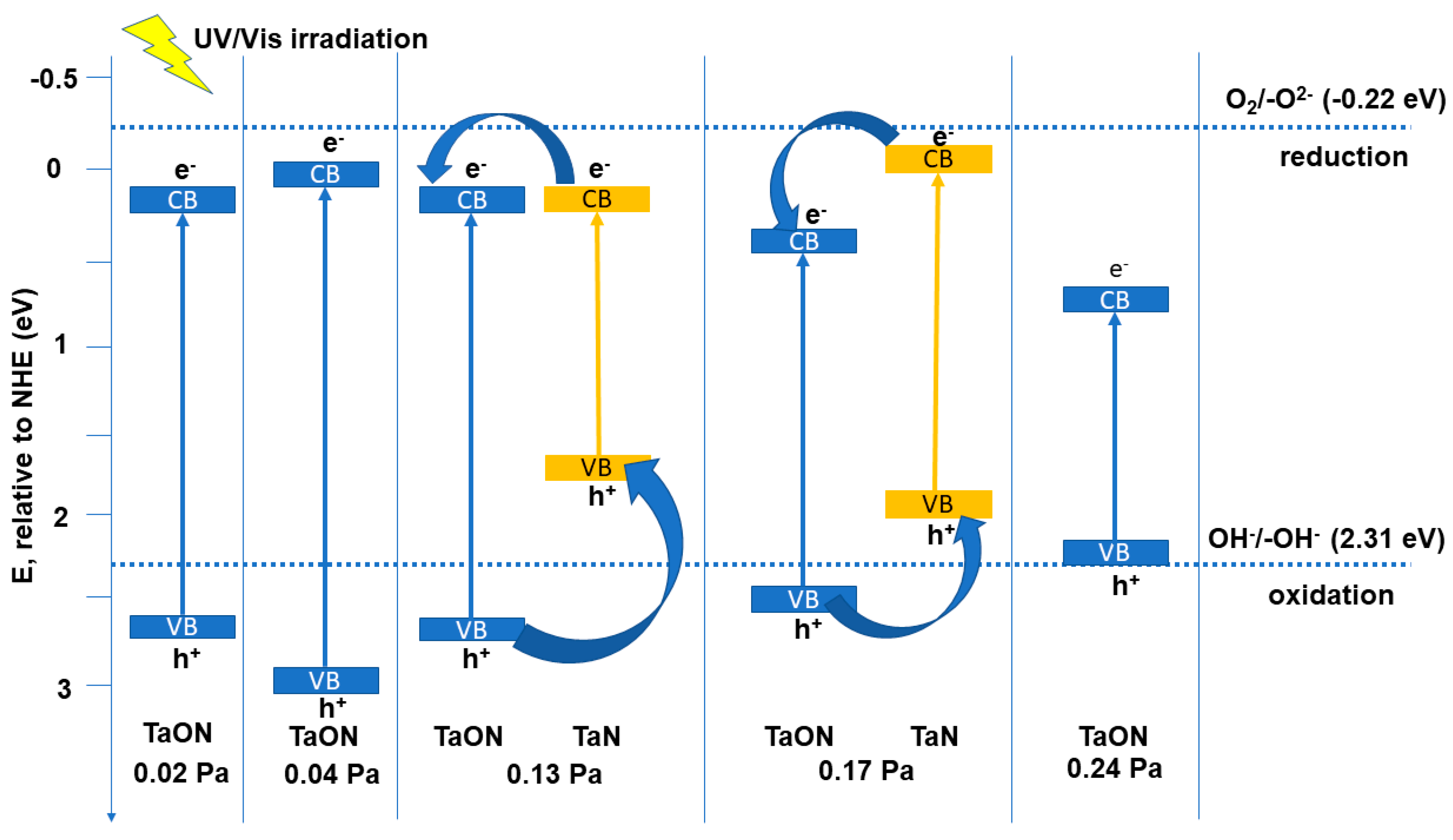
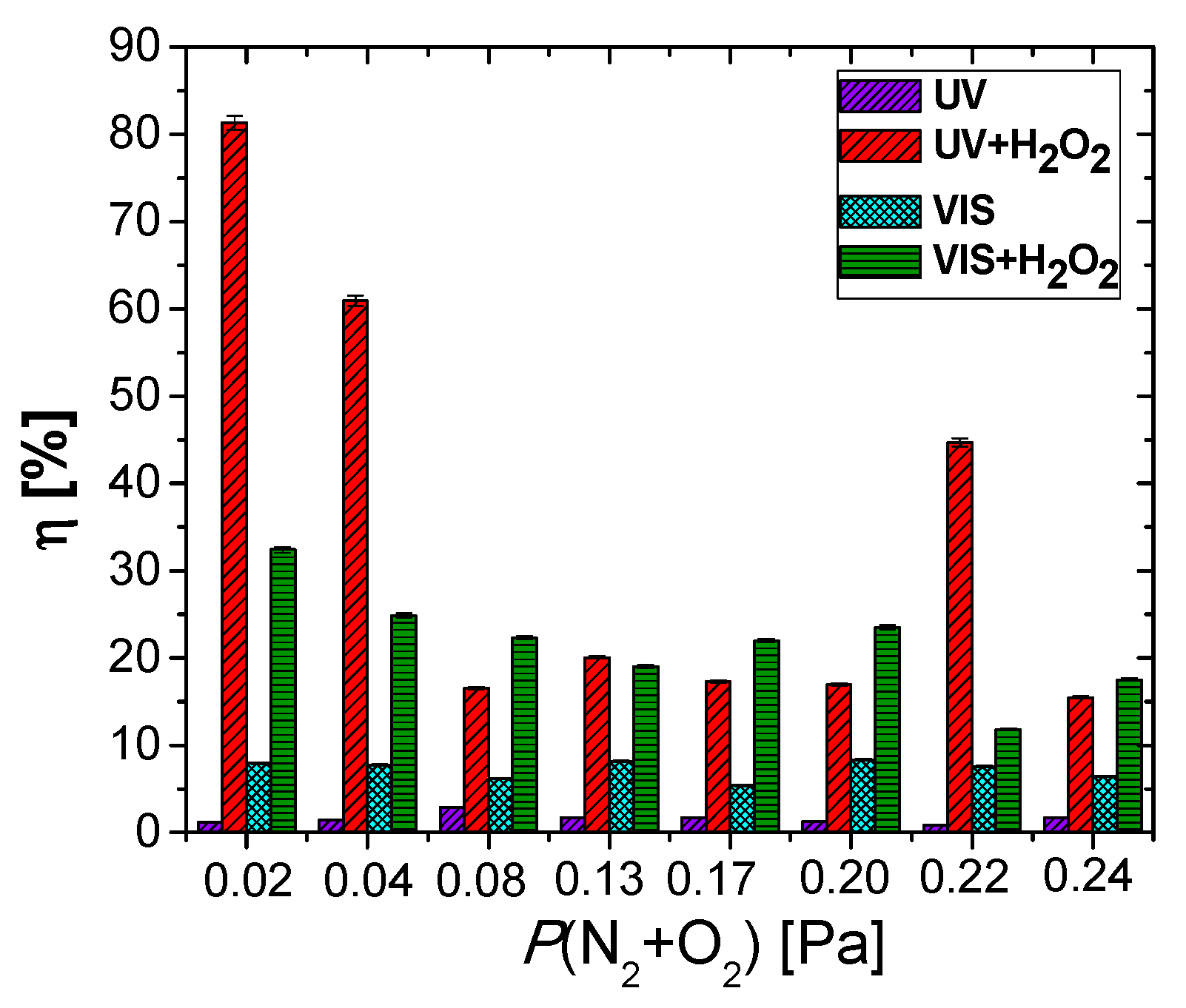
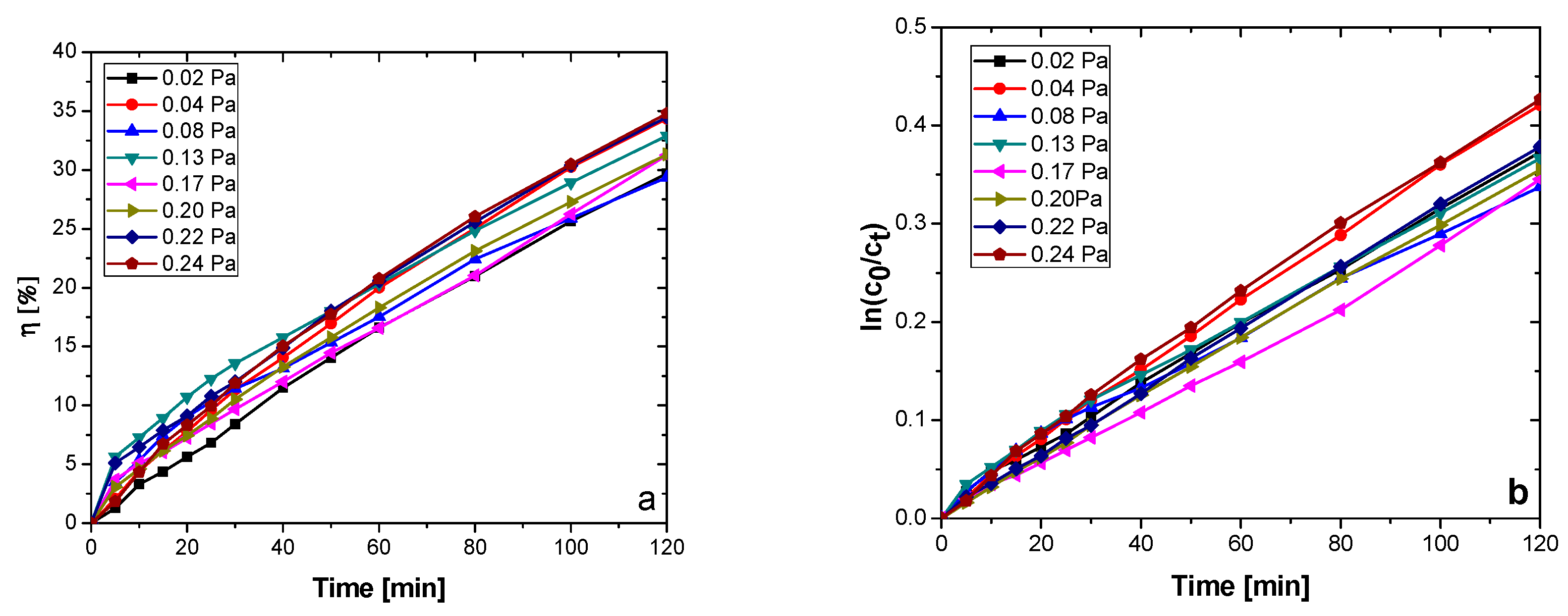
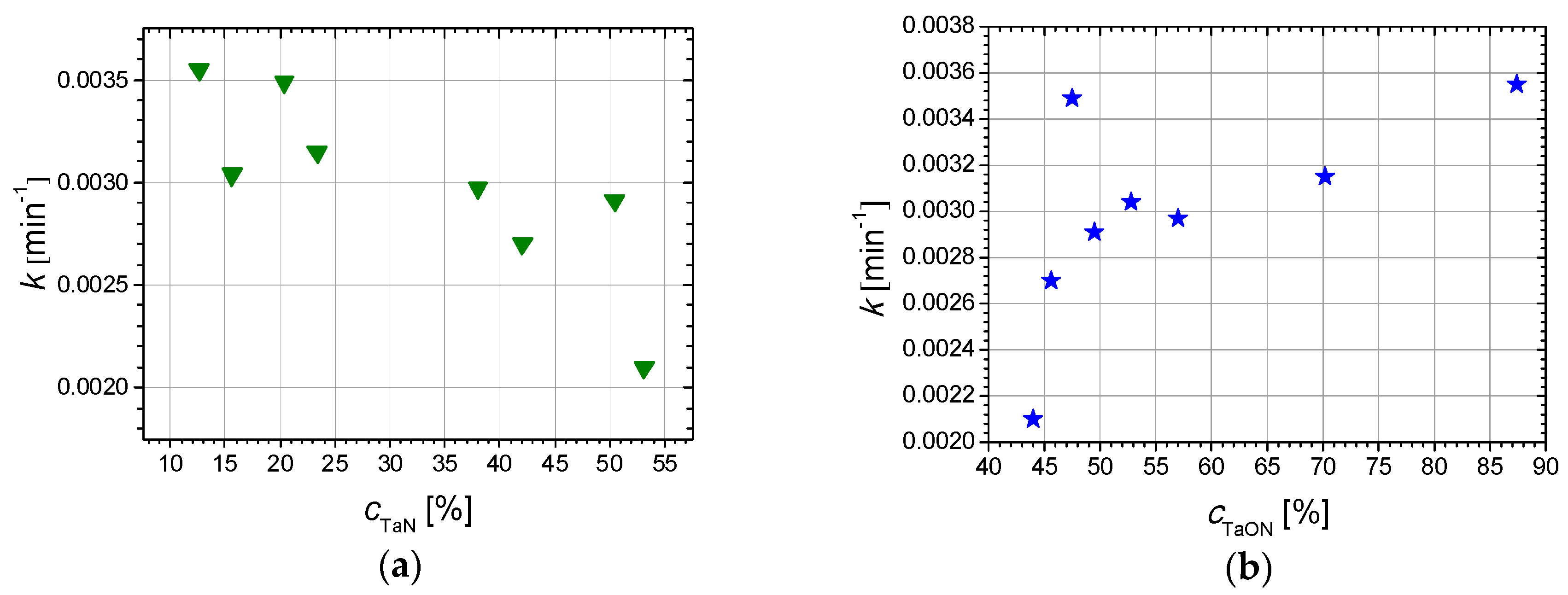

| Sample | Reactive Gas Flow (sccm) | Partial Pressure N2 + O2 (Pa) | Sputtering Pressure (Pa) |
|---|---|---|---|
| B2 | 2.5 | 0.02 | 0.41 |
| B3 | 5 | 0.04 | 0.43 |
| B4 | 10 | 0.08 | 0.47 |
| B5 | 15 | 0.13 | 0.50 |
| B6 | 20 | 0.17 | 0.54 |
| B7 | 22.5 | 0.20 | 0.57 |
| B8 | 25 | 0.22 | 0.59 |
| B9 | 30 | 0.24 | 0.64 |
| Sample | n | R2 | Eg (eV) | EU (meV) | Edge Potentials | ||
|---|---|---|---|---|---|---|---|
| EVB (eV) | ECB (eV) | ||||||
| 0.02 Pa | 1.863 | 0.996 | 2.265 | TaOxNy | 147.43 | 2.61 | 0.35 |
| 0.04 Pa | 1.947 | 0.993 | 2.665 | TaOxNy | 175.67 | 2.81 | 0.15 |
