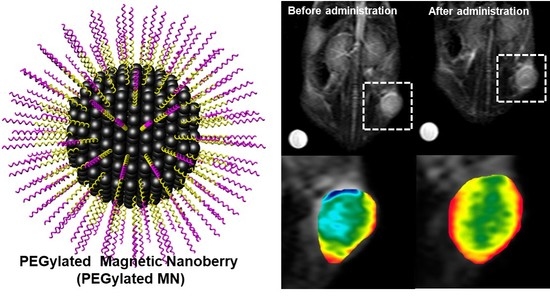
PEGylated Magnetic Nano-Assemblies as Contrast Agents for Effective T2-Weighted MR Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetic Nanocrystals (MNCs)
2.3. Synthesis of mPEG-PLA by Ring-Opening Polymerization
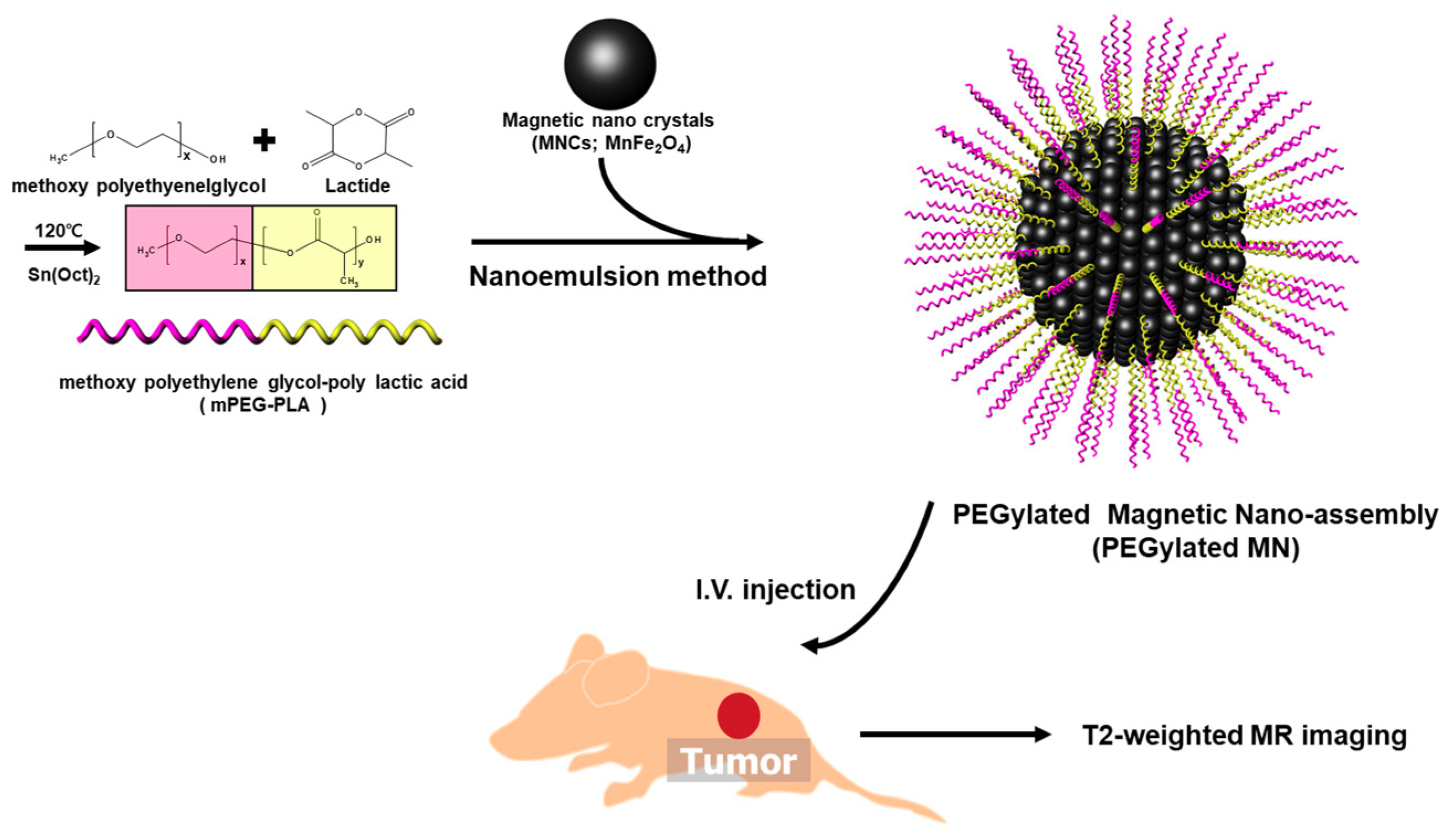
2.4. Preparation of PEGylated Magnetic Nano-Assemblies (PEGylated MNs)
2.5. Biocompatibility Tests
2.6. In Vivo (Mouse Xenograft Tumor) Model Procedure
2.7. MR Imaging Procedure
3. Results and Discussion
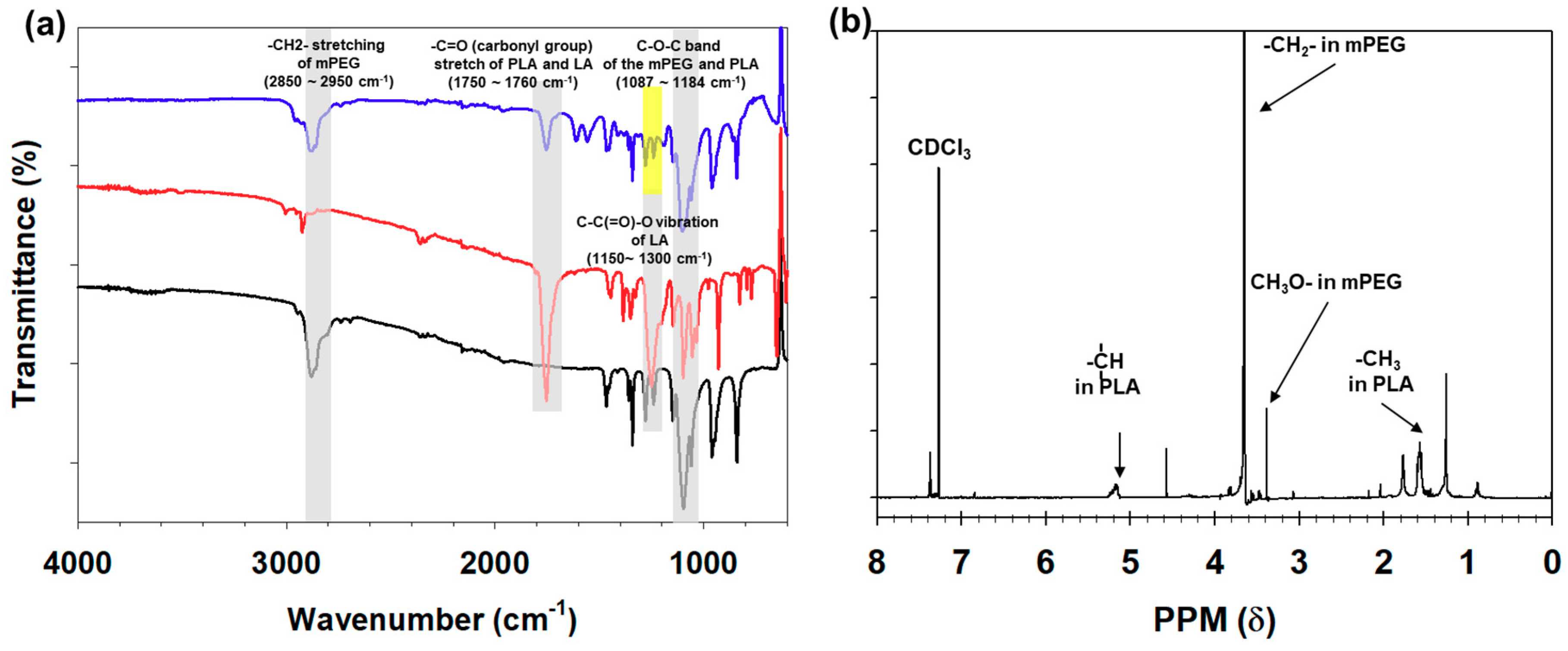
3.1. Synthesis and Characterization of mPEG-PLA
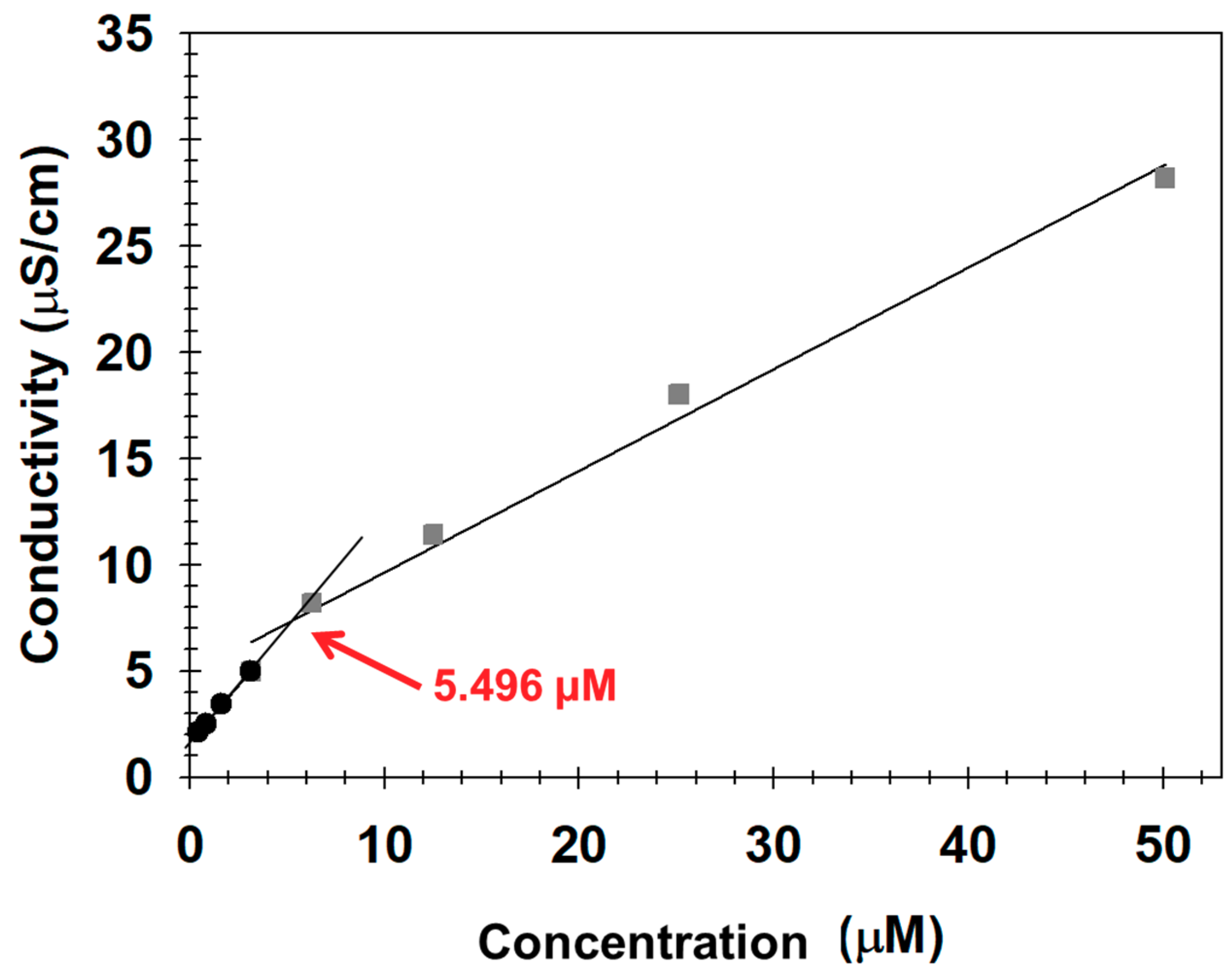
3.2. Formulation and Characterization of PEGylated MNs
3.3. Biocompatibility of the PEGylated MNs
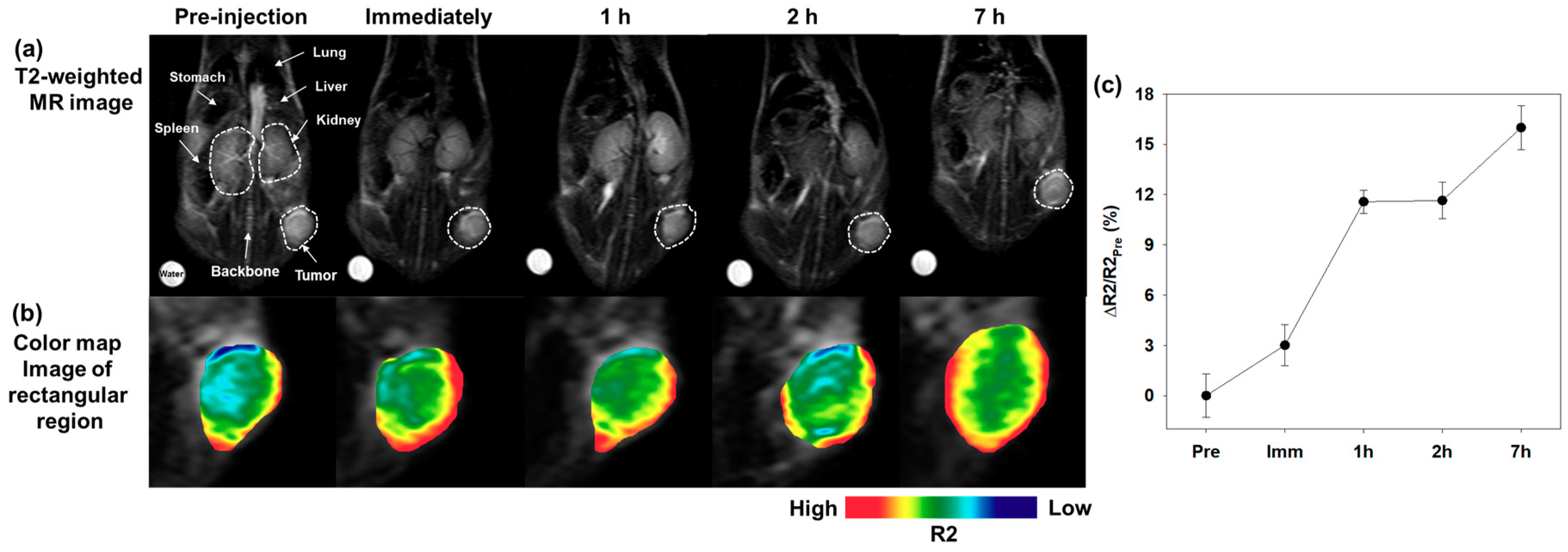
3.4. In Vivo MR Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arami, H.; Khandhar, A.P.; Tomitaka, A.; Yu, E.; Goodwill, P.W.; Conolly, S.M.; Krishnan, K.M. In vivo multimodal magnetic particle imaging (MPI) with tailored magneto/optical contrast agents. Biomaterials 2015, 52, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, B.; Lim, E.K.; Choi, Y.; Choi, J.; Kim, E.; Jang, E.; Park, H.S.; Suh, J.S.; Huh, Y.M.; et al. Magnetic nanoclusters engineered by polymer-controlled self-assembly for the accurate diagnosis of atherosclerotic plaques via magnetic resonance imaging. Macromol. Biosci. 2014, 14, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Huh, Y.M.; Jun, Y.W.; Seo, J.W.; Jang, J.T.; Song, H.T.; Kim, S.; Cho, E.J.; Yoon, H.G.; Suh, J.S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Jeong, J.