Three-Dimensionally Porous Li-Ion and Li-S Battery Cathodes: A Mini Review for Preparation Methods and Energy-Storage Performance
Abstract
:1. Introduction
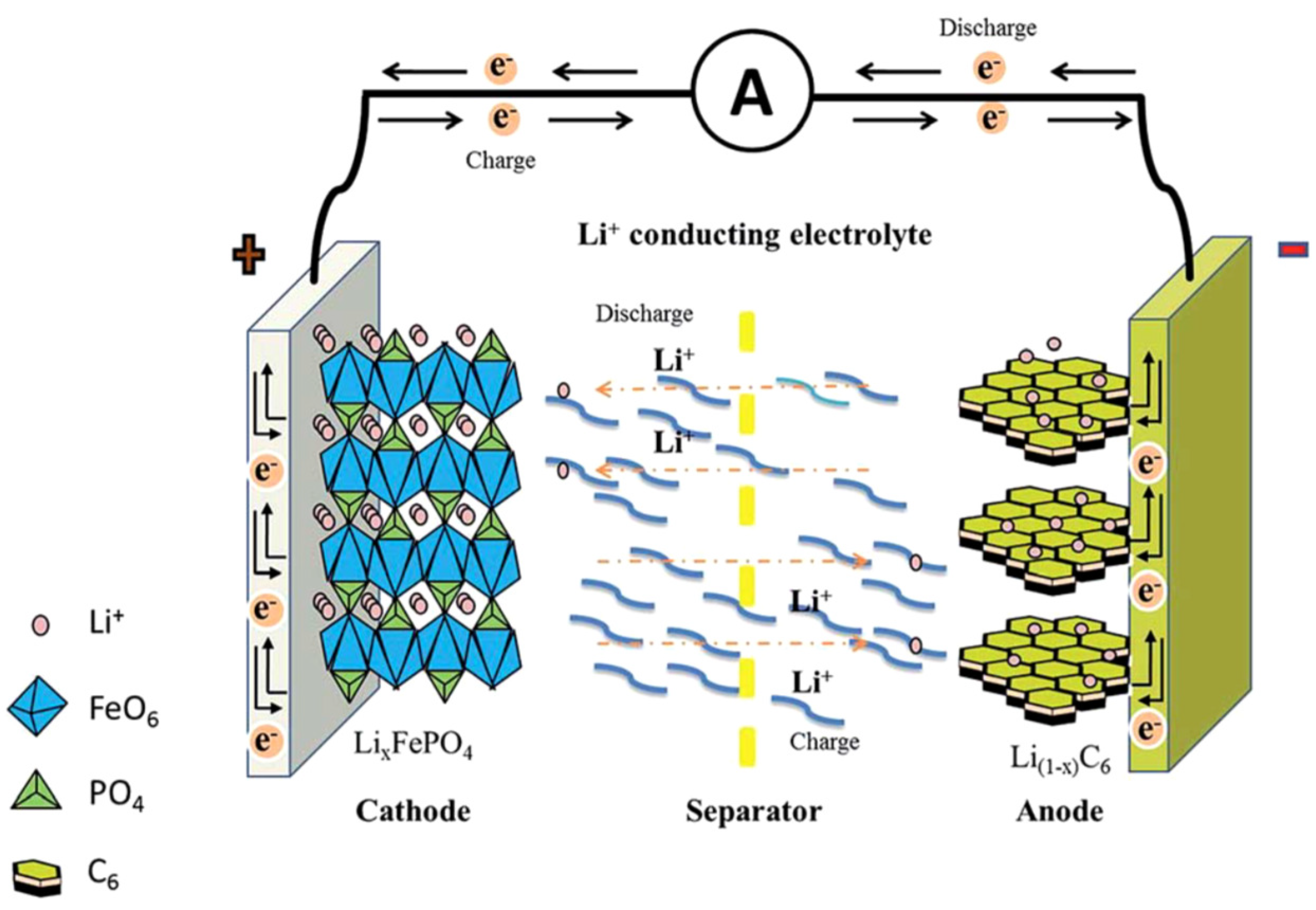
2. Challenges of Li-Ion and Li-S Battery Cathodes
3. Methods for Making 3D Porous Li-Ion Battery Cathodes
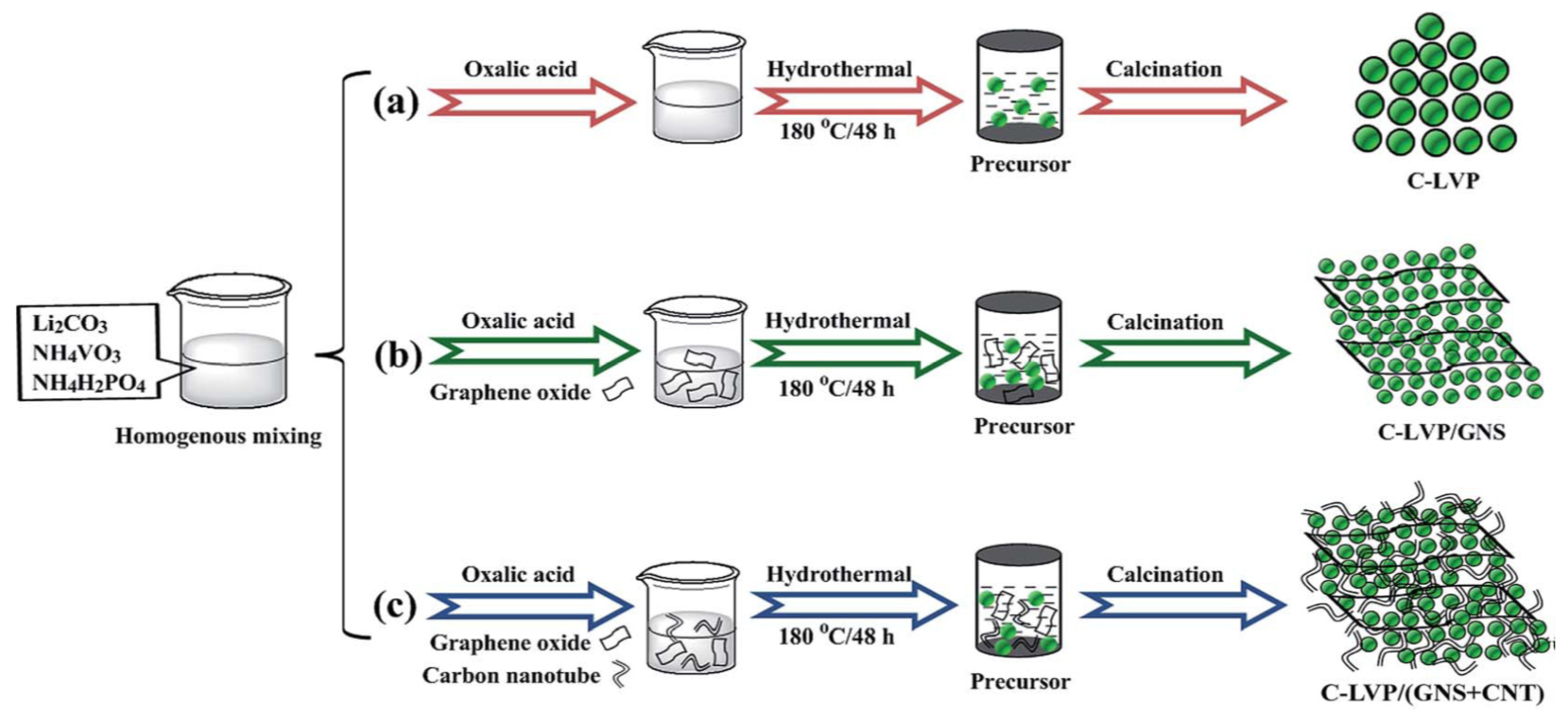
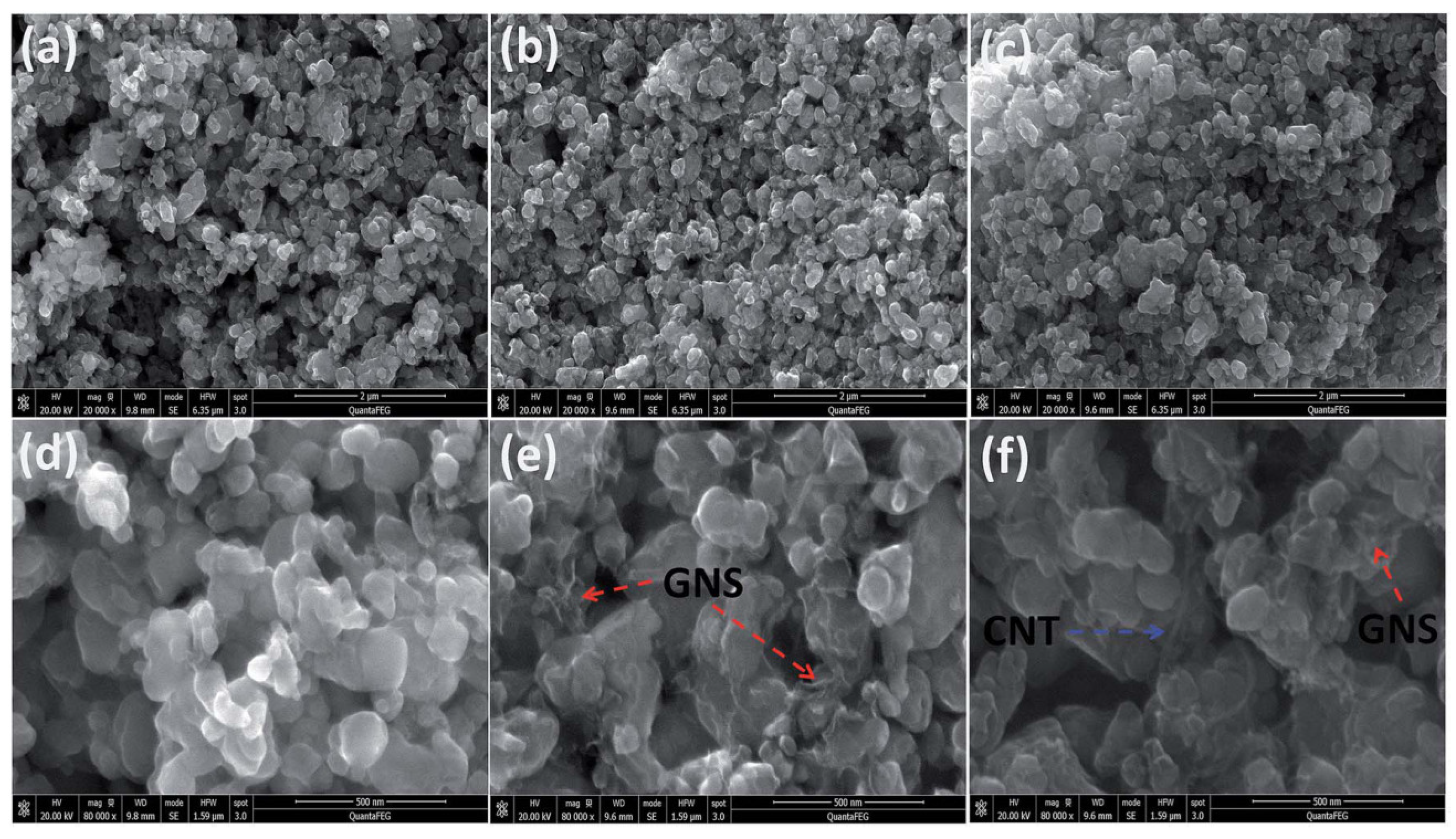
3.1. Hydrothermal Synthesis
3.2. Sol-Gel Method
3.3. Solid-Based Approcah
3.4. Other Methods
4. Methods for Constructing 3D Porous Li-S Battery Cathodes and Their Comparison
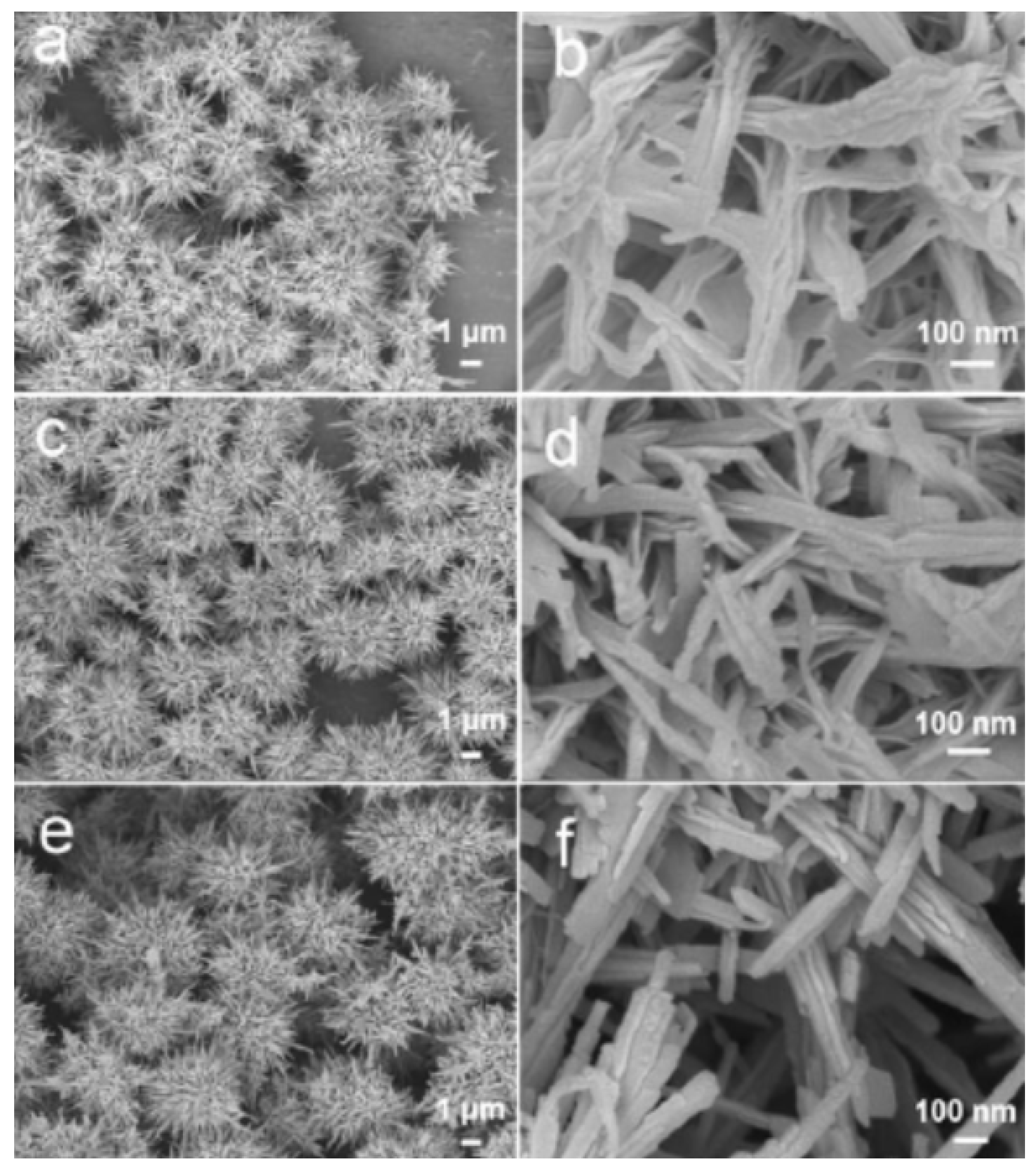
4.1. 3D Porous S Cathodes for Li-S Batteries
4.2. Brief Comparison between Li-Ion and Li-S Batteries
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Seh, Z.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.J.; Sun, Y.K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, D.; Ge, M.; Yu, X.; Chu, Y.; Huang, X.; Zhang, X.; Yin, Y.; Yang, X.; Guo, Y.; Gu, L.; Wan, L. High-capacity cathode material with high voltage for Li-ion batteries. Adv. Mater. 2018, 30, 1705575. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Li, X.H.; Chen, J.S. Surface and Interface Engineering of Electrode Materials for Lithium-Ion Batteries. Adv. Mater. 2015, 27, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Xia, X.-H.; Xie, D.; Yao, Z.J.; Zhong, Y.; Zhan, J.Y.; Wang, D.H.; Wu, J.B.; Wang, X.L.; Tu, J.P. Encapsulating silicon nanoparticles into mesoporous carbon forming pomegranatestructured microspheres as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2017, 5, 11197–11203. [Google Scholar] [CrossRef]
- Wang, X.F.; Feng, Z.J.; Huang, J.T.; Deng, W.; Li, H.B.; Zhang, H.S.; Wen, Z.H. Graphene-decorated carbon-coated LiFePO4 nanospheres as a high-performance cathode material for lithium-ion batteries. Carbon 2018, 127, 149–157. [Google Scholar] [CrossRef]
- Li, C.X.; Xi, Z.C.; Guo, D.X.; Chen, X.J.; Yin, L.W. Chemical immobilization effect on lithium polysulfides for lithium–sulfur batteries. Small 2018, 14, 1701986. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.W.; Zhao, D.L.; Yao, R.R.; Li, C.; Cheng, X.W.; Hu, T. Polyaniline@spherical ordered mesoporous carbon/sulfur nanocomposites for high-performance lithium-sulfur batteries. Int. J. Hydrogen Energy 2018, 43, 10502–10510. [Google Scholar] [CrossRef]
- Liang, X.Q.; Wang, J.J.; Zhang, S.Y.; Wang, L.Y.; Wang, W.F.; Li, L.Y.; Wang, H.F.; Huang, D.; Zhou, W.Z.; Guo, J. Fabrication of uniform Si-incorporated SnO2 nanoparticles on graphene sheets as advanced anode for Li-ion batteries. Appl. Surf. Sci. 2019, 476, 28–35. [Google Scholar] [CrossRef]
- Sun, W.W.; Tao, X.C.; Du, P.P.; Wang, Y. Carbon-coated mixed-metal sulfide hierarchical structure: MOF-derived synthesis and lithium-storage performances. Chem. Eng. J. 2019, 366, 622–630. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Tang, L.; Zhang, L.; Chen, X.; Mao, Y.C.; Sun, K.N. Well-developed capacitive-capacity of metal-organic framework derived Co3O4 films in Li ion battery anodes. J. Alloy. Compd. 2018, 746, 277–284. [Google Scholar] [CrossRef]
- Li, N.; Weng, Z.; Wang, Y.; Li, F.; Cheng, H.-M.; Zhou, H. An aqueous dissolved polysulfide cathode for lithium–sulfur batteries. Energy Environ. Sci. 2014, 7, 3307–3312. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, P.; Shu, H.B.; Man, X.L.; Li, F.; Huang, J.; Dong, Q.M.; Chao, D.L. Borophene as efficient sulfur hosts for lithium–sulfur batteries: Suppressing shuttle effect and improving conductivity. J. Phys. Chem. C 2017, 121, 15549–15555. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Bai, Z.; Song, X.; Xiong, D.; Zhao, M.; Li, D.; Lu, S. A review of atomic layer deposition providing high performance lithium sulfur batteries. J. Power Sources 2017, 338, 34–48. [Google Scholar] [CrossRef]
- Daniele, D.L.; Roberta, V.; Jusef, H. Lithium-ion batteries for sustainable energy storage: Recent advances towards new cell configurations. Green Chem. 2017, 19, 3442–3467. [Google Scholar]
- He, Y.B.; Chang, Z.; Wu, S.C.; Zhou, H.S. Effective strategies for long-cycle life lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 6155–6182. [Google Scholar] [CrossRef]