| 0.08 Pa | 1.817 | 0.964 | 2.508 | TaOxNy | 259.47 | 2.73 | 0.22 |
| 0.13 Pa | 1.913 | 0.979 | 2.411 | TaOxNy | 182.19 | 2.68 | 0.27 |
| 1.921 | 0.962 | 1.429 | TaN | 1.69 | 0.25 | ||
| 0.17 Pa | 1.828 | 0.983 | 1.979 | TaOxNy | 331.61 | 2.46 | 0.49 |
| 1.835 | 0.967 | 1.864 | TaN | 1.90 | -0.03 | ||
| 0.20 Pa | 2.008 | 0.997 | 1.956 | TaOxNy | 485.96 | 2.46 | 0.51 |
| 0.22 Pa | 1.996 | 0.984 | 1.951 | TaOxNy | 484.16 | 2.45 | 0.50 |
| 0.24 Pa | 0.501 | 0.970 | 1.436 | TaOxNy | 499.97 | 2.19 | 0.76 |
| MB Photodegradation Kinetic | Contact Angle Kinetic | ||||
|---|---|---|---|---|---|
| Sample | k (min−1) | R2 | θ0 (°) | kθ (min−1) | R2 |
| 0.02 Pa | 0.00304 | 0.998 | 84.18 | 0.0303 | 0.982 |
| 0.04 Pa | 0.00349 | 0.998 | 71.84 | 0.0185 | 0.977 |
| 0.08 Pa | 0.00270 | 0.991 | 70.77 | 0.0062 | 0.942 |
