Fast and Simple Fabrication of Superhydrophobic Coating by Polymer Induced Phase Separation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
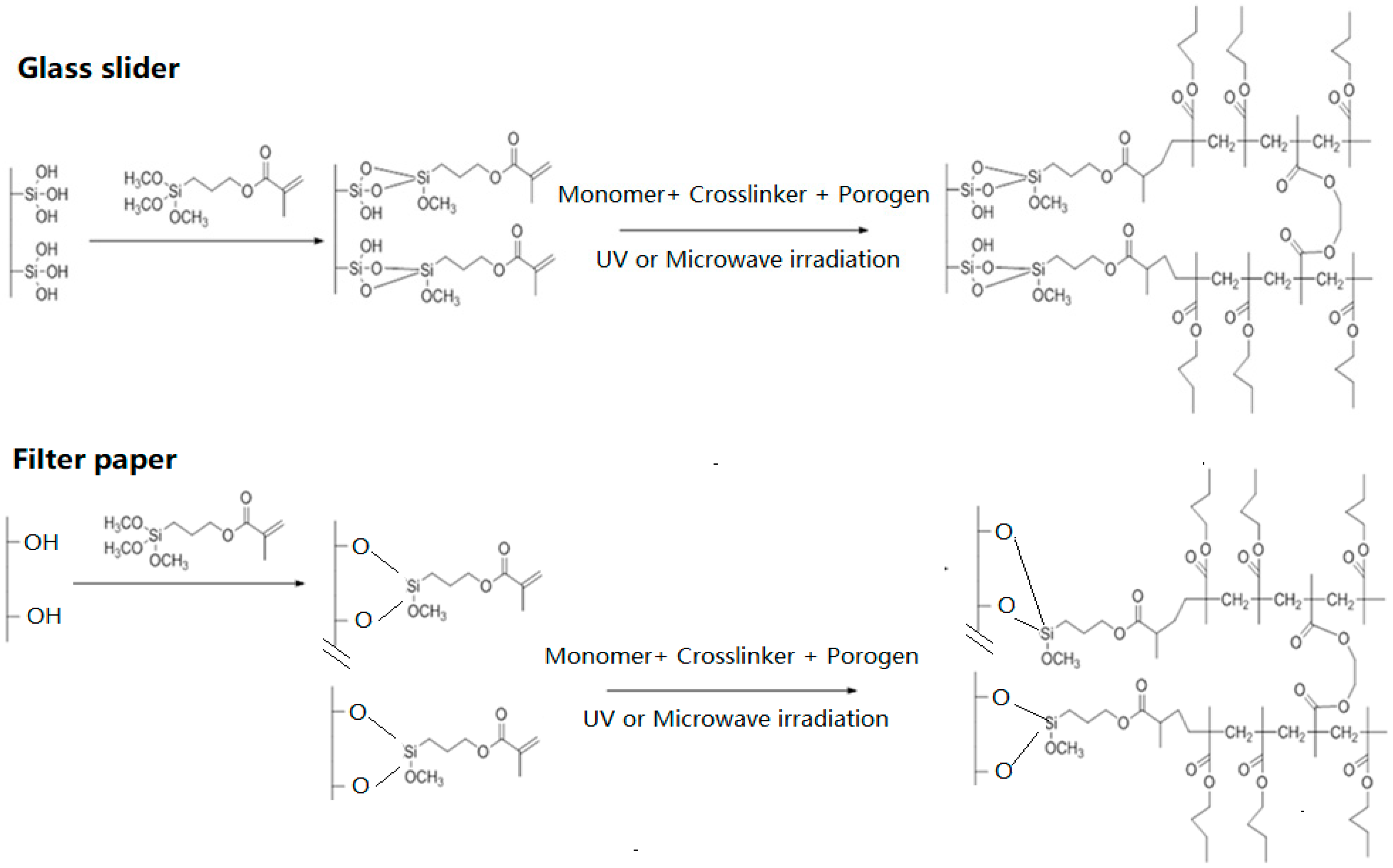
3.1. Preparation of the Monolithic Coatings by Microwave and UV Irradiations
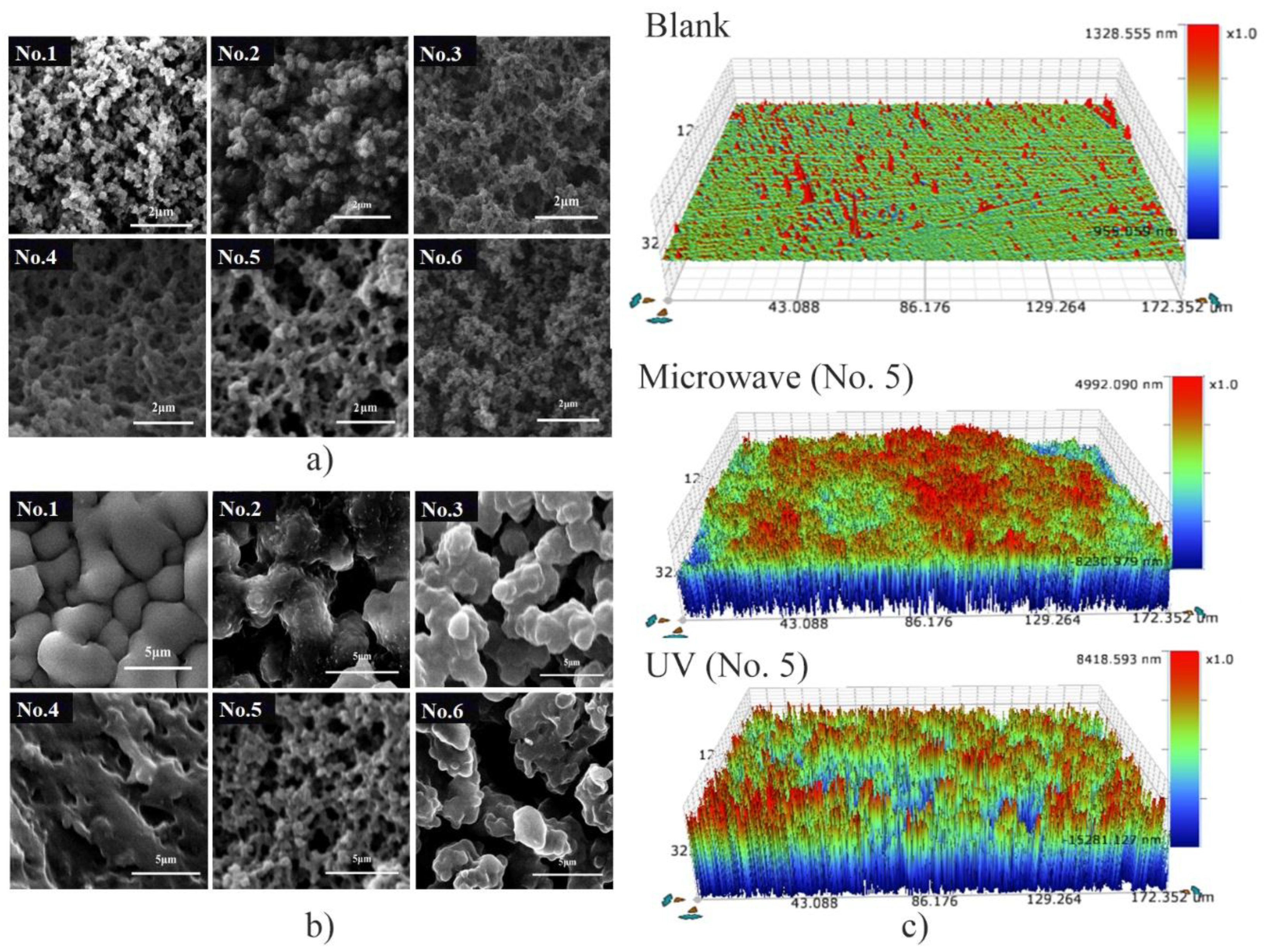
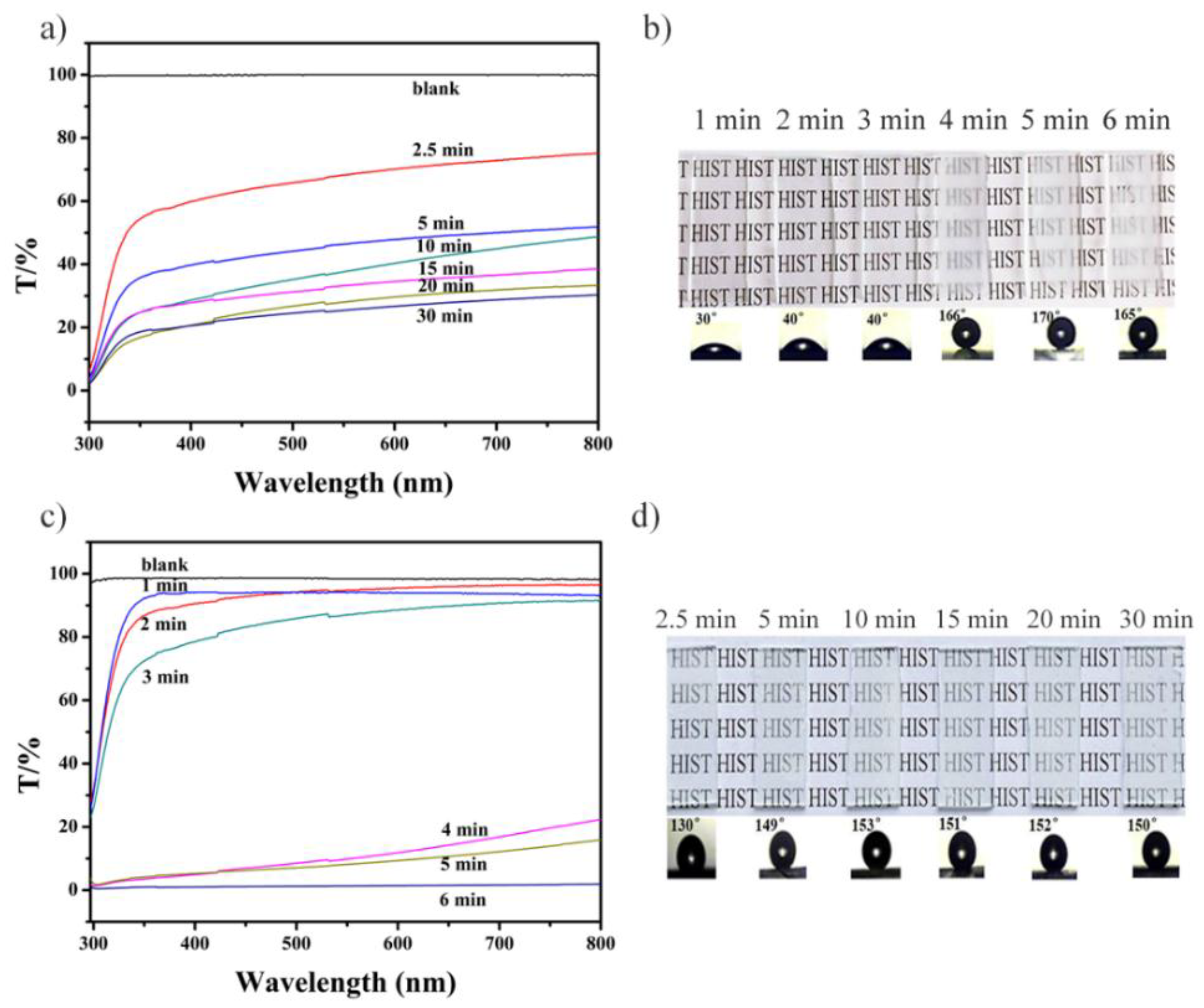
3.2. Characterization and Evaluation
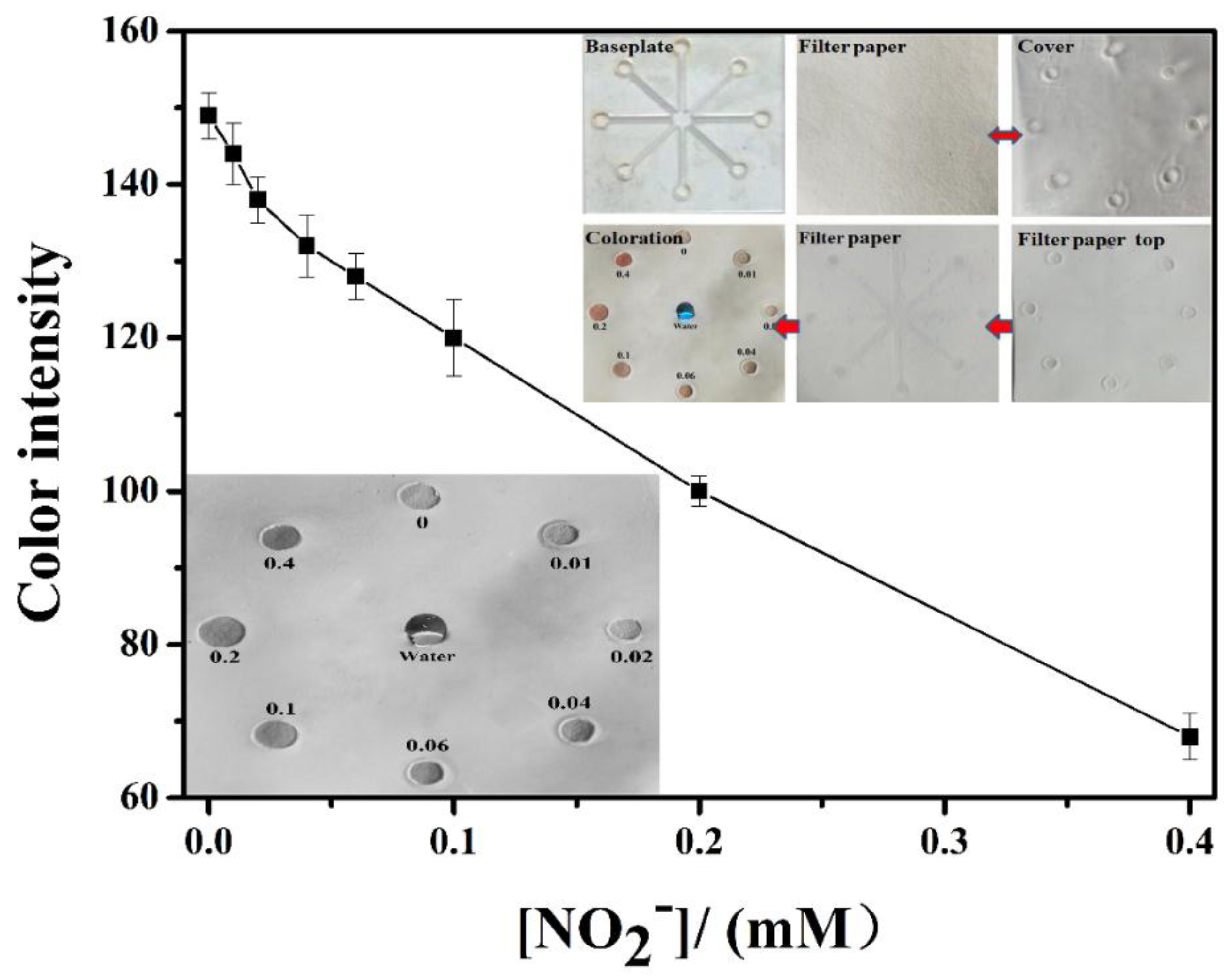
3.3. Application of the Prepared Filter Paper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, C.; He, Z.; Xie, X.; Mai, X.; Li, Y.; Li, T.; Zhao, M.; Yan, C.; Liu, H.; Wujcik, E.; et al. Controllable Cross-Linking Anion Exchange Membranes with Excellent Mechanical and Thermal Properties. Macromol. Mater. Eng. 2018, 303, 1700462–1700469. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-titania nanocomposite coating: Fabrication and corrosion resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Wang, C.; Mo, B.; He, Z.; Xie, X.; Zhao, C.; Zhang, L.; Shao, Q.; Guo, X.; Wujcik, E.; Guo, Z. Hydroxide ions transportation in polynorbornene anion exchange membrane. Polymer 2018, 138, 363–368. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, J.; Wang, F.; Liu, L.; Liu, H.; Yan, C.; Guo, Z. Sol-gel Synthesized Hexagonal Boron Nitride/Titania Nanocomposites with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2018, 465, 154–163. [Google Scholar] [CrossRef]