-R.; Shin, S.-C.; Kim, J.-C.; Chang, Y.-H.; Chang, Y.-M.; Kim, J.-D. Nanoparticles of magnetic ferric oxides encapsulated with poly(D,L latide-co-glycolide) and their applications to magnetic resonance imaging contrast agent. J. Magn. Magn. Mater. 2004, 272–276, 2432–2433. [Google Scholar] [CrossRef]
- Li, Z.; Yin, S.; Cheng, L.; Yang, K.; Li, Y.; Liu, Z. Magnetic targeting enhanced theranostic strategy based on multimodal imaging for selective ablation of cancer. Adv. Funct. Mater. 2014, 24, 2312–2321. [Google Scholar] [CrossRef]
- Lim, E.K.; Huh, Y.M.; Yang, J.; Lee, K.; Suh, J.S.; Haam, S. pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Adv. Mater. 2011, 23, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Kang, B.; Choi, Y.; Jang, E.; Han, S.; Lee, K.; Suh, J.S.; Haam, S.; Huh, Y.M. Gadolinium-based nanoparticles for highly efficient T1-weighted magnetic resonance imaging. Nanotechnology 2014, 25, 245103–245112. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Kim, B.; Choi, Y.; Ro, Y.; Cho, E.J.; Lee, J.H.; Ryu, S.H.; Suh, J.S.; Haam, S.; Huh, Y.M. Aptamer-conjugated magnetic nanoparticles enable efficient targeted detection of integrin alphavbeta3 via magnetic resonance imaging. J. Biomed. Mater. Res. A 2014, 102, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Kim, H.O.; Jang, E.; Park, J.; Lee, K.; Suh, J.S.; Huh, Y.M.; Haam, S. Hyaluronan-modified magnetic nanoclusters for detection of CD44-overexpressing breast cancer by MR imaging. Biomaterials 2011, 32, 7941–7950. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Yang, J.; Dinney, C.P.; Suh, J.S.; Huh, Y.M.; Haam, S. Self-assembled fluorescent magnetic nanoprobes for multimode-biomedical imaging. Biomaterials 2010, 31, 9310–9319. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Yang, J.; Park, M.Y.; Park, J.; Suh, J.S.; Yoon, H.G.; Huh, Y.M.; Haam, S. Synthesis of water soluble PEGylated magnetic complexes using mPEG-fatty acid for biomedical applications. Colloids Surf. B Biointerfaces 2008, 64, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Jang, E.; Kim, B.; Choi, J.; Lee, K.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Dextran-coated magnetic nanoclusters as highly sensitive contrast agents for magnetic resonance imaging of inflammatory macrophages. J. Mater. Chem. 2011, 21, 12473–12478. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Wuang, S.C.; Neoh, K.G.; Kang, E.T.; Pack, D.W.; Leckband, D.E. HER-2-mediated endocytosis of magnetic nanospheres and the implications in cell targeting and particle magnetization. Biomaterials 2008, 29, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lim, E.K.; Lee, H.J.; Park, J.; Lee, S.C.; Lee, K.; Yoon, H.G.; Suh, J.S.; Huh, Y.M.; Haam, S. Fluorescent magnetic nanohybrids as multimodal imaging agents for human epithelial cancer detection. Biomaterials 2008, 29, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Heo, D.; Lee, E.; Kim, E.; Lim, E.K.; Lee, Y.H.; Haam, S.; Suh, J.S.; Huh, Y.M.; Yang, J.; et al. Galactosylated manganese ferrite nanoparticles for targeted MR imaging of asialoglycoprotein receptor. Nanotechnology 2013, 24, 475103. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of monodisperse iron oxide nanocrystal formation by “heating-up” process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of monodisperse spherical nanocrystals. Angew. Chem. Int. Ed. Engl. 2007, 46, 4630–4660. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Chung, B.H. Preparation of pyrenyl-based multifunctional nanocomposites for biomedical applications. Nat. Protoc. 2016, 11, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Sajomsang, W.; Choi, Y.; Jang, E.; Lee, H.; Kang, B.; Kim, E.; Haam, S.; Suh, J.-S.; Chung, S.J.; et al. Chitosan-based intelligent theragnosis nanocomposites enable pH-sensitive drug release with MR-guided imaging for cancer therapy. Nanoscale Res. Lett. 2013, 8, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Yang, J.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Self-labeled magneto nanoprobes using tri-aminated polysorbate 80 for detection of human mesenchymal stem cells. J. Mater. Chem. 2009, 19, 8958–8963. [Google Scholar] [CrossRef]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, S. PLA-PEG coated multifunctional imaging probe for targeted drug delivery. Mol. Pharm. 2015, 12, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, B.; Yin, X.; Zhang, Y.; Hu, D.; Hu, Z.; Liu, M.; Pan, Y.; Zhao, J.; Li, H.; et al. Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems. J. Biotechnol. 2007, 130, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; MacKay, J.A.; Francis, C.; Szoka, J. Low-pH-sensitive PEG-stabilized plasmid-lipid nanoparticles: Preparation and characterization. Bioconj. Chem. 2003, 14, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Abdollahi, M.; Emamgholi Zadeh, E.; Khodabakhshi, K.; Badeli, A.; Bagheri, H.; Hosseinkhani, S. mPEG-PLA and PLA-PEG-PLA nanoparticles as new carriers for delivery of recombinant human Growth Hormone (rhGH). Sci. Rep. 2018, 8, 9854. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Tabata, Y. PEGylation enhances tumor targeting of plasmid DNA by an artificial cationized protein with repeated RGD sequences, Pronectin. J. Control. Release 2004, 97, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Tabata, Y. Ultrasound enhances in vivo tumor expression of plasmid DNA by PEG-introduced cationized dextran. J. Control. Release 2005, 108, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.K.; Park, J.S.; Byun, Y.; Kim, C.K. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials 2009, 30, 5751–5756. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Park, T.G. Stereocomplex formation between enantiomeric PLA-PEG-PLA triblock copolymers. J. Appl. Polym. Sci. 2000, 75, 1615–1623. [Google Scholar] [CrossRef]
- Park, T.G.; Yoo, H.S. Dexamethasone nano-aggregates composed of PEG-PLA-PEG triblock copolymers for anti-proliferation of smooth muscle cells. Int. J. Pharm. 2006, 326, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hong, H.-Y.; Ren, T.-B.; Teng, X.-R. Preparation and characterization of magnetic PLA-PEG composite particles. Mater. Lett. 2005, 59, 2655–2658. [Google Scholar] [CrossRef]
- Tobío, M.; Sa’nchez, A.; Vila, A.; Soriano, I.; Evora, C.; Vila-Jato, J.L.; Alonso, M.J. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids Surf. B 2000, 18, 315–323. [Google Scholar] [CrossRef]
