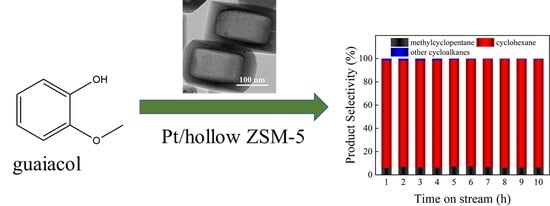
Hollow MFI Zeolite Supported Pt Catalysts for Highly Selective and Stable Hydrodeoxygenation of Guaiacol to Cycloalkanes
Abstract
1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Evaluation
3. Results and Discussion
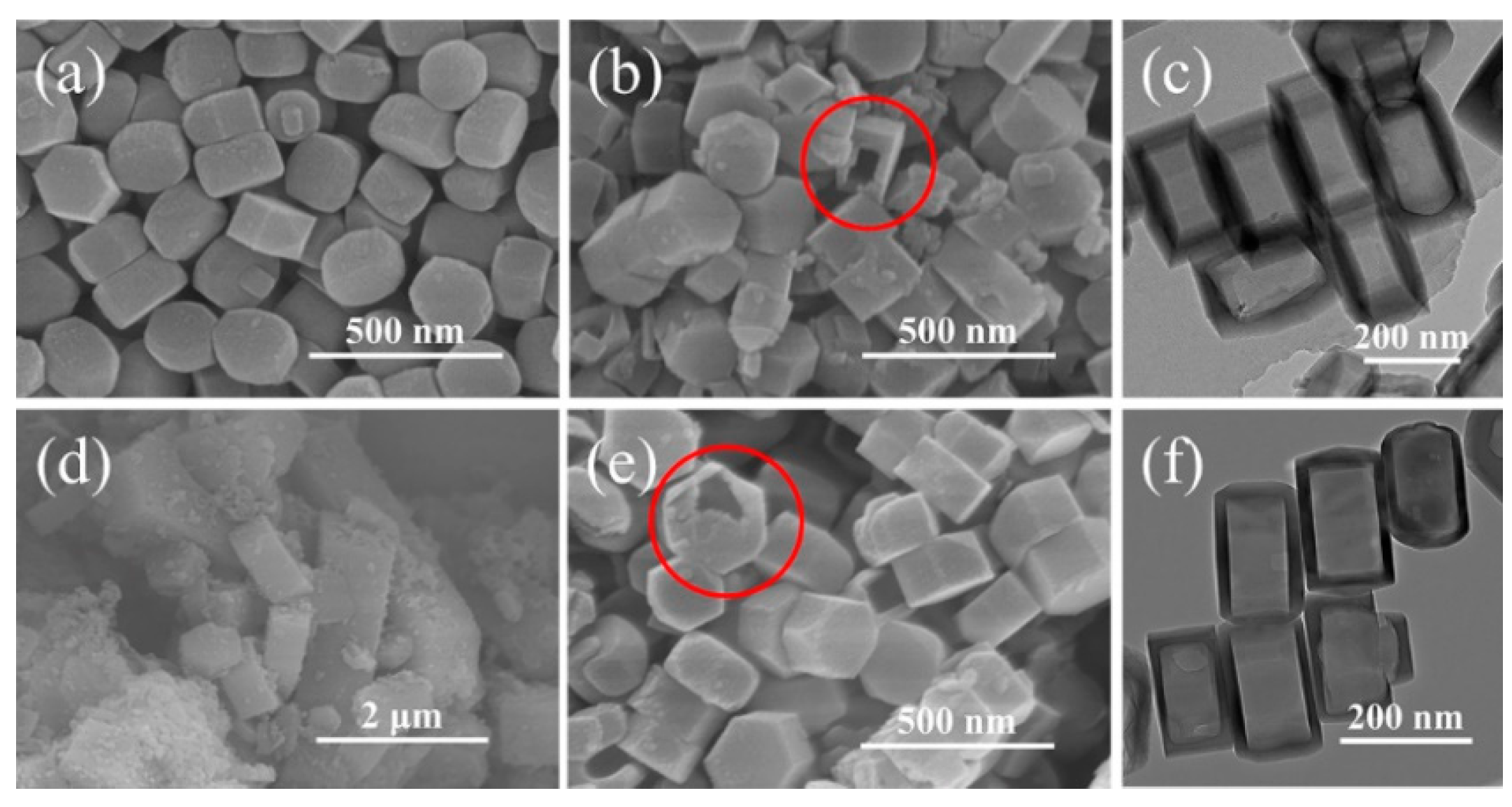
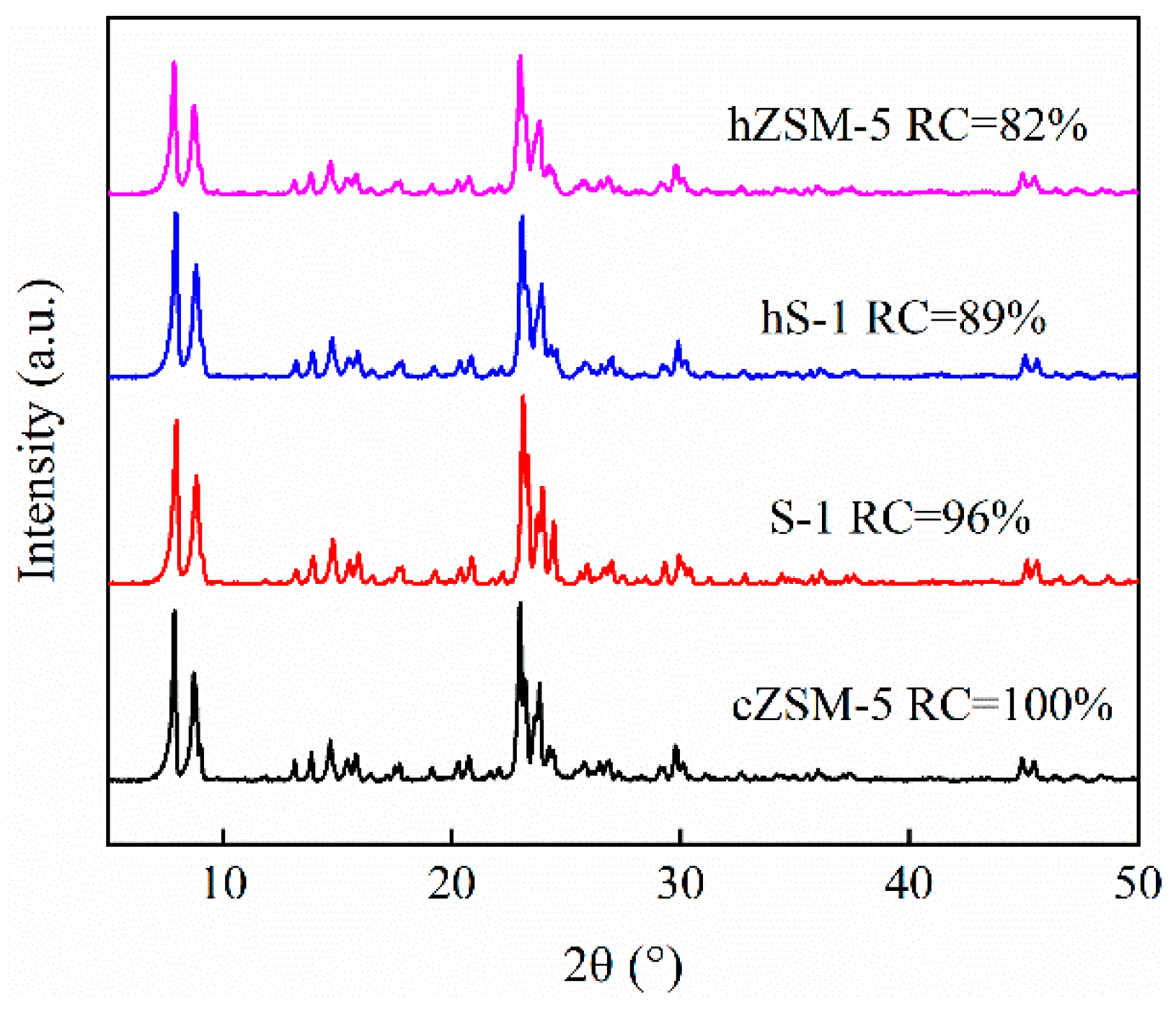
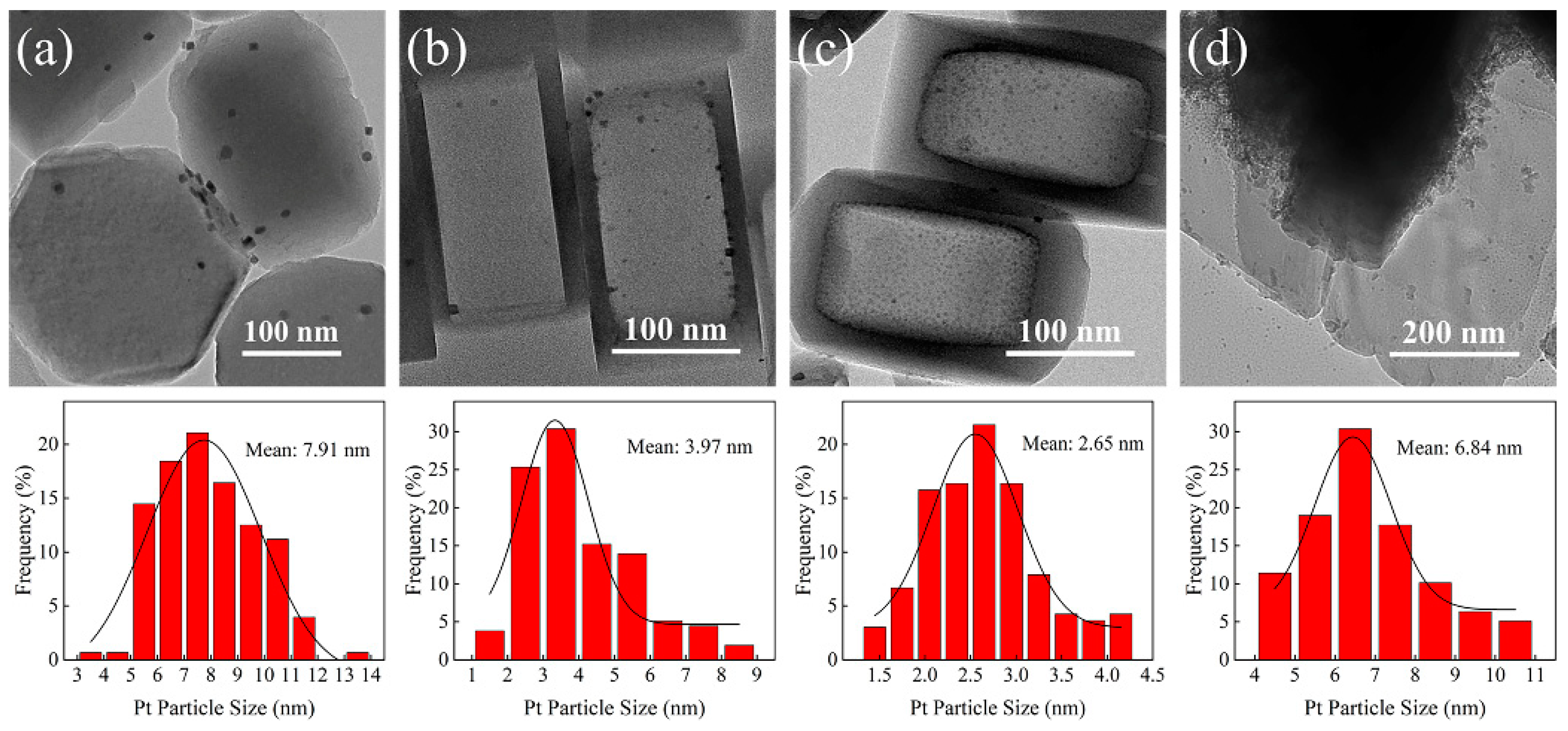
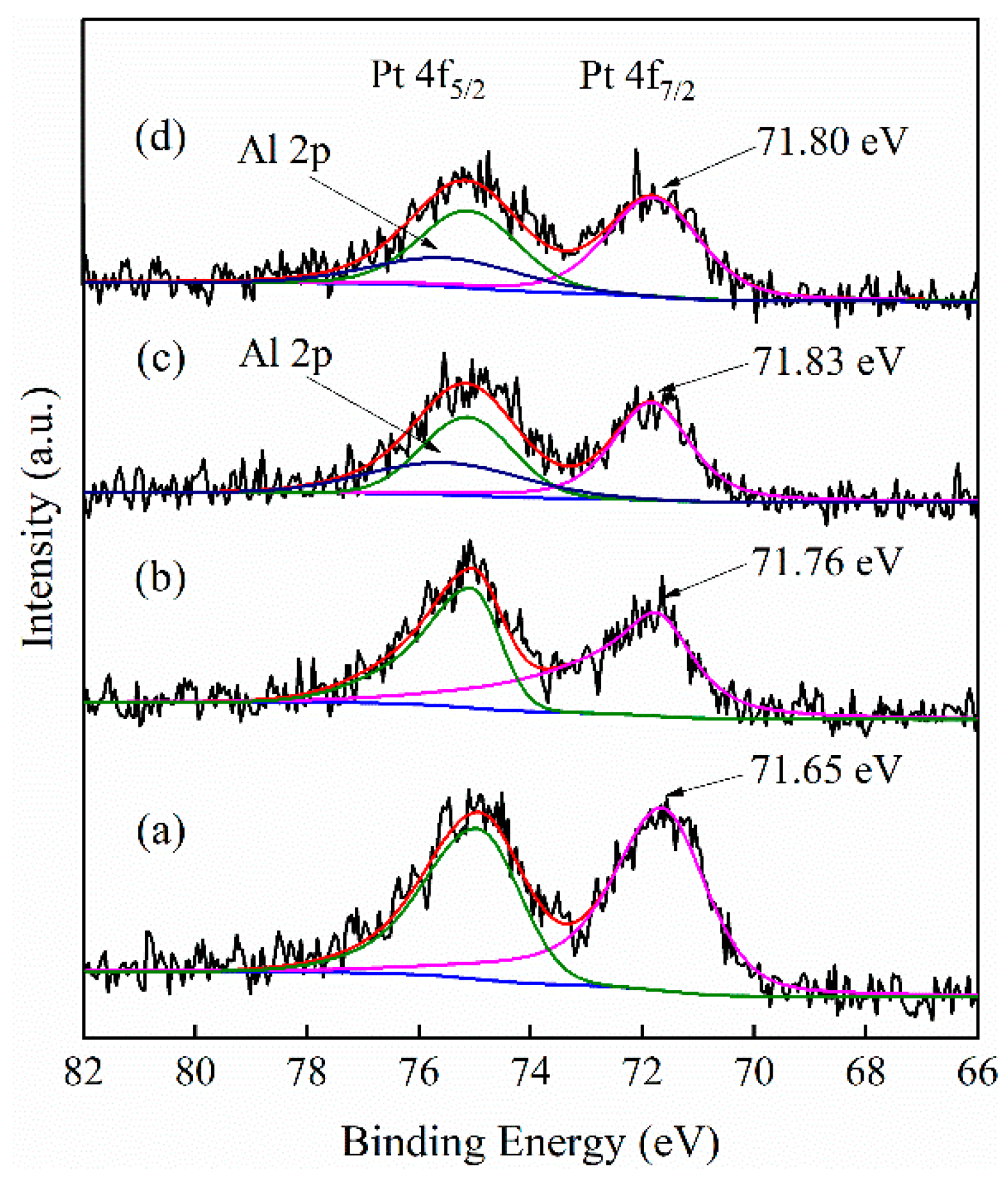
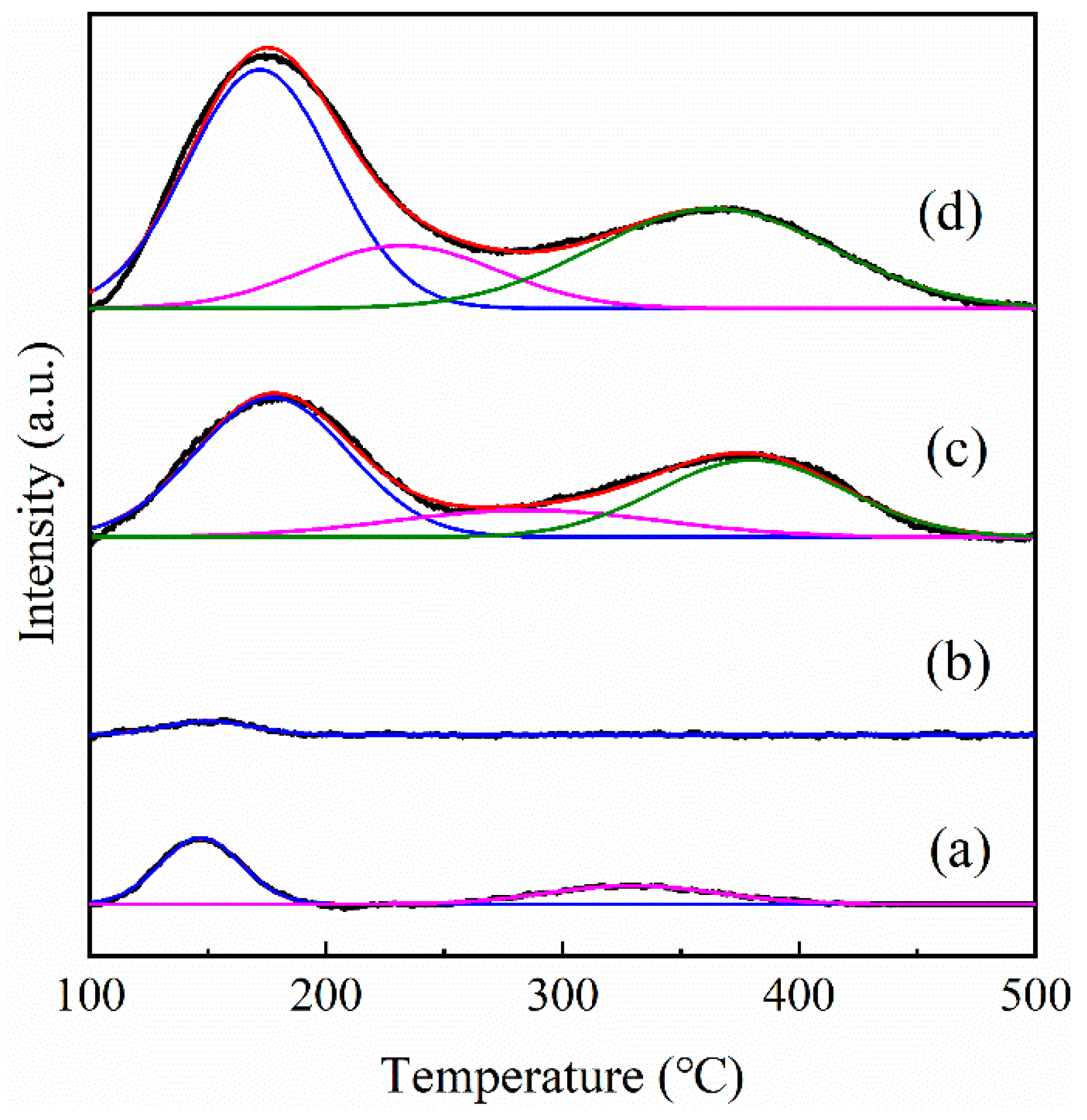
3.1. Catalyst Properties
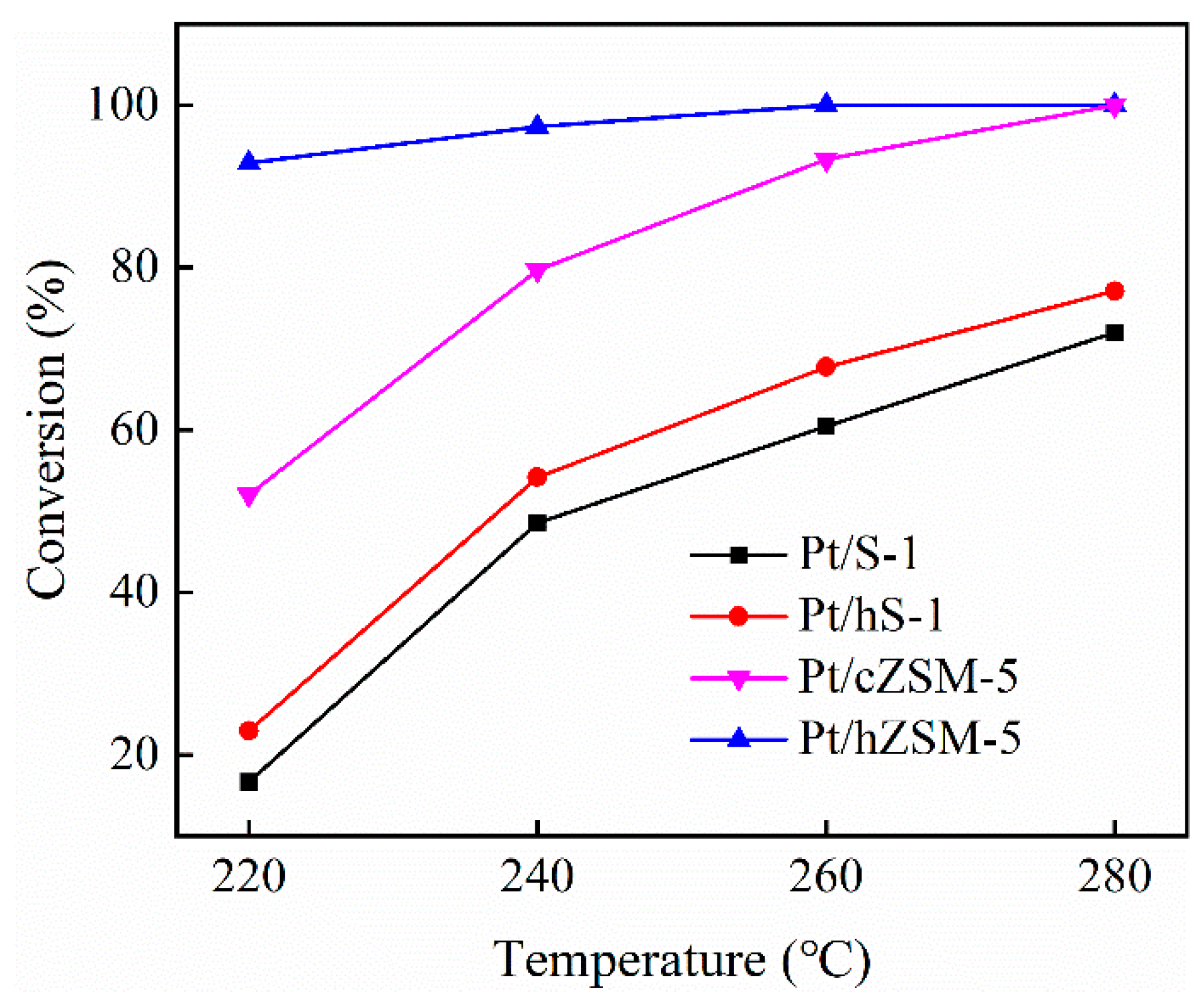
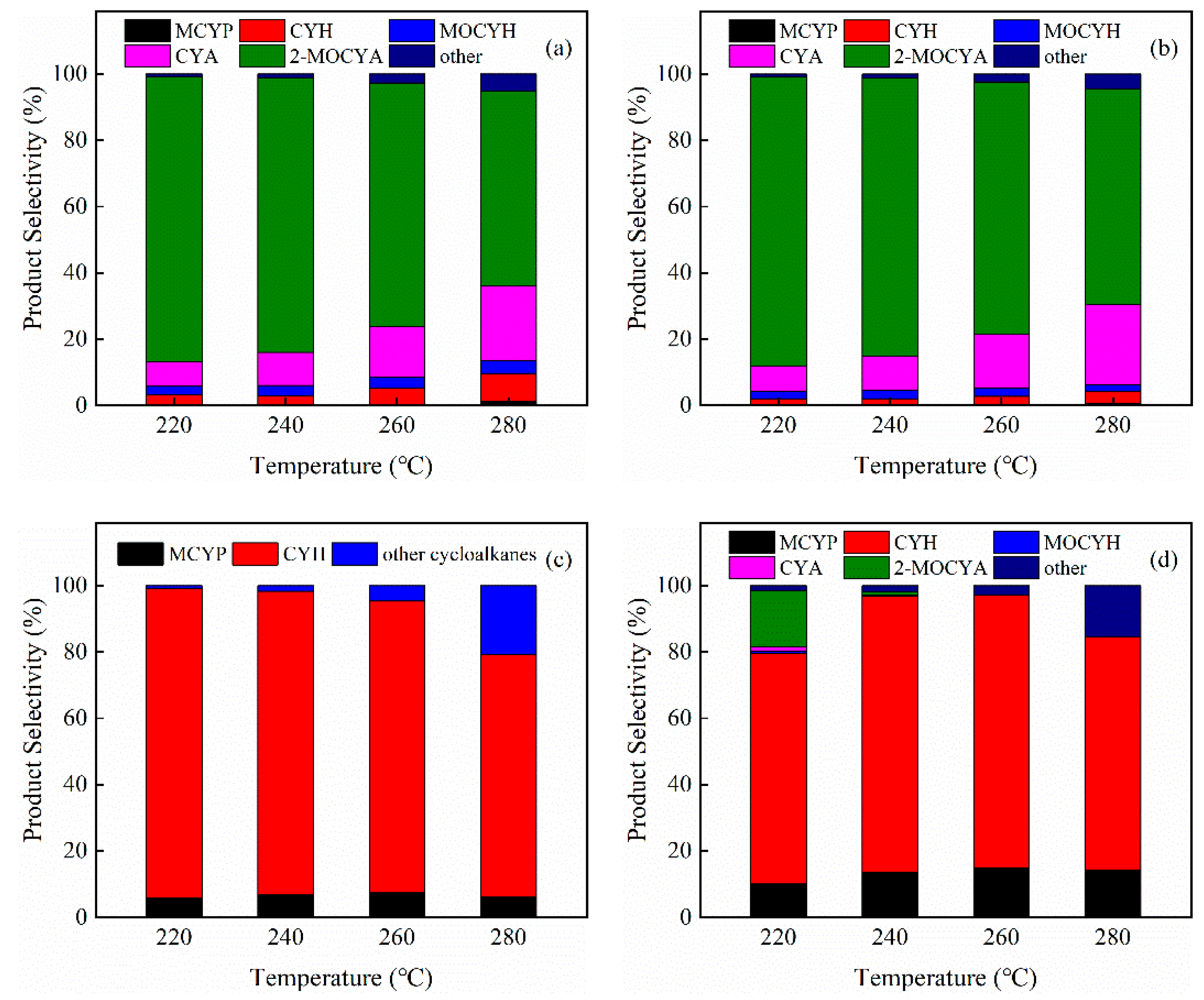
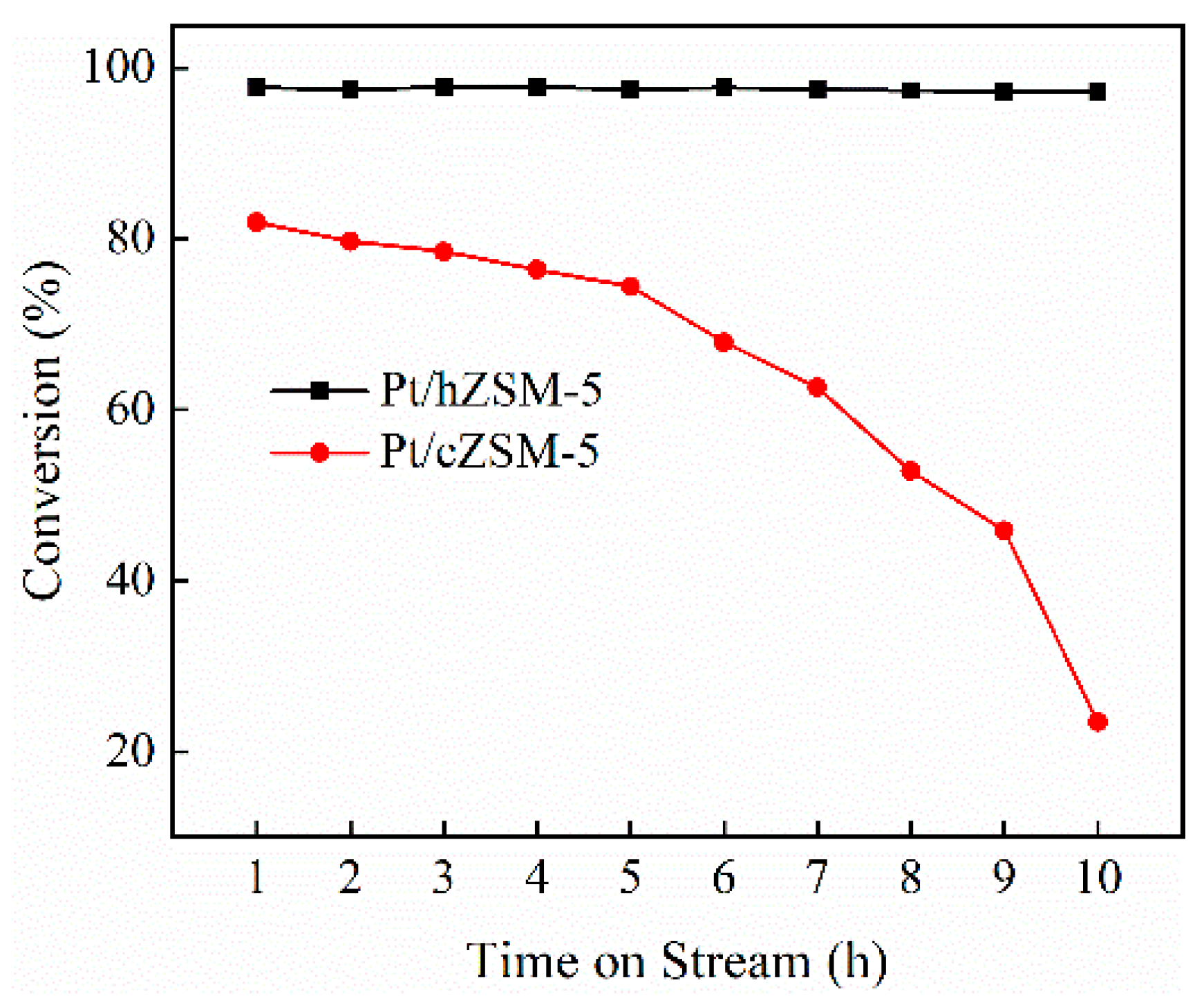
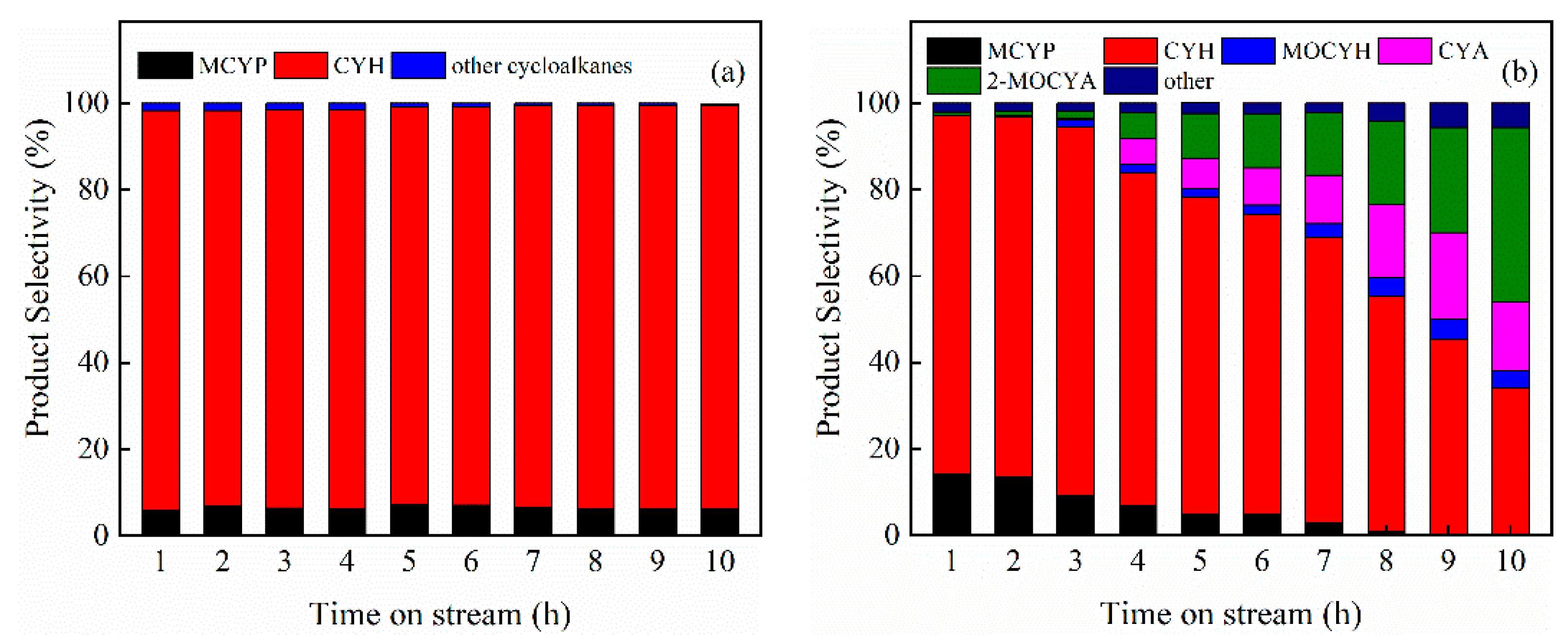
3.2. Hydrodeoxygenation of Guaiacol
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xing, E.; Wu, K.; Wang, J.; Yang, M.; Wu, Y. Recent progress on upgrading of bio-oil to hydrocarbons over metal/zeolite bifunctional catalysts. Catal. Sci. Technol. 2017, 7, 2385–2415. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Danuthai, T.; Dewiyanti, S.; Wang, C.; Borgna, A. Hydrodeoxygenation of guaiacol over carbon-supported metal catalysts. ChemCatChem 2013, 5, 3041–3049. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Du, H.; Wei, B.; Zhu, J.; Li, M.; Shan, Y.; Song, C. Catalytic hydrodeoxygenation of guaiacol over palladium catalyst on different titania supports. Energy Fuels 2017, 31, 10858–10865. [Google Scholar] [CrossRef]
- Zhao, C.; Lercher, J.A. Upgrading pyrolysis oil over Ni/HZSM-5 by cascade reactions. Angew. Chem. Int. Ed. Engl. 2012, 51, 5935–5940. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, Y.; Sun, Z.; Li, X.; Wang, A.; Camaioni, D.M.; Lercher, J.A. Ni3P as a high-performance catalytic phase for the hydrodeoxygenation of phenolic compounds. Green Chem. 2018, 20, 609–619. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Influence of water in the deactivation of a sulfided NiMo/γ-Al2O3 catalyst during hydrodeoxygenation. J. Catal. 1994, 146, 281–291. [Google Scholar] [CrossRef]
