One-Step Hydrothermal Synthesis of Yellow and Green Emitting Silicon Quantum Dots with Synergistic Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of Silicon QDs
2.3. MTT Method
3. Results and Discussion
3.1. The Optimization of the Synthesis Environment for SiQDs
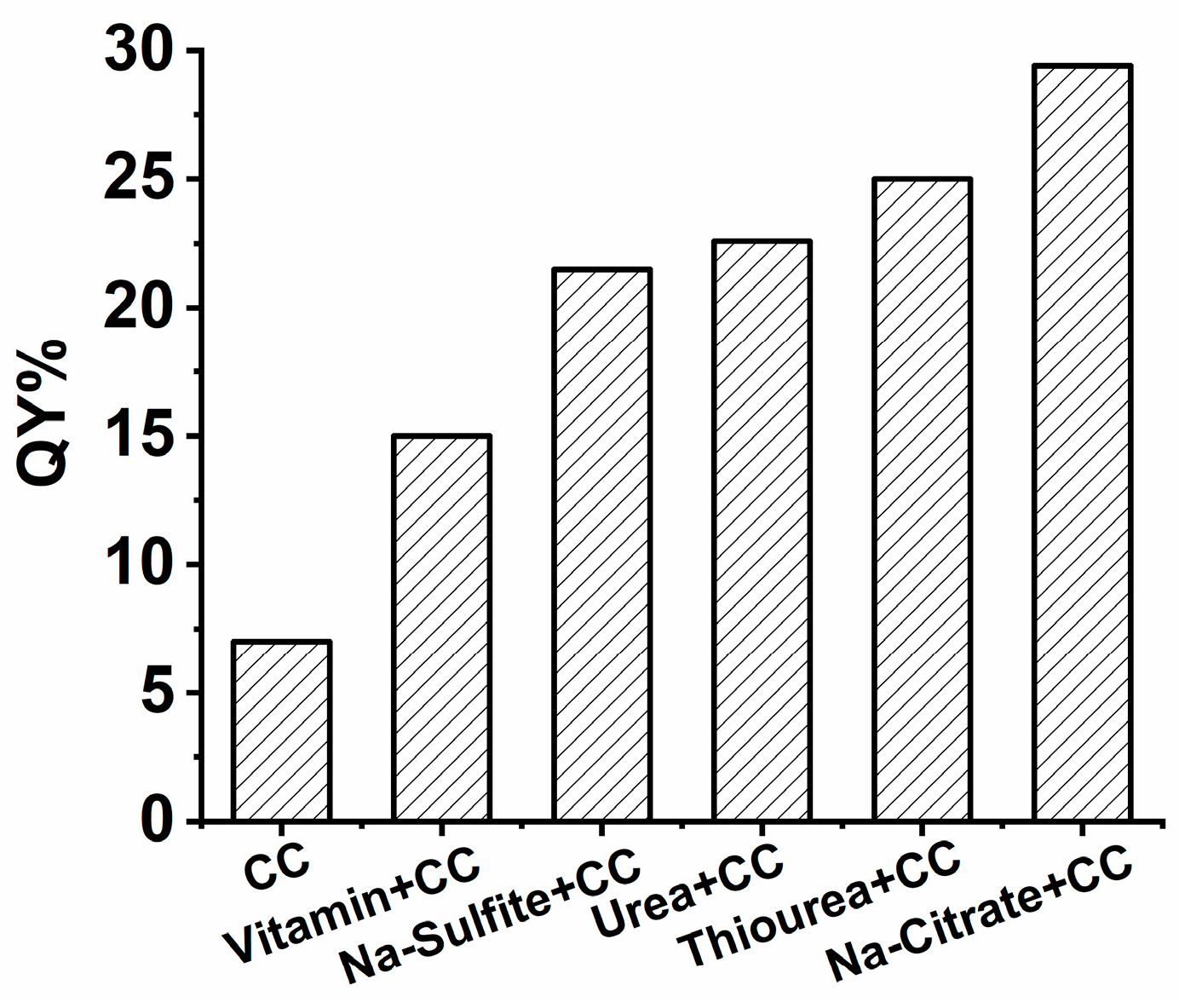
3.1.1. Filtration of the Proper Selection of Reducing Reagents for SiQDs Preparation
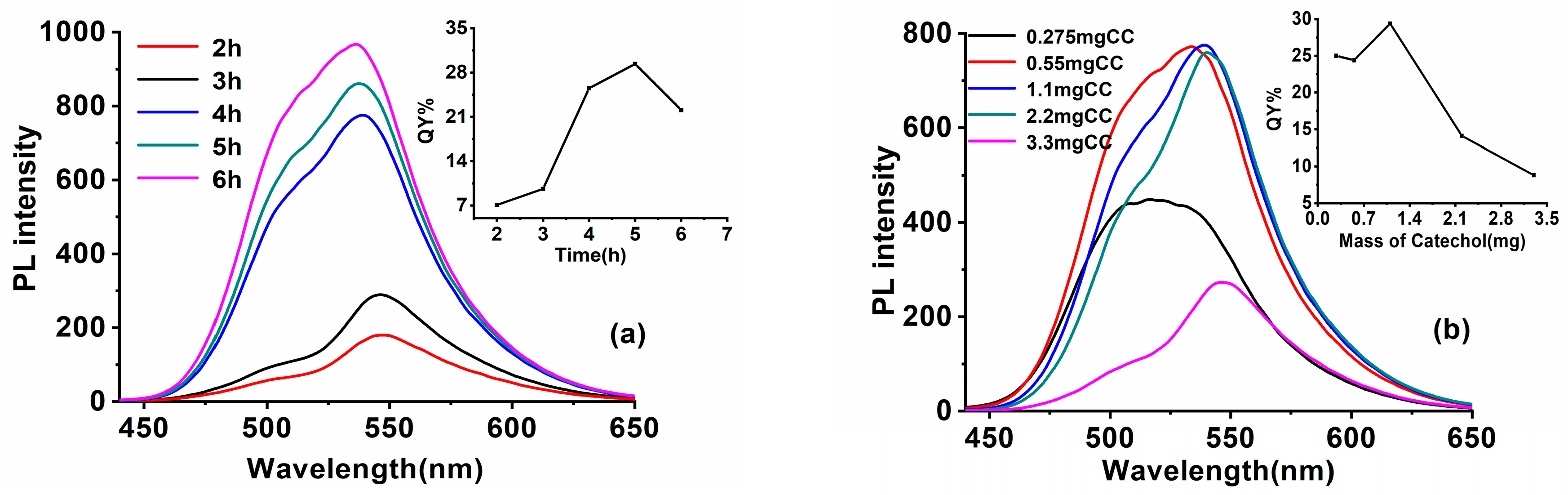
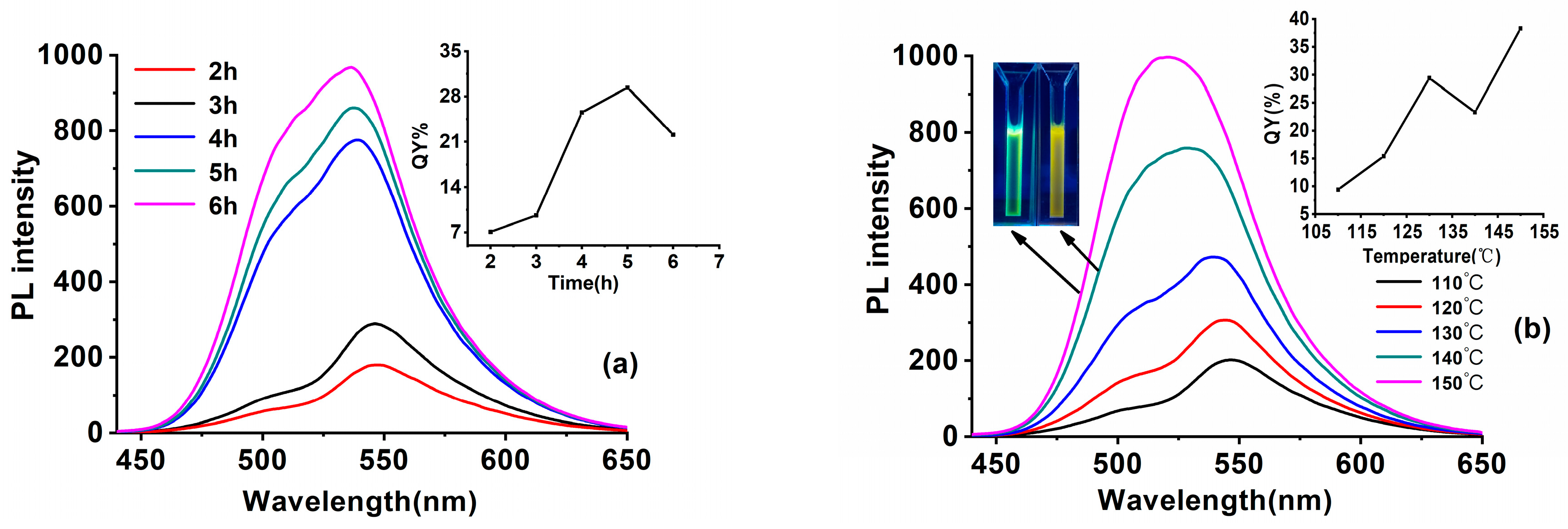
3.1.2. The Impact of Synthesis Parameters upon the Optical Properties of SiQDs
3.2. Characterization of SiQDs and Mechanism Discussion
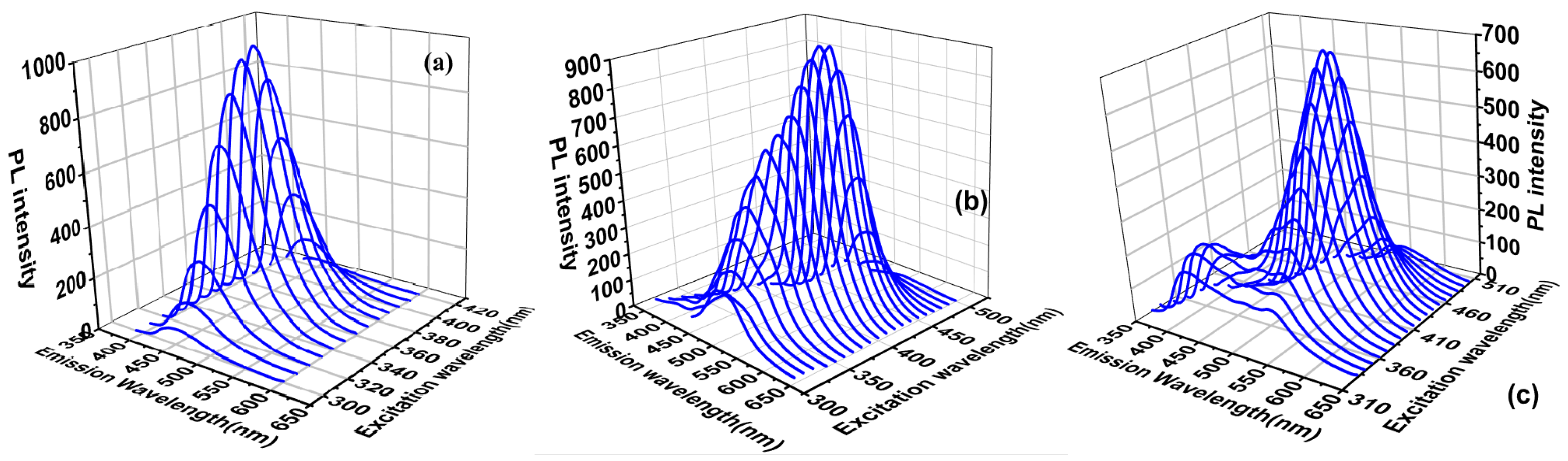
3.2.1. Three Dimensional Fluorescence Spectra
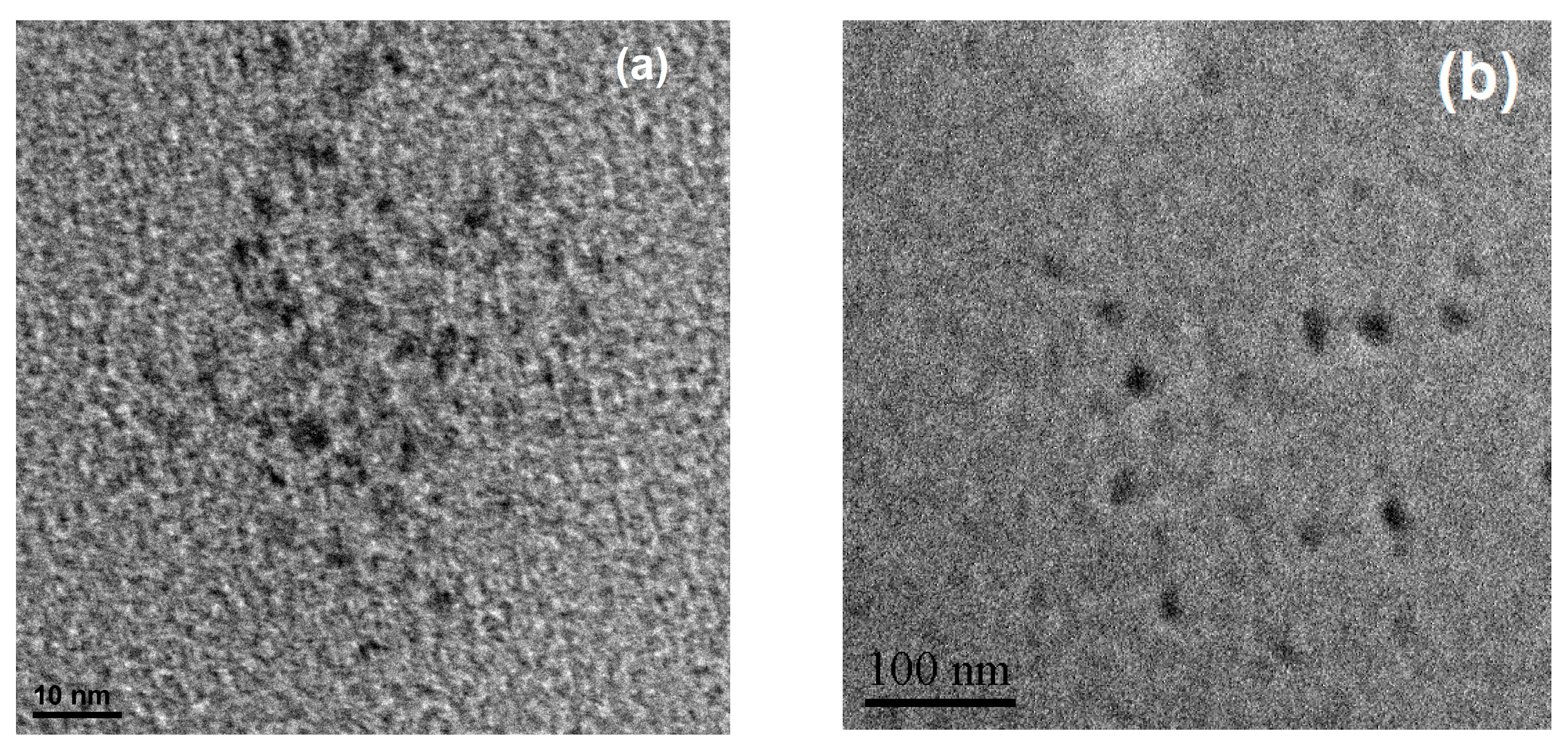
3.2.2. HRTEM Imaging
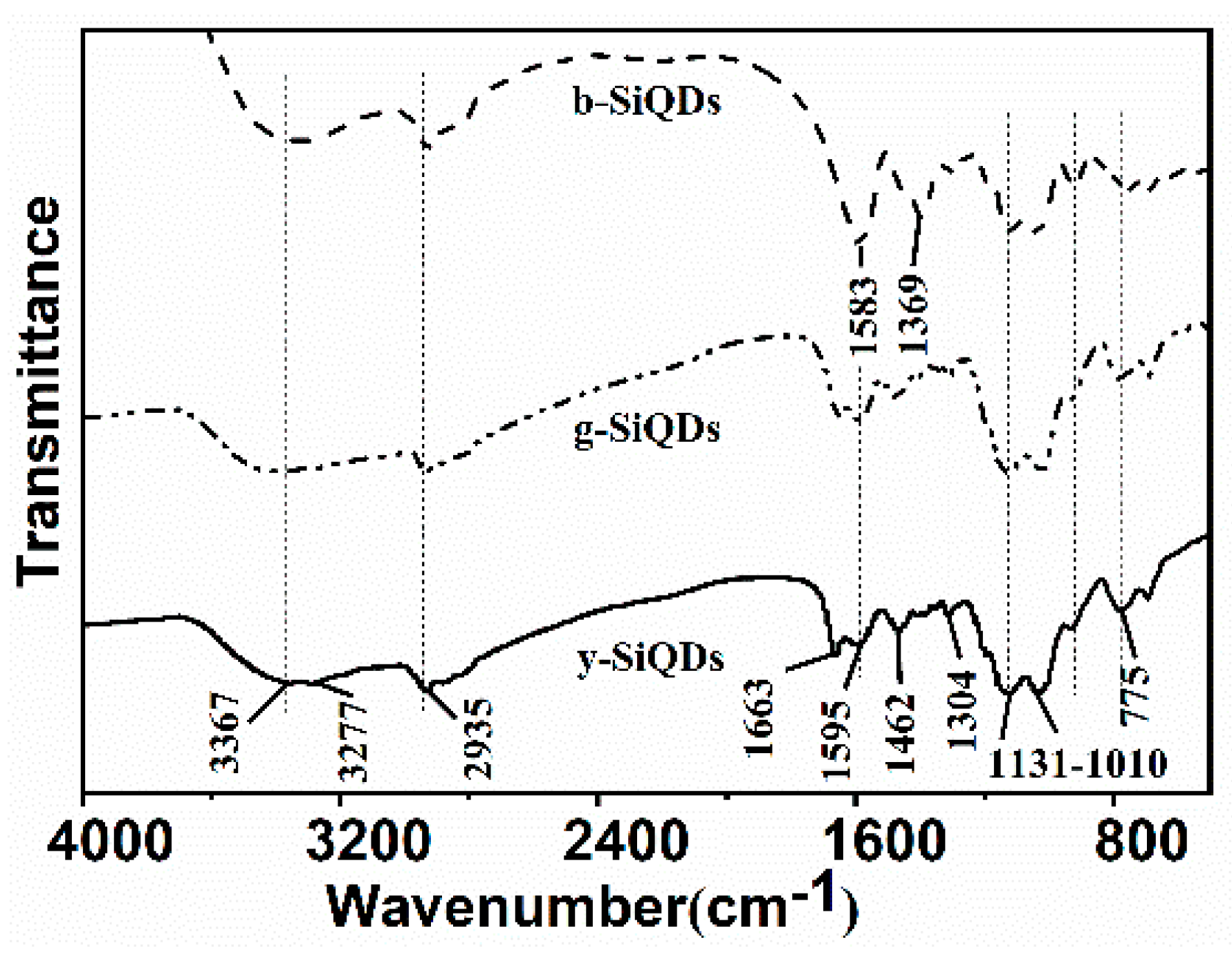
3.2.3. FT-IR Spectrometer
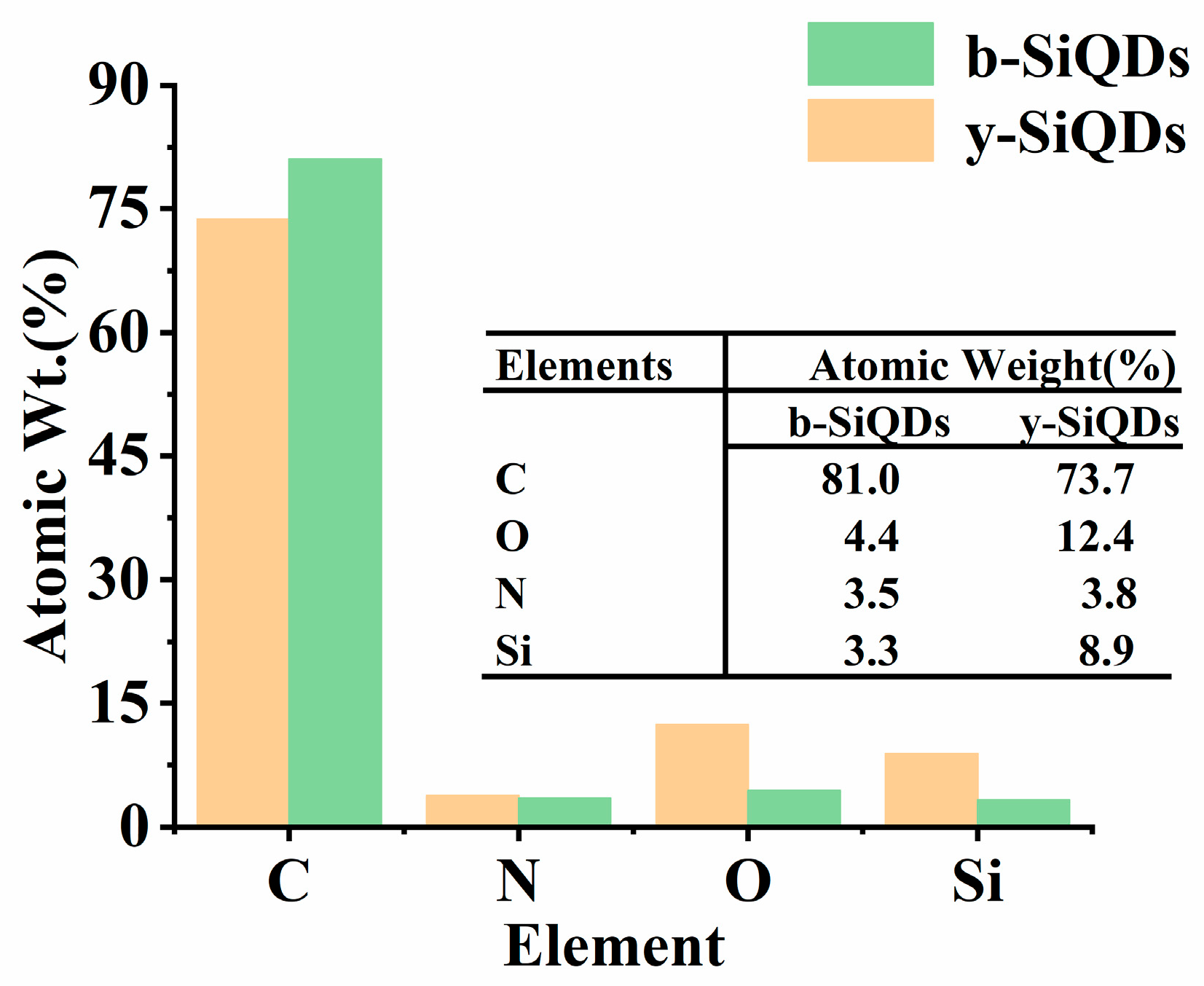
3.2.4. EDS Spectrum
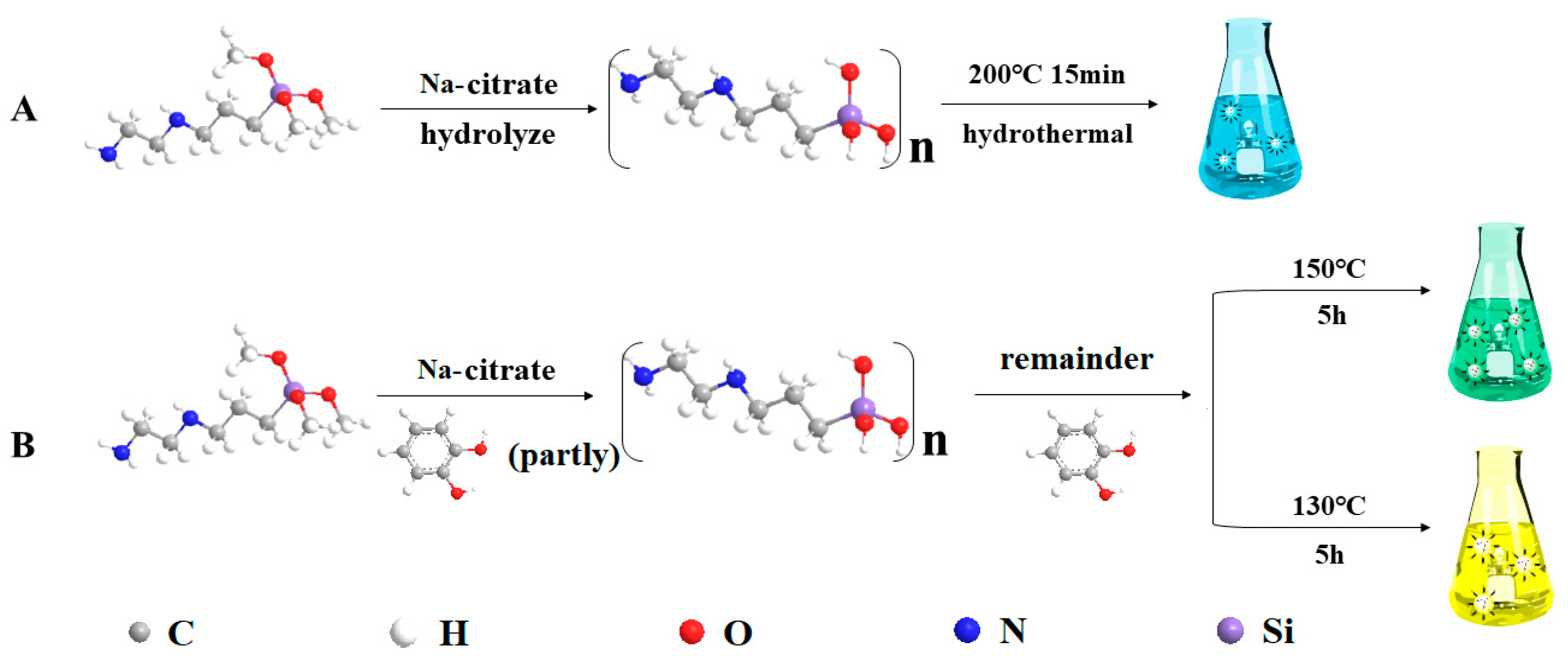
3.2.5. Study on the Synthesis Mechanism
3.3. Application of the Prepared SiQDs
3.3.1. Optical Stability of the SiQDs
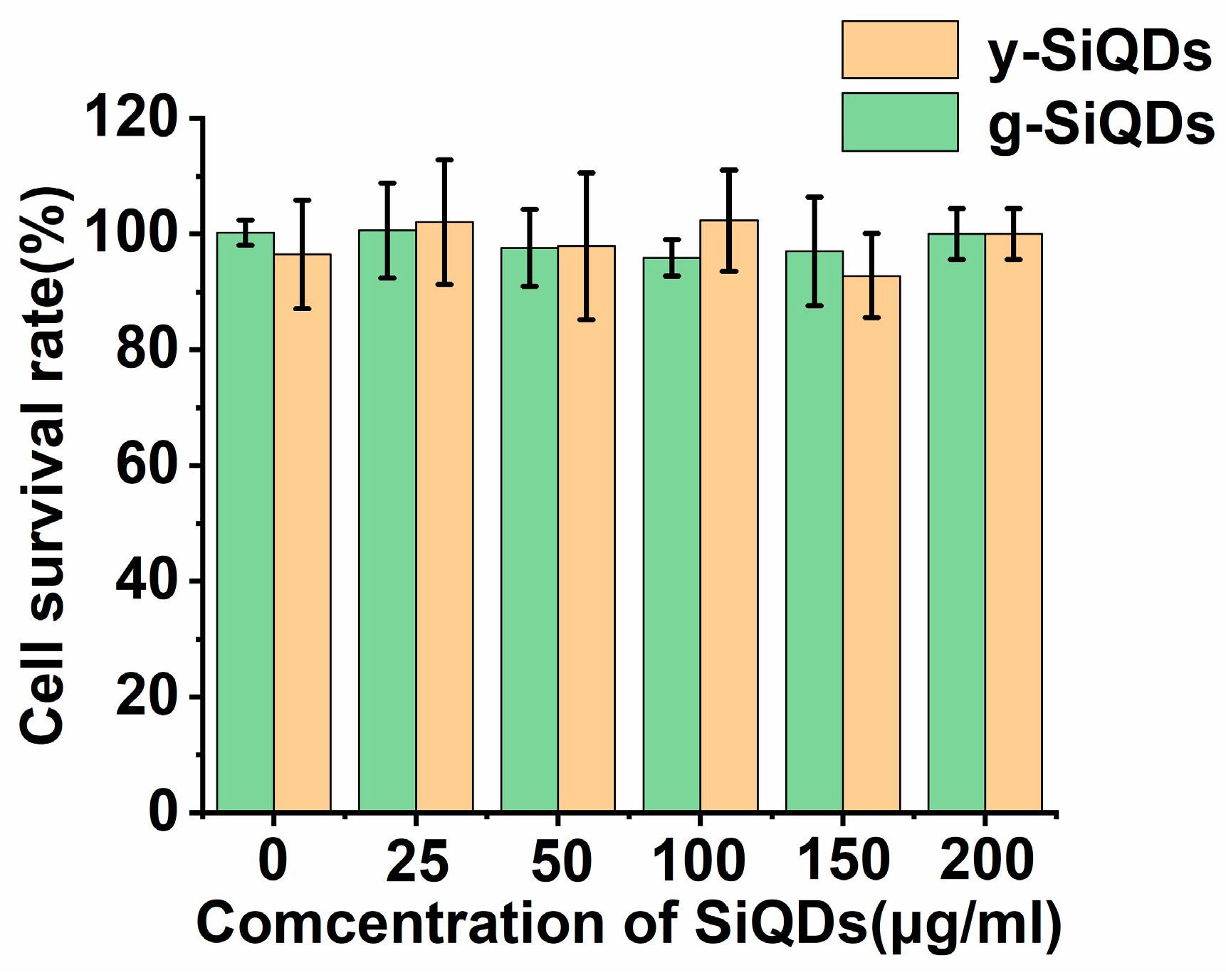
3.3.2. Cytotoxicity of the SiQDs
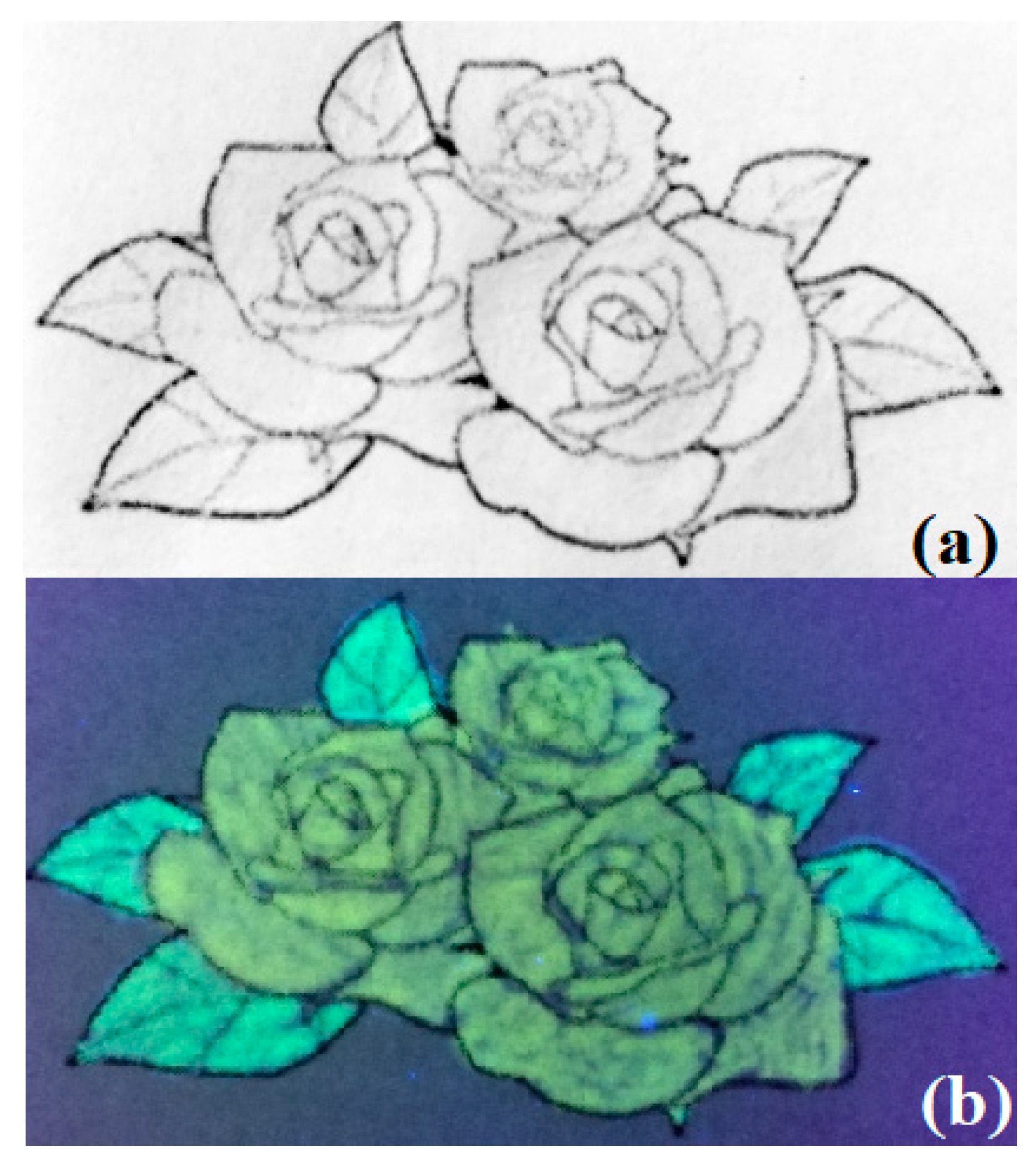
3.3.3. Fluorescence Ink
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shan, C.; Wu, K.; Yen, H.-J.; Narvaez Villarrubia, C.; Nakotte, T.; Bo, X.; Zhou, M.; Wu, G.; Wang, H.-L. Graphene Oxides Used as a New “Dual Role” Binder for Stabilizing Silicon Nanoparticles in Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 15665–15672. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Guo, X.X.; Liu, X.H.; Chen, G.; Wang, H.J.; Zhang, N.; Wang, J.; Qiu, G.Z.; Ma, R.Z. Interconnected silicon nanoparticles originated from halloysite nanotubes through the magnesiothermic reduction: A high-performance anode material for lithium-ion batteries. Appl. Clay Sci. 2018, 162, 499–506. [Google Scholar] [CrossRef]
- Tang, M.M.; Ji, X.Y.; Xu, H.; Zhang, L.; Jiang, A.R.; Song, B.; Su, Y.Y.; He, Y. Photostable and Biocompatible Fluorescent Silicon Nanoparticles-Based Theranostic Probes for Simultaneous Imaging and Treatment of Ocular Neovascularization. Anal. Chem. 2018, 90, 8188–8195. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, Z.H.; Hu, Y.L.; Liu, H.F.; Sun, Y.Q.; Meng, H.M.; Wang, Y.W.; Qu, L.B.; Lin, Y.H. One-Pot Green Synthesis of Ultrabright N-Doped Fluorescent Silicon Nanoparticles for Cellular Imaging by Using Ethylenediaminetetraacetic Acid Disodium Salt as an Effective Reductant. ACS Appl. Mater. Interfaces 2018, 10, 27979–27986. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.G.; Zhao, M.Y.; Deng, D.; Ye, X.; Zhang, F.; Chen, H.; Kong, J.L. An intelligent and biocompatible photosensitizer conjugated silicon quantum dots-MnO2 nanosystem for fluorescence imaging-guided efficient photodynamic therapy. J. Mater. Chem. B 2018, 6, 4592–4601. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, Y.Z.; Li, W.; Xiao, C.; Liu, D.F.; Dong, C.; Zhang, M.; Makila, E.; Kemell, M.; Salonen, J.; et al. Multifunctional Nanohybrid Based on Porous Silicon Nanoparticles, Gold Nanoparticles, and Acetalated Dextran for Liver Regeneration and Acute Liver Failure Theranostics. Adv. Mater. 2018, 30, 1703393–1703403. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Song, Y.; Zhu, C.Z.; Fu, S.F.; Shi, Q.R.; Sun, Y.M.; Jia, B.Z.; Du, D.; Xu, Z.L.; Lin, Y.H. Fluorescent silicon nanoparticles-based ratiometric fluorescence immunoassay for sensitive detection of ethyl carbamate in red wine. Sens. Actuators B Chem. 2018, 255, 2742–2749. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Ren, G.J.; Tang, M.Y.; Chai, F.; Qu, F.Y.; Wang, C.G.; Su, Z.M. Fluorescent silicon nanoparticles for sensing Hg2+ and Ag+ as well visualization of latent fingerprints. Dyes Pigments 2018, 149, 686–695. [Google Scholar] [CrossRef]
- Tu, C.C.; Hoo, J.H.; Bohringer, K.F.; Lin, L.Y.; Cao, G.Z. Red-emitting silicon quantum dot phosphors in warm white LEDs with excellent color rendering. Opt. Express 2014, 22, A276–A281. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Liu, Y.; Tsang, C.H.A.; Ma, D.D.D.; Fan, X.; Wong, N.B.; Lee, S.T. Water-soluble silicon quantum dots with wavelength-tunable photoluminescence. Adv. Mater. 2009, 21, 661–664. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Yong, K.T.; Roy, I.; Xu, G.X.; Prasad, P.N.; Swihart, M.T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Dohnalova, K.; Poddubny, A.N.; Prokofiev, A.A.; de Boer, W.D.A.M.; Umesh, C.P.; Paulusse, J.M.J.; Zuilhof, H.; Gregorkiewicz, T. Surface brightens up Si quantum dots: direct bandgap-like size-tunable emission. Light Sci. Appl. 2013, 2, 47–53. [Google Scholar] [CrossRef]
- Xu, X.L.; Ma, S.Y.; Xiao, X.C.; Hu, Y.; Zhao, D. The preparation of high-quality water-soluble silicon quantum dots and their application in the detection of formaldehyde. RSC Adv. 2016, 6, 98899–98907. [Google Scholar] [CrossRef]
- Ma, S.D.; Chen, Y.L.; Feng, J.; Liu, J.J.; Zuo, X.W.; Chen, X.G. One-Step Synthesis of Water-Dispersible and Biocompatible Silicon Nanoparticles for Selective Heparin Sensing and Cell Imaging. Anal. Chem. 2016, 88, 10474–10481. [Google Scholar] [CrossRef]
- Wang, J.; Ye, D.X.; Liang, G.H.; Chang, J.; Kong, J.L.; Chen, J.Y. One-step synthesis of water-dispersible silicon nanoparticles and their use in fluorescence lifetime imaging of living cells. J. Mater. Chem. B 2014, 2, 4338–4345. [Google Scholar] [CrossRef]
- Han, Y.X.; Chen, Y.L.; Feng, J.; Liu, J.J.; Ma, S.D.; Chen, X.G. One-Pot Synthesis of Fluorescent Silicon Nanoparticles for Sensitive and Selective Determination of 2,4,6-Trinitrophenol in Aqueous Solution. Anal. Chem. 2017, 89, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Gupta, S.K.; Naaz, F.; Singh, A.; Singh, V.K.; Verma, R.; Singh, N.; Singh, R.K. Synthesis, antibacterial activity, synergistic