- Han, Y.; Dong, L.; Feng, J.; Li, D.; Li, X.; Liu, S. Cobalt oxide modified porous carbon anode enhancing electrochemical performance for Li-ion batteries. Electrochim. Acta 2015, 167, 246–253. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Han, Y.; Gao, T.; Shao, J.; Zuo, Z.; Liu, W.; Shi, Q.; Zheng, H. Robust 3D nanowebs assembled from interconnected and sandwich-like C@Fe3O4@C coaxial nanocables for enhanced Li-ion storage. J. Mater. Chem. A 2016, 4, 10314–10320. [Google Scholar] [CrossRef]
- Ren, J.; Ren, R.P.; Lv, Y.K. A flexible 3D graphene@CNT@MoS2 hybrid foam anode for high-performance lithium-ion battery. Chem. Eng. J. 2018, 353, 419–424. [Google Scholar] [CrossRef]
- Huang, Z.D.; Liu, X.M.; Oh, S.W.; Zhang, B.; Ma, P.C.; Kim, J.K. Microscopically porous, interconnected single crystal LiNi1/3Co1/3Mn1/3O2 cathode material for Lithium ion batteries. J. Mater. Chem. 2011, 21, 10777–10784. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Lu, J.; Wu, F.; Li, L.; Chen, J.; Tan, G.; Ye, Y.; Amine, K. Graphene-based three-dimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 2013, 13, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.H.; Zhou, Y.K.; Tu, X.F.; Zhang, Z.T.; Du, G.D. Well-dispersed LiFePO4 nanoparticles anchored on a three-dimensional graphene aerogel as high-performance positive electrode materials for lithium-ion batteries. J. Power Sources 2017, 340, 40–50. [Google Scholar] [CrossRef]
- Yao, M.; Okuno, K.; Iwaki, T.; Kato, M.; Tanase, S.; Emura, K.; Sakai, T. LiFePO4-based electrode using micro-porous current collector for high power lithium ion battery. J. Power Sources 2007, 173, 545–549. [Google Scholar] [CrossRef]
- Du, Y.H.; Tang, Y.F.; Huang, F.Q.; Chang, C.K. Preparation of three-dimensional free-standing nano-LiFePO4/graphene composite for high performance lithium ion battery. RSC Adv. 2016, 6, 52279–52283. [Google Scholar] [CrossRef]
- Fu, F.; Tang, J.Y.; Yao, Y.Z.; Shao, M.H. Hollow Porous Hierarchical-Structured 0.5Li2MnO3·0.5LiMn0.4Co0.3Ni0.3O2 as a High-Performance Cathode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 25654–25659. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Chou, S.L.; Zhou, C.F.; Gu, Q.F.; Liu, H.K.; Dou, S.X. Three-dimensional-network Li3V2(PO4)3/C composite as high rate lithium ion battery cathode material and its compatibility with ionic liquid electrolytes. J. Power Sources 2014, 246, 124–131. [Google Scholar] [CrossRef]
- Cui, K.; Li, Y.K. Monoclinic Li3V2(PO4)3/C nanocrystals co-modified with graphene nanosheets and carbon nanotubes as a three-dimensional-network cathode material for rechargeable lithium-ion batteries. RSC Adv. 2016, 6, 8431–8439. [Google Scholar] [CrossRef]
- Chao, D.; Xia, X.; Liu, J.; Fan, Z.; Ng, C.F.; Lin, J.; Zhang, H.; Shen, Z.X.; Fan, H.J. Lithium-Ion Batteries: A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: A high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv. Mater. 2014, 26, 5733. [Google Scholar] [CrossRef]
- Gao, X.T.; Liu, Y.T.; Zhu, X.D.; Yan, D.J.; Wang, C.; Feng, Y.J.; Sun, K.N. V2O5 nanoparticles confined in Three−Dimensionally organized, porous Nitrogen−Doped graphene frameworks: Flexible and Free−Standing cathodes for high performance lithium storage. Carbon 2018, 140, 218–226. [Google Scholar] [CrossRef]
- Pan, A.Q.; Wu, H.B.; Yu, L.; Zhu, T.; Lou, X.W. Synthesis of hierarchical three-dimensional vanadium oxide microstructures as high-capacity cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 3874–3879. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sun, Z.; Gao, M.; Tan, Z.; Zhang, B.; Su, D.S. Porous V2O5-SnO2/CNTs composites as high performance cathode materials for lithium-ion batteries. J. Energy Chem. 2013, 22, 347–355. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Q.; Goodman, M.D.; Zhu, H.; Kim, J.; Krueger, N.A.; Ning, H.; Huang, X.; Liu, J.; Terrones, M.; Braun, P.V. Graphene sandwiched mesostructured Li-ion battery electrodes. Adv. Mater. 2016, 28, 7696–7702. [Google Scholar] [CrossRef] [PubMed]
- Park, B.G.; Kim, S.; Kim, I.-D.; Park, Y.J. Structural and electrochemical performance of three-dimensional LiMn2O4 thin film. J. Mater. Sci. 2010, 45, 3947–3953. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, D.; Liu, Y.P.; Han, W.W.; Chu, H.Q.; Rui, X.H. Integrated charge transfer in Li3V2(PO4)3/C for high-power Li-ion batteries. Int. J. Electrochem. Sci. 2017, 12, 9925–9932. [Google Scholar] [CrossRef]