- Bui, V.N.; Laurenti, D.; Afanasiev, P.; Geantet, C. Hydrodeoxygenation of guaiacol with CoMo catalysts. Part I: Promoting effect of cobalt on HDO selectivity and activity. Appl. Catal. B Environ. 2011, 101, 239–245. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Runnebaum, R.C.; Block, D.E.; Gates, B.C. Catalytic conversion of guaiacol catalyzed by platinum supported on alumina: Reaction network including hydrodeoxygenation reactions. Energy Fuels 2011, 25, 3417–3427. [Google Scholar] [CrossRef]
- Hong, D.Y.; Miller, S.J.; Agrawal, P.K.; Jones, C.W. Hydrodeoxygenation and coupling of aqueous phenolics over bifunctional zeolite-supported metal catalysts. Chem. Commun. 2010, 46, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Kaila, R.K.; Honkela, M.L.; Slioor, R.; Krause, A.O.I. Hydrodeoxygenation of guaiacol on noble metal catalysts. Catal. Today 2009, 147, 239–246. [Google Scholar] [CrossRef]
- Zhu, X.; Lobban, L.L.; Mallinson, R.G.; Resasco, D.E. Bifunctional transalkylation and hydrodeoxygenation of anisole over a Pt/HBeta catalyst. J. Catal. 2011, 281, 21–29. [Google Scholar] [CrossRef]
- Lee, H.W.; Jun, B.R.; Kim, H.; Kim, D.H.; Jeon, J.-K.; Park, S.H.; Ko, C.H.; Kim, T.-W.; Park, Y.-K. Catalytic hydrodeoxygenation of 2-methoxy phenol and dibenzofuran over Pt/mesoporous zeolites. Energy 2015, 81, 33–40. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, A.; Liu, S.; Yao, Y.; Sun, Z.; Li, X.; Liu, Y.; Wang, Y.; Camaioni, D.M.; Lercher, J.A. Hydrodeoxygenation of phenolic compounds to cycloalkanes over supported nickel phosphides. Catal. Today 2019, 319, 48–56. [Google Scholar] [CrossRef]
- Berenguer, A.; Bennett, J.A.; Hunns, J.; Moreno, I.; Coronado, J.M.; Lee, A.F.; Pizarro, P.; Wilson, K.; Serrano, D.P. Catalytic hydrodeoxygenation of m-cresol over Ni2P/hierarchical ZSM-5. Catal. Today 2018, 304, 72–79. [Google Scholar] [CrossRef]
- Koike, N.; Hosokai, S.; Takagaki, A.; Nishimura, S.; Kikuchi, R.; Ebitani, K.; Suzuki, Y.; Oyama, S.T. Upgrading of pyrolysis bio-oil using nickel phosphide catalysts. J. Catal. 2016, 333, 115–126. [Google Scholar] [CrossRef]
- Ochoa, E.; Torres, D.; Moreira, R.; Pinilla, J.L.; Suelves, I. Carbon nanofiber supported Mo2C catalysts for hydrodeoxygenation of guaiacol: The importance of the carburization process. Appl. Catal. B Environ. 2018, 239, 463–474. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Smith, K.J.; Kim, C.S. Hydrodeoxygenation of 2-methoxyphenol over Ru, Pd, and Mo2C catalysts supported on carbon. Energy Fuels 2017, 31, 6378–6388. [Google Scholar] [CrossRef]
