Phosphate Ion-Modified RuO2/Ti3C2 Composite as a High-Performance Supercapacitor Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
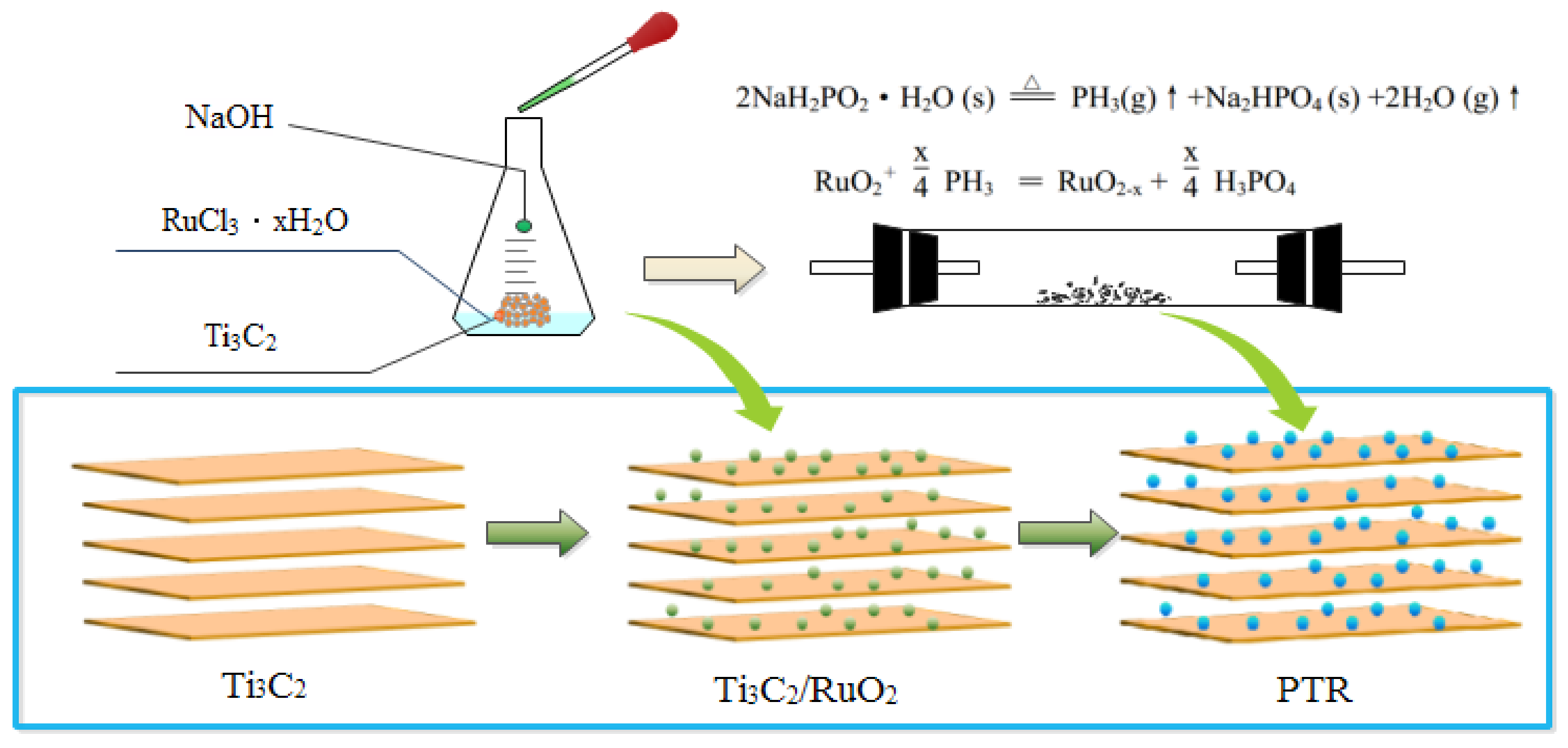
2.2. Synthesis of Phosphate Ion-Modified RuO2/Ti3C2
2.3. Electrochemical Characterization
2.4. Material Characterization
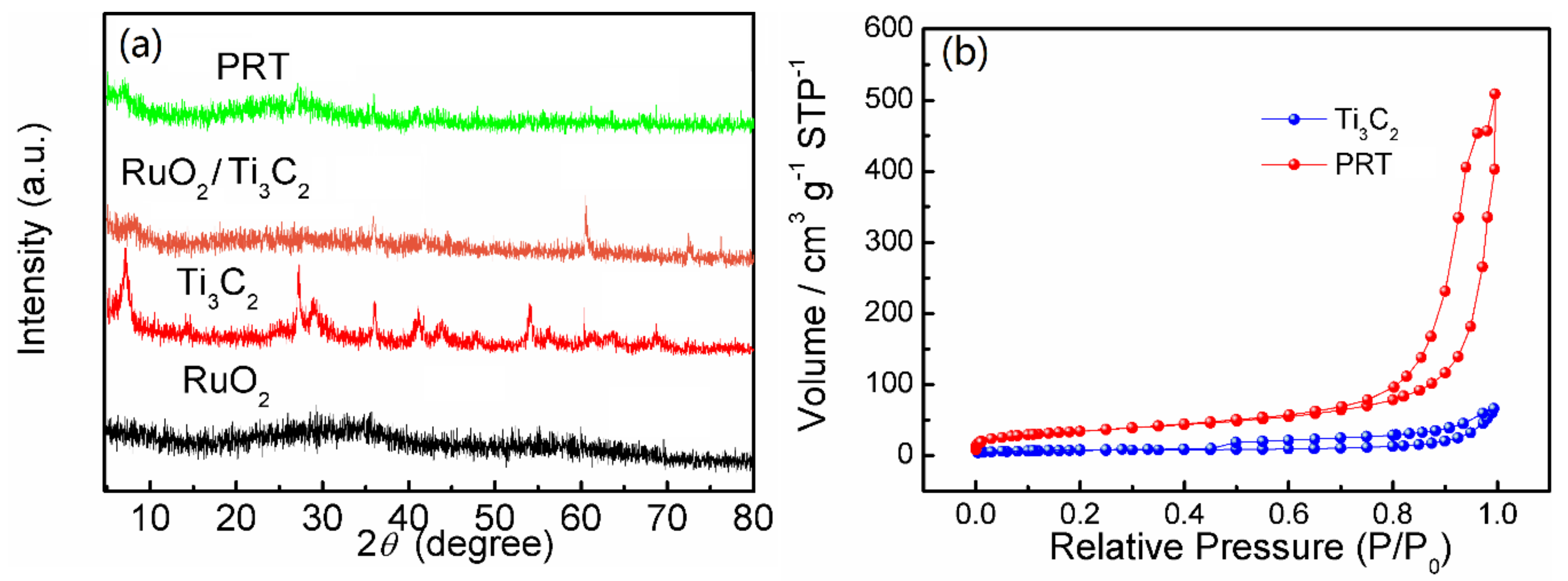
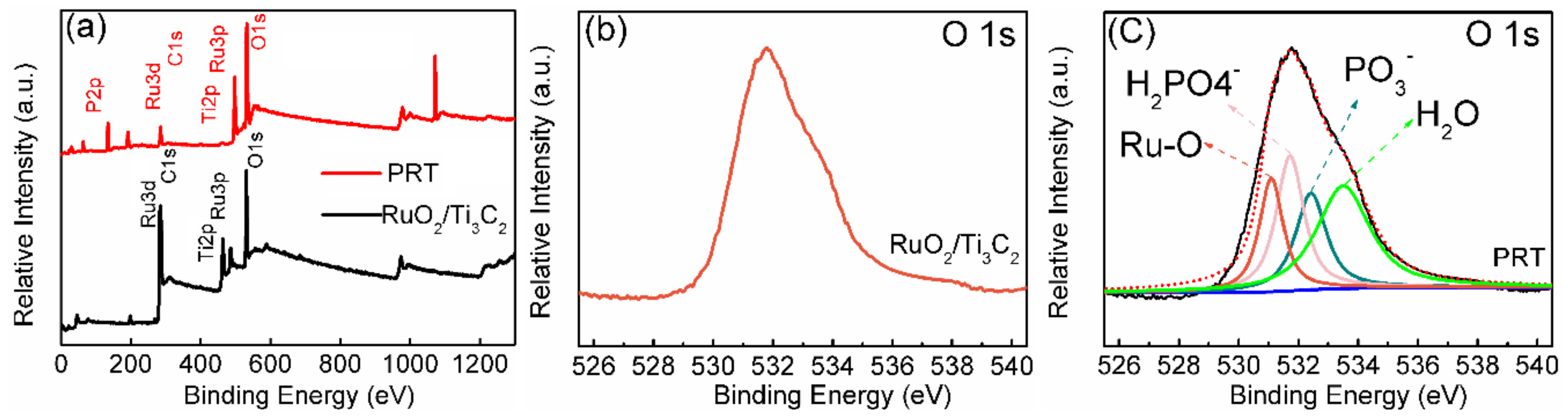
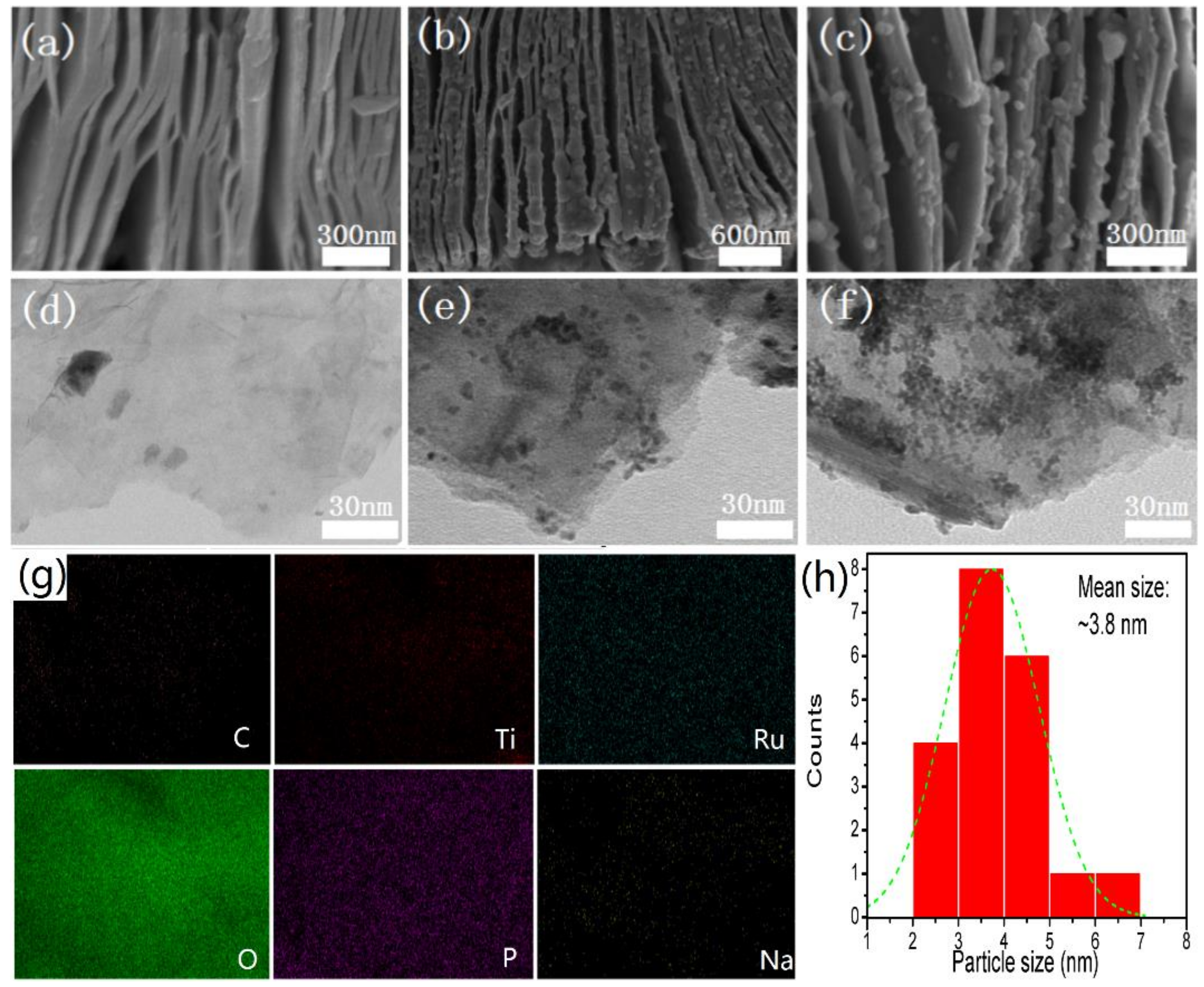
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A new family of promising hydrogen storage medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, L.; Zhou, A.; Wang, B.; Wang, X.; Lian, W.; Hu, Q.; Qin, G.; Liu, X. Synthesis and Electrochemical Properties of Two-Dimensional RGO/Ti(3)C(2)Tx Nanocomposites. Nanomaterials (Basel) 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, X.; Xu, C.; Jiang, W.; Zhang, Y.; Gao, D.; Bi, J.; Wang, Y. Palladium Supported on Titanium Carbide: A Highly Efficient, Durable, and Recyclable Bifunctional Catalyst for the Transformation of 4-Chlorophenol and 4-Nitrophenol. Nanomaterials (Basel) 2018, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Myung, Y.; Jung, S.; Tung, T.T.; Tripathi, K.M.; Kim, T. Graphene-Based Aerogels Derived from Biomass for Energy Storage and Environmental Remediation. ACS Sustain. Chem. Eng. 2019, 7, 3772–3782. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Cho, C.P. Mixed-Phase MnO(2)/N-Containing Graphene Composites Applied as Electrode Active Materials for Flexible Asymmetric Solid-State Supercapacitors. Nanomaterials (Basel) 2018, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, J.; Li, W.