- Li, J.Z.; Luo, S.H.; Ding, X.Y.; Wang, Q.; He, P. Three-Dimensional Honeycomb-Structural LiAlO2-Modified LiMnPO4 Composite with Superior High Rate Capacity as Li-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 10786–10795. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, V.; Krishnaswamy, S.; Raman, S.; Panigrahi, P.; Lee, J.; Nagarajan, G.S. Enhanced electrochemical performance of LiCoBO3 cathode material for next generation Lithium-ion batteries. Appl. Surf. Sci. 2018, 449, 421–425. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, X.; Xu, H.; Xie, M.; Deng, S.; Wang, H.; Liu, J.; Yan, H. Facile synthesis of porous LiMn2O4 spheres as cathode materials for high-power lithium ion batteries. J. Power Sources 2013, 226, 140–148. [Google Scholar] [CrossRef]
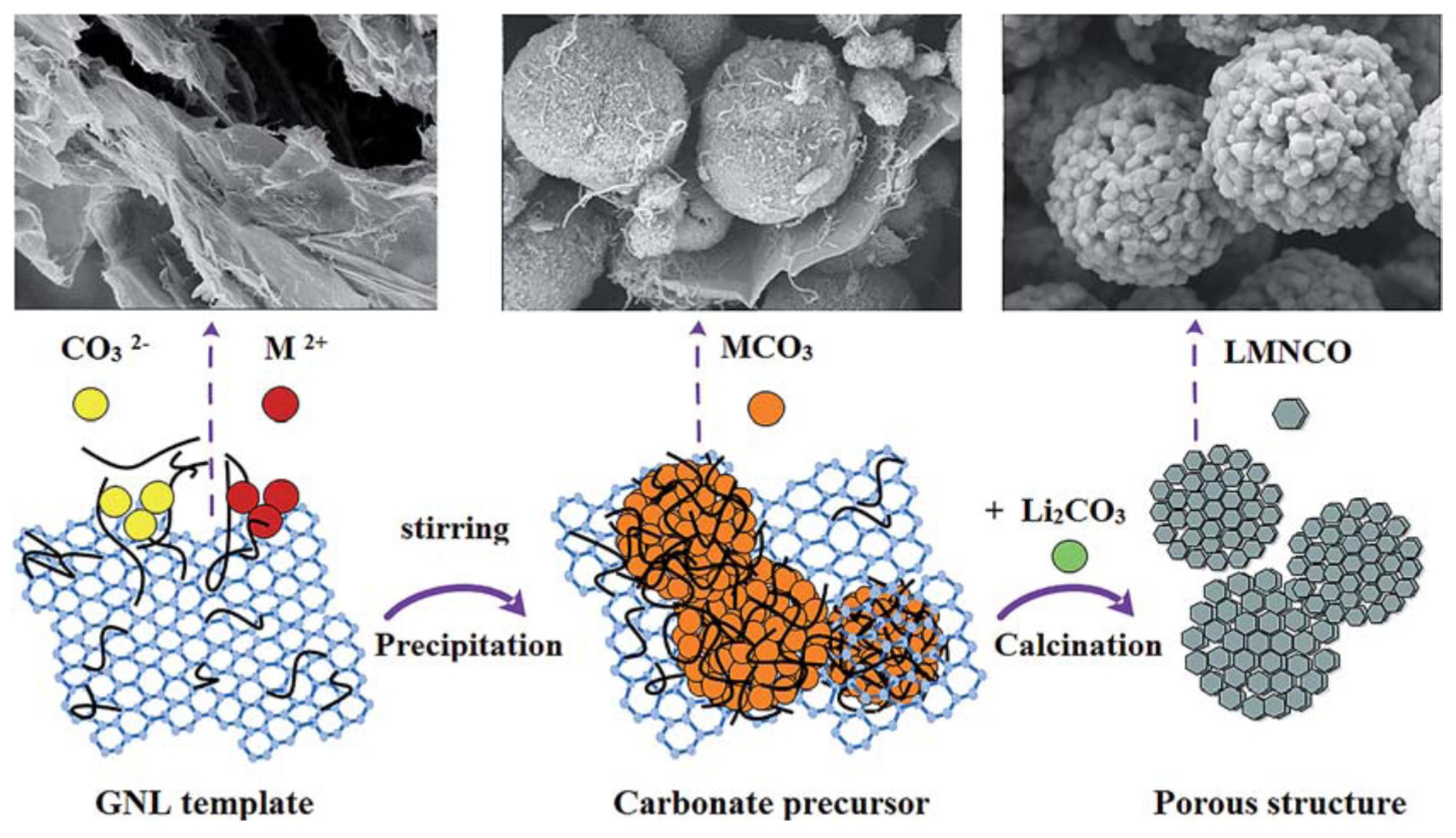
- Huang, Y.; Hou, X.; Ma, S.; Zou, X.; Wu, Y.; Hu, S.; Shao, Z.; Liu, X. Template GNL-assisted synthesis of porous Li1.2Mn0.534Ni0.133Co0.133O2: Towards high performance cathodes for lithium ion batteries. RSC Adv. 2015, 5, 25258–25265. [Google Scholar] [CrossRef]
- Biasi, L.D.; Lieser, G.; Drager, C.; Indris, S.; Rana, J.; Schumacher, G.; Monig, R.; Ehrenberg, H.; Binder, J.R.; Gebwein, H. LiCaFeF6: A zero-strain cathode material for use in Li-ion batteries. J. Power Sources 2017, 362, 192–201. [Google Scholar] [CrossRef]
- Baster, D.; Paziak, P.; Ziąbka, M.; Wazny, G.; Molenda, J. LiNi0.6Co0.4-zTizO2—New cathode materials for Li-ion batteries. Solid State Ion. 2018, 320, 118–125. [Google Scholar] [CrossRef]
- Doherty, C.M.; Caruso, R.A.; Smarsly, B.M.; Drummond, C.J. Colloidal crystal templating to produce hierarchically porous LiFePO4 electrode materials for high power lithium ion batteries. Chem. Mater. 2009, 21, 2895–2903. [Google Scholar] [CrossRef]
- Tu, X.; Zhou, Y.; Song, Y. Freeze-drying synthesis of three-dimensional porous LiFePO4 modified with well-dispersed nitrogen-doped carbon nanotubes for high-performance lithium-ion batteries. Appl. Surf. Sci. 2017, 400, 329–338. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, J.; Wang, G.F.; Liu, S.S.; Tan, M.; Liu, X.Q.; Komarneni, S. Facile synthesis of orthorhombic LiMnO2 nanorods by in-situ carbothermal reduction: Promising cathode material for Li ion batteries. Ceram. Int. 2017, 43, 10585–10589. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, X.; Yue, K.; Wu, Y.; Zhuang, J.; Lu, W. Synthesis and electrochemical property of LiMn2O4 porous hollow nanofiber as cathode for lithium-ion batteries. Nanoscale Res. Lett. 2017, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.X.; Pi, Z.C.; Zhai, H.A.; Li, J.Q.; Chen, L.L.; Shen, S.Q.; Xi, X.M.; Xiao, K.S. Three-dimensional Li3V2(PO4)3/C nanowire and nanofiber hybrid membrane as a self-standing, binder-free cathode for lithium ion batteries. RSC Adv. 2016, 6, 71574–71580. [Google Scholar] [CrossRef]
- Li, Y.H.; Xiang, K.X.; Shi, C.F.; Zhou, W.; Zhu, Y.R.; Chen, H. Frogegg-like Li3V2(PO4)3/carbon composite with three dimensional porous structure and its improved electrochemical performance in lithium ion batteries. Mater. Lett. 2017, 204, 104–107. [Google Scholar] [CrossRef]
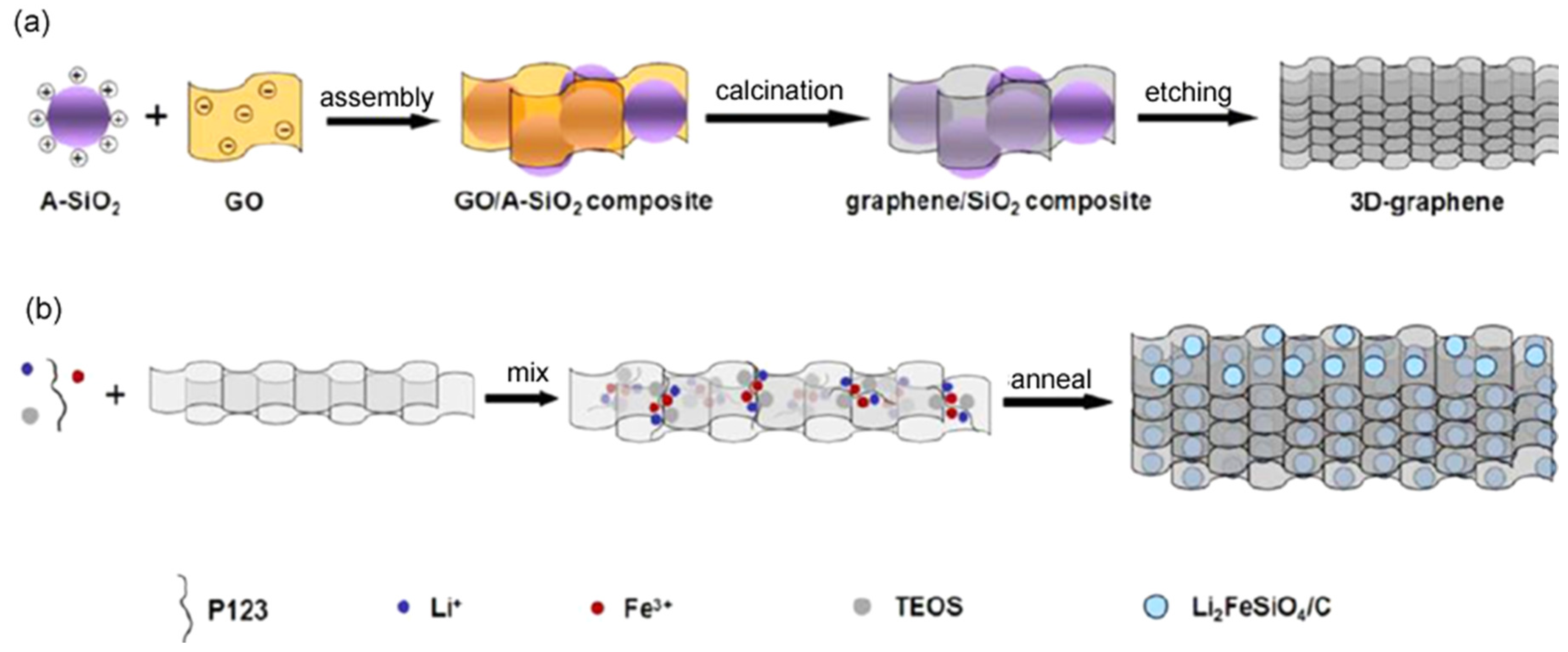
- Zhu, H.; Wu, X.Z.; Zan, L.; Zhang, Y.X. Three-dimensional macroporous graphene-Li2FeSiO4 composite as cathode material for lithium-ion batteries with superior electrochemical performances. ACS Appl. Mater. Interfaces 2014, 6, 11724–11733. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.H.; Zhang, X.Y.; Zhang, X.; Yang, L.; Zhang, X.Y.; Chen, H.S.; Yang, M.; Fang, D.N. Free-standing and flexible LiMnTiO4/carbon nanotube cathodes for high performance lithium ion batteries. J. Power Sources 2016, 321, 120–125. [Google Scholar] [CrossRef]
- Liu, J.; Yin, L.; Yang, X.Q.; Khalifah, P.G. Li3VP3O9N as a Multielectron Redox Cathode for Li-Ion Battery. Chem. Mater. 2018, 30, 4609–4616. [Google Scholar] [CrossRef]
- Deng, Y.L.; Mou, J.R.; Wu, H.L.; Jiang, N.; Zheng, Q.J.; Lam, K.H.; Xu, C.G.; Lin, D.M. A superior Li2SiO3-composited LiNi0.5Mn1.5O4 cathode for high-voltage and high-performance lithium-ion batteries. Electrochim. Acta 2017, 235, 19–31. [Google Scholar] [CrossRef]
- Du, Y.H.; Tang, Y.F.; Chang, C.K. Enhanced electrochemical performance from 3D G/LiFePO4/G sandwich cathode material. J. Phys. Chem. Solids 2017, 107, 36–41. [Google Scholar] [CrossRef]
- Wen, B.H.; Liu, J.; Chernova, N.A.; Wang, X.Y.; Janssen, Y.; Omenya, F.; Khalifah, P.G.; Whittingham, M.S. Li3Mo4P5O24: A two-electron cathode for lithium-ion batteries with three-dimensional diffusion pathways. Chem. Mater. 2016, 28, 2229–2235. [Google Scholar] [CrossRef]