- Ghampson, I.T.; Sepúlveda, C.; Garcia, R.; Radovic, L.R.; Fierro, J.L.G.; DeSisto, W.J.; Escalona, N. Hydrodeoxygenation of guaiacol over carbon-supported molybdenum nitride catalysts: Effects of nitriding methods and support properties. Appl. Catal. A Gen. 2012, 439–440, 111–124. [Google Scholar] [CrossRef]
- Ghampson, I.T.; Sepúlveda, C.; Garcia, R.; Frederick, B.G.; Wheeler, M.C.; Escalona, N.; DeSisto, W.J. Guaiacol transformation over unsupported molybdenum-based nitride catalysts. Appl. Catal. A Gen. 2012, 413–414, 78–84. [Google Scholar] [CrossRef]
- Gao, D.; Schweitzer, C.; Hwang, H.T.; Varma, A. Conversion of guaiacol on noble metal catalysts: Reaction performance and deactivation studies. Ind. Eng. Chem. Res. 2014, 53, 18658–18667. [Google Scholar] [CrossRef]
- Bouxin, F.P.; Zhang, X.; Kings, I.N.; Lee, A.F.; Simmons, M.J.H.; Wilson, K.; Jackson, S.D. Deactivation study of the hydrodeoxygenation of p-methylguaiacol over silica supported rhodium and platinum catalysts. Appl. Catal. A Gen. 2017, 539, 29–37. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Li, C.-L.; Wan, H.-P.; Lee, H.-T.; Liu, C.-F. Catalytic hydrodeoxygenation of guaiacol on Rh-based and sulfided CoMo and NiMo catalysts. Energy Fuels 2011, 25, 890–896. [Google Scholar] [CrossRef]
- Wu, S.-K.; Lai, P.-C.; Lin, Y.-C.; Wan, H.-P.; Lee, H.-T.; Chang, Y.-H. Atmospheric hydrodeoxygenation of guaiacol over alumina-, zirconia-, and silica-supported nickel phosphide catalysts. ACS Sustain. Chem. Eng. 2013, 1, 349–358. [Google Scholar] [CrossRef]
- Shu, R.; Lin, B.; Wang, C.; Zhang, J.; Cheng, Z.; Chen, Y. Upgrading phenolic compounds and bio-oil through hydrodeoxygenation using highly dispersed Pt/TiO2 catalyst. Fuel 2019, 239, 1083–1090. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, X.; Wang, H.; Han, J.; Ge, Q.; Zhu, X. Effect of strong metal-support interaction of Pt/TiO2 on hydrodeoxygenation of m-cresol. ChemistrySelect 2018, 3, 10364–10370. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, J.; Li, M.; Shan, Y.; He, M.; Song, C. TiO2-modified Pd/SiO2 for catalytic hydrodeoxygenation of guaiacol. Energy Fuels 2016, 30, 6671–6676. [Google Scholar] [CrossRef]