- Li, S.; Jasim, A.; Zhao, W.; Fu, L.; Ullah, M.; Shi, Z.; Yang, G. Fabrication of pH-Electroactive Bacterial Cellulose/Polyaniline Hydrogel for the Development of a Controlled Drug Release System. ES Mater. Manuf. 2018, 1, 41–49. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Sun, X.; Yu, H.; Yan, F.; Meguellati, K.; Cheng, Z.; Zhang, H.; Yang, Y.-W. Facile surface functionalization of upconversion nanoparticles with phosphoryl pillar [5] arenes for controlled cargo release and cell imaging. Chem. Commun. 2018, 54, 12990–12993. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.S.; Buie, C.R. Antiwetting Fabric Produced by a Combination of Layer-by-Layer Assembly and Electrophoretic Deposition of Hydrophobic Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 20100–20110. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; An, S.; Latthe, S.S.; Lee, C.; Hong, S.; Yoon, S.S. Electrospun Polystyrene Nanofiber Membrane with Superhydrophobicity and Superoleophilicity for Selective Separation of Water and Low Viscous Oil. ACS Appl. Mater. Interfaces 2013, 5, 10597–10604. [Google Scholar] [CrossRef] [PubMed]
- Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, B.; Qin, X.; Wang, Y.; Liu, C.; Shao, Q.; Wang, N.; Zhang, J.; Shen, C.; Guo, Z. Superhydrophobic/Superoleophilic polycarbonate/carbon nanotubes porous monolith for selective oil adsorption from water. ACS Sustain. Chem. Eng. 2018, 6, 13747–13755. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, L.; Liu, X.; Wu, L.; Dai, K.; Liu, C.; Shen, C.; Guo, X.; Zheng, G.; Guo, Z. Superhydrophobic Shish-kebab Membrane with Self-cleaning and Oil/Water Separation Properties. ACS Sustain. Chem. Eng. 2018, 6, 9866–9875. [Google Scholar] [CrossRef]
- Kang, H.; Cheng, Z.; Lai, H.; Ma, H.; Liu, Y.; Mai, X.; Wang, Y.; Shao, Q.; Xiang, L.; Guo, X.; Guo, Z. Superlyophobic Anti-corrosive and Self-cleaning Titania Robust Mesh Membrane with Enhanced Oil/Water Separation, Separation and Purification Technology. Sep. Purif. Technol. 2018, 201, 193–204. [Google Scholar] [CrossRef]
- Nischang, I.; Causon, T.J. Porous polymer monoliths: From their fundamental structure to analytical engineering applications. TrAC Trends Anal. Chem. 2016, 75, 108–117. [Google Scholar] [CrossRef]
- Levkin, P.A.; Svec, F.; Fréchet, J.M.J. Porous Polymer Coatings: A Versatile Approach to Superhydrophobic Surfaces. Adv. Funct. Mater. 2009, 19, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Porcar, R.; Nuevo, D.; García-Verdugo, E.; Lozano, P.; Sanchez-Marcano, J.; Burguete, M.I.; Luis, S.V. New porous monolithic membranes based on supported ionic liquid-like phases for oil/water separation and homogenous catalyst immobilisation. Chem. Commun. 2018, 54, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Sato, A. Micro/nanotextured polymer coatings fabricated by UV curing-induced phase separation: Creation of superhydrophobic surfaces. J. Mater. Chem. 2012, 22, 8613–8621. [Google Scholar] [CrossRef]