- Ding, Z.P.; Liu, J.T.; Ji, R.; Zeng, X.H.; Yang, S.L.; Pan, A.Q.; Ivey, D.G.; Wei, W.F. Three-dimensionally ordered macroporous Li2FeSiO4/C composite as a high performance cathode for advanced lithium ion batteries. J. Power Sources 2016, 329, 297–304. [Google Scholar] [CrossRef]
- Peng, T.; Guo, W.; Zhang, Q.; Zhang, Y.G.; Chen, M.; Wang, Y.H.; Yan, H.L.; Lu, Y.; Luo, Y.S. Uniform coaxial CNT@Li2MnSiO4@C as advanced cathode material for lithium-ion battery. Electrochim. Acta 2018, 291, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Z.L.; Zhou, H.C.; Tang, J.P.; Sun, H.X.; Ding, J.; Zhou, X.Y. A cathode material based on the iron fluoride with an ultra-thin Li3FeF6 protective layer for high-capacity Li-ion batteries. J. Power Sources 2017, 363, 244–250. [Google Scholar] [CrossRef]
- Bao, L.; Xu, G.; Sun, X.L.; Zeng, H.; Zhao, R.Y.; Yang, X.; Shen, G.; Han, G.R.; Zhou, S.X. Mono-dispersed LiFePO4@C core-shell [001] nanorods for a high power Li-ion battery cathode. J. Alloy. Compd. 2017, 708, 685–693. [Google Scholar] [CrossRef]
- Ludwig, J.; Marino, C.; Haering, D.; Stinner, C.; Gasteiger, H.A.; Nilges, T. Morphology-controlled microwave-assisted solvothermal synthesis of high-performance LiCoPO4 as a high-voltage cathode material for Li-ion batteries. J. Power Sources 2017, 342, 214–223. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Wang, H.; Xie, Z.Q.; Ellis, S.; Kuai, X.X.; Guo, J.; Zhu, X.; Wang, Y.; Gao, L.J. Tailorable electrochemical performance of spinel cathode materials via in-situ integrating a layered Li2MnO3 phase for lithium-ion batteries. J. Power Sources 2016, 333, 43–52. [Google Scholar] [CrossRef]
- Thayumanasundaram, S.; Rangasamy, V.S.; Seo, J.W.; Locquet, J.P. A combined approach: Polyol synthesis of nanocrystalline Li2FeSiO4, doping multi-walled carbon nanotubes, and ionic liquid electrolyte to enhance cathode performance in Li-ion batteries. Electrochim. Acta 2017, 258, 1044–1052. [Google Scholar] [CrossRef]
- Yang, X.J.; Wang, X.L.; Wang, K.Y.; Chang, G.L. Improved Li-storage performance of CNTs-decorated LiVPO4F/C cathode material for electrochemical energy storage. Ceram. Int. 2018, 44, 3825–3829. [Google Scholar] [CrossRef]
- Wi, S.G.; Kim, J.W.; Lee, S.H.; Kang, J.H.; Kim, K.H.; Park, K.; Kim, K.S.; Nam, S.H.; Kim, C.J.; Park, B.W. Synthesis of LiMn0.8Fe0.2PO4 Mesocrystals for High-Performance Li-Ion Cathode Materials. Electrochim. Acta 2016, 216, 203–210. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.S.; Yu, W.J.; Zhang, J.F.; An, C.S. Facile synthesis of carbon-encapsulated LiMnBO3 composite by the sol-gel method as a lithium-ion battery cathode material. J. Alloy. Compd. 2017, 704, 343–347. [Google Scholar] [CrossRef]
- Jiang, C.H.; Tang, Z.L.; Wang, S.T.; Zhang, Z.T. A truncated octahedral spinel LiMn2O4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries. J. Power Sources 2017, 357, 144–148. [Google Scholar] [CrossRef]
- Chang, C.Y.; Huang, Z.P.; Tian, R.S.; Jiang, X.Y.; Li, C.S.; Feng, J.J. Targeted partial surface modification with nano-SiO2@Li2CoPO4F as high-voltage cathode material for LIBs. J. Power Sources 2017, 364, 351–358. [Google Scholar] [CrossRef]
- Yu, H.Y.; Su, Z.; Wang, L. Synthesis and electrochemical properties of LiVP2O7/C as novel cathode material for lithium ion batteries. Ceram. Int. 2017, 43, 17116–17120. [Google Scholar] [CrossRef]
- Zhang, B.; Ming, L.; Tong, H.; Zhang, J.F.; Zheng, J.C.; Wang, X.W.; Li, H.; Cheng, L. Ni-doping to improve the performance of LiFeBO3/C cathode material for lithium-ion batteries. J. Alloy. Compd. 2018, 740, 382–388. [Google Scholar] [CrossRef]
- Wang, P.P.; Xu, C.Y.; Wang, L.; Zhang, B.Y.; Zhen, L. Electrochemical behavior and structural stability of LiV3O8 microrods as cathode for lithium-ion batteries. Ceram. Int. 2016, 42, 18747–18755. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Chen, Z.L.; Zhang, X.H.; Wu, D.Y.; Li, J. P-doping Li2CoSiO4/C cathode material: A joint experimental and theoretical study. Electrochim. Acta 2018, 264, 166–172. [Google Scholar] [CrossRef]
- Lim, S.C.; Chae, M.S.; Heo, J.W.; Hong, S.T. Electrochemical lithium intercalation chemistry of condensed molybdenum metal cluster oxide: LiMo4O6. J. Solid State Chem. 2017, 254, 90–95. [Google Scholar] [CrossRef]
