Comparative Study of Pleurotus ostreatus Mushroom Grown on Modified PAN Nanofiber Mats
Abstract
1. Introduction
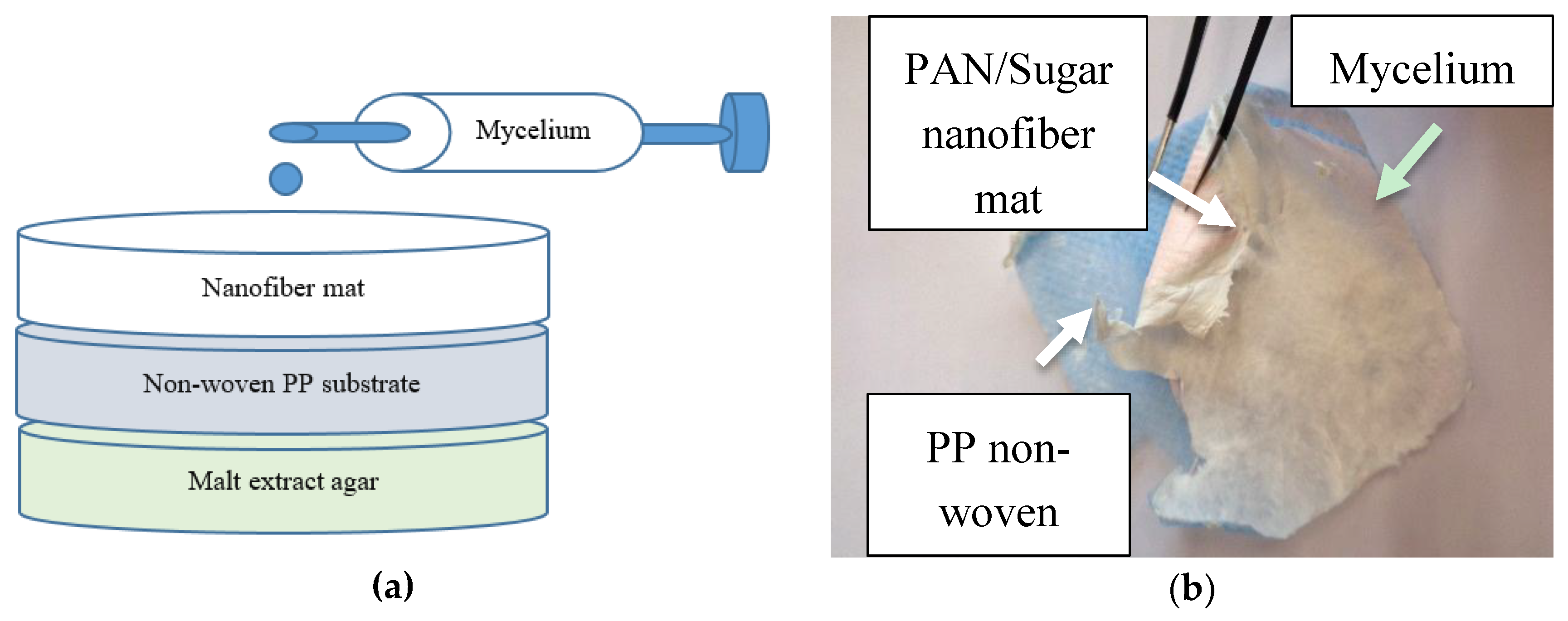
2. Materials and Methods
- (A)
- In a solution of 16% PAN in DMSO (18 g), 20 g saccharose (food grade, Pfeiffer & Langen GmbH & Co. KG, Cologne, Germany) were dissolved prior to electrospinning.
- (B)
- 11.6% PAN + 13% poloxamer “Lutrol F 68”, 7680-9510 Da, 2 × 40% hydrophilic parts, sol–gel transition temperature approx. 45 °C [46] (BASF, Ludwigshafen am Rhein, Germany).
- (C)
- 16% PAN in DMSO, stabilized at 280 °C for 1 h after electrospinning, heating rate 1 K/min, in a B150 muffle furnace (Nabertherm, Lilienthal, Germany).