- Venkatraman, S.S.; Jie, P.; Min, F.; Freddy, B.Y.; Leong-Huat, G. Micelle-like nanoparticles of PLA-PEG-PLA triblock copolymer as chemotherapeutic carrier. Int. J. Pharm. 2005, 298, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Vila, A.; Gill, H.; McCallion, O.; Alonso, M.J. Transport of PLA-PEG particles across the nasal mucosa: Effect of particle size and PEG coating density. J. Control. Release 2004, 98, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent advances in PEG-PLA block copolymer nanoparticles. Int. J. Nanomed. 2010, 5, 1057–1065. [Google Scholar]
- Xiong, F.; Hu, K.; Yu, H.; Zhou, L.; Song, L.; Zhang, Y.; Shan, X.; Liu, J.; Gu, N. A functional iron oxide nanoparticles modified with PLA-PEG-DG as tumor-targeted MRI contrast agent. Pharm. Res. 2017, 34, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, T.-I.; Lee, J.; Lim, E.-K.; Hyung, W.; Lee, C.-H.; Song, Y.J.; Suh, J.-S.; Yoon, H.-G.; Huh, Y.-M.; et al. Synthesis of ultrasensitive magnetic resonance contrast agents for cancer imaging using PEG-fatty acid. Chem. Mater. 2007, 19, 3870–3876. [Google Scholar] [CrossRef]
- Lim, E.K.; Haam, S.; Lee, K.; Huh, Y.M. Design and synthesis of biofunctionalized metallic/magnetic nanomaterials. Methods Mol. Biol. 2011, 751, 583–595. [Google Scholar] [PubMed]