-H.; Zhang, X.-Y.; Xu, H.-Z.; Li, K.; Kai, Y.; Sun, L.-S.; Li, C.-Q.; Liu, F.-Q. Synthesis of Ag nanoparticles decorated MnO2/sulfonated graphene composites with 3D macroporous structure for high performance capacitors electrode materials. RSC Adv. 2016, 6, 94682–94686. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, J.; Li, H.; Zhao, X.S. A high-performance asymmetric supercapacitor fabricated with graphene-based electrodes. Energy Environ. Sci. 2011, 4, 4009–4015. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, Y.; Hu, Y.; Chen, W.; Li, C. Nanostructured Ternary Nanocomposite of rGO/CNTs/MnO2 for High-Rate Supercapacitors. ACS Sustain. Chem. Eng. 2014, 2, 70–74. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.; Lai, G.; Wei, M.; Jiang, X.; Bai, L. Nitrogen-Doped Hierarchically Porous Carbons Derived from Polybenzoxazine for Enhanced Supercapacitor Performance. Nanomaterials (Basel) 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, B.; Gu, Y.; Xiong, Z.; Sun, J.; Zhao, X.S. Graphene-based electrodes for electrochemical energy storage. Energy Environ. Sci. 2013, 6, 1388–1414. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutierrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.W. Mesoporous Transition Metal Oxides for Supercapacitors. Nanomaterials (Basel) 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.S.; Tripathi, K.M.; Kim, B.N.; You, I.