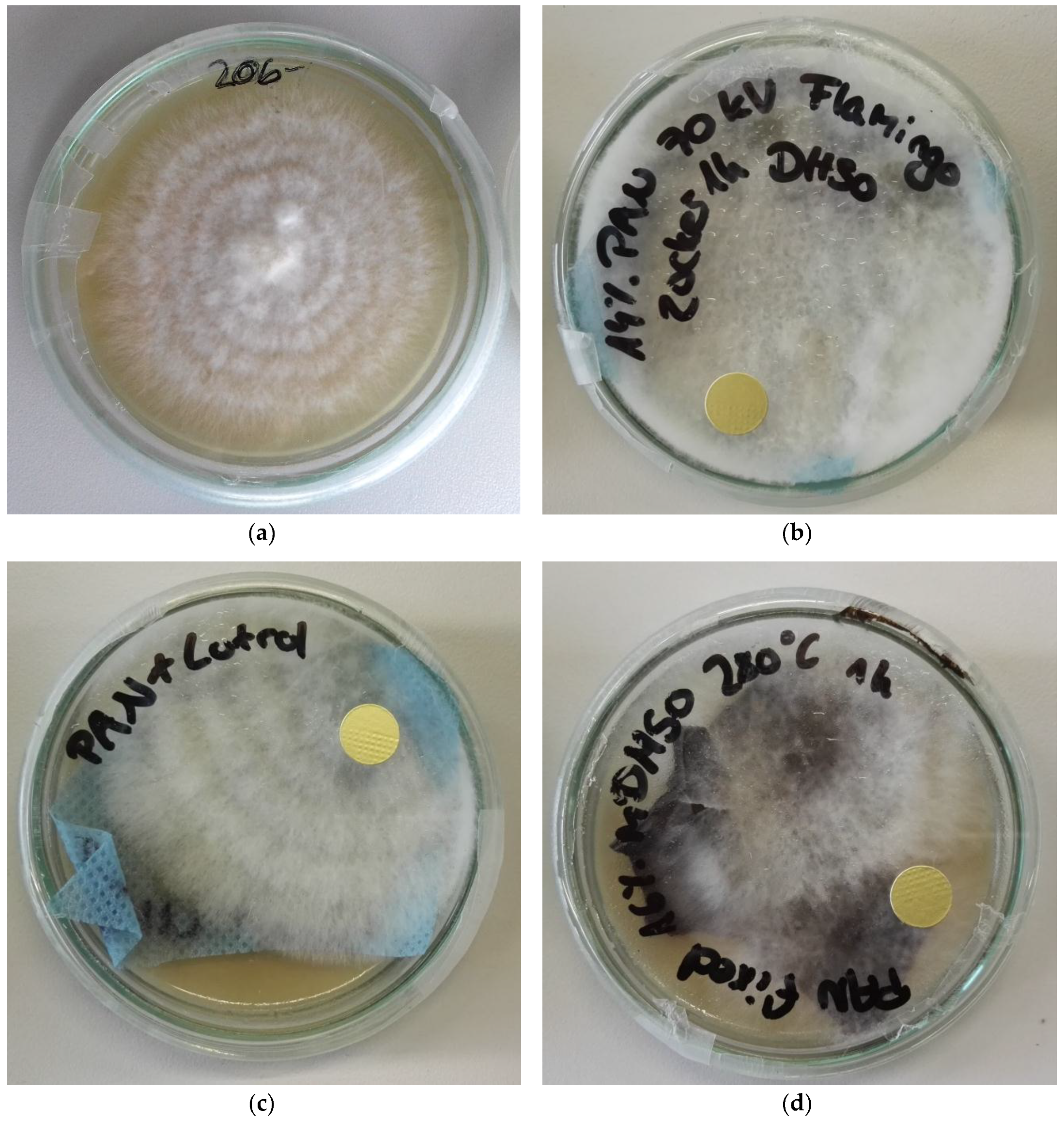
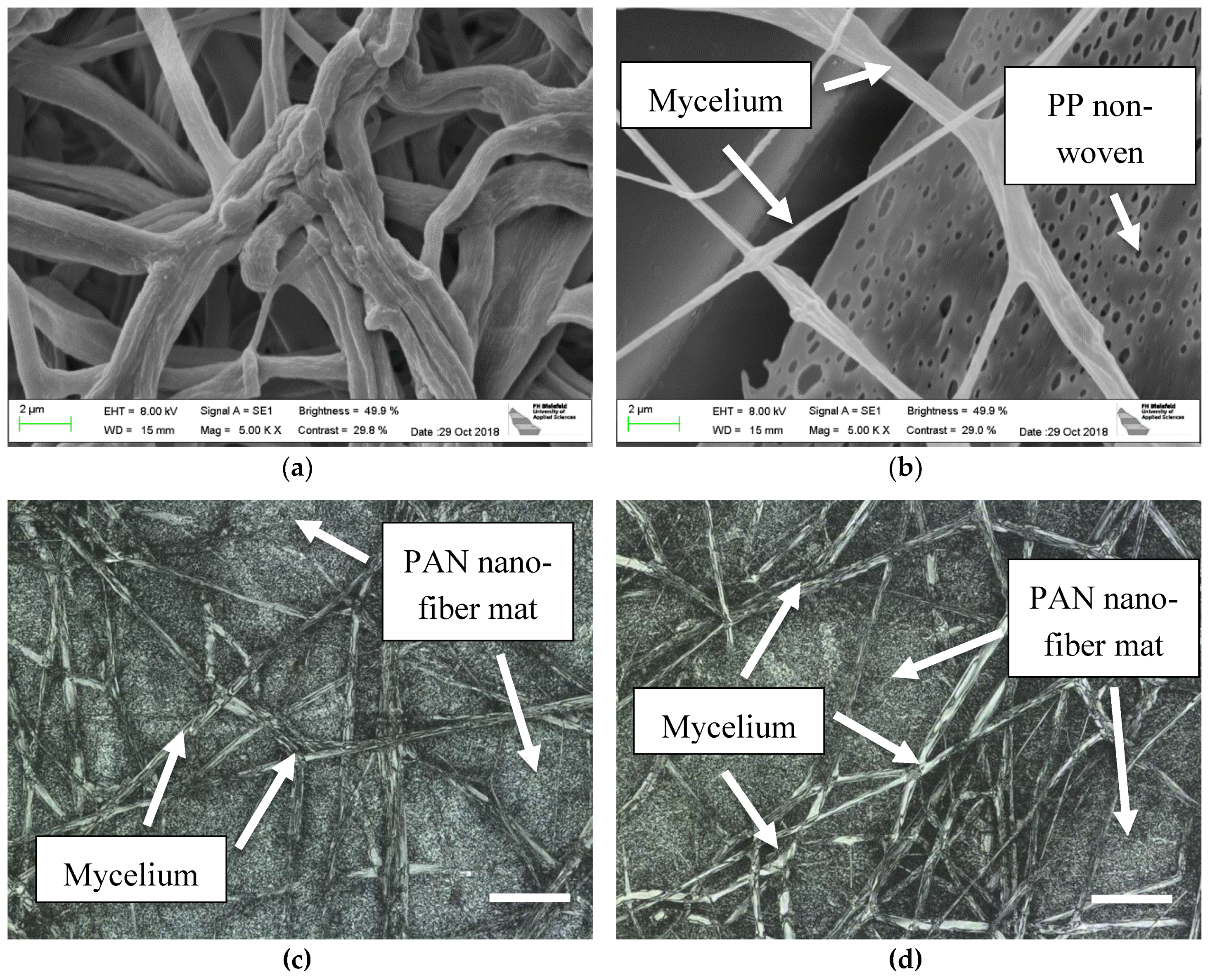
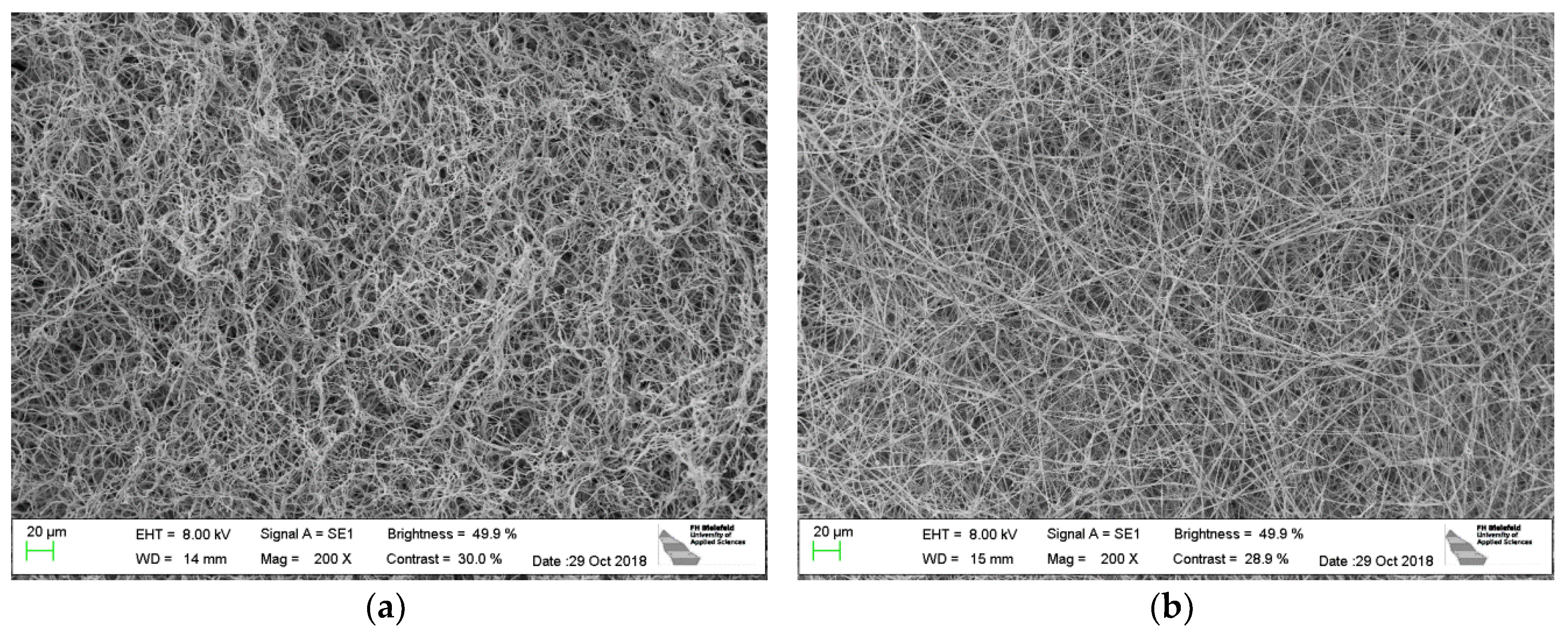
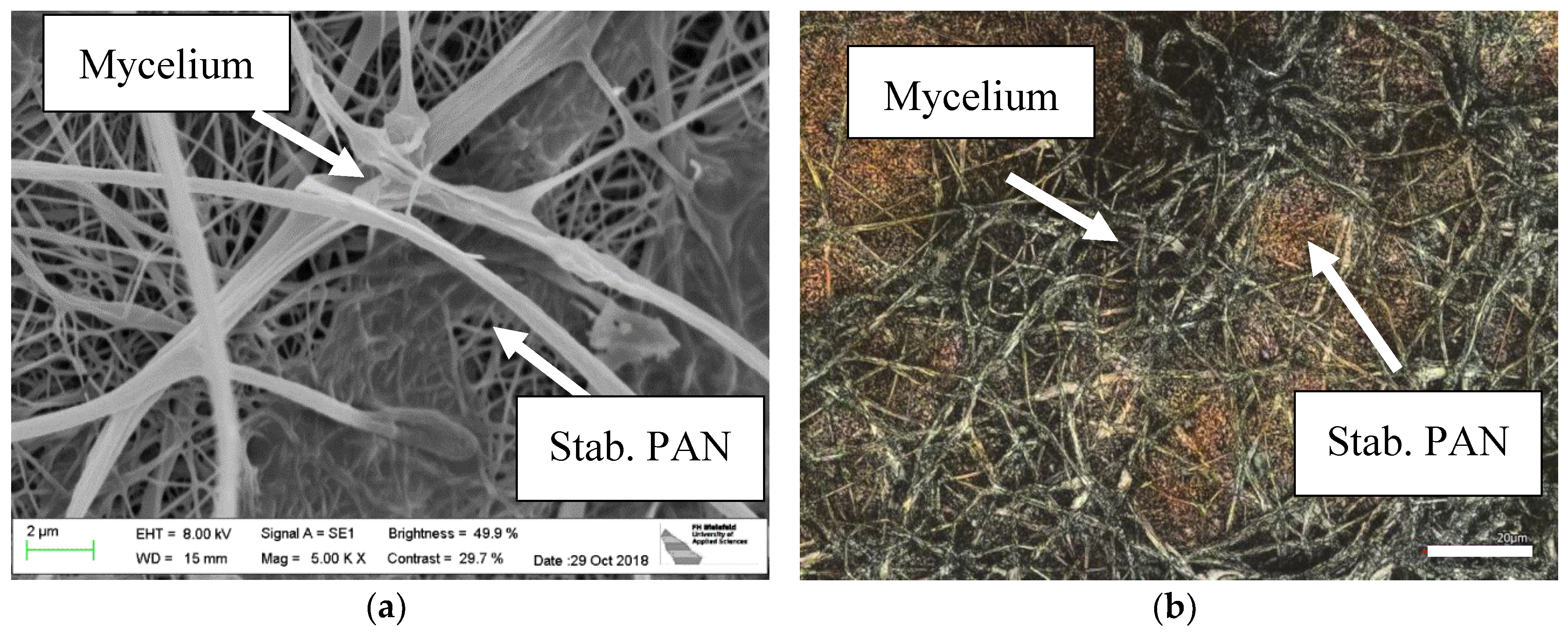
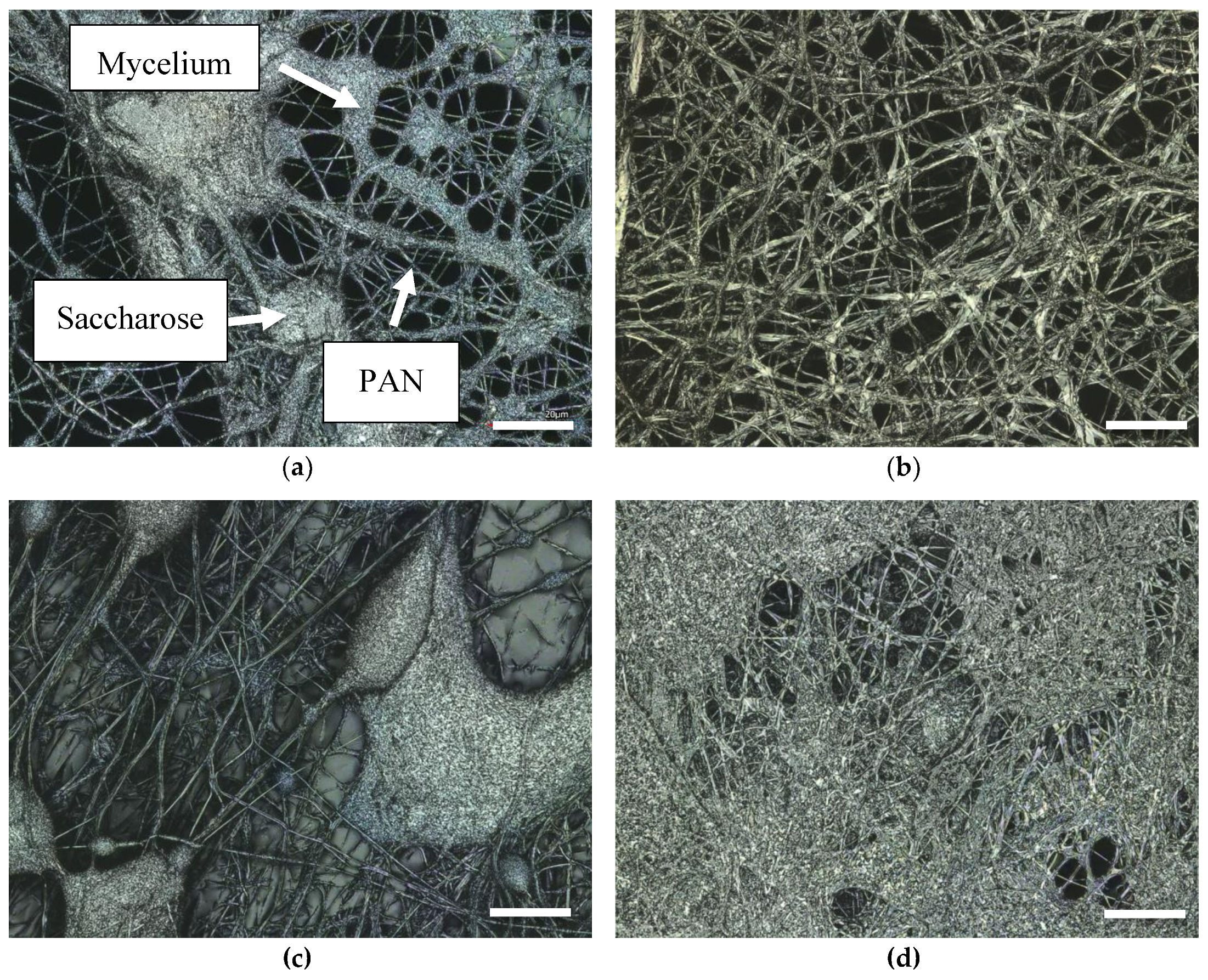
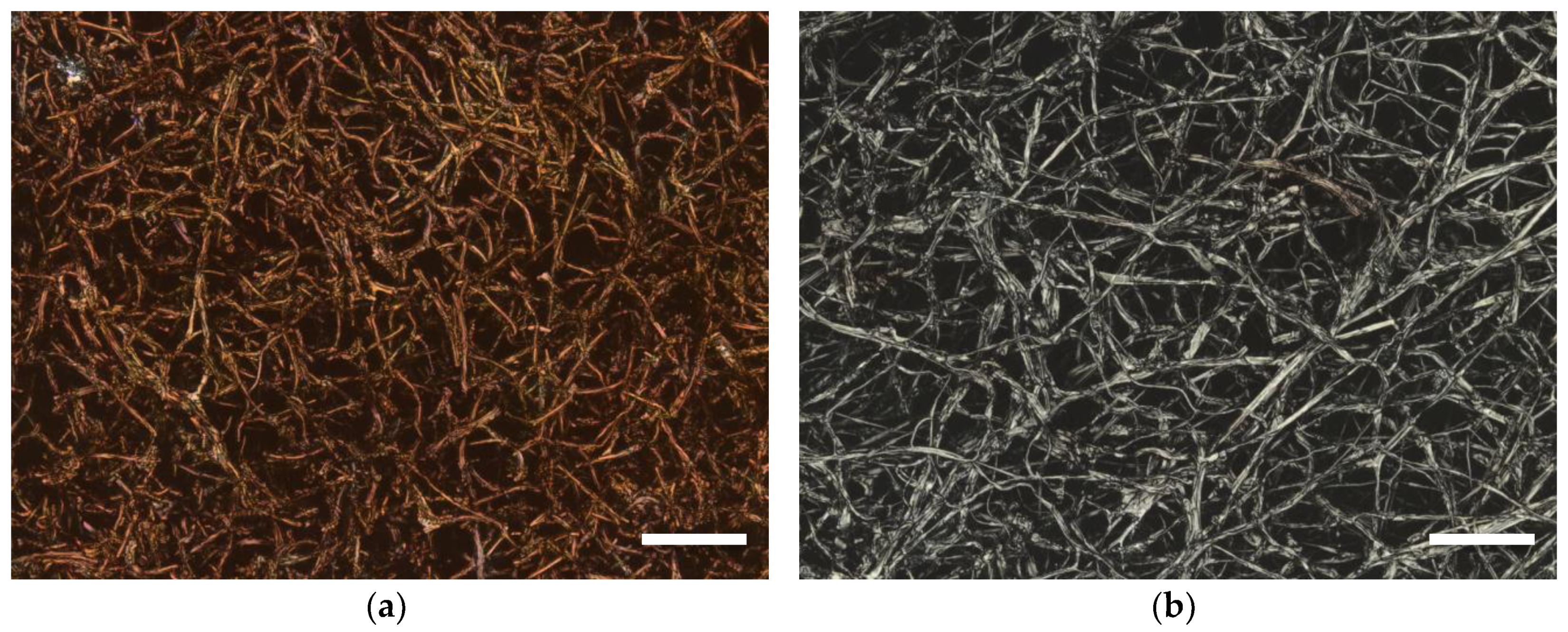
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adeli-Sardou, M.; Yaghoobi, M.M.; Torkzadeh-Mahani, M.; Dodel, M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nanofibers for skin tissue regeneration. Int. J. Biol. Macromol. 2019, 124, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Ki, G.H. Anisotropically Aligned Cell-Laden Nanofibrous Bundle Fabricated via Cell Electrospinning to Regenerate Skeletal Muscle Tissue. Small 2018, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Erdem, R.; Yuksek, M.; Sancak, E.; Atak, O.; Erginer, M.; Kabasakal, L.; Beyit, A. Electrospinning of single and multilayered scaffolds for tissue engineering applications. J. Text. Inst. 2017, 108, 935–946. [Google Scholar] [CrossRef]
- Klinkhammer, K.; Seiler, N.; Grafahrend, D.; Gerardo-Nava, J.; Mey, J.; Brook, G.A.; Möller, M.; Dalton, P.D.; Klee, D. Deposition of Electrospun Fibers on Reactive Substrates for In Vitro Investigations. Tissue Eng. Part C-Methods 2009, 15, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Mashayekhan, S.; Baniasadi, H.; Ramazani, A.; Ansarizadeh, M. Design and fabrication of conductive nanofibrous scaffolds for neural tissue engineering: Process modeling via response surface methodology. J. Biomater. Appl. 2018, 33, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Jahani, H.; Jalilian, F.A.; Wu, C.Y.; Kaviani, S.; Soleimani, M.; Abassi, N.; Lou, K.L.; Hosseinkhani, H. Controlled surface morphology and hydrophilicity of polycaprolactone towards selective differentiation of mesenchymal stem cells to neural like cells. J. Biomed. Mater. Res. A 2015, 103, 1875–1881. [Google Scholar] [CrossRef]