- He, Y.; Bie, Y.; Lehtonen, J.; Liu, R.; Cai, J. Hydrodeoxygenation of guaiacol as a model compound of lignin-derived pyrolysis bio-oil over zirconia-supported Rh catalyst: Process optimization and reaction kinetics. Fuel 2019, 239, 1015–1027. [Google Scholar] [CrossRef]
- Resende, K.A.; Teles, C.A.; Jacobs, G.; Davis, B.H.; Cronauer, D.C.; Jeremy Kropf, A.; Marshall, C.L.; Hori, C.E.; Noronha, F.B. Hydrodeoxygenation of phenol over zirconia supported Pd bimetallic catalysts. The effect of second metal on catalyst performance. Appl. Catal. B Environ. 2018, 232, 213–231. [Google Scholar] [CrossRef]
- Leiva, K.; Martinez, N.; Sepulveda, C.; García, R.; Jiménez, C.A.; Laurenti, D.; Vrinat, M.; Geantet, C.; Fierro, J.L.G.; Ghampson, I.T.; et al. Hydrodeoxygenation of 2-methoxyphenol over different Re active phases supported on SiO2 catalysts. Appl. Catal. A Gen. 2015, 490, 71–79. [Google Scholar] [CrossRef]
- Nie, L.; Resasco, D.E. Kinetics and mechanism of m-cresol hydrodeoxygenation on a Pt/SiO2 catalyst. J. Catal. 2014, 317, 22–29. [Google Scholar] [CrossRef]
- Shafaghat, H.; Rezaei, P.S.; Daud, W.M.A.W. Catalytic hydrodeoxygenation of simulated phenolic bio-oil to cycloalkanes and aromatic hydrocarbons over bifunctional metal/acid catalysts of Ni/HBeta, Fe/HBeta and NiFe/HBeta. J. Ind. Eng. Chem. 2016, 35, 268–276. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Baillie, B.P.; Augustine, E.; Hall, J.; Bollas, G.M.; Valla, J.A. Nickel impregnated mesoporous USY zeolites for hydrodeoxygenation of anisole. Microporous Mesoporous Mater. 2018, 261, 18–28. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Yu, M.J.; Ko, C.H.; Jeon, J.K.; Jae, J.; Park, S.H.; Jung, S.C.; Park, Y.K. Catalytic hydrodeoxygenation of bio-oil model compounds over Pt/HY catalyst. Sci. Rep. 2016, 6, 28765. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Park, R.-S.; Kim, H.; Park, S.H.; Jung, S.-C.; Jeon, J.-K.; Kim, S.C.; Park, Y.-K. Hydrodeoxygenation of guaiacol over Pt loaded zeolitic materials. J. Ind. Eng. Chem. 2016, 37, 18–21. [Google Scholar] [CrossRef]