- Zhou, H.M.; Zhao, X.X.; Yin, C.J.; Li, J. Regeneration of LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. Electrochim. Acta 2018, 291, 142–150. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, X.Y.; Bai, H.L.; Guo, J.M.; Xiang, M.W.; Su, C.W.; Liu, X.F.; Bai, W.; Wang, R. Investigating the enhanced kinetics of LiNi0.08Mn1.92O4 cathode material by regulating calcination temperature for long life lithium-ion battery. Vacuum 2018, 158, 223–230. [Google Scholar] [CrossRef]
- Huang, Z.-G.; Li, J.-T.; Wang, K.; Ren, W.-F.; Lu, Y.-Q.; Deng, L.; Huang, L.; Sun, S.-G. Synthesis of LiFe0.4Mn0.4Co0.2PO4/C cathode material of lithium ion battery with enhanced electrochemical performance. J. Alloy. Compd. 2019, 782, 413–420. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, Y.; Xue, L.; Cheng, Y.; Hu, Z.; Liu, X. Understanding the roles of Ti on the structure and electrochemical performances of Li2Ru1-xTixO3 cathode materials for Li-ion batteries. J. Energy Chem. 2019, 33, 9–16. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; He, Y.; Lu, K. Li4Ti5O12 epitaxial coating on LiNi0.5Mn1.5O4 surface for improving the electrochemical performance through solvothermal-assisted processing. J. Alloy. Compd. 2019, 779, 978–984. [Google Scholar] [CrossRef]
- Tang, C.; Li, B.Q.; Zhang, Q.; Zhu, L.; Wang, H.F.; Shi, J.L.; Wei, F. CaO-templated growth of hierarchical porous graphene for high-power lithium–sulfur battery applications. Adv. Funct. Mater. 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Su, D.W.; Cortie, M.; Wang, G.X. Fabrication of N-doped graphene–carbon nanotube hybrids from prussian blue for lithium–sulfur batteries. Adv. Energy Mater. 2017, 7, 1602014. [Google Scholar] [CrossRef]
- Ummethala, R.; Fritzsche, M.; Jaumann, T.; Balach, J.; Oswald, S.; Nowak, R.; Sobczak, N.; Kaban, I.; Rümmeli, M.H.; Giebeler, L. Lightweight, free-standing 3D interconnected carbon nanotube foam as a flexible sulfur host for high performance lithium-sulfur battery cathodes. Energy Storage Mater. 2018, 10, 206–215. [Google Scholar] [CrossRef]
- Lu, S.; Chen, Y.; Wu, X.; Wang, Z.; Li, Y. Three-dimensional sulfur/graphene multifunctional hybrid sponges for lithium-sulfur batteries with large areal mass loading. Sci. Rep. 2014, 4, 4629. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Wei, Y.Q.; Ren, J.; Zhang, W.L.; Zhang, C.W.; Zhang, Y.G. Three-dimensionally ordered hierarchically porous polypyrrole loading sulfur as high-performance cathode for lithium/sulfur batteries. Polymer 2018, 137, 261–268. [Google Scholar] [CrossRef]
- Li, C.X.; Yu, J.Y.; Xue, S.L.; Cheng, Z.H.; Sun, G.Q.; Zhang, J.; Huang, R.D.; Qu, L.T. Wood-inspired multi-channel tubular graphene network for high-performance lithium-sulfur batteries. Carbon 2018, 139, 522–530. [Google Scholar] [CrossRef]
- Gu, X.X.; Tong, C.J.; Wen, B.; Liu, L.M.; Lai, C.; Zhang, S.Q. Ball-milling synthesis of ZnO@sulphur/carbon nanotubes and Ni(OH)2@sulphur/carbon nanotubes composites for high-performance lithium-sulphur batteries. Electrochim. Acta 2016, 196, 369–376. [Google Scholar] [CrossRef]
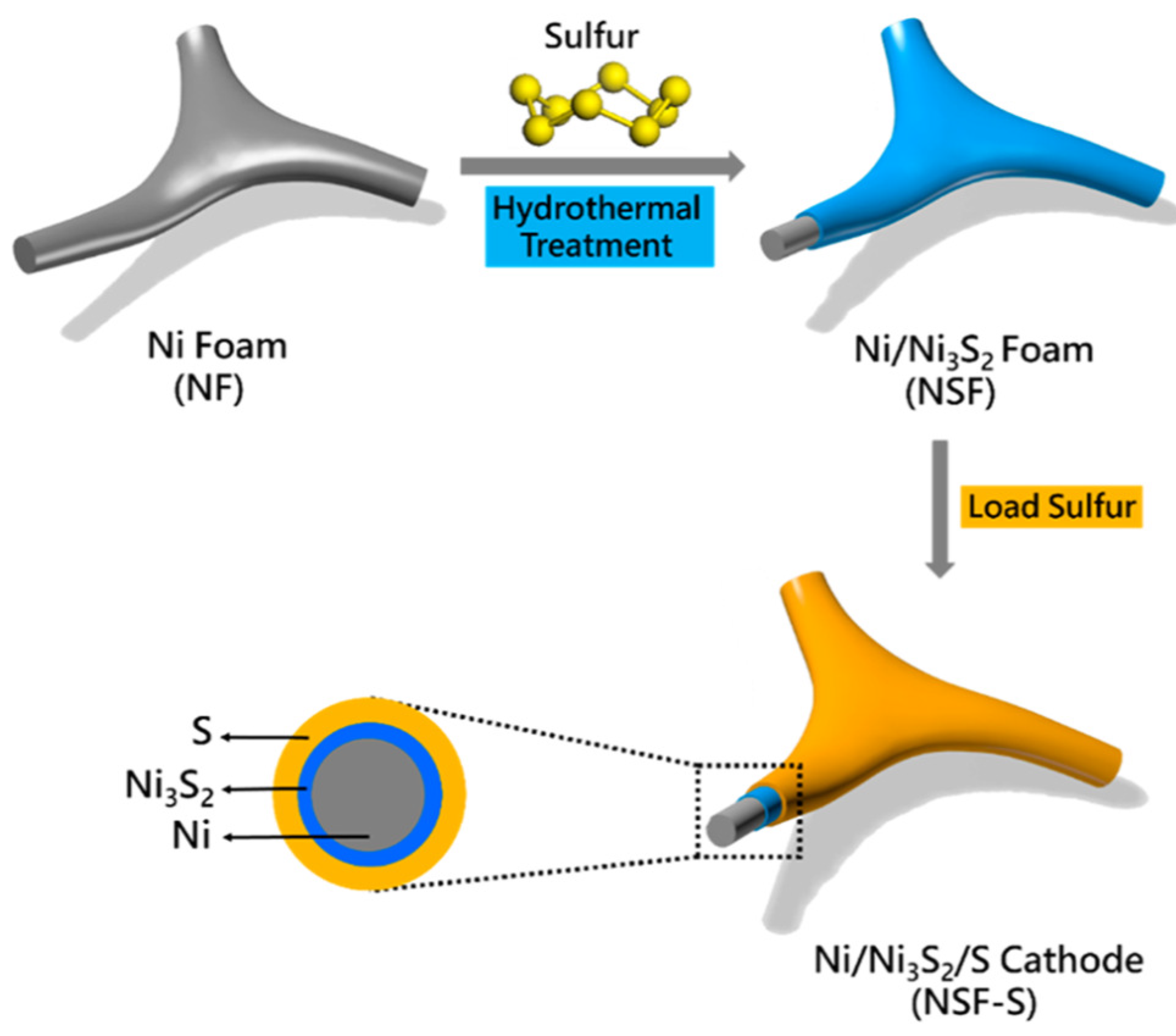
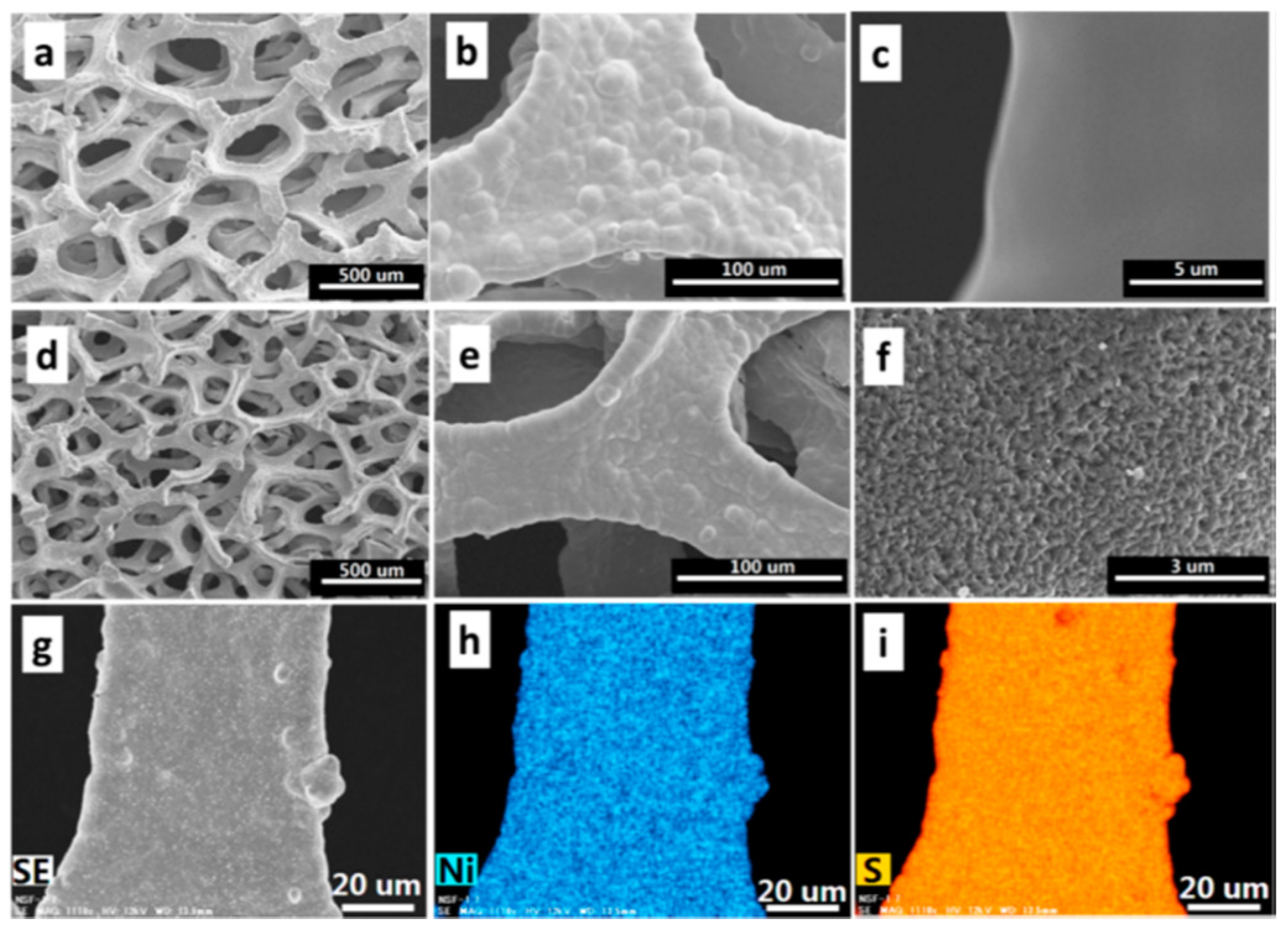
- Li, Z.; Zhang, S.G.; Zhang, J.H.; Xu, M.; Tatara, R.; Dokko, K.; Watanabe, M. Three-Dimensionally Hierarchical Ni/Ni3S2/S Cathode for Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2017, 9, 38477–38485. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Z.; Zhao, W.; Kong, L.; Zhang, L.; Zhu, X.Y.; Shao, Y.L.; Ding, F.; Zhang, Q.; Sun, J.Y.; Liu, Z.F. Synchronous immobilization and conversion of polysulfides on a VO2–VN binary host targeting high sulfur load Li–S batteries. Energy Environ. Sci. 2018, 11, 2620–2630. [Google Scholar] [CrossRef]
- Li, N.; Gan, F.Y.; Wang, P.; Chen, K.H.; Chen, S.Y.; He, X. In situ synthesis of 3D sulfur-doped graphene/sulfur as a cathode material for lithium-sulfur batteries. J. Alloy. Compd. 2018, 754, 64–71. [Google Scholar] [CrossRef]
- Wu, R.; Chen, S.G.; Deng, J.H.; Huang, X.; Song, Y.J.; Gan, R.Y.; Wan, X.J.; Wei, Z.D. Hierarchically porous nitrogen-doped carbon as cathode for lithium–sulfur batteries. J. Energy Chem. 2018, 27, 1661–1667. [Google Scholar] [CrossRef]
- Ji, P.H.; Shang, B.; Peng, Q.M.; Hu, X.B.; Wei, J.W. α-MoO3 spheres as effective polysulfides adsorbent for high sulfur content cathode in lithium-sulfur batteries. J. Power Sources 2018, 400, 572–579. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.Y.; Chen, X.B.; Wang, L.; Ye, Z.Z. Powder metallurgy template growth of 3D N-doped graphene foam as binder-free cathode for high-performance lithium/sulfur battery. Carbon 2018, 137, 368–378. [Google Scholar] [CrossRef]