- Dalton, P.D.; Klinkhammer, K.; Salber, J.; Klee, D.; Möller, M. Direct in Vitro Electrospinning with Polymer Melts. Biomacromolecules 2006, 7, 686–690. [Google Scholar] [CrossRef]
- Roche, R.; Yalcinkaya, F. Electrospun Polyacrylonitrile Nanofibrous Membranes for Point-of-Use Water and Air Cleaning. ChemistryOpen 2019, 8, 97–103. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, M.J.; Zhang, H.N.; Quan, Z.Z.; Wang, R.W.; Yin, X.H. Sandwich-structures fibrous membranes with low filtration resistance for effective PM2.5 capture via one-step needleless electrospinning. Mater. Res. Express 2019, 6, 035027. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fiber. Fabr. 2019, 14, 1558925018824901. [Google Scholar] [CrossRef]
- Luu, Y.K.; Kim, K.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Subbiah, B.T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Ramakrishna, C.S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Cui, D.W.; Zhou, S.; Li, X.; Weng, J. Drug-loaded biodegradable polymeric nanofibers prepared by electrospinning. Tissue Eng. 2006, 12, 1070. [Google Scholar]
- Barnes, E.C.P.; Sell, S.A.; Knapp, D.C.; Walpoth, B.H.; Brand, D.D.; Bowlin, G.L. Preliminary investigation of electrospun collagen and polydioxanone for vascular tissue engineering applications. Int. J. Electrospun Nanofiber Appl. 2007, 1, 73–87. [Google Scholar]
- Welle, F.A.; Kroger, M.; Doring, M.; Niederer, K.; Pindel, E.; Chronakis, S. Electrospun aliphatic polycarbonates as tailored tissue scaffold materials. Biomaterials 2007, 28, 2211–2219. [Google Scholar] [CrossRef]
- Mei, L.; Ren, Y.M.; Gu, Y.C.; Li, X.L.; Wang, C.; Du, Y.; Fan, R.R.; Gao, X.; Chen, H.F.; Tong, A.P.; et al. Strengthened and Thermally Resistant Poly(lactic acid)-Based Composite Nanofibers Prepared via Easy Stereocomplexation with Antibacterial Effects. ACS Appl. Mater. Inter. 2018, 10, 42992–43002. [Google Scholar] [CrossRef]
- He, J.J.; Wang, W.; Shi, R.S.; Zhang, W.J.; Yang, X.; Shi, W.X.; Cui, F.Y. High speed water purification and efficient phosphate rejection by active nanofibrous membrane for microbial contamination and regrowth control. Chem. Eng. J. 2018, 337, 428–435. [Google Scholar] [CrossRef]