- Hunns, J.A.; Arroyo, M.; Lee, A.F.; Escola, J.M.; Serrano, D.; Wilson, K. Hierarchical mesoporous Pd/ZSM-5 for the selective catalytic hydrodeoxygenation of m-cresol to methylcyclohexane. Catal. Sci. Technol. 2016, 6, 2560–2564. [Google Scholar] [CrossRef]
- Ohta, H.; Yamamoto, K.; Hayashi, M.; Hamasaka, G.; Uozumi, Y.; Watanabe, Y. Low temperature hydrodeoxygenation of phenols under ambient hydrogen pressure to form cyclohexanes catalysed by Pt nanoparticles supported on H-ZSM-5. Chem. Commun. 2015, 51, 17000–17003. [Google Scholar] [CrossRef] [PubMed]
- Roldugina, E.A.; Naranov, E.R.; Maximov, A.L.; Karakhanov, E.A. Hydrodeoxygenation of guaiacol as a model compound of bio-oil in methanol over mesoporous noble metal catalysts. Appl. Catal. A Gen. 2018, 553, 24–35. [Google Scholar] [CrossRef]
- Dongil, A.B.; Ghampson, I.T.; García, R.; Fierro, J.L.G.; Escalona, N. Hydrodeoxygenation of guaiacol over Ni/carbon catalysts: Effect of the support and Ni loading. RSC Adv. 2016, 6, 2611–2623. [Google Scholar] [CrossRef]
- Sun, J.; Karim, A.M.; Zhang, H.; Kovarik, L.; Li, X.S.; Hensley, A.J.; McEwen, J.-S.; Wang, Y. Carbon-supported bimetallic Pd–Fe catalysts for vapor-phase hydrodeoxygenation of guaiacol. J. Catal. 2013, 306, 47–57. [Google Scholar] [CrossRef]
- Fu, T.; Qi, R.; Wang, X.; Wan, W.; Li, Z. Facile synthesis of nano-sized hollow ZSM-5 zeolites with rich mesopores in shell. Microporous Mesoporous Mater. 2017, 250, 43–46. [Google Scholar] [CrossRef]
- Groen, J.C.; Bach, T.; Ziese, U.; Paulaime-van Donk, A.M.; de Jong, K.P.; Moulijn, J.A.; Pérez-Ramírez, J. Creation of hollow zeolite architectures by controlled desilication of Al-zoned ZSM-5 crystals. J. Am. Chem. Soc. 2005, 127, 10792–10793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hua, Z.; Liu, Z.; Li, Y.; Guo, L.; Bu, W.; Cui, X.; Ruan, M.; Chen, H.; Shi, J. Direct fabrication of mesoporous zeolite with a hollow capsular structure. Chem. Commun. 2009, 7578–7580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Y.; He, T.; Hu, H.; Wu, J. Hydrodeoxygenation of dibenzofuran over noble metal supported on mesoporous zeolite. Catal. Commun. 2011, 12, 1201–1205. [Google Scholar] [CrossRef]