- Hao, Q.Y.; Cui, G.L.; Tian, Y.; Tan, T.Z.; Zhang, Y.G. Three-dimensional S/CeO2/rGO composites as cathode materials for lithium–sulfur batteries. Materials 2018, 11, 1720. [Google Scholar] [CrossRef] [PubMed]
- He, J.R.; Chen, Y.F.; Lv, W.Q.; Wen, K.C.; Li, P.J.; Qi, F.; Wang, Z.G.; Zhang, W.L.; Li, Y.R.; Qin, W.; He, W.D. Highly-flexible 3D Li2S/graphene cathode for high-performance lithium sulfur batteries. J. Power Sources 2016, 327, 474–480. [Google Scholar] [CrossRef]
- Li, C.X.; Xi, Z.C.; Dong, S.H.; Ge, X.L.; Li, Z.Q.; Wang, C.X.; Yin, L.W. CNTs/MOFs-derived carbon/Al2(OH)2.76F3.24/S cathodes for high-performance lithium-sulfur batteries. Energy Storage Mater. 2018, 12, 341–351. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.G.; Kaiser, M.R.; Gao, X.W.; Konstantinov, K.; Tandiono, R.; Wang, Z.X.; Liu, H.K.; Dou, S.X.; Wang, J.Z. Split-half-tubular polypyrrole@sulfur@polypyrrole composite with a novel three-layer-3D structure as cathode for lithium/sulfur batteries. Nano Energy 2015, 11, 587–599. [Google Scholar] [CrossRef]
- Gong, Y.; Fu, C.P.; Zhang, G.P.; Zhou, H.H.; Kuang, Y.F. Three-dimensional porous C3N4 nanosheets@reduced graphene oxide network as sulfur hosts for high performance lithium-sulfur batteries. Electrochim. Acta 2017, 256, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.A.; Li, Q.; Zhang, K.; Chen, W.; Lai, Y.Q.; Li, J. Titanium-dioxide-grafted carbon paper with immobilized sulfur as a flexible free-standing cathode for superior lithium–sulfur batteries. J. Power Sources 2015, 290, 159–167. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, L.Y.; Ding, Z.Q.; He, Y. Ultrafine Nd2O3 nanoparticles doped carbon aerogel to immobilize sulfur for high performance lithium–sulfur batteries. J. Electroanal. Chem. 2017, 799, 617–624. [Google Scholar] [CrossRef]
- Deng, N.P.; Ju, J.G.; Yan, J.; Zhou, X.H.; Qin, Q.Q.; Zhang, K.; Liang, Y.Y.; Li, Q.X.; Kang, W.M.; Cheng, B. CeF3-doped porous carbon nanofibers as sulfur immobilizers in cathode material for high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 12626–12638. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Lu, Q.; Jiang, S.X.; Huang, C.; Wang, X.Y.; Wu, B.; Xiang, K.X.; Wu, Y.T. MnO2 nanosheets grown on the internal/external surface of N-doped hollow porous carbon nanospheres as the sulfur host of advanced lithium-sulfur batteries. Chem. Eng. J. 2018, 335, 831–842. [Google Scholar] [CrossRef]
- Zhang, J.; You, C.Y.; Zhang, W.H.; Wang, J.; Guo, S.H.; Yang, R.; Xu, Y.H. Conductive bridging effect of TiN nanoparticles on the electrochemical performance of TiN@CNT-S composite cathode. Electrochim. Acta 2017, 250, 159–166. [Google Scholar] [CrossRef]
- Iqbal, A.; Ali Ghazi, Z.; Muqsit Khattak, A.; Ahmad, A. Efficient sulfur host based on NiCo2O4 hollow microtubes for advanced Li-S batteries. J. Solid State Chem. 2017, 256, 189–195. [Google Scholar] [CrossRef]
- Li, X.L.; Chu, L.B.; Wang, Y.Y.; Pan, L.S. Anchoring function for polysulfide ions of ultrasmall SnS2 in hollow carbon nanospheres for high performance lithium–sulfur batteries. Mater. Sci. Eng. B 2016, 205, 46–54. [Google Scholar] [CrossRef]
- Li, C.C.; Liu, X.B.; Zhu, L.; Huang, R.Z.; Zhao, M.W.; Xu, L.Q.; Qian, Y.T. Conductive and polar titanium boride as a sulfur host for advanced lithium–sulfur batteries. Chem. Mater. 2018, 30, 6969–6977. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.X.; Hao, Z.X.; Liu, X.X.; Xiang, J.W.; Zhang, Z.R.; Huang, Y.H.; Xie, J. Free-standing Mn3O4@CNF/S paper cathodes with high sulfur loading for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 13406–13412. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.M.; Noh, H.J.; Lee, J.H.; Lee, H.K.; Kim, H.T. Li2S/Carbon nanocomposite strips from a low-temperature conversion of Li2SO4 as high- performance lithium-sulfur cathodes. J. Mater. Chem. A 2018, 6, 6617–6624. [Google Scholar] [CrossRef]
- Chen, A.; Liu, W.F.; Hu, H.; Chen, T.; Ling, B.L.; Liu, K.Y. Facile preparation of ultrafine Ti4O7 nanoparticle-embedded porous carbon for high areal capacity lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 20083–20092. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, N.; Zhu, C.C.; Gao, H.; Zhang, X.T. Rationally designing S/Ti3C2Tx as a cathode material with an interlayer for high-rate and long-cycle lithium-sulfur batteries. Nanoscale 2018, 10, 16935–16942. [Google Scholar] [CrossRef] [PubMed]