- Xiao, L.; Li, J.; Mieszkin, S.; Di Fino, A.; Clare, A.S.; Callow, M.E.; Callow, J.A.; Grunze, M.; Rosenhahn, A.; Levkin, P.A. Slippery Liquid-Infused Porous Surfaces Showing Marine Antibiofouling Properties. ACS Appl. Mater. Interfaces 2013, 5, 10074–10080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Ye, X.-W.; Tian, M.-K.; Qu, L.-B.; Choi, S.-H.; Gopalan, A.I.; Lee, K.-P. Novel method to prepare polystyrene-based monolithic columns for chromatographic and electrophoretic separations by microwave irradiation. J. Chromatogr. A 2008, 1188, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Fan, L.-Q.; Gong, W.-J.; Zhang, Y.-P.; Qu, L.-B.; Lee, K.-P. Rapid Preparation and Characterization of Methacrylate-Based Monoliths for Chromatographic and Electrophoretic Separation. J. Chromatogr. Sci. 2010, 48, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Hoshian, S.; Jokinen, V.; Franssila, S. Robust hybrid elastomer/metal-oxide superhydrophobic surfaces. Soft Matter 2016, 12, 6526–6535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Wang, X.; Lin, T. Fluoroalkyl Silane Modified Silicone Rubber/Nanoparticle Composite: A Super Durable, Robust Superhydrophobic Fabric Coating. Adv. Mater. 