-K.; Park, B.J.; Han, Y.H.; Kim, T. Three-dimensionally assembled Graphene/α-MnO2 nanowire hybrid hydrogels for high performance supercapacitors. Mater. Res. Bull. 2017, 96, 395–404. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.H.; Yoon, S.B.; Kim, H.K.; Park, S.H.; Kim, K.B. In situ chemical synthesis of ruthenium oxide/reduced graphene oxide nanocomposites for electrochemical capacitor applications. Nanoscale 2013, 5, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; El-Kady, M.F.; Wang, Y.; Wang, L.; Shao, Y.; Marsh, K.; Ko, J.M.; Kaner, R.B. Direct preparation and processing of graphene/RuO2 nanocomposite electrodes for high-performance capacitive energy storage. Nano Energy 2015, 18, 57–70. [Google Scholar] [CrossRef]
- Gui, Z.; Gillette, E.; Duay, J.; Hu, J.; Kim, N.; Lee, S.B. Co-electrodeposition of RuO2-MnO2 nanowires and the contribution of RuO2 to the capacitance increase. Phys. Chem. Chem. Phys. PCCP 2015, 17, 15173–15180. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.R.; Bulakhe, R.N.; Pusawale, S.N.; Sartale, S.D.; Lokhande, C.D. Polyaniline–RuO2 composite for high performance supercapacitors: Chemical synthesis and properties. RSC Adv. 2015, 5, 28687–28695. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, L.; Xiao, H.; Ma, Y.; Chao, L.; Xie, Q. Facile electrochemical preparation of a composite film of ruthenium dioxide and carboxylated graphene for a high performance supercapacitor. RSC Adv. 2016, 6, 33666–33675. [Google Scholar] [CrossRef]