- He, J.R.; Chen, Y.F.; Lv, W.Q.; Wen, K.C.; Xu, C.; Zhang, W.L.; Li, Y.R.; Qin, W.; He, W.D. From metal–organic framework to Li2S@C–Co–N nanoporous architecture: A high-capacity cathode for lithium–sulfur batteries. ACS Nano 2016, 10, 10981–10987. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.Q.; Nie, H.G.; Yang, Z.; Hua, W.X.; Ruan, C.P.; Chan, D.; Ge, M.Z.; Chen, X.A.; Huang, S.M. 3D CNTs/graphene-S-Al3Ni2 cathodes for high-sulfur-loading and long-life lithium–sulfur batteries. Adv. Sci. 2018, 5, 1800026. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, X. Bamboo-like Co3O4 nanofiber as host materials for enhanced lithium-sulfur battery performance. J. Alloy. Compd. 2019, 777, 688–692. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, Q.; Zhang, P.; Zheng, W.; Chen, J.; Zhou, A.; Tian, W.; Zhang, W.; Sun, Z. Self-assembled 3D MnO2 nanosheets@delaminated-Ti3C2 aerogel as sulfur host for lithium–sulfur battery cathodes. ACS Appl. Energy Mater. 2019, 2, 705–714. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Yang, P.; Zhang, H.; Du, C.; Xu, J.; Song, K. S@TiO2 nanospheres loaded on PPy matrix for enhanced lithium-sulfur batteries. J. Alloy. Compd. 2019, 783, 279–285. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, J.; Mei, T.; Zhou, S.; Hou, B.; Li, J.; Li, J.; Wang, J.; Qian, J.; Wang, X. Strong lithium polysulfides chemical trapping of TiC-TiO2/S composite for long-cycle lithium-sulfur batteries. Electrochim. Acta 2019, 298, 43–51. [Google Scholar] [CrossRef]
- Daniel, P.; Tabor, L.M.R.; Semion, K.S.; Christoph, K.; Dennis, S.; Joseph, H.M.; Shyam, D.; Muratahan, A.; Carlos, O.; Hermann, T.; et al. Accelerating the discovery of materials for clean energy in the era of smart automation. Nat. Rev. Mater. 2018, 3, 5–20. [Google Scholar]
- Garg, A.; Peng, X.B.; Le, M.L.P.; Pareek, K.; Chind, C.M.M. Design and analysis of capacity models for lithium-ion battery. Measurement 2018, 120, 114–120. [Google Scholar] [CrossRef]
- Li, J.; Zou, L.L.; Tian, F.; Dong, X.W.; Zou, Z.Q.; Yang, H. Parameter identification of lithium-ion batteries model to predict discharge behaviors using heuristic algorithm. J. Electrochem. Soc. 2016, 163, A1646–A1652. [Google Scholar] [CrossRef]
- Garg, A.; Vijayaraghavan, V.; Zhang, J.; Li, S.; Liang, X.Y. Design of robust battery capacity model for electric vehicle by incorporation of uncertainties. Int. J. Energy Res. 2017, 41, 1436–1451. [Google Scholar] [CrossRef]


| Cathode Materials | Preparation Method | Cycling Rate | Cycle Number | Capacity (mAh g−1) | References |
|---|---|---|---|---|---|
| Li3VP3ON | Solid-solid ion-exchange method | 20 C | 50 | 70 | [51] |
| Li3V2(PO4)3/C | Sol-gel method | 30 C | 35 | 85 | [36] |
| LiAlO2-LiMnPO4/C | Sol-gel method | 10 C | 100 | 105 | [37] |
| (1 − x) LiNi0.5Mn1.5O4- xLi2SiO3 | Sol-gel method | 2 C | 50 | 150.3 | [52] |
| G/LiFePO4/G | Hydrothermal method | 10 C | 100 | 124 | [53] |
| Li3Mo4P5O24 | - | 50 C | 20 | 110 | [54] |
| Li2FeSiO4/C | - | 10 C | 420 | 239 | [55] |
| LiCaFeF6 | Solid-state reaction | 20 C | 20 | 112 | [41] |
| CNT@Li2MnSiO4@C | - | 0.2 C | 50 | 227 | [56] |
| Li3FeF6 | Sol-gel Mechanical stirring | 50 mA g−1 | 100 | 174 | [57] |
| LiFePO4@C | Hydrothermal | 10 C | 500 | 117 | [58] |
| LiMnO2 | In-situ carbothermal reduction method | 0.1 C | 40 | 165.3 | [48] |
| LiCoPO4 | Microwave-assisted solvothermal | 0.1 C | 20 | 141 | [59] |
| Li2MnO3 | Sol-gel method | 0.1 C | 100 | 225 | [60] |
| Li2FeSiO4 | Polyol method | 20 C | 50 | 270 | [61] |
| LiVPO4F/C | Sol-gel method | 10 C | 20 | 121.1 | [62] |
| LiMn0.8Fe0.2PO4 | Solvothermal method | 3 C | 35 | 171 | [63] |
| LiMnBO3@C | Sol-gel method | 0.05 C | 50 | 159.7 | [64] |
| LiMn2O4 | Hydrothermal method | 0.2 C | 1000 | 143.4 | [65] |
| LiMnTiO4 | Vacuum filtration method | 0.5 C | 50 | 161 | [50] |
| Nano-SiO2@Li2CoPO4F | Hydrothermal method | 2 C | 60 | 79.4 | [66] |
| LiVP2O7/C | Sol-gel method | 0.05 C | 50 | 102.3 | [67] |
| LiFeBO3/C | Spray-drying | 0.05 C | 105 | 201.5 | [68] |
| LiV3O8 | High-temperature calcination | 60 mA g−1 | 100 | 212.8 | [69] |
| Li2CoSiO4/C | Hydrothermal method | 36 mA g−1 | 100 | 144 | [70] |