- Wang, K.; Huang, X.; Li, D. Hollow ZSM-5 zeolite grass ball catalyst in methane dehydroaromatization: One-step synthesis and the exceptional catalytic performance. Appl. Catal. A Gen. 2018, 556, 10–19. [Google Scholar] [CrossRef]
- Dai, R.; Zheng, Z.; Sun, C.; Li, X.; Wang, S.; Wu, X.; An, X.; Xie, X. Pt nanoparticles encapsulated in a hollow zeolite microreactor as a highly active and stable catalyst for low-temperature ethanol steam reforming. Fuel 2018, 214, 88–97. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Y.; Zhang, J.; Wang, Y.; Gutierrez, O.Y.; Wang, S.; Li, Z.; Jin, P.; Wang, S.; Ma, X.; et al. A nitrogen-doped PtSn nanocatalyst supported on hollow silica spheres for acetic acid hydrogenation. Chem. Commun. 2018, 54, 8818–8821. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Zhang, T.; Wang, S.; Yao, P.; Feng, W.; Lin, Y.; Xu, J. Effect of TS-1 treatment by tetrapropyl ammonium hydroxide on cyclohexanone ammoximation. Catal. Commun. 2014, 50, 59–62. [Google Scholar] [CrossRef]
- Li, S.; Aquino, C.; Gueudré, L.; Tuel, A.; Schuurman, Y.; Farrusseng, D. Diffusion-driven selectivity in oxidation of CO in the presence of propylene using zeolite nano shell as membrane. ACS Catal. 2014, 4, 4299–4303. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, S.; Zhang, A.; Song, C.; Shi, C.; Guo, X. Hollow zeolite encapsulated Ni–Pt bimetals for sintering and coking resistant dry reforming of methane. J. Mater. Chem. A 2015, 3, 16461–16468. [Google Scholar] [CrossRef]
- Wang, Y.; Tuel, A. Nanoporous zeolite single crystals: ZSM-5 nanoboxes with uniform intracrystalline hollow structures. Microporous Mesoporous Mater. 2008, 113, 286–295. [Google Scholar] [CrossRef]
- Long, R.; Yang, R.T. Pt/MCM-41 catalyst for selective catalytic reduction of nitric oxide with hydrocarbons in the presence of excess oxygen. Catal. Lett. 1998, 52, 91–96. [Google Scholar] [CrossRef]
- Mohan, V.; Raghavendra, C.; Pramod, C.V.; Raju, B.D.; Rama Rao, K.S. Ni/H-ZSM-5 as a promising catalyst for vapour phase hydrogenation of levulinic acid at atmospheric pressure. RSC Adv. 2014, 4, 9660–9668. [Google Scholar] [CrossRef]
- Mei, C.; Liu, Z.; Wen, P.; Xie, Z.; Hua, W.; Gao, Z. Regular HZSM-5 microboxes prepared via a mild alkaline treatment. J. Mater. Chem. 2008, 18, 3496–3500. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. Developments and structures of mesopores in alkaline-treated ZSM-5 zeolites. Adsorption 2006, 12, 309–316. [Google Scholar] [CrossRef]
- Tao, H.; Yang, H.; Liu, X.; Ren, J.; Wang, Y.; Lu, G. Highly stable hierarchical ZSM-5 zeolite with intra- and inter-crystalline porous structures. Chem. Eng. J. 2013, 225, 686–694. [Google Scholar] [CrossRef]
- Abu Bakar, N.H.H.; Bettahar, M.M.; Abu Bakar, M.; Monteverdi, S.; Ismail, J.; Alnot, M. PtNi catalysts prepared via borohydride reduction for hydrogenation of benzene. J. Catal. 2009, 265, 63–71. [Google Scholar] [CrossRef]
- Raddi de Araujo, L.R.; Schmal, M. The calcination effects on Pt/HZSM-5 catalysts in the aromatization of propane. Appl. Catal. A Gen. 2000, 203, 275–284. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Zheng, X.; Meng, X.; Xiao, F.S. Importance of platinum particle size for complete oxidation of toluene over Pt/ZSM-5 catalysts. Chem. Commun. 2015, 51, 5936–5938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Wang, S. Hydrodeoxygenation of bio-oil over Pt-based supported catalysts: Importance of mesopores and acidity of the support to compounds with different oxygen contents. RSC Adv. 2013, 3, 12635–12640. [Google Scholar] [CrossRef]
- Lee, C.R.; Yoon, J.S.; Suh, Y.-W.; Choi, J.-W.; Ha, J.-M.; Suh, D.J.; Park, Y.-K. Catalytic roles of metals and supports on hydrodeoxygenation of lignin monomer guaiacol. Catal. Commun. 2012, 17, 54–58. [Google Scholar] [CrossRef]
- Shi, L.; Liu, G.; Guo, H. Efficient Pt/Silicalite-1 catalyst for isomerization of n-heptane. Catal. Commun. 2017, 101, 111–115. [Google Scholar] [CrossRef]
- Lanzafame, P.; Barbera, K.; Perathoner, S.; Centi, G.; Aloise, A.; Migliori, M.; Macario, A.; Nagy, J.B.; Giordano, G. The role of acid sites induced by defects in the etherification of HMF on Silicalite-1 catalysts. J. Catal. 2015, 330, 558–568. [Google Scholar] [CrossRef]