- Nikolic, L.; Ristic, I.; Adnadjevic, B.; Nikolic, V.; Jovanovic, J.; Stankovic, M. Novel microwave-assisted synthesis of poly(D,L-lactide): The influence of monomer/initiator molar ratio on the product properties. Sensors 2010, 10, 5063–5073. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, T.; Wang, G.; Li, H.; Qian, Z.; Yang, M. Fe3O4 nanoparticles-loaded PEG-PLA polymeric vesicles as labels for ultrasensitive immunosensors. Biomaterials 2010, 31, 7332–7339. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.-W.; Huh, Y.-M.; Choi, J.-S.; Lee, J.-H.; Song, H.-T.; Yoon, S.; Kim, K.-S.; Shin, J.-S.; Suh, J.-S.; Cheon, J. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Jensen, C.; Charity, N.; Towner, R.; Mao, C. Oil phase evaporation-induced self-assembly of hydrophobic nanoparticles into spherical clusters with controlled surface chemistry in an oil-in-water dispersion and comparison of behaviors of individual and clustered iron oxide nanoparticles. J. Am. Chem. Soc. 2010, 132, 17724–17732. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Yang, J.; Suh, J.S.; Huh, Y.M.; Haam, S. Synthesis of aminated polysorbate 80 for polyplex-mediated gene transfection. Biotechnol. Prog. 2010, 26, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.R.; Tan, I.M.; Ismail, L.; Mushtaq, M.; Nadeem, M.; Sagir, M. Static adsorption of anionic surfactant onto crushed Berea sandstone. J. Petrol. Explor. Prod. Technol. 2013, 3, 195–201. [Google Scholar] [CrossRef]
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.N.; Lim, E.-K.; Kim, T.; Kim, M.; Choi, Y.; Kim, B.; Lee, M.; Oh, A.; Jin, J.; Chae, Y.; et al. A highly crystalline manganese-doped iron oxide nanocontainer with predesigned void volume and shape for theranostic applications. Adv. Mater. 2013, 25, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Atae, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wum, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef] [PubMed]