| LiMo4O6 | Ion-exchange method | 0.05 C | 50 | 36.3 | [71] |
| LiNi0.5Co0.2Mn0.3O2 | - | 0.1 C | 100 | 164.6 | [72] |
| LiCoBO3 | Sol-gel method | 10 C | 52 | 98 | [41] |
| LiNi0.08Mn1.92O4 | Solution combustion method | 1 C | 1000 | 95.7 | [73] |
| LiNi0.6Co0.4-zTizO2 | Solid-state method | 20 | 50 | 100 | [42] |
| LiFe0.4Mn0.4Co0.2PO4/C | coprecipitation-and-milling method | 1 C | 100 | 104.7 | [74] |
| Li2Ru0.8Ti0.2O3 | 100 mA g−1 | 90 | 196.1 | [75] | |
| Li4Ti5O12- LiNi0.5Mn1.5O4 | Solvothermal method | 0.5 C | 100 | 122.6 | [76] |
| Cathode Materials | Preparation Method | Cycling Rate | Cycle Number | Capacity (mAh g−1) | S/E (Weight Ratio) | References |
|---|---|---|---|---|---|---|
| 3D sulfur-doped graphene | One-pot wet chemical method | 0.5 C | 350 | 785 | 80% | [86] |
| Hierarchically porous nitrogen-doped carbon | - | 0.1 C | 300 | 1355 | 69% | [87] |
| α-MoO3 | - | 0.5 C | 400 | 912.8 | 68% | [88] |
| Porous polypyrrole loading sulfur | Chemical polymerization method | 0.1 C | 100 | 751 | 59% | [81] |
| Wood inspired multi-channel tubular graphene | Template-directed chemical vapor deposition | 0.1 C | 500 | 1390 | 70% | [82] |
| 3D N-doped graphene foam | Annealing method in chemical vapor deposition | 0.2 C | 200 | 819 | 2.05 mg cm−2 | [89] |
| S/CeO2/RGO (reduced graphene oxide) | Hydrothermal method | 0.1 C | 20 | 792 | 64% | [90] |
| Li2S/graphene | Infiltration method | 0.1 C | 300 | 894.7 | [91] | |
| CNTs/MOFs-C/Al2(OH)2.76F3.24/S | - | 500 mA g−1 | 300 | 889 | 72% | [92] |
| Polypyrrole@sulfur@polypyrrole | Chemical precipitation method | 50 mA g−1 | 50 | 554 | 66% | [93] |
| Porous C3N4 nanosheets@RGO | - | 0.5 C | 800 | 680 | 68% | [94] |
| Titanium-dioxide-grafted carbon paper | - | 0.5 C | 200 | 850 | 40% | [95] |
| Nd2O3 nanoparticles doped carbon | Acid catalyzing method | 0.2 C | 100 | 1082 | 56% | [96] |
| CeF3-doped porous carbon nanofibers | Electroblown spinning technique and carbonization process | 0.5 C | 500 | 901.2 | 75% | [97] |
| NHCSs@MnO2/S | - | 0.5 C | 1000 | 1249 | 70% | [98] |
| ZnO@S/CNT | Ball-milling method | 160 mA g−1 | 70 | 942 | 45% | [83] |
| TiN@CNT-S | - | 0.05 C | 80 | 1269 | 5.4 mg cm−2 | [99] |
| NiCo2O4 | Solvothermal method | 0.2 C | 200 | 1274 | 27% | [100] |
| S/AHCNS-SnS2 | - | 0.2 C | 200 | 924 | 64% | [101] |
| Ni/Ni3S2/S | Hydrothermal method | 4 mA cm−1 | 150 | 441 | 71% | [84] |
| TiB2/S | Melting-diffusion method | 1 C | 100 | 837 | 70% | [102] |
| Mn3O4@CNF/S | 0.1 C | 100 | 993 | 50% | [103] | |
| Li2S/carbon | Icy water bathing method | 0.2 C | 150 | 595 | - | [104] |
| Ti4O7 nanoparticle-embedded porous carbon | - | 0.2 C | 1000 | 1445 | 77% | [105] |
| S/Ti3C2Tx | Melt-diffusion method | 5 C | 1500 | 608 | 80% | [106] |
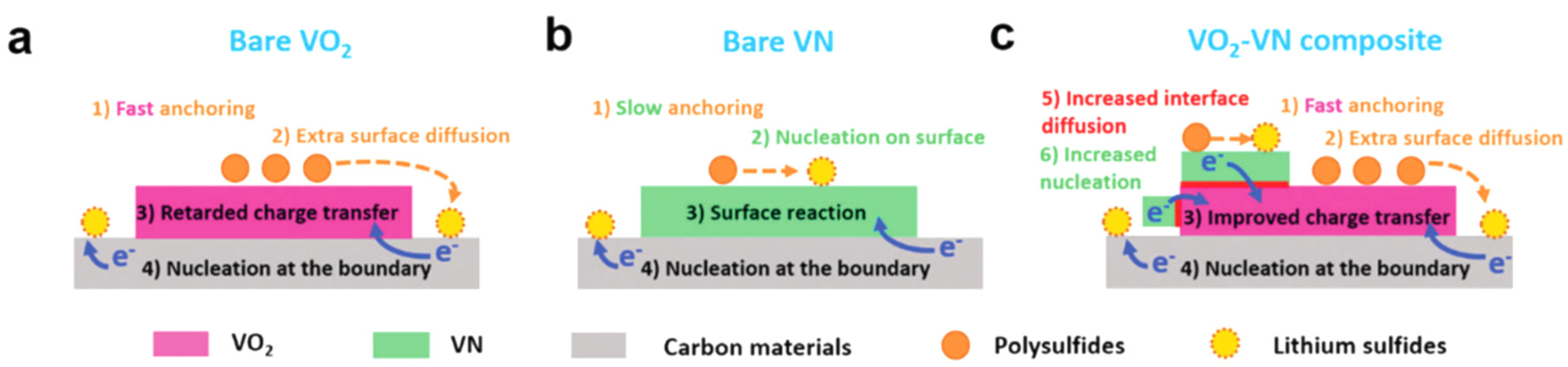
| VO2-VN | Hydrothermal | 1 C | 800 | 1105 | 62% | [85] |
| Li2S@C-Co-N | Liquid infiltration-evaporation method | 1 C | 300 | 950.6 | - | [107] |
| 3D CNTs/Graphene-S-Al3Ni2 | -- | 1 C | 800 | 496 | 65% | [108] |
| Bamboo-like Co3O4 | Hydrothermal method | 1 C | 300 | 796 | 72.6% | [109] |
| MnO2-Ti3C2 | Electrostatic self-assembly approach | 2 C | 500 | 844.5 | 70% | [110] |
| S@TiO2/PPy | - | 0.2 C | 50 | 745.6 | 72.4% | [111] |
| TiC-TiO2/S | - | 0.5 C | 500 | 714 | 1.1 mg cm−2 | [112] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Long, J.; Du, S.; Sun, B.; Zhu, S.; Li, J. Three-Dimensionally Porous Li-Ion and Li-S Battery Cathodes: A Mini Review for Preparation Methods and Energy-Storage Performance. Nanomaterials 2019, 9, 441. https://doi.org/10.3390/nano9030441
Liu J, Long J, Du S, Sun B, Zhu S, Li J. Three-Dimensionally Porous Li-Ion and Li-S Battery Cathodes: A Mini Review for Preparation Methods and Energy-Storage Performance. Nanomaterials. 2019; 9(3):441. https://doi.org/10.3390/nano9030441
Chicago/Turabian StyleLiu, Jinyun, Jiawei Long, Sen Du, Bai Sun, Shuguang Zhu, and Jinjin Li. 2019. "Three-Dimensionally Porous Li-Ion and Li-S Battery Cathodes: A Mini Review for Preparation Methods and Energy-Storage Performance" Nanomaterials 9, no. 3: 441. https://doi.org/10.3390/nano9030441
APA StyleLiu, J., Long, J., Du, S., Sun, B., Zhu, S., & Li, J. (2019). Three-Dimensionally Porous Li-Ion and Li-S Battery Cathodes: A Mini Review for Preparation Methods and Energy-Storage Performance. Nanomaterials, 9(3), 441. https://doi.org/10.3390/nano9030441