- Gao, Z.D.; Zhu, X.; Li, Y.H.; Zhou, X.; Song, Y.Y.; Schmuki, P. Carbon cladded TiO2 nanotubes: Fabrication and use in 3D-RuO2 based supercapacitors. Chem. Commun. 2015, 51, 7614–7617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, X.; Pan, C.; Sun, Q.; Song, W.; Fang, L.; Chen, Q.; Banks, C.E. A carbon quantum dot decorated RuO2 network: Outstanding supercapacitances under ultrafast charge and discharge. Energy Environ. Sci. 2013, 6, 3665–3675. [Google Scholar] [CrossRef]
- Ma, H.; Kong, D.; Xu, Y.; Xie, X.; Tao, Y.; Xiao, Z.; Lv, W.; Jang, H.D.; Huang, J.; Yang, Q.H. Disassembly-Reassembly Approach to RuO2/Graphene Composites for Ultrahigh Volumetric Capacitance Supercapacitor. Small 2017, 13, 1701026. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Tian, J.; Shan, Z.; Chen, K.; Liao, W. Hydrothermal synthesis of hydrous ruthenium oxide/graphene sheets for high-performance supercapacitors. Electrochim. Acta 2013, 99, 219–224. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, H.; Yu, X.; Shan, D.; Ye, T.; Huang, Z.; Kuang, Y. Synthesis of RuO2 decorated quasi graphene nanosheets and their application in supercapacitors. RSC Adv. 2014, 4, 11197–11205. [Google Scholar] [CrossRef]
- Amir, F.Z.; Pham, V.H.; Dickerson, J.H. Facile synthesis of ultra-small ruthenium oxide nanoparticles anchored on reduced graphene oxide nanosheets for high-performance supercapacitors. RSC Adv. 2015, 5, 67638–67645. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, X.; Guo, R.; Lang, J.; Chen, J.; Yan, X. Carbon encapsulated RuO2 nano-dots anchoring on graphene as an electrode for asymmetric supercapacitors with ultralong cycle life in an ionic liquid electrolyte. J. Mater. Chem. A 2016, 4, 8180–8189. [Google Scholar] [CrossRef]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.-Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Naslund, L.A.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. Publ. Am. Chem. Soc. 2014, 26, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Kurra, N.; Ahmed, B.; Gogotsi, Y.; Alshareef, H.N. MXene-on-Paper Coplanar Microsupercapacitors. Adv. Energy Mater. 2016, 6, 1601372. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Akuzum, B.; Kurra, N.; Zhao, M.-Q.; Alhabeb, M.; Anasori, B.; Kumbur, E.C.; Alshareef, H.N.; Ger, M.-D.; Gogotsi, Y. All-MXene (2D titanium carbide) solid-state microsupercapacitors for on-chip energy storage. Energy Environ. Sci. 2016, 9, 2847–2854. [Google Scholar] [CrossRef]
- Xiong, J.; Pan, L.; Wang, H.; Du, F.; Chen, Y.; Yang, J.; Zhang, C. Synergistically enhanced lithium storage performance based on titanium carbide nanosheets (MXene) backbone and SnO2 quantum dots. Electrochim. Acta 2018, 268, 503–511. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Anjum, D.; Alshareef, H.N. Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications. ACS Appl. Mater. interfaces 2016, 8, 18806–18814. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Wan, L.; Sun, S.; Chen, Q.; Sun, J.; Xia, Q.; Xia, H. Phosphate Ion Functionalized Co3O4 Ultrathin Nanosheets with Greatly Improved Surface Reactivity for High Performance Pseudocapacitors. Adv. Mater. 2017, 29, 1604167. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Dong, Y.; Wu, Z.-S.; Zheng, S.; Wang, X.; Sen, W.; Sun, C.; Qin, J.; Shi, X.; Bao, X. Alkalized Ti 3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries. Nano Energy 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H.N. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef]
- Xu, S.; Wei, G.; Li, J.; Ji, Y.; Klyui, N.; Izotov, V.; Han, W. Binder-free Ti 3C2Tx MXene electrode film for supercapacitor produced by electrophoretic deposition method. Chem. Eng. J. 2017, 317, 1026–1036. [Google Scholar] [CrossRef]
- Ghosh, K.; Balog, E.R.M.; Sista, P.; Williams, D.J.; Kelly, D.; Martinez, J.S.; Rocha, R.C. Temperature-dependent morphology of hybrid nanoflowers from elastin-like polypeptides. APL Mater. 2014, 2, 021101. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, M.-Q.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Jo, W.J.; Jang, J.W.; Kong, K.J.; Kang, H.J.; Kim, J.Y.; Jun, H.; Parmar, K.P.; Lee, J.S. Phosphate doping into monoclinic BiVO4 for enhanced photoelectrochemical water oxidation activity. Angew. Chem. 2012, 51, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, Y.; Yu, M.; Liu, P.; Tong, Y.; Cheng, F.; Lu, X. Recent Smart Methods for Achieving High-Energy Asymmetric Supercapacitors. Small Method. 2018, 2, 1700230. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel-Cobalt Layered Double Hydroxide Nanosheets for High-performance Supercapacitor Electrode Materials. Adv. Funct. Mater. 2014, 24, 934–942. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y. Rapid synthesis of graphene/amorphous α-MnO2 composite with enhanced electrochemical performance for electrochemical capacitor. Mater. Sci. Eng. B 2015, 194, 41–47. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, H.; Yang, J.; Qin, Q.; Fan, H.; Wei, C.; Zheng, W. Solvothermal Synthesis of Three-Dimensional Hierarchical CuS Microspheres from a Cu-Based Ionic Liquid Precursor for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 21735–21744. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene–MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Xu, X.; Pei, L.; Yang, Y.; Shen, J.; Ye, M. Facile synthesis of NiWO4/reduced graphene oxide nanocomposite with excellent capacitive performance for supercapacitors. J. Alloys Compd. 2016, 654, 23–31. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Qian, L.; Xiao, J.; Wang, S.; Liu, Y. Facile Synthesis of 3D MnO2–Graphene and Carbon Nanotube–Graphene Composite Networks for High-Performance, Flexible, All-Solid-State Asymmetric Supercapacitors. Adv. Energy Mater. 2014, 4, 1400064. [Google Scholar] [CrossRef]
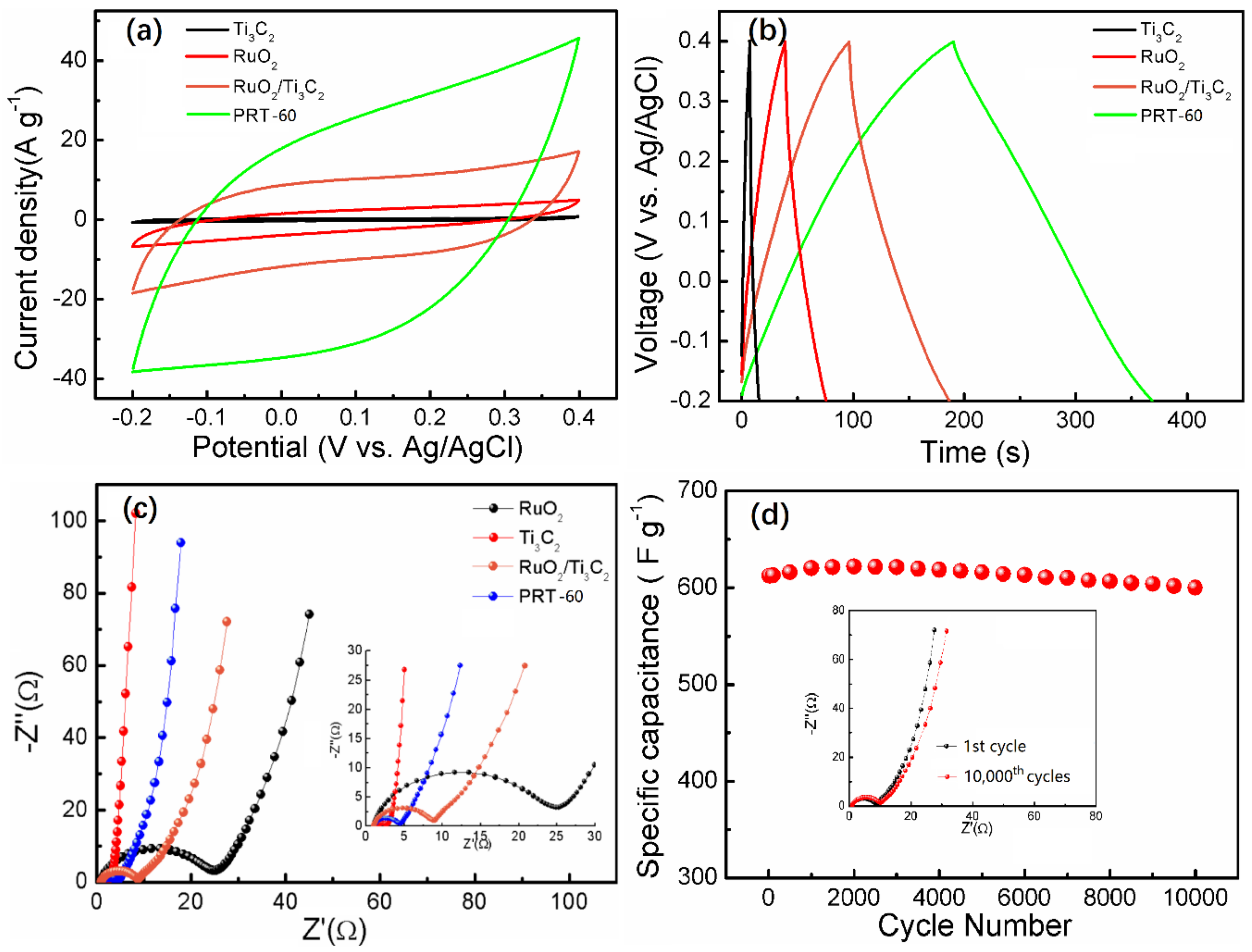
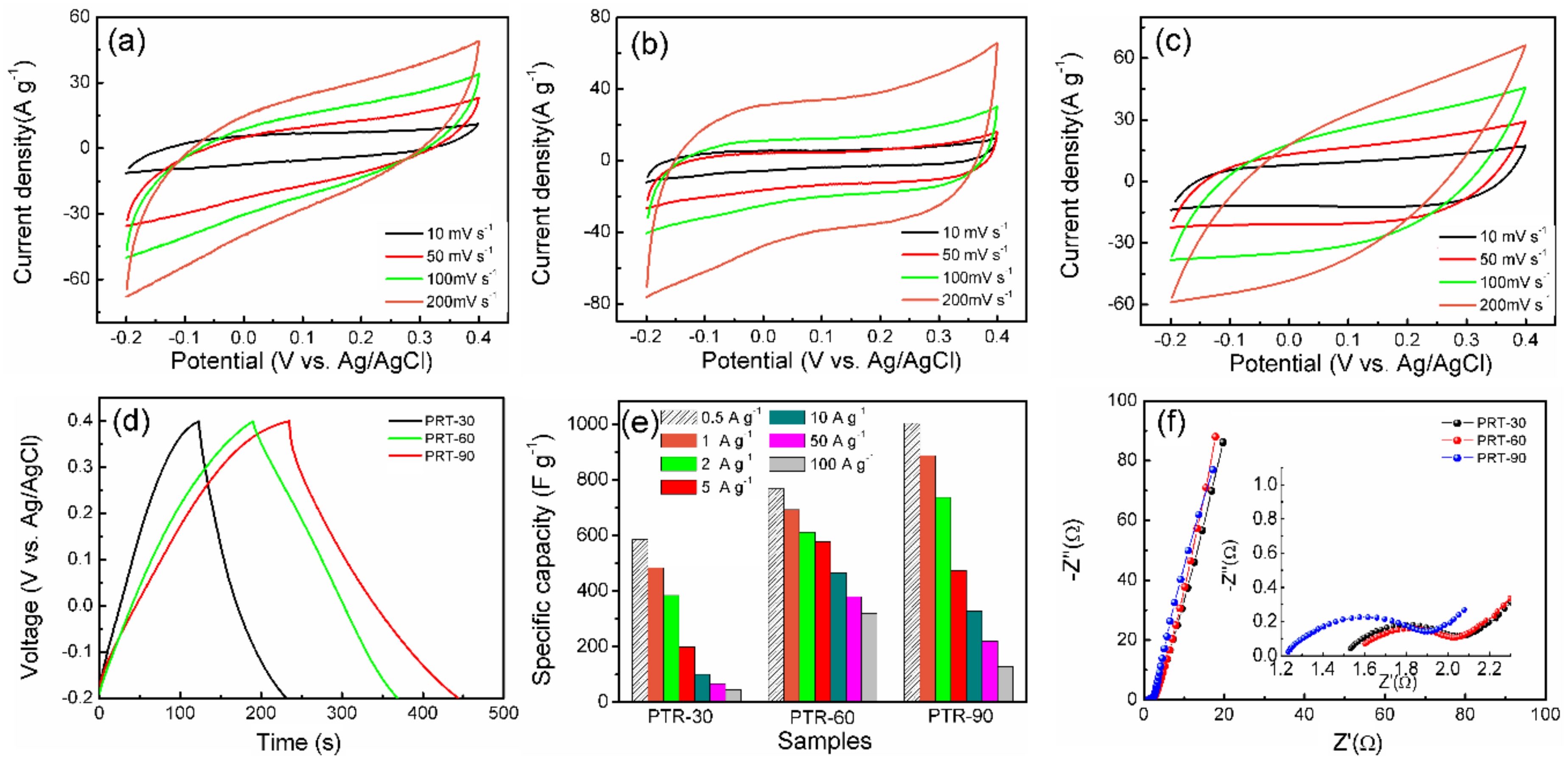
| Samples | Specific Capacitance (F g−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0.5 A g−1 | 1 A g−1 | 2 A g−1 | 5 A g−1 | 10 A g−1 | 50 A g−1 | 100 A g−1 | |