- Wirth, E.; Sabantina, L.; Weber, M.O.; Finsterbusch, K.; Ehrmann, A. Preliminary Study of Ultrasonic Welding as a Joining Process for Electrospun Nanofiber Mats. Nanomaterials 2018, 8, 746. [Google Scholar] [CrossRef]
- Lv, W.Y.; Mei, Q.Q.; Xiao, J.L.; Du, M.; Zheng, Q. 3D Multiscale Superhydrophilic Sponges with Delicately Designed Pore Size for Ultrafast Oil/Water Separation. Adv. Funct. Mater. 2017, 27, 1704293. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Mazinani, S.; Heydari, V. Electrospinning of zein/propolis nanofibers; antimicrobial properties and morphology investigation. J. Mater. Sci. 2018, 29, 165. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Kim, E.H.; Kim, S.H.; Kim, Y.H.; Oh, W.; Lee, J.T.; Jang, Y.A.; Sabina, Y.; Ji, B.C.; Yeum, J.H. Electrospinning Fabrication of Poly(vinyl alcohol)/Coptis chinensis Extract Nanofibers for Antimicrobial Exploits. Nanomaterials 2018, 8, 734. [Google Scholar] [CrossRef]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun Fiber Pads of Cellulose Acetate and Essential Oils with Antimicrobial Activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef]
- Buchholz, V.; Molnar, M.; Wang, H.; Reich, S.; Agarwal, S.; Fischer, M.; Greiner, A. Protection of Vine Plants against Esca Disease by Breathable Electrospun Antifungal Nonwovens. Macromol. Biosci. 2016, 16, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Quiros, J.; Gonzalo, S.; Jalvo, B.; Boltes, K.; Perdigon-Melon, J.A.; Rosal, R. Electrospun cellulose acetate composites containing supported metal nanoparticles for antifungal membranes. Sci. Total Environ. 2016, 563, 912–920. [Google Scholar] [CrossRef]
- Ohkawa, K.; Kim, H.; Lee, K. Biodegradation of electrospun poly(epsilon-caprolactone) non-woven fabrics by pure-cultured soil filamentous fungi. J. Polym. Environ. 2004, 12, 211–218. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Maydenov, M.; Kuzmanova, J.; Rashkov, I. Electrospun biohybrid materials for plant biocontrol containing chitosan and Trichoderma viride spores. J. Bioact. Compat. Polym. 2011, 26, 48–55. [Google Scholar] [CrossRef]
- Parveen, A. Effect of Electrospun Nanofibers on Growth Behavior of Fungal Cells. Ph.D. Thesis, North Carolina A&T State University, Greensboro, CA, USA, 2018. [Google Scholar]
- Kortei, N.K.; Odamtten, G.T.; Obodai, M.; Wiafe-Kwagyan, M.; Dzomeku, M. Comparative bioconversion of gamma irradiated and steam sterilized “wawa“ sawdust (Triplochiton scleroxylon L.) by mycelia of oyster mushrooms (Pleurotus ostreatus Jadq. Ex. Fr. Kummer). Int. Food Res. J. 2018, 25, 943–950. [Google Scholar]
- Parola, S.; Chiodaroli, L.; Orlandi, V.; Vannini, C.; Panno, L. Lentinula edodes and Pleurotus ostreatus: Functional food with antioxidant antimicrobial activity and an important source of Vitamin D and medicinal compounds. Funct. Foods Health Dis. 2017, 7, 773–794. [Google Scholar] [CrossRef]
- Vieira, F.R.; Nogueira de Andrade, M.C. Optimization of substrate preparation for oyster mushroom (Pleurotus ostreatus) cultivation by studying different raw materials and substrate preparation conditions (composting: Phases I and II). World J. Microbiol. Biotechnol. 2016, 32, 190. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.H.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. High temperature induced disruption of the cell wall integrity and structure in Pleurotus ostreatusk mycelia. Appl. Microbiol. Biotechnol. 2018, 102, 6627–6636. [Google Scholar] [CrossRef] [PubMed]
- Sabantina, L.; Hes, L.; Mirasol, J.R.; Cordero, T.; Ehrmann, A. Water Vapor Permeability through PAN nanofiber Mat with Varying Membrane-Like Areas. Fibres Text. East. Eur. 2019, 27, 12–15. [Google Scholar] [CrossRef]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Polyacrylonitrile (PAN) nanofibers spun with copper nanoparticles: An anti-Escherichia coli membrane for water treatment. Appl. Microbiol. Biotechnol. 2018, 102, 7171–7181. [Google Scholar] [CrossRef] [PubMed]
- Pourbaghi, R.; Zarrebini, M.; Semnani, D.; Pourazar, A.; Akbari, N.; Shamsfar, R. Evaluation of polyacrylonitrile electrospun nano-fibrous mats as leukocyte removal filter media. J. Biomed. Mater. Res. B 2018, 106, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Natu, M.V.; de Sousa, H.C.; Gil, M.H. Electrospun Drug-Eluting Fibers for Biomedical Applications. Act. Implant Scaffold Tissue Eng. 2011, 8, 57–85. [Google Scholar]
- Pan, J.F.; Liu, N.H.; Sun, H.; Xu, F. Preparation and Characterization of Electrospun PLCL/Poloxamer Nanofibers and Dextran/Gelatin Hydrogels for Skin Tissue Engineering. PLoS ONE 2014, 9, e112885. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Y.; Liu, N.H.; Yang, X.R.; Feng, Z.H.; Yi, F.Z. Adiposed-derived stem cells seeded on PLCL/P123 electrospun nanofibrous scaffold enhance wound healing. Biomed. Mater. 2014, 9, 035012. [Google Scholar] [CrossRef]
- Böttjer, R.; Grothe, T.; Ehrmann, A. Functional Nanofiber Mats for Medical and Biotechnological Applications. In Narrow and Smart Textiles; Kyosev, Y., Mahltig, B., Schwarz-Pfeiffer, A., Eds.; Springer International Publishing: Basel, Switzerland, 2018. [Google Scholar]
- Sabantina, L.; Rodríguez-Cano, M.Á.; Klöcker, M.; García-Mateos, F.J.; Ternero-Hidalgo, J.J.; Mamun, A.; Beermann, F.; Schwakenberg, M.; Voigt, A.-L.; Rodríguez Mirasol, J.; et al. Fixing PAN nanofiber mats during stabilization for carbonization and creating novel metal/carbon composites. Polymers 2018, 10, 735. [Google Scholar] [CrossRef]
- Sabantina, L.; Mirasol, J.R.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Investigation of Needleless Electrospun PAN Nanofiber Mats. AIP Conf. Series 2018, 1952, 020085. [Google Scholar]
- Grothe, T.; Wehlage, D.; Böhm, T.; Remche, A.; Ehrmann, A. Needleless Electrospinning of PAN Nanofibre Mats. Tekstilec 2017, 60, 290–295. [Google Scholar] [CrossRef]
- Brown, V.K.; Robinson, J.; Stevenson, D.E. A note on the toxicity and solvent properties of dimethyl sulphoxide. J. Pharm. Pharmacol. 1963, 15, 688–692. [Google Scholar] [CrossRef]
- Kligman, A.M. Topical Pharmacology and Toxicology of Dimethyl Sulfoxide—Part 1. JAMA 1965, 193, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Wehlage, D.; Böttjer, R.; Grothe, T.; Ehrmann, A. Electrospinning water-soluble/insoluble polymer blends. AIMS Mater. Sci. 2018, 5, 190–200. [Google Scholar] [CrossRef]
- Khateb, K.A.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Ma, F.Y.; Zhang, X.Y.; Zhang, J.S. Carbohydrate changes during growth and fruiting in Pleurotus ostreatus. Fungal Biol. 2016, 120, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Hoa, H.T.; Wang, C.-L. The Effects of Temperature and Nutritional Conditions on Mycelium Growth of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.D.; Bebber, D.; Boddy, L. Mycelial networks: Structure and dynamics. In British Mycological Society Symposia Series; Boddy, L., Frankland, J.C., van West, P., Eds.; Academic Press: Cambridge, MA, USA, 2008; Volume 28, pp. 3–18. [Google Scholar]
- Sabantina, L.; Wehlage, D.; Klöcker, M.; Mamun, A.; Grothe, T.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Stabilization of electrospun PAN/gelatin nanofiber mats for carbonization. J. Nanomater. 2018, 2018, 6131085. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Y.; Yu, J.; Ji, H.; Liu, A. Characterization of Se-enriched Pleurotus ostreatus polysaccharides and their antioxidant effects in vitro. Int. J. Biol. Macromol. 2018, 111, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Dzulkefli, N.A.; Zainol, N. Data on modeling mycelium growth in Pleurotus sp. cultivation by using agricultural wastes via two level factorial analysis. Data Brief 2018, 20, 1710–1720. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Kupryashina, M.; Gorshkov, V.; Ageeva, M.; Gogolev, Y.; Nikitina, V. Alteration in the ultrastructural morphology of mycelial hyphae and the dynamics of transcriptional activity of lytic enzyme genes during basidiomycete morphogenesis. J. Microbiol. 2017, 55, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Mykchaylova, O.B.; Bisko, N.A.; Sukhomlyn, M.M.; Lomberg, M.L.; Pasaylyuk, M.V.; Petrichuk, Y.V.; Gryganskye, A.P. Biological peculiarities of a rare medicinal mushroom Fomitopsis officinalis (Fomitopsidaceae, Polyporales) on agar media and plant substrates. Regul. Mech. Biosyst. 2017, 8, 469–475. [Google Scholar]
- Bellou, S.; Makri, A.; Triantaphyllidou, I.E.; Papanikolaou, S.; Aggelis, G. Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 2014, 160, 807–817. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabantina, L.; Kinzel, F.; Hauser, T.; Többer, A.; Klöcker, M.; Döpke, C.; Böttjer, R.; Wehlage, D.; Rattenholl, A.; Ehrmann, A. Comparative Study of Pleurotus ostreatus Mushroom Grown on Modified PAN Nanofiber Mats. Nanomaterials 2019, 9, 475. https://doi.org/10.3390/nano9030475
Sabantina L, Kinzel F, Hauser T, Többer A, Klöcker M, Döpke C, Böttjer R, Wehlage D, Rattenholl A, Ehrmann A. Comparative Study of Pleurotus ostreatus Mushroom Grown on Modified PAN Nanofiber Mats. Nanomaterials. 2019; 9(3):475. https://doi.org/10.3390/nano9030475
Chicago/Turabian StyleSabantina, Lilia, Franziska Kinzel, Thomas Hauser, Astrid Többer, Michaela Klöcker, Christoph Döpke, Robin Böttjer, Daria Wehlage, Anke Rattenholl, and Andrea Ehrmann. 2019. "Comparative Study of Pleurotus ostreatus Mushroom Grown on Modified PAN Nanofiber Mats" Nanomaterials 9, no. 3: 475. https://doi.org/10.3390/nano9030475
APA StyleSabantina, L., Kinzel, F., Hauser, T., Többer, A., Klöcker, M., Döpke, C., Böttjer, R., Wehlage, D., Rattenholl, A., & Ehrmann, A. (2019). Comparative Study of Pleurotus ostreatus Mushroom Grown on Modified PAN Nanofiber Mats. Nanomaterials, 9(3), 475. https://doi.org/10.3390/nano9030475