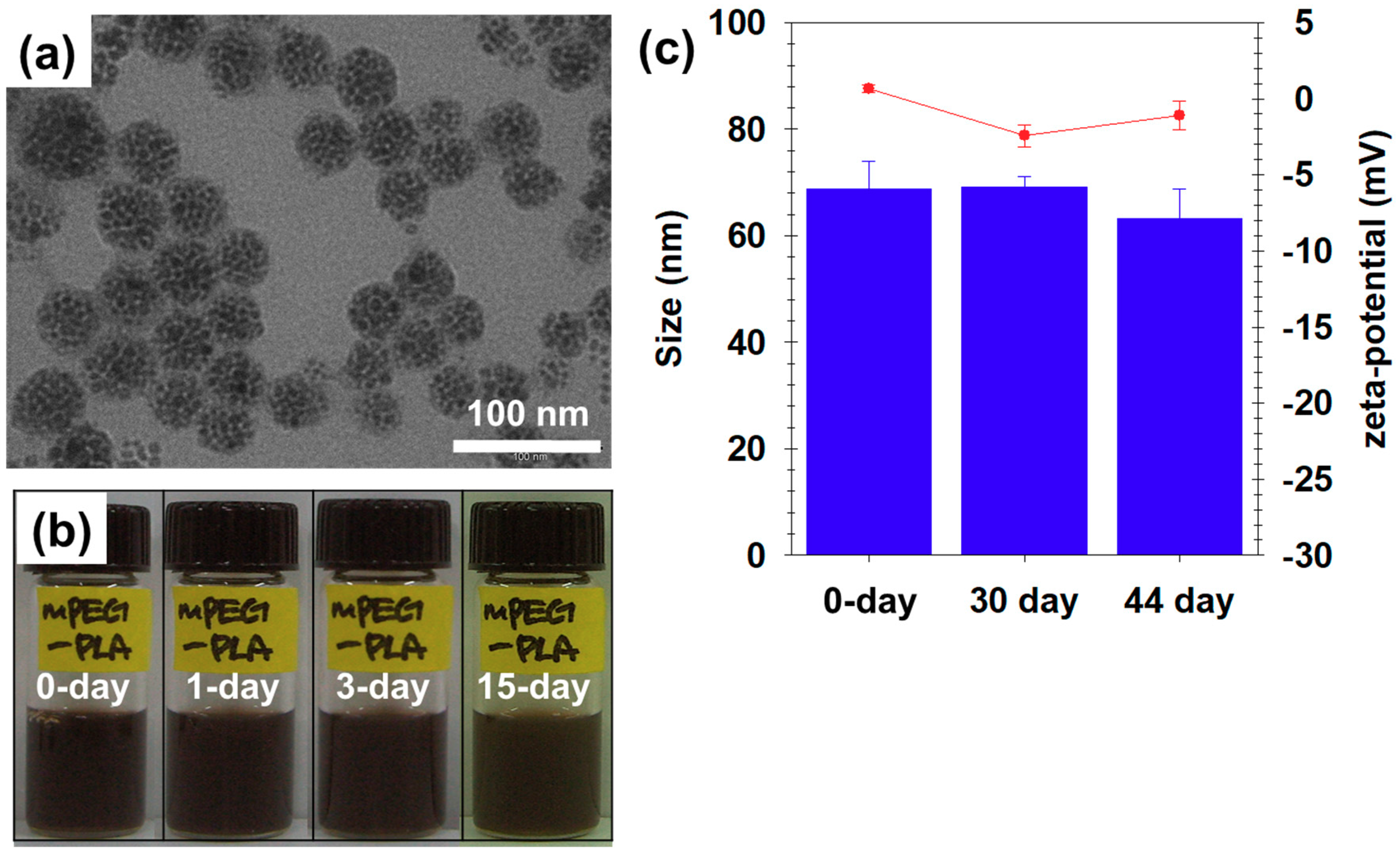
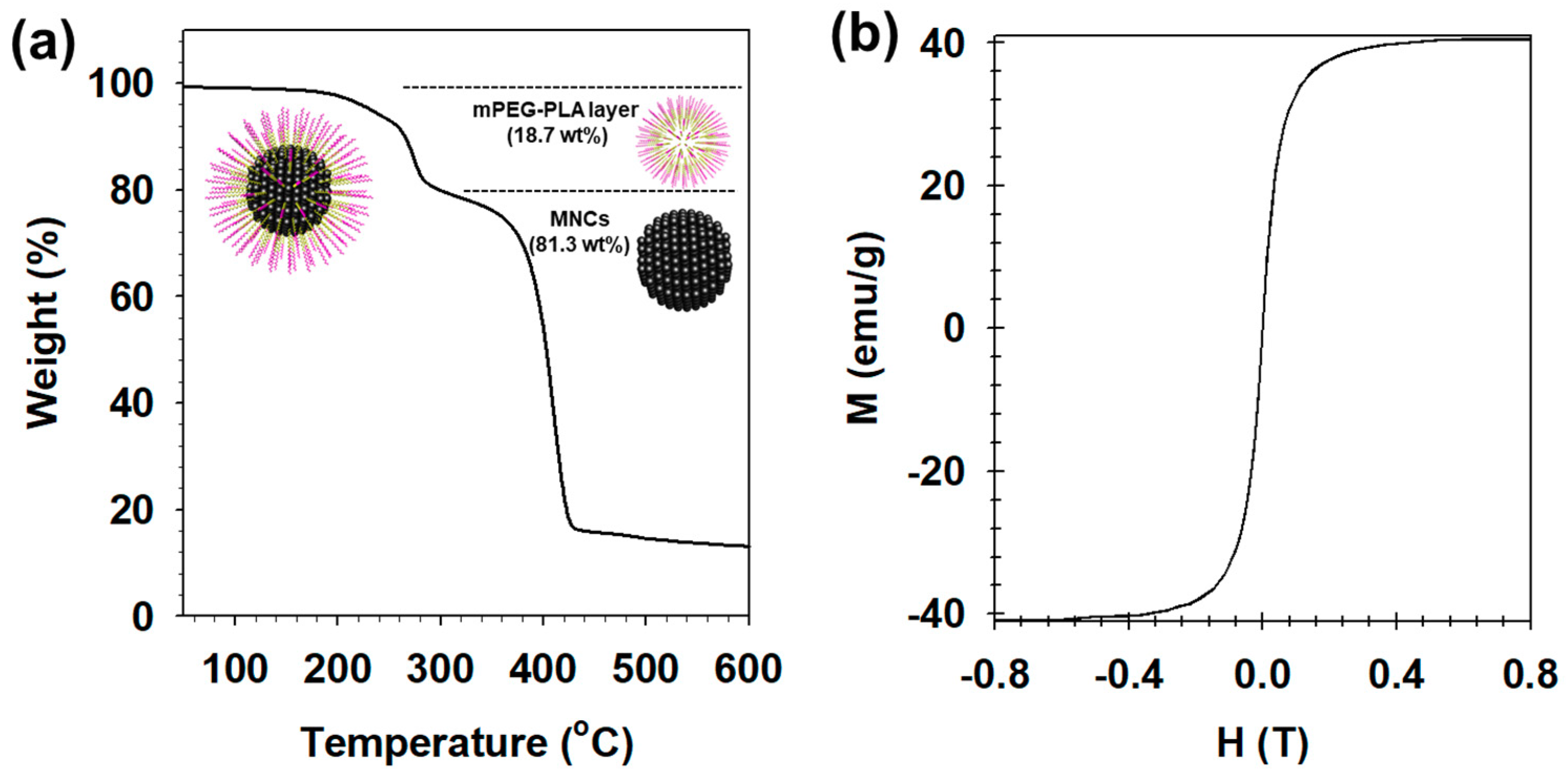
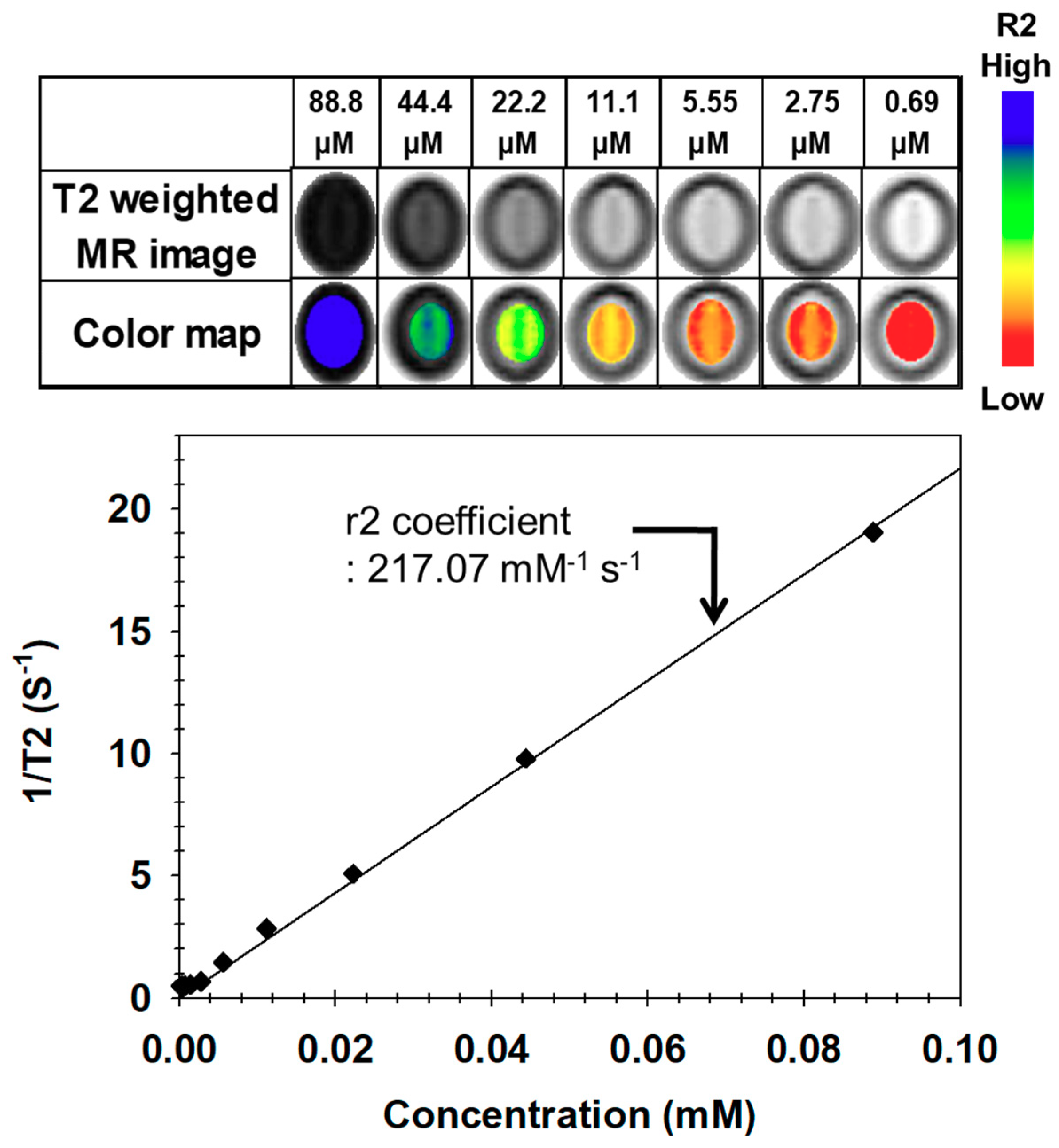
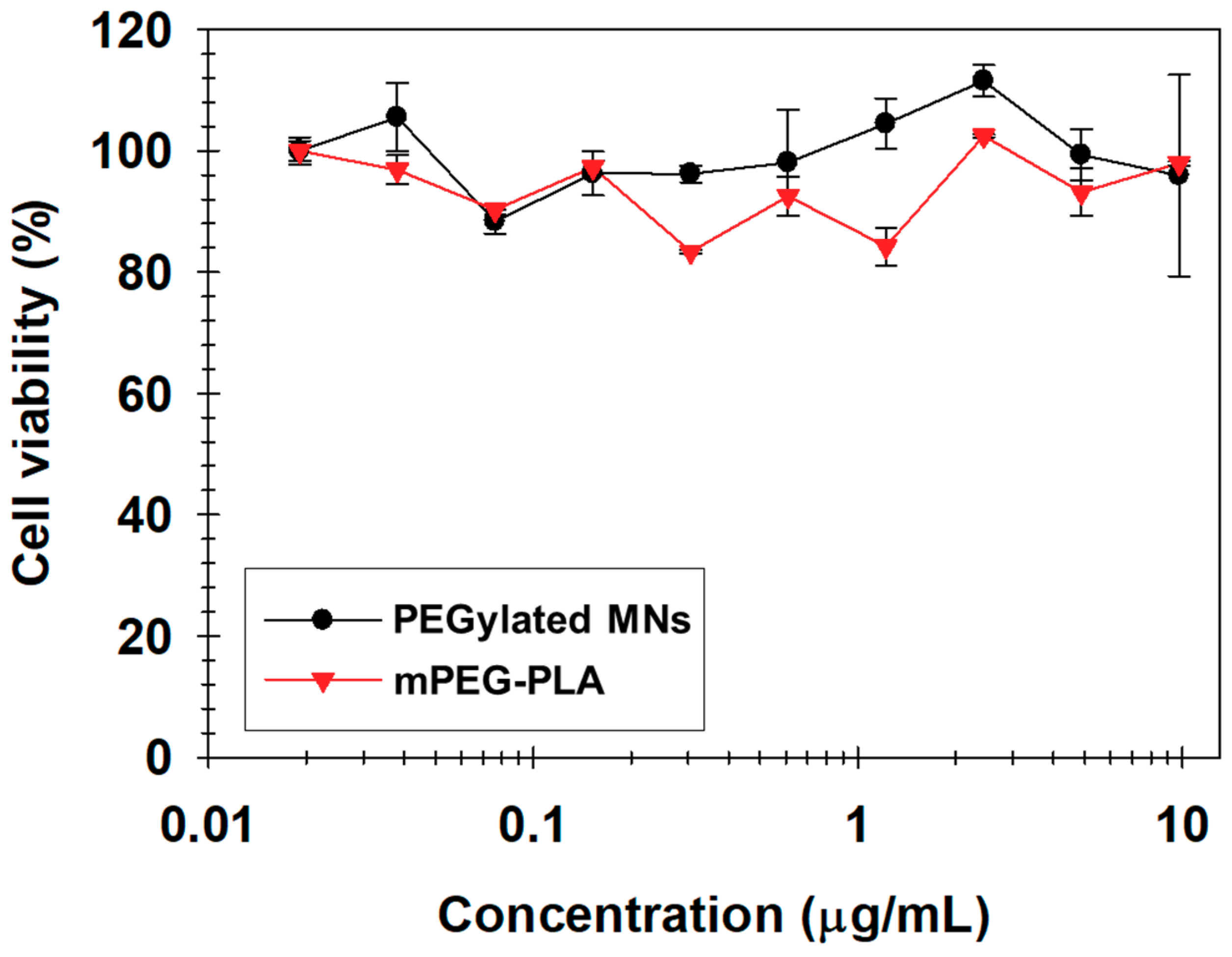
| Time | Size (nm) | PDI a | Zeta (mV) |
|---|---|---|---|
| 0 day | 68.8 ± 5.1 | 0.38 | 0.7 ± 0.3 |
| 33 days | 69.2 ± 2.0 | 0.06 | −2.4 ± 0.7 |
| 44 days | 63.2 ± 5.6 | 0.49 | −1.1 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Lim, J.; Son, H.-y.; Choi, Y.; Kang, T.; Jung, J.; Huh, Y.-M.; Haam, S.; Lim, E.-K. PEGylated Magnetic Nano-Assemblies as Contrast Agents for Effective T2-Weighted MR Imaging. Nanomaterials 2019, 9, 410. https://doi.org/10.3390/nano9030410
Kang B, Lim J, Son H-y, Choi Y, Kang T, Jung J, Huh Y-M, Haam S, Lim E-K. PEGylated Magnetic Nano-Assemblies as Contrast Agents for Effective T2-Weighted MR Imaging. Nanomaterials. 2019; 9(3):410. https://doi.org/10.3390/nano9030410
Chicago/Turabian StyleKang, Byunghoon, Jaewoo Lim, Hye-young Son, Yuna Choi, Taejoon Kang, Juyeon Jung, Yong-Min Huh, Seungjoo Haam, and Eun-Kyung Lim. 2019. "PEGylated Magnetic Nano-Assemblies as Contrast Agents for Effective T2-Weighted MR Imaging" Nanomaterials 9, no. 3: 410. https://doi.org/10.3390/nano9030410
APA StyleKang, B., Lim, J., Son, H.-y., Choi, Y., Kang, T., Jung, J., Huh, Y.-M., Haam, S., & Lim, E.-K. (2019). PEGylated Magnetic Nano-Assemblies as Contrast Agents for Effective T2-Weighted MR Imaging. Nanomaterials, 9(3), 410. https://doi.org/10.3390/nano9030410