2012, 24, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.; Emelyanenko, A.M.; Pashinin, A.S. Analysis of Long-Term Durability of Superhydrophobic Properties under Continuous Contact with Water. ACS Appl. Mater. Interfaces 2010, 2, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, I.; Bernagozzi, I.; Antonini, C.; Marengo, M. Assessing durability of superhydrophobic surfaces. Surf. Innov. 2014, 3, 49–60. [Google Scholar] [CrossRef]
- Lopez-Ruiz, N.; Curto, V.F.; Erenas, M.M.; Benito-Lopez, F.; Diamond, D.; Palma, A.J.; Capitan-Vallvey, L.F. Smartphone-Based Simultaneous pH and Nitrite Colorimetric Determination for Paper Microfluidic Devices. Anal. Chem. 2014, 86, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kwok, H.; Li, X.; Yu, H.-Z. Superhydrophobic Substrates from Off-the-Shelf Laboratory Filter Paper: Simplified Preparation, Patterning, and Assay Application. ACS Appl. Mater. Interfaces 2017, 9, 39728–39735. [Google Scholar] [CrossRef] [PubMed]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper−Plastic Hybrid Microfluidic Device for Smartphone-Based Colorimetric Analysis of Urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ma, C.; Hu, X.; Chen, H. Method for Fabrication of Paper-Based Microfluidic Devices by Alkylsilane Self-Assembling and UV/O3-Patterning. Anal. Chem. 2013, 85, 1327–1331. [Google Scholar] [CrossRef] [PubMed]

| Mixture No. | Monomer | Crosslinker | Porogen | Percentage of Porogen (%) | Average WCAs (°) | |
|---|---|---|---|---|---|---|
| BMA (μL) | EDMA (μL) | Butanediol (μL) | 1-Propanol (μL) | UV | ||
| 1 | 600 | 400 | 156 | 244 | 29 | 94° |
| 2 | 600 | 400 | 233 | 367 | 38 | 100° |
| 3 | 600 | 400 | 350 | 550 | 47 | 107° |
| 4 | 600 | 400 | 506 | 794 | 57 | 95° |
| 5 | 600 | 400 | 700 | 1100 | 64 | 109° |
| 6 | 600 | 400 | 1167 | 1833 | 75 | 95° |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-P.; Li, P.-P.; Liu, P.-F.; Zhang, W.-Q.; Wang, J.-C.; Cui, C.-X.; Li, X.-J.; Qu, L.-B. Fast and Simple Fabrication of Superhydrophobic Coating by Polymer Induced Phase Separation. Nanomaterials 2019, 9, 411. https://doi.org/10.3390/nano9030411
Zhang Y-P, Li P-P, Liu P-F, Zhang W-Q, Wang J-C, Cui C-X, Li X-J, Qu L-B. Fast and Simple Fabrication of Superhydrophobic Coating by Polymer Induced Phase Separation. Nanomaterials. 2019; 9(3):411. https://doi.org/10.3390/nano9030411
Chicago/Turabian StyleZhang, Yu-Ping, Pan-Pan Li, Peng-Fei Liu, Wan-Qing Zhang, Ji-Chao Wang, Cheng-Xing Cui, Xiang-Jun Li, and Ling-Bo Qu. 2019. "Fast and Simple Fabrication of Superhydrophobic Coating by Polymer Induced Phase Separation" Nanomaterials 9, no. 3: 411. https://doi.org/10.3390/nano9030411
APA StyleZhang, Y.-P., Li, P.-P., Liu, P.-F., Zhang, W.-Q., Wang, J.-C., Cui, C.-X., Li, X.-J., & Qu, L.-B. (2019). Fast and Simple Fabrication of Superhydrophobic Coating by Polymer Induced Phase Separation. Nanomaterials, 9(3), 411. https://doi.org/10.3390/nano9030411