| 0.13 Pa | 0.00210 | 0.993 | 71.79 | 0.0054 | 0.972 |
| 0.17 Pa | 0.00291 | 0.998 | 67.52 | 0.0030 | 0.960 |
| 0.20 Pa | 0.00297 | 0.999 | 61.86 | 0.0032 | 0.879 |
| 0.22 Pa | 0.00315 | 0.999 | 61.33 | 0.0017 | 0.865 |
| 0.24 Pa | 0.00355 | 0.996 | 68.43 | 0.0101 | 0.990 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristea, D.; Cunha, L.; Gabor, C.; Ghiuta, I.; Croitoru, C.; Marin, A.; Velicu, L.; Besleaga, A.; Vasile, B. Tantalum Oxynitride Thin Films: Assessment of the Photocatalytic Efficiency and Antimicrobial Capacity. Nanomaterials 2019, 9, 476. https://doi.org/10.3390/nano9030476
Cristea D, Cunha L, Gabor C, Ghiuta I, Croitoru C, Marin A, Velicu L, Besleaga A, Vasile B. Tantalum Oxynitride Thin Films: Assessment of the Photocatalytic Efficiency and Antimicrobial Capacity. Nanomaterials. 2019; 9(3):476. https://doi.org/10.3390/nano9030476
Chicago/Turabian StyleCristea, Daniel, Luis Cunha, Camelia Gabor, Ioana Ghiuta, Catalin Croitoru, Alexandru Marin, Laura Velicu, Alexandra Besleaga, and Bogdan Vasile. 2019. "Tantalum Oxynitride Thin Films: Assessment of the Photocatalytic Efficiency and Antimicrobial Capacity" Nanomaterials 9, no. 3: 476. https://doi.org/10.3390/nano9030476
APA StyleCristea, D., Cunha, L., Gabor, C., Ghiuta, I., Croitoru, C., Marin, A., Velicu, L., Besleaga, A., & Vasile, B. (2019). Tantalum Oxynitride Thin Films: Assessment of the Photocatalytic Efficiency and Antimicrobial Capacity. Nanomaterials, 9(3), 476. https://doi.org/10.3390/nano9030476