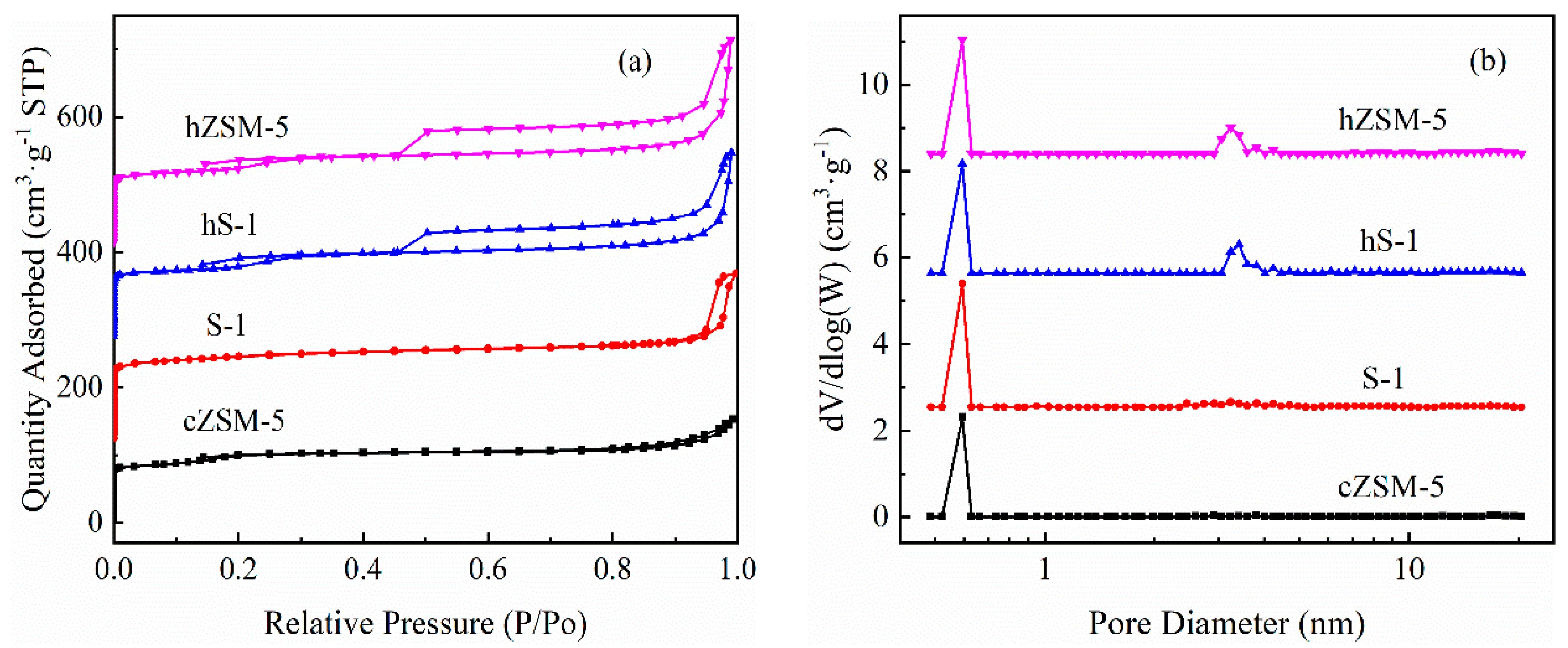
| Samples | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | ||||
|---|---|---|---|---|---|---|
| SBET a | Smicro. b | Sext. c | Vtotal d | Vmicro. b | Vmeso. e | |
| cZSM-5 | 332.04 | 218.34 | 113.70 | 0.1920 | 0.1366 | 0.0554 |
| S-1 | 408.85 | 289.98 | 118.87 | 0.2411 | 0.1421 | 0.0990 |
| hS-1 | 372.22 | 142.03 | 230.19 | 0.2483 | 0.0715 | 0.1768 |
| hZSM-5 | 386.96 | 157.88 | 229.08 | 0.2585 | 0.0804 | 0.1781 |
| Samples | Si/Al a | Peak Temperature (°C) | Amount of Acid Sites (μmol·g−1) | CO Uptake (μmol·g−1) | Dispersion b (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak I | Peak II | Peak III | Weak | Medium | Strong | Total | ||||
| Pt/cZSM-5 | 80 | 172 | 232 | 364 | 208.03 | 72.61 | 144.1 | 424.74 | 7.98 | 15.56 |
| Pt/S-1 | — | 147 | — | 329 | 32.44 | — | 19.15 | 51.59 | 6.79 | 13.24 |
| Pt/hS-1 | — | 150 | — | — | 7.08 | — | — | 7.08 | 14.01 | 27.32 |
| Pt/hZSM-5 | 175 | 177 | 285 | 380 | 128.37 | 43.8 | 87.75 | 259.93 | 17.36 | 33.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Feng, F.; Yuan, G.; Zhang, X.; Wang, Q. Hollow MFI Zeolite Supported Pt Catalysts for Highly Selective and Stable Hydrodeoxygenation of Guaiacol to Cycloalkanes. Nanomaterials 2019, 9, 362. https://doi.org/10.3390/nano9030362
Niu X, Feng F, Yuan G, Zhang X, Wang Q. Hollow MFI Zeolite Supported Pt Catalysts for Highly Selective and Stable Hydrodeoxygenation of Guaiacol to Cycloalkanes. Nanomaterials. 2019; 9(3):362. https://doi.org/10.3390/nano9030362
Chicago/Turabian StyleNiu, Xiaopo, Fuxiang Feng, Gang Yuan, Xiangwen Zhang, and Qingfa Wang. 2019. "Hollow MFI Zeolite Supported Pt Catalysts for Highly Selective and Stable Hydrodeoxygenation of Guaiacol to Cycloalkanes" Nanomaterials 9, no. 3: 362. https://doi.org/10.3390/nano9030362
APA StyleNiu, X., Feng, F., Yuan, G., Zhang, X., & Wang, Q. (2019). Hollow MFI Zeolite Supported Pt Catalysts for Highly Selective and Stable Hydrodeoxygenation of Guaiacol to Cycloalkanes. Nanomaterials, 9(3), 362. https://doi.org/10.3390/nano9030362