| PRT-30 | 585.04 | 484.63 | 384.62 | 199.91 | 100.25 | 66.92 | 45.85 |
| PRT-60 | 768.03 | 693.02 | 612.72 | 578.02 | 466.87 | 380.64 | 320.83 |
| PRT-90 | 1004.3 | 888.54 | 737.59 | 474.42 | 328.67 | 220.52 | 128.65 |
| Material | Capacitance (F g−1) | Cycle Life (Cycles) | Reference |
|---|---|---|---|
| Reduced graphene oxide sheets modified with RuO2 | 400 | 2500 | [9] |
| RuO2/reduced graphene oxide nanocomposites | 489 | 1000 | [19] |
| RuO2 deposited on the surface of graphene sheets | 551 | 2000 | [27] |
| Reduced graphene oxide–RuO2 hybrid materials | 509 | 2000 | [29] |
| Phosphate ion-modified RuO2/Ti3C2 composite | 693 | 10,000 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Liu, F.; Li, W. Phosphate Ion-Modified RuO2/Ti3C2 Composite as a High-Performance Supercapacitor Material. Nanomaterials 2019, 9, 377. https://doi.org/10.3390/nano9030377
Zhao J, Liu F, Li W. Phosphate Ion-Modified RuO2/Ti3C2 Composite as a High-Performance Supercapacitor Material. Nanomaterials. 2019; 9(3):377. https://doi.org/10.3390/nano9030377
Chicago/Turabian StyleZhao, Jie, Faqian Liu, and Weihua Li. 2019. "Phosphate Ion-Modified RuO2/Ti3C2 Composite as a High-Performance Supercapacitor Material" Nanomaterials 9, no. 3: 377. https://doi.org/10.3390/nano9030377
APA StyleZhao, J., Liu, F., & Li, W. (2019). Phosphate Ion-Modified RuO2/Ti3C2 Composite as a High-Performance Supercapacitor Material. Nanomaterials, 9(3), 377. https://doi.org/10.3390/nano9030377