effect, cytotoxicity, docking and molecular dynamics of benzimidazole analogues. Comput. Biol. Chem. 2018, 76, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Chu, X.J.; Sun, P.Y.; Feng, X.J.; Huang, W.W.; Liu, H.X.; Ma, Y.B. Synergy effects of Polyinosinic-polycytidylic acid, CpG oligodeoxynucleotide, and cationic peptides to adjuvant HPV E7 epitope vaccine through preventive and therapeutic immunization in a TC-1 grafted mouse model. Hum. Vaccines Immunother. 2018, 14, 931–940. [Google Scholar] [CrossRef]
- Zhao, R.L.; He, Y.M. Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice. J. Ethnopharmacol. 2018, 210, 287–295. [Google Scholar] [CrossRef]
- Zhan, Y.Q.; Zhang, J.M.; Wan, X.Y.; Long, Z.H.; He, S.J.; He, Y. Epoxy composites coating with Fe3O4 decorated graphene oxide: Modified bio-inspired surface chemistry, synergistic effect and improved anti-corrosion performance. Appl. Surf. Sci. 2018, 436, 756–767. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Li, Y.; Wang, S.; Lei, A. The synergistic effect of self-assembly and visible-light induced the oxidative C–H acylation of N-heterocyclic aromatic compounds with aldehydes. Chem. Commun. 2018, 54, 5744–5747. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Zhou, Y.X.; Wang, H.; Lu, J.; Uchida, T.; Xu, Q.; Yu, S.H.; Jiang, H.L. Multifunctional PdAg@ MIL-101 for one-pot cascade reactions: combination of host–guest cooperation and bimetallic synergy in catalysis. ACS Catal. 2015, 5, 2062–2069. [Google Scholar] [CrossRef]
- Anastasaki, A.; Nikolaou, V.; Zhang, Q.; Burns, J.; Samanta, S.R.; Waldron, C.; Haddleton, A.J.; McHale, R.; Fox, D.; Percec, V.; et al. Copper(II)/Tertiary Amine Synergy in Photoinduced Living Radical Polymerization: Accelerated Synthesis of omega-Functional and alpha,omega-Heterofunctional Poly(acrylates). J. Am. Chem. Soc. 2014, 136, 1141–1149. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Soriano, M.L.; Benitez-Martinez, S.; Valcarcel, M. Photoluminescent sensing hydrogel platform based on the combination of nanocellulose and S,N-codoped graphene quantum dots. Sens. Actuators B Chem. 2017, 245, 946–953. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, S.G.; Mo, S.; Li, N.; Ju, Y.J.; Ling, Y.; Li, N.B.; Luo, H.Q. Highly selective detection of p-nitrophenol using fluorescence assay based on boron, nitrogen co-doped carbon dots. Talanta 2018, 184, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Yue, T.; Xiao, X.C.; Cheng, H.; Zhao, D. A proof of concept study of preparing ultra bright silicon quantum dots based on synergistic effect of reductants. J. Lumin. 2018, 201, 77–84. [Google Scholar] [CrossRef]
- Wu, F.G.; Zhang, X.D.; Kai, S.Q.; Zhang, M.Y.; Wang, H.Y.; Myers, J.N.; Weng, Y.X.; Liu, P.D.; Gu, N.; Chen, Z. One-Step Synthesis of Superbright Water-Soluble Silicon Nanoparticles with Photoluminescence Quantum Yield Exceeding 80%. Adv. Mater. Interfaces 2015, 2, 1500360–1500371. [Google Scholar] [CrossRef]
- Ghosh, B.; Shirahata, N. Colloidal silicon quantum dots: synthesis and luminescence tuning from the near-UV to the near-IR range. Sci. Technol. Adv. Mater. 2014, 15, 014207–014221. [Google Scholar] [CrossRef]
- Kumar, A.; Grewal, A.S.; Singh, V.; Narang, R.; Pandita, D.; Lather, V. Synthesis, Antimicrobial Activity and QSAR Studies of Some New Sparfloxacin Derivatives. Pharm. Chem. J. 2018, 52, 444–454. [Google Scholar] [CrossRef]
- Selim, Y.; Abd El-Azim, M.H.M. Conventional and Microwave-Activated the Synthesis of a Novel Series of Imidazoles, Pyrimidines, and Thiazoles Candidates. J. Heterocycl. Chem. 2018, 55, 1403–1409. [Google Scholar] [CrossRef]
- Oleynik, I.V.; Oleynik, I.I. Design of Postmetallocene Catalytic Systems of Arylimine Type for Olefin Polymerization: XVIII. Synthesis of N-Arylsalicylaldimine Ligands Containing meta-or para-Diallylamino Group and Their Titanium(IV) Complexes. Russ. J. Org. Chem. 2018, 54, 537–544. [Google Scholar] [CrossRef]
- Musa, W.J.; Duengo, S.; Situmeang, B. Isolation and characterization triterpenoid compound from leaves mangrove plant (Sonnertia Alba) and antibacterial activity test. Int. Res. J. Pharm. 2018, 9, 85–89. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.G.; Chao, Y.M.; Bao, Y.P. Anti-cancer activities of allyl isothiocyanate and its conjugated silicon quantum dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; McVey, B.F.P.; Robinson, A.B.; Longatte, G.; O’Mara, P.B.; Tan, V.T.G.; Thordarson, P.; Tilley, R.D.; Gaus, K.; Gooding, J.J. Protease sensing using nontoxic silicon quantum dots. J. Biomed. Opt. 2017, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Wang, H.Y.; Zhong, Y.L.; Chu, B.B.; Su, Y.Y.; He, Y. Fluorescent and magnetic anti-counterfeiting realized by biocompatible multifunctional silicon nanoshuttle-based security ink. Nanoscale 2018, 10, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wei, C.; Ma, W.; Li, J.; Xiao, X.; Zhao, D. One-Step Hydrothermal Synthesis of Yellow and Green Emitting Silicon Quantum Dots with Synergistic Effect. Nanomaterials 2019, 9, 466. https://doi.org/10.3390/nano9030466
Zhang Z, Wei C, Ma W, Li J, Xiao X, Zhao D. One-Step Hydrothermal Synthesis of Yellow and Green Emitting Silicon Quantum Dots with Synergistic Effect. Nanomaterials. 2019; 9(3):466. https://doi.org/10.3390/nano9030466
Chicago/Turabian StyleZhang, Zhixia, Chunjin Wei, Wenting Ma, Jun Li, Xincai Xiao, and Dan Zhao. 2019. "One-Step Hydrothermal Synthesis of Yellow and Green Emitting Silicon Quantum Dots with Synergistic Effect" Nanomaterials 9, no. 3: 466. https://doi.org/10.3390/nano9030466
APA StyleZhang, Z., Wei, C., Ma, W., Li, J., Xiao, X., & Zhao, D. (2019). One-Step Hydrothermal Synthesis of Yellow and Green Emitting Silicon Quantum Dots with Synergistic Effect. Nanomaterials, 9(3), 466. https://doi.org/10.3390/